Abstract
Aluminum phosphide (AlP) poisoning had high morbidities and mortalities with absence of a standardized approach for the treatment. The present study investigated the efficiency of GIT decontamination methods and Coenzyme Q10(Co Q10) (Ubiquinone) in improving the outcome of acute AlP poisoning. A total of 90 patients were included and all patients received immediately supportive measures, then they distributed into three equal groups: In group I, gastric lavage was done using KMNO4 solution (1:10 000); group II received 250–500 ml liquid paraffin oil orally; group III received 300 mg of Co Q10 dissolved in liquid paraffin. Co Q10 was continued in a dose of 200 mg/day every 12 h. Follow-up blood pressure, arterial blood gases, serum troponin level and need for intubation revealed that the best improvement was in group III followed by group II. The percentage of survivors was 76.67% in group III and 70% of the patients had no residual effects. In group II, the survivors were 63.33%, and 36.67% of the cases discharged without sequelae. The survivors in group I constituted 26.67% and only 16.67% of the patients had no residual effects. GIT decontamination with aqueous solutions in acute AlP poisoning should be avoided. Rapid oral intake of any available oil as a prehospital treatment or immediately on hospital admission could critically improve the outcome of acute AlP poisoning. Besides, the addition of Co Q10 to the oil further improve patients’ prognosis. Highlights
Acute aluminum phosphide (AlP) poisoning is associated with high mortalities.
The appropriate method of GIT decontamination in acute AlP poisoning is controversy.
Conventional gastric lavage was associated with poor prognosis in acute AlP poisoning.
GIT decontamination using liquid paraffin oil improved outcome of acute AlP poisoning.
Coenzyme Q10 ameliorated AlP toxicity with improvement of cardiac functions.
Keywords: aluminum phosphide, coenzyme Q10, paraffin oil, rice table
Introduction
Aluminum phosphide (AlP) becomes a popular suicidal agent because of its high toxicity and availability. Therefore, the number of AlP fatalities is rising at an alarming rate in agricultural countries. This escalating trend is expected to continue in the lack of an effective treatment [1].
Aluminum phosphide is a phosphine-generating pesticide that is extensively used. It is considered an ideal pesticide because it is highly potent against a broad spectrum of insects, cost-effective, and leaves little residue on food grains [2].
After ingestion of AlP tablets or pellets, phosphine is rapidly liberated upon contact with gastric juice. Deadly phosphine gas is rapidly absorbed and exerts its cytotoxicity through disrupting mitochondrial function and free radical generation [3].
Clinically, acute AlP poisoning characterized by rapidly progressive cardiogenic shock along with severe metabolic acidosis. Proper supportive measures often fail to save patients’ lives. Therefore, management of acute AlP toxicity is one of the most frustrating and challenging tasks [4].
In addition to the basic supportive treatment, the physicians are trying to ameliorate AlP toxicity either by reducing phosphine liberation in the stomach or by alleviation of phosphine-induced cellular dysfunction [4, 5]. Antioxidants were also tried clinically to alleviate phosphine-induced oxidative stress such as N-acetyl cysteine (NAC) [6], vitamin C [7], vitamin E [8] and coenzyme Q10 [9].
Considering GIT decontamination, conventional gastric lavage in acute AlP is done using water or saline. Besides, potassium permanganate (KMNO4), charcoal or sodium bicarbonate (NaHCO3) could be added to these aqueous solutions [8, 10]. By reviewing recent literature, laboratory experiments demonstrated that AlP is highly water-soluble with an immediate release of phosphine, whereas the AlP tablet preserves its integrity in the oily medium [11]. Therefore, oils were suggested as alternatives to aqueous solutions in gastric decontamination in cases suffering from acute AlP poisoning. It was hypothesized that surrounding AlP with an oily medium might decrease phosphine liberation that could be associated with a better prognosis [12, 13].
Coenzyme Q10(Co Q10) (Ubiquinone) is an antioxidant that could increase energy production at the mitochondrial level in cardiomyocytes that enhance myocardial contractility [9]. Besides the absence of an effective antidote, there is no standardized approach for the treatment of acute AlP poisoning. The physicians follow their experiences rather than an evidence-based protocol. To the moment, the appropriate method of gastric decontamination in acute AlP poisoning is a matter of controversy [12, 13]. Also, the efficiency of Co Q10 in the alleviation of acute AlP poisoning needs further scientific verification. Therefore, the present study investigated the efficiency of GIT decontamination methods and Co Q10 in improving the outcome of acute AlP poisoning.
Subjects and Methods
The study included a total of 90 patients with acute AlP poisoning admitted to Alexandria Main University Hospital (AMUH) within the first 6 h after oral intake of firmly sealed AlP-containing products (Rice tablets). The diagnosis based on the history of the ingestion of AlP along with the presence of a poison label or bottle, the clinical picture of acute phosphine toxicity supported the diagnosis. The current research did not include patients inhaled AlP or ingested expired AlP tablets that were previously left in the open air. Also, those who received any treatment before admission or had chronic cardiorespiratory diseases were excluded from the study.
Before starting the study, ethical approval was obtained from the Research Ethics Committee of Faculty of Medicine, Alexandria University (IRB NO: 00012098, FWA NO: 00018699, Serial protocol NO: 0105627). This Ethics Committee is constituted and operates according to ICH GCP Guidelines and applicable local and institutional regulations and guidelines that govern the Ethics Committees operation. Informed consent was obtained from all patients or their relatives.
All AlP poisoned patients were subjected to the following [10, 14]: First, a detailed history taking (personal and medical, AlP exposure data). Second, thorough clinical assessment (glasgow coma scale (GCS), vital signs and general examination). Third, laboratory investigations that included: arterial blood gases (ABG), complete blood count, serum sodium and potassium levels, blood glucose level, liver functions (bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT)), renal functions (urea, creatinine, blood urea nitrogen (BUN)) and cardiac enzymes (CK-MB, troponin). Fourth, electrocardiogram (ECG) and electrocardiography. Fifth, calculation of simplified acute physiology score II (SAPS II): it consists of the assessment of 16 clinical and laboratory parameters and the inclusion of the worse t values of these parameters within the first 24 h [15].
The supportive measures were tailored according to the patient’s condition that includes: oxygen; fluid therapy according to central venous pressure; NaHCO3 infusion for the treatment of metabolic acidosis; vasopressor therapy in case of severe hypotension (SBP < 90 mmHg); inotrope therapy according to the echocardiographic examination and ejection fraction; endotracheal intubation and mechanical ventilation in case of refractory shock state or development of acute respiratory distress syndrome [14].
Regard gastric decontamination method and the use of antioxidant, the following three strategies were implemented:
Group I (potassium permanganate group): the gastric decontamination was done to the patients by repeated gastric wash through a gastric tube using KMNO4 solution (1:10 000) [10].
Group II (paraffin oil group): gastric decontamination was done by aspiration of gastric content through nasogastric tube whenever possible then 250–500 ml liquid paraffin oil was added to be left in the stomach or orally administered on those refusing insertion of a nasogastric tube. Liquid paraffin oil composed of saturated hydrocarbons obtained from petroleum [13].
Group III (paraffin and coenzyme Q10 group): besides gastric decontamination using liquid paraffin oil, the patients received 300 mg of Co Q10 as an antioxidant therapy after being dissolved in the paraffin oil [9, 13]. Then, Co Q10 was continued in a dose of 200 mg/day every 12 h [9]. The preparation used was puritan’s pride Q-SORB Co Q10 100 mg each soft gel capsule contains 100 mg Co Q 10 together with: rice bran oil, gelatin, vegetable glycerin, < 2% soy lecithin, titanium dioxide color [16].
By reviewing the recent literature, the potentially hazardous effects of using the aqueous-based solution in GIT decontamination in acute AlP poisoning were appreciated. Therefore, for ethical considerations, the data concerning the 30 patients belonging to group I were retrospectively collected from hospital records in the duration from the first of June 2017 till the end of May 2018 according to the availability of full data and considered as a historical control [17].
The patients with acute AlP poisoning admitted to the hospital in the duration from the first of June 2018 till the end of May 2019 were randomly distributed into groups II and III (each included 30 patients) [18].
Follow-up of all patients’ parameters during their hospital stay was done. Then, the outcome was assessed regarding: survival and mortality percentages in each group, presence of residual sequelae on discharge and duration of hospital and ICU stay.
Statistical methodology
The allocation sequence was generated using a permuted block randomization technique and the block size was variable. In this study, double-blinded approach was adopted. Masking/blinding was employed to participants and statisticians who were blinded to the group allocation of patients [18].
Data were collected in a designed sheet and analyses by SPSS (version 21). Kolmogorov–Smirnov test of normality was used. The normally distributed data were analyzed using Student’s t-test, paired t-test and one-way (ANOVA) test [19]. Chi-square χ2 test was used to analyze qualitative variables and Monte Carlo correction was carried out when indicated. Post-Hoc multiple comparisons and the pair-wise comparison were done using the Bonferroni method [20].
Results
The study included a total of 90 patients admitted to Alexandria Main University Hospital within 6 h following the ingestion of unexpired AlP-containing pesticides. The mean age of the patients was within the third decade of life. Nearly three-quarters (73.3%) of the patients were females and most of the cases (96.67%) were residents of rural areas. All patients received the basic life supportive measure on admission and during the period of hospitalization according to the progress of their conditions. The patients categorized according to the method of GIT decontamination and the use of Co Q10 into three equal groups.
On admission data (Table 1)
Table 1.
Base line data (on admission) of acute aluminum phosphide poisoned patients in the three treatment groups
| On admission data | Group I | Group II | Group III | Test of significance |
|---|---|---|---|---|
| Age (years) | 18–50 (23.67 ± 9.34) | 18–50 (22.83 ± 7.03) | 18–35 (21.47 ± 4.98) | F = 0.688 (P = 0.505) |
| Number of ingested tablets | 0.25–3.00 (0.97 ± 0.50) | 0.25–2.00 (0.83 ± 0.36) | 0.25–3.00 (1.15 ± 0.73) | F = 2.609 (P = 0.079) |
| Time till hospitalization (hours) | 1.00–4 (2.63a,b,c ± 0.93) | 1.00–6 (3.22a,b ± 1.30) | 0.50–5 (2.45a,c ± 1.05) | F = 4.154 (P = 0.019*) |
| Pulse (60–100 beats/min) | 70–200 (113.10 ± 28.77) | 50–150 (108.33 ± 24.26) | 20–130 (96.47 ± 31.27) | F = 2.758 (P = 0.069) |
| SBP (90–120 mmHg). | 60–150 (91.58 ± 18.34) | 50–140 (91.43 ± 24.14) | 70–120 (90.00 ± 12.33) | F = 0.052 (P = 0.949) |
| DBP (60–80 mmHg) | 30–80 (61.58 ± 12.59) | 20–90 (56.19 ± 17.46) | 30–80 (59.23 ± 12.30) | F = 0.726 (P = 0.488) |
| MBP (70–100 mmHg) | 40.00–103.33 (71.58 ± 13.81) | 30.00–106.67 (67.94 ± 19.05) | 43.33–93.33 (69.49 ± 11.88) | F = 0.294 (P = 0.746) |
| Respiratory rate (12–20 breath/min) | 12–40 (22.20 ± 7.02) | 12–40 (23.60 ± 8.23) | 10–40 (20.17 ± 5.62) | F = 1.805 (P = 0.171) |
| Temperature (36.1–37.2°C) | 35–37 (36.27 ± 0.69) | 35–38 (36.57 ± 0.77) | 35–38 (36.63 ± 0.76) | F = 2.066 (P = 0.133) |
| Glasgow Coma Scale (15) | 7–15 (12.93 ± 2.85) | 7–15 (13.40 ± 2.04) | 3–15 (12.97 ± 2.75) | F = 0.307 (P = 0.736) |
| pH (7.35–7.45) | 7.05–7.48 (7.31 ± 0.13) | 7.11–7.54 (7.37 ± 0.10) | 6.80–7.53 (7.37 ± 0.14) | F = 2.614 (P = 0.079) |
| PaCO₂ (35–45 mmHg). | 13–40 (25.27 ± 6.58) | 18–42 (27.87 ± 7.84) | 17–44 (26.97 ± 7.42) | F = 0.983 (P = 0.378) |
| PaO2(70–100 mmHg). | 23–134 (78.31 ± 31.54) | 7–461 (96.03 ± 84.00) | 12–161 (97.10 ± 38.83) | F = 1.049 (P = 0.355) |
| HCO₃(22–26 mEq/l) | 7.00–22.60 (13.65a ± 4.58) | 6.00–22.30 (16.03b.c ± 4.47) | 6.60–23.60 (16.50b.c ± 4.27) | F = 3.535 (P = 0.033*) |
| Oxygen saturation (90–95%) | 33–100 (90.97 ± 13.61) | 5–100 (80.60 ± 28.01) | 11–99 (89.83 ± 19.56) | F = 2.152 (P = 0.122) |
| Hemoglobin (12–15 g/dl) | 11.00–17.30 (12.83 ± 1.40a.b.c) | 9.40–16.00 (12.10 ± 1.27a.b) | 10.80–17.00 (13.09 ± 1.64a.c) | F = 3.828 (P = 0.026*) |
| RBCs (4.5–5.5 × 106 cell/ml) | 3.50–6.17 (4.44 ± 0.57) | 3.50–6.13 (4.48 ± 0.66) | 3.00–9.33 (4.63 ± 1.07) | F = 0.486 (P = 0.617) |
| WBCs (4–11 × 103 cell/ul) | 3.70–21.39 (11.21 ± 4.47) | 1.71–21.75 (10.01 ± 4.22) | 4.00–22.20 (9.30 ± 4.81) | F = 1.376 (P = 0.258) |
| Platelets (150–400 × 103 cell/ul) | 100.00–382.00 (239.23 ± 62.15) | 127.00–330.00 (219.60 ± 51.62) | 141.00–371.00 (236.40 ± 56.01) | F = 1.049 (P = 0.355) |
| Serum sodium (135–145 mmol/l) | 134–154 (142.10 ± 5.19) | 129–158 (140.93 ± 5.69) | 115–145 (139.00 ± 6.01) | F = 2.313 (P = 0.105) |
| Serum potassium (3.5–5.0 mmol/l) | 3.00–4.70 (3.69 ± 0.42) | 2.40–4.50 (3.65 ± 0.61) | 2.70–4.50 (3.60 ± 0.44) | F = 0.266 (P = 0.767) |
| Blood glucose level (70–130 mg/dl) | 66 –300 (146.90 ± 67.66) | 50–330 (137.77 ± 74.80) | 58–380 (127.33 ± 71.74) | F = 0.563 (P = 0.571) |
| Urea (15–45 mg/dl) | 5–60 (18.79 ± 10.42) | 6–51 (20.37 ± 11.11) | 9–62 (23.50 ± 13.97) | F = 1.210 (P = 0.303) |
| Creatinine (0.7–1.3 mg/dl) | 0.20–1.80 (0.93 ± 0.42) | 0.40–1.90 (0.93 ± 0.40) | 0.40–1.50 (0.86 ± 0.34) | F = 0.277 (P = 0.759) |
| BUN (7–20 mg/dl) | 5–31 (13.40 ± 5.21) | 3–28 (13.40 ± 6.26) | 5–29 (13.57 ± 6.05) | F = 0.008 (P = 0.992) |
| Bilirubin (0.1–1.2 mg/dl) | 0.20–4.20 (1.02 ± 0.72) | 0.40–2.50 (0.98 ± 0.48) | 0.30–3.10 (1.06 ± 0.58) | F = 0.132 (P = 0.876) |
| ALT (16–63 U/l) | 9–192 (36.07 ± 45.82) | 8–119 (29.97 ± 26.53) | 11–1000 (67.10 ± 181.72) | F = 0.996 (P = 0.373) |
| AST (15–37 U/l) | 9.00–280.00 (45.77 ± 61.93) | 14.00–167.00 (33.63 ± 28.53) | 13.00–3000 (143.47 ± 542.47) | F = 1.092 (P = 0.340) |
| Troponin (Up to 0.05 ng/ml) | 0.00–7.60 (1.28 ± 1.65) | 0.00–16.50 (2.13 ± 3.93) | 0.00–11.50 (1.11 ± 2.18) | F = 1.170 (P = 0.315) |
| CK–MB (Up to 5 ng/ml) | 0.00–39.00 (13.64 ± 10.88) | 0.00–31.00 (9.87 ± 7.78) | 0.00–30.00 (9.93 ± 8.96) | F = 1.618 (P = 0.204) |
| Abnormal ECG | 33.3% | 30.0% | 33.3% | χ2 = 9.74 (P = 0.484) |
| Ejection fraction (%) | 15.00–49.00 (26.91 ± 10.89) | 15.00–50.00 (33.20 ± 10.32) | 15.00–50.00 (35.44 ± 11.96) | F = 2.036 (P = 0.144) |
| Score points | 13–59 (37.23 ± 12.13) | 9–60 (31.53 ± 15.93) | 13–79 (32.10 ± 18.02) | F = 1.223 (P = 0.299) |
| SAPSII mortality rate (%) | 1.10–66.10 (25.26 ± 17.34) | 0.80–68.10 (19.99 ± 22.26) | 1.10–91.90 (20.82 ± 26.48) | F = 0.483 (P = 0.619) |
Bonferroni method: “a” for group I, “b” for group II and “c” for group III.
F-test (ANOVA).
χ2: Chi-square test.
*Statistically significant at P ≤ 0.05.
Normal ranges are between brackets.
The comparability among the three groups was ensured regarding their baseline data to guarantee statistical inference. On admission data belonging to the patients included: demographic data, data of exposure, clinical findings, laboratory and SAPS II score. The Bonferroni method was used in statistical analysis, superscript letters are assigned by default as “a” for group I, “b” for group II and “c” for group III.
The 34 parameters of the studied patients in the three treatment groups were compared on admission. No significant difference was observed among the groups in 31 parameters. However, the time till hospitalization was significantly longer in group II than group III. Hemoglobin level was higher in group III than group II. The bicarbonate level was significantly lower in group I than the two other groups.
Follow-up data
Blood pressure (Table 2)
Table 2.
Follow-up of the blood pressure among acute aluminum phosphide poisoned patients in the treatment groups (8 h interval for 24 h)
| Group I | Group II | Group III | Test of significance | |
|---|---|---|---|---|
| SBP (mmHg) | ||||
| On admission | ||||
| N | 19 | 21 | 26 | F = 0.052 |
| Minimum–Maximum (Mean) | 60–150 (91.58 ± 18.34) | 50–140 (91.43 ± 24.14) | 70–120 (90.00 ± 12.33) | (p = 0.949) |
| 8 h | ||||
| N | 18 | 21 | 26 | F = 2.966 |
| Minimum–Maximum (Mean) | 60–150 (92.22 ± 18.65) | 50–130 (100.48 ± 18.84) | 70–120 (88.85 ± 12.43) | (p = 0.059) |
| 16 h | ||||
| N | 24 | 23 | 27 | F = 6.041 |
| Minimum–Maximum (Mean) | 20.0–120 (76.3a ± 22.8) | 70–140 (93.9b,c ± 20.6) | 70.0–120.0 (93.3b,c ± 16.9) | P = 0.004* |
| 24 h | ||||
| N | 16 | 26 | 23 | F = 1.122 |
| Minimum–Maximum (Mean) | 60–130 (93.8 ± 17.5) | 70.0–150.0 (101.9 ± 19.8) | 70.0–140.0 (101.3 ± 17.1) | P = 0.332 |
| Test of significance | F = 1.968 | F = 2.600 | F = 4.807 | |
| P = 0.143 | P = 0.062 | P = 0.005* | ||
| DBP (mmHg) | ||||
| On admission | ||||
| N | 19 | 21 | 26 | F = 0.726 |
| Minimum–Maximum (Mean) | 30.00–80.00 (61.58 ± 12.59) | 20.00–90.00 (56.19 ± 17.46) | 30.00–80.00 (59.23 ± 12.30) | P = 0.488 |
| 8 h | ||||
| N | 18 | 21 | 26 | F = 2.715 |
| Minimum–Maximum (Mean) | 30.00–90.00 (61.11 ± 12.78) | 20.00–80.00 (64.76 ± 13.65) | 40.00–80.00 (56.54 ± 10.18) | P = 0.074 |
| 16 h | ||||
| N | 24 | 23 | 27 | F = 2.996 |
| Minimum–Maximum (Mean) | 30.0–80.0 (50.8 ± 15.3) | 30.0–90.0 (59.6 ± 15.8) | 40.0–80.0 (60.0 ± 13.3) | P = 0.056 |
| 24 h | ||||
| N | 16 | 26 | 23 | F = 2.825 |
| Minimum–Maximum (Mean) | 40.0–70.0 (58.1 ± 9.1) | 30.0–100.0 (65.4 ± 15.6) | 50.0–100.0 (68.7 ± 14.2) | P = 0.067 |
| Test of significance | F = 3.010 | F = 3.271 | F = 6.913 | |
| P = 0.048* | P = 0.028* | P = 0.001* | ||
| MBP (mmHg) | ||||
| On admission | ||||
| N | 19 | 21 | 26 | F = 0.294 |
| Minimum–Maximum (Mean) | 40.00–103.33 (71.58 ± 13.81) | 30.00–106.67 (67.94 ± 19.05) | 43.33–93.33 (69.49 ± 11.88) | P = 0.746 |
| 8 h | ||||
| N | 18 | 21 | 26 | F = 2.981 |
| Minimum–Maximum (Mean) | 40.00–110.00 (71.48 ± 14.38) | 30.00–96.67 (76.67 ± 15.02) | 50.00–93.33 (67.31 ± 10.11) | P = 0.058 |
| 16 h | ||||
| N | 24 | 23 | 27 | F = 4.706 |
| Minimum–Maximum (Mean) | 36.67–90.00 (59.31a ± 15.45) | 43.33–106.67 (71.01b,c ± 16.74) | 50.00–93.33 (71.11b,c ± 14.23) | P = 0.012* |
| 24 h | ||||
| N | 16 | 26 | 23 | F = 2.076 |
| Minimum–Maximum (Mean) | 46.67–90.00 (70.00 ± 11.16) | 43.33–113.33 (77.56 ± 16.72) | 56.67–113.33 (79.57 ± 14.92) | P = 0.134 |
| Test of significance | F = 3.084 | F = 3.200 | F = 6.482 | |
| P = 0.044* | P = 0.030* | P = 0.001* | ||
Bonferroni method: “a” for group I, “b” for group II and “c” for group III.
F-test (ANOVA).
*Statistically significant at P ≤ 0.05.
By comparing the systolic blood pressure (SBP) of the three groups during follow-up (at 8, 16 and 24 h), no statistically significant difference was present except at 16 h (P were 0.059, 0.004 and 0.332, respectively). There was a significant difference between groups I and II and also between groups I and III, nevertheless, no statistically significant difference was present between groups II and III.
Also, a statistically significant improvement of SBP was observed in group III (P value = 0.005). On the other hand, no significant improvement was noticed as regard SBP within the group I and group II (P values were 0.143. and 0.062, respectively).
Regarding follow-up diastolic blood pressure (DBP) over 24 h, no statistically significant difference among groups was present (P values were 0.074, 0.056, and 0.067, respectively). However, there was a statistically significant improvement of DBP within groups II, and III (P values were 0.028, and 0.001 respectively), whereas a significant deterioration was observed in DBP in group I (P = 0.048).
On comparing the mean blood pressure (MBP) in the three groups at 8, 16 and 24 h, there was no statistically significant difference except at 16 h (P value = 0.058, 0.012 and 0.134, respectively). There was also a statistically significant improvement of MBP within groups II, and III (P value = 0.030 and 0.001 respectively), whereas a significant deterioration was noticed in MBP in group I (P = 0.044).
Arterial blood gases (ABG) (Table 3)
Table 3.
Follow-up ABG of acute aluminum phosphide poisoned patients in the three treatment groups (8 h interval for 24 h)
| Group I | Group II | Group III | Test of significance | |
|---|---|---|---|---|
| pH | ||||
| On admission | ||||
| N | 30 | 30 | 30 | F = 2.614 |
| Minimum–Maximum (Mean) | P = 0.079 | |||
| 8 h | ||||
| N | 30 | 30 | 30 | F = 3.415 |
| Minimum–Maximum (Mean) | 7.05–7.52 (7.30a,b ± 0.13) | 6.90–7.54 (7.35a,b,c ± 0.13) | 7.04–7.50 (7.38b,c ± 0.11) | P = 0.037* |
| 16 h | ||||
| N | 29 | 29 | 29 | F = 9.835 |
| Minimum–Maximum (Mean) | 6.94–7.54 (7.27a,b ± 0.16) | 7.01–7.46 (7.33a,b,c ± 0.11) | 7.02–7.60 (7.42b,c ± 0.12) | P = 0.001* |
| 24 h | ||||
| N | 22 | 26 | 25 | F = 2.779 |
| Minimum–Maximum (Mean) | 6.90–7.54 (7.29 ± 0.17) | 7.00–7.60 (7.36 ± 0.15) | 7.02–7.60 (7.40 ± 0.15) | P = 0.069 |
| Test of significance | F = 0.772 | F = 0.389 | F = 1.493 | |
| P = 0.514 | P = 0.711 | P = 0.233 | ||
| PaCO2 | ||||
| On admission | ||||
| N | 30 | 30 | 30 | F = 0.983 |
| Minimum–Maximum (Mean) | 13.00–40.00 (25.27 ± 6.58) | 18.00–42.00 (27.87 ± 7.84) | 17.00–44.00 (26.97 ± 7.42) | P = 0.378 |
| 8 h | ||||
| N | 30 | 30 | 30 | F = 318 |
| Minimum–Maximum (Mean) | 14.00–44.00 (27.83 ± 7.55) | 9.00–42.00 (28.66 ± 8.35) | 14.00–45.00 (27.03 ± 7.73) | P = 0.728 |
| 16 h | ||||
| N | 29 | 29 | 29 | F = 1.107 |
| Minimum–Maximum (Mean) | 14.00–47.00 (27.10 ± 9.19) | 10.00–40.00 (30.17 ± 7.12) | 11.00–41.00 (29.21 ± 7.64) | P = 0.335 |
| 24 h | ||||
| N | 22 | 26 | 25 | F = 2.013 |
| Minimum–Maximum (Mean) | 14.00–42.00 (24.50 ± 7.02) | 8.70–42.00 (27.26 ± 9.69) | 18.00–50.00 (29.40 ± 7.93) | P = 0.141 |
| Test of significance | F = 1.547 | F = 1.502 | F = 1.788 | |
| P = 0.211 | P = 0.233 | P = 0.177 | ||
| HCO3 | ||||
| On admission | ||||
| N | 30 | 30 | 30 | F = 3.535 |
| Minimum–Maximum (Mean) | 7.00–22.60 (13.65a ± 4.58) | 6.00–22.30 (16.03b.c ± 4.47) | 6.60–23.60 (16.50b.c ± 4.27) | P = 0.033* |
| 8 h | ||||
| N | 30 | 30 | 30 | F = 2.092 |
| Minimum–Maximum (Mean) | 7.10–24.00 (14.57 ± 5.03) | 7.00–25.50 (16.55 ± 4.55) | 6.60–27.50 (16.98 ± 4.99) | P = 0.130 |
| 16 h | ||||
| N | 29 | 29 | 29 | F = 8.231 |
| Minimum–Maximum (Mean) | 4.00–27.40 (13.15a ± 5.58) | 8.60–27.00 (17.48b,c ± 4.67) | 5.00–28.50 (18.86b,c ± 6.40) | P = 0.01* |
| 24 h | ||||
| N | 22 | 26 | 25 | F = 5.056 |
| Minimum–Maximum (Mean) | 3.20–27.40 (13.45a ± 7.66) | 5.50–26.90 (18.39b,c ± 6.58) | 8.00–26.00 (19.08b,c ± 5.38) | P = 0.009* |
| Test of significance | F = 1.153 | F = 2.955 | F = 3.533 | |
| P = 0.320 | P = 0.038* | P = 0.030* | ||
Bonferroni method: “a” for group I, “b” for group II and “c” for group III.
F-test (ANOVA).
*Statistically significant at P ≤ 0.05.
pH: on comparing the follow-up pH in the three groups, a significant improvement was observed in group III over the group I at 8 h and 16 h (P = 0.037 and 0.001). Nevertheless, there was no statistically significant difference between either groups I and II or groups II and III at the same intervals. At 24 h, no significant difference was elicited among the three groups (P = 0.069). Besides, no significant improvement was noticed in pH within groups.
PaCO2: regarding PaCO2, there was no statistically significant difference among the three groups either at 8, 16 and 24 h follow-up with P values = 0.728, 0.335 and 0.141, respectively. Similarly, no significant difference was present as regard PaCO2 within each group.
Bicarbonate (HCO3): on comparing the follow-up of mean HCO₃ level in the three groups, no statistically significant difference among groups at 8 h could be observed (P value = 0.130). On the other hand, there was a significant improvement of both groups II and III over group I at 16 and 24 h (P = 0.01 and 0.009, respectively). Besides, there was a statistically significant improvement of HCO₃ level within group II (P = 0.038) and group III (P = 0.030) over the first 24 h that could not be observed within the group I during the first day after admission (P = 0.320).
Serum troponin level (Fig. 1)
Figure 1.
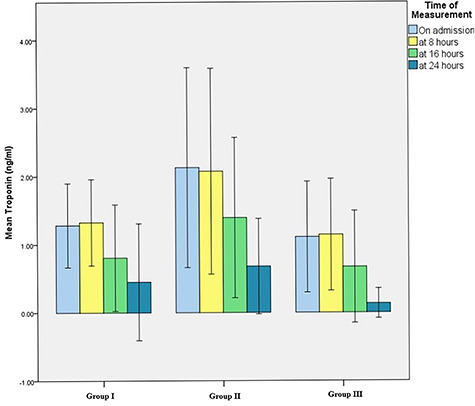
Serum troponin levels (on admission and 24 h follow-up) of acute aluminum phosphide poisoned patients in the three treatment groups.
The normal serum troponin level is up to 0.05 ng/ml. in the current work, there was no statistically significant difference among the three groups concerning troponin level either on admission or after 8, 16 and 24 h where P values were 0.315, 0.396, 0.501 and 0.277, respectively. Also, there was no statistically significant decrease in mean serum troponin levels within groups I and II (P = 0.566 and 0.082).
Concerning group III, despite the marked improvement in mean serum troponin level over the first 24 h (after 8 h from admission it was 1.14 ± 2.20 ng/ml, at 16 h it decreased to become 0.6716 ± 1.9005 ng/ml to reach 0.13 ± 0.45 ng/ml after 24 h. However, the change did not reach a statistically significant difference (P = 0.054).
Need for supportive measures (Table 4)
Table 4.
Supportive measures provided to acute aluminum phosphide poisoned patients in the three treatment groups
| Supportive measures | Group I | Group II | Group III | Test of significance |
|---|---|---|---|---|
| Fluids therapy | 18 (60.00%) | 16 (53.33%) | 14 (46.67%) | χ2 = 1.071 (P = 0.585) |
| NaHCO3 | 30 (100.00%) | 29 (96.67%) | 29 (96.67%) | χ2 = 1.023 (P = 1.000) |
| Vasopressors | 27 (90.00%) | 22 (73.33%) | 20 (66.67%) | χ2 = 4.845 (P = 0.089) |
| Inotrops | 16 (53.33%) | 6 (20.00%) | 2 (6.67%) | χ2 = 17.727 (P = 0.001*) |
χ2: Chi-square test.
*Statistically significant at P ≤ 0.05.
Considering the need for fluid therapy, 60% of group I (N = 18), 53.33% (N = 16) of group II and 46.67% (N = 14) of groups III received intravenous fluids according to their central venous pressure, the difference among groups was statistically insignificant (P = 0.585).
Sodium bicarbonate was administered parentally in 100% (N = 30) of group I and 96.67% (N = 29) of groups II and III to correct metabolic acidosis, the difference among groups was statistically not significant (P = 1.000).
Vasopressors were used in the management of severely hypotensive patients (SBP < 90 mmHg). In group I, 90% (N = 27) needed vasopressors, nevertheless, 73.33% (N = 22) of groups II and 66.67% (N = 20) of groups III received vasopressors. The difference among groups was statistically insignificant (P = 0.089).
Inotropes were administered to 53.33% (N = 16) of group I and 20% (N = 6) of groups II and 6.67% (N = 2) of groups III to enhance myocardial contractility, the difference among groups was statistically significant (P = 0.001). A pairwise comparison was used to elucidate the difference among groups. The number of patients that need inotropes in group I was significantly higher than those in group II (χ2 = 7.177 P = 0.001). Similarly, there was a significant difference between groups I and III regarding the need for inotropes (χ2 = 15.5, 56, P = 0.001). Nevertheless, there was no significant difference between groups II and III regarding the number of patients who received inotropes during their course of treatment (χ2 = 1.298, P = 0.255).
Need for intubation
Considering the need for intubation, 80% of group I (N = 24) were intubated, whereas 56.7% (N = 17) and 40% (N = 12) of groups II and III, respectively, were intubated, the difference among the groups was statistically significant (P = 0.007).
Outcome
Primary endpoint (mortality and survival percentages)
Table 5 revealed that the overall mortality among all patients was 44.44% (N = 40). More than half of the fatalities (55%) (N = 22) were recorded in group I, 27.5% (N = 11) was in group II while 17.5% (N = 7) was in group III. The cause of death in all fatalities in the current study was progressive cardiogenic and hypotensive shock.
Table 5.
Outcome of acute aluminum phosphide poisoning in the three treatment groups
| Outcome | Group I | Group II | Group III | Total |
|---|---|---|---|---|
| Survived without sequelae | ||||
| N | 5 | 11 | 21 | 37 |
| Percent within outcome | 13.51 | 29.73 | 56.76 | 100.00 |
| Percent within group | 16.67 | 36.67 | 70.00 | 41.11 |
| Survived with sequelae | ||||
| N | 3 | 8 | 2 | 13 |
| Percent within outcome | 23.08 | 61.54 | 15.38 | 100.00 |
| Percent within group | 10.00 | 26.67 | 6.67 | 14.44 |
| Died | ||||
| N | 22 | 11 | 7 | 40 |
| Percent within outcome | 55.00 | 27.50 | 17.50 | 100.00 |
| Percent within group | 73.33 | 36.67 | 23.33 | 44.44 |
| Total number | 30 | 30 | 30 | 90 |
| Test of significance | χ2 = 24.414 P = 0.001* | |||
χ2: Chi-square test.
*Statistically significant at P ≤ 0.05.
By analyzing the mortality percentage within each group, nearly three-quarters (73.33%) of cases in group I died, one-third (36.67%) in group II and less than one-quarter (23.33%) in group III had a fatal outcome (Fig. 2). There was a highly statistically significant difference among the three groups regarding the outcome of acute AlP poisoning (P = 0.001).
Figure 2.
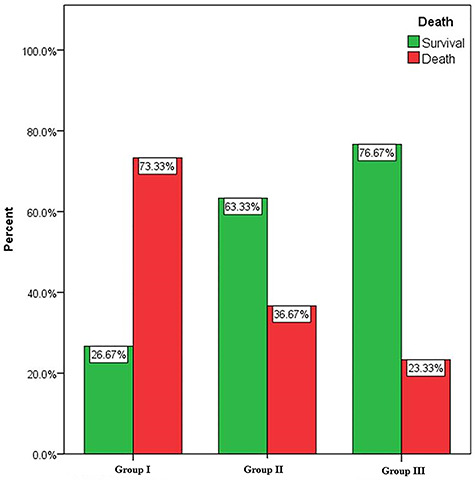
Survival and mortality percentages of acute aluminum phosphide poisoned patients in the three treatment groups.
Secondary endpoint
Residual sequelae among survivors (Table 5). More than one-third (41.11%) (N = 37) of all studied patients survived without residual toxic effects. Regarding completely cured patients, more than half of them (56.76%) (N = 21) were belonging to group III, 29.73% (N = 11) was from group II and 13.51% (N = 5) was from group I.
By analysis of the outcome within each group, nearly three-quarters (70%) of group III survived without sequelae. Whereas, 36.67% of group II and 16.67% of group I were discharged without residual effects. The residual toxic effects among AlP poisoning survivors could be cardiac, hepatic, or renal impairment.
Hospital and ICU stay duration
As regards the duration of hospital stay for all patients, the mean values were 31.9 ± 33.9, 77.4 ± 84.7 and 78.3 ± 80.4 h in groups I, II and III, respectively. There was a statistically significant difference between groups I and II and between groups I and III (P = 0.017).
All patients in the current study were admitted to ICU except for two patients in group II. Considering ICU stay duration, the mean values were 24.7 ± 22.5, 51.0 ± 62.7 and 49.9 ± 56.8 h in groups I, II and III, respectively. There was a statistically significant difference between groups I and II and between groups I and III, with P value = 0.021.
Discussion
In the last few years, the number of AlP fatalities had dramatically increased in agricultural countries in the absence of an effective antidote to the moment. Thus, different researches aimed to ameliorate aluminum phosphide toxicity either through GIT decontamination to decrease the liberated phosphine or counteracting the lethal effects of absorbed phosphine [8, 21].
Theoretically, AlP is highly water-soluble and phosphine gas is rapidly liberated upon its presence in aqueous solution. Also, many in vitro studies demonstrated the rapid dissolution of rice tablets in aqueous solutions but on the other hand, they preserve their integrity in an oily medium [11]. Therefore, oils were proposed as alternatives in gastric decontamination instead of using a water-based solutions in gastric lavage. It was postulated that the presence of AlP within an oily medium limits the liberation of phosphine with subsequent better prognosis. Therefore, the administration of oil was recommended by some researchers and physicians [12, 13].
According to clinical experience in AMUH, it was noticed that patients who dissolve rice tablets in water before the deliberate intake might present with less severe manifestation than swallowing the tablet with water that is associated with rapid fatal intoxication. Despite the various studies conducted on AlP, the proper method of GIT decontamination in acute AlP poisoning is still a matter of debate. Moreover, the potential benefits or hazards of each method and its possible association with the patient outcome are not scientifically verified.
Thus, the current study evaluated the efficiency of aqueous versus oily solutions in GIT decontamination following oral intake of AlP. Besides, the potential effect of Co Q10 in improving the outcome of acute AlP poisoning was investigated.
Ninety patients with acute AlP poisoning were included in this study. All patients were properly assessed and received supportive care. According to the method of GIT decontamination and the use of Co Q10 the patients categorized into three equal groups (each included 30 patients). In group I, gastric lavage was done using water or saline together with KMNO4 solution. Because of ethical considerations, the data of patients belonging to the group I retrieved from hospital records and considered as a historical control group. Only cases with complete clinical and laboratory data were included in the current study. The other 60 AlP poisoned patients were prospectively included and they were randomly distributed into groups II and III.
The validity of historical control in clinical studies is well-established for years [17, 22]. Often such retrospective data are of choice when the current treatment carries potential benefits to the participants or researchers hypothesize hazardous effects of the conventional regimen. The inherited limitation of the inclusion of historical control with clinical studies is selection bias. Therefore, in the current work, such limitation was considered to ensure statistical inference [17, 22].
For statistical validation of the current results, the selection bias was avoided through two ways: first, those patients who were included in group I as a historical control has complete medical data with consideration of the same inclusion and exclusion criteria; second, the comparability among the groups was ensured regarding their baseline data on admission. The similarity of the baseline characteristics of patients in three groups allow the success of randomization and eliminate selection bias. When important on admission parameters are matched, any difference in outcome among groups was likely a real effect of management strategies.
Considering the statistical analysis of the baseline data in the present study, 31 out of 34 parameters were matched with no statistically significant difference among the three studied groups. The three parameters that had a statistically significant difference among groups were the duration lapsed till hospitalization, serum hemoglobin, and bicarbonate levels. However, the SAPS II score had no significant difference among the studied groups that could justify initial similarity in the severity of the cases included in the studied groups. It is worth mentioning that SAPS II score included the evaluation of 16 clinical and laboratory parameters that were assessed by obtaining the worst value within the first 24 h. Thus, SAPS II score is a valuable tool in the assessment of critical cases and it is of great reliability in the evaluation of acute AlP poisoned patients as mentioned by Abd Elghany et al. [15].
In the current study, aluminum phosphide was intentionally ingested in 94.4% of the cases and females constituted 73% of all studied patients. Similarly, females constituted 92% of the AlP poisoned patients in the studies conducted by Saha et al. [23]. Female dominance in cases of acute AlP poisoning could be explained by the tendency of females for self-poisoning at such a young age for actual suicidal intent or just to gain sympathy as mentioned by Khan et al. [24].
The number of ingested tablets in all cases ranged from 0.25 to 3 tablets with no significant difference regarding the mean number of ingested tablets among the three studied groups. The amount taken is considered slightly less than that recorded by Soltaninejad et al. [25] who reported that the range of ingested AlP tablets was 0.5–4 tablets. Regarding time passed since exposure, it ranged from 0.5 to 6 h for all studied cases. All cases that arrived after 6 h were excluded from the study that was in concordance with Halvae et al. [8].
Considering the level of consciousness, GCS revealed that most of the studied patients were conscious at the time of admission. However, the conscious level deteriorated later along with the progress of profound shock state and brain hypoxia that was in agreement with Louriz et al. [26].
Tachypnea has been observed in some patients either as a compensatory mechanism for metabolic acidosis or due to the development of pulmonary edema that was in concordance with Ghassemi Toussi [27].
The cardiotoxic effects of AlP were evident in the current study, such as hypotension, arrhythmias, decreased ejection fraction and elevated cardiac enzymes. Profound hypotension could be due to phosphine-induced cardiogenic shock. Besides, the hypovolemic shock could occur as a result of the extensive vascular wall insufficiency and intravascular fluid loss. Arrhythmias, decreased ejection fraction and elevated cardiac enzymes point to the direct toxic effects of phosphine on the myocardium [28, 29].
Considering acid-base status, severe metabolic acidosis was obvious in most of the AlP poisoned cases due to phosphine-induced cytotoxicity. Also, marked hypotension with subsequent poor tissue perfusion leads to more acidosis. Unfortunately, metabolic acidosis might aggravate cardiac impairment that might introduce the cases into a vicious circle that ended only by patient death [30].
The outcome results were promising, the best outcome was among AlP poisoned patients who received Co Q10 along with liquid paraffin oil (group III), followed by those who received paraffin oil only (group II). Nevertheless, the worst prognosis was in those who had conventional gastric lavage using an aqueous solution (group I).
Interestingly, in group III, the survivors constituted 76.67% and 70% of patients in this group had no residual sequelae. However, in group II, the survivors constituted 63.33%, and 36.67% discharged without sequelae. Whereas in group I, the survivors constituted 26.67% and only 16.67% had no residual effects.
Out of 90 patients, 44 cases died that constituted 40% of the studied patients. However, more than half of the fatalities (55%) were in group I. The CVS collapse was responsible for all AlP fatalities recorded in the present study that was in agreement with Mehrpour and Gurjar [31] and Sheta et al. [10]. Therefore, the improvement of CVS functions could be responsible for the favorable outcomes in patients belonging to groups II and III.
The duration of hospital stay in the current study was significantly longer in group III followed by group II and the shortest duration was in the group I and that could be explained by the higher percentage of survivors in groups II and III while most of the cases in group I died a short time after admission. Similarly, the duration of ICU stay was longer in groups II and III than in group I due to the higher number of deaths in this group.
The follow-up in the current study revealed that the better recovery in CVS functions and amelioration of metabolic acidosis was better in group III followed by group II, nevertheless, deterioration was noticed in group I.
SBP significantly increased in group III (P = 0.005), a noticeably improved in group II (P = 0.062) but not improved in group I (0.143) when compared at baseline and for 24 h (8 h interval) following treatment. Follow-up of DBP revealed significant increase in DBP in groups II and III; the highest significance was in group III (P = 0.001) followed by group II (P = 0.028) , whereas a significant deterioration was observed in DBP in group I (P = 0.048). Similarly, MBP significantly improved in groups II and III and deteriorated in group I during 24 h follow up.
The serum troponin level was used in the current study as a cardio-specific marker for the follow-up of cardiac function. Soltaninejad et al. [25] confirmed that serum cardiac troponin is a reliable indicator of myocardial injury in acute AlP poisoning. During the follow-up, the serum troponin level decreased in the three treatment groups. This supports that myocardial injury following acute AlP poisoning could be reversible in response to treatment. The marked improvement in troponin level was noticed in group III that may reflect the potential therapeutic value of Co Q10 in the alleviation of AlP-induced cardiotoxicity.
Other than the CVS parameters, ABG were regularly assessed at 8 h interval for 24 h. Metabolic acidosis was significantly ameliorated. pH had a significant improvement between treatment groups I and III at 8 h and 16 h. However, at 24 h it was noticed that the mean pH was 7.4 in group III which was better than that in groups II and I (7.36 and 7.29, respectively) that was in concordance with Halvae et al. (2017) who reported an improvement of metabolic acidosis following the use antioxidant in treatment of acute AlP poisoning.
Regarding bicarbonate, there was significant improvement within groups II and III over 24 h follow-up (8 h interval) whereas no significant improvement in group I was noticed. The significant difference among groups on baseline bicarbonate challenges the comparability. Abdel hady et al. [32] also reported increase bicarbonate levels in patients receiving N-acetylcysteine.
Endotracheal intubation was indicated in AlP poisoning due to profound acidosis, massive myocardial suppression, pulmonary edema, aspiration or disturbed sensorium [26, 27]. Therefore, the high percentage (80%) of intubated patients in group I could point to the deterioration of the general condition of the patients in this group. In groups II and III, 56.7% and 40% of patients, respectively, were intubated that might denote the relatively better outcome in these groups.
In view of the current results, it is obvious that replacing the aqueous solution with liquid paraffin oil for GIT decontamination succeeded to increase the percentage of the survivors and improve the quality of their lives. Moreover, the addition of Co Q10 to paraffin oil further improved the patients’ prognosis.
The present work clarified that conventional gastric lavage using aqueous solutions in acute AlP was associated with high fatality. Therefore, the current results provide solid evidence against the use of the water-based solution in GIT decontamination in cases of acute AlP poisoning. The liquid paraffin oil is proposed as an alternative to conventional gastric lavage using an aqueous solution. It is hypothesized that paraffin oil minimizes the phosphine liberation by preventing the reaction of AlP with water and gastric acid and decreases phosphine absorption from gastric mucosa. Besides, paraffin oil enhances peristalsis thus fastens phosphides excretion from GIT [13, 14].
Many studies used different kinds of oils in gastric decontamination for cases of AlP poisoning, such as coconut, castor, sunflower and olive oil [12–14]. It seems that researchers tend to utilize available oil in their communities; however, some oils have additional antioxidant properties, such as olive and coconut oils [12–14]. In the current study, paraffin oil was used for gastric decontamination because it is readily available as a pharmaceutical preparation in Egypt.
The antioxidants were proposed as promising agents to alleviate phosphine-induced oxidative stress. Coenzyme Q10 might be an antioxidant of choice in the management of acute phosphine toxicity. Besides the antioxidant properties of Co Q10, it could revive cellular energy production and selectively improve cardiac systolic function. Marashi et al. [9] published a review article and they highly recommend the administration of Co Q10 as an antioxidant in AlP poisoning.
Clinical studies implemented various antioxidants along with different GIT decontamination methods in the management of AlP poisoning, as shown in Table 6. However, variation in sample size and initial poisoning severity greatly challenge the comparability among different researches. Nevertheless, the results of other studies might point to the use of antioxidants as beneficial remedies in acute AlP poisoning management that is in agreement with the current study.
Table 6.
Percentage of deaths from acute aluminum phosphide poisoning among different clinical studies
| Linical study | Sample size | GIT decontamination | Antioxidant therapy | Deaths (%) |
|---|---|---|---|---|
| Tehrani et al. [33] | 15 Control group | NaHCO3+ KMNO4 + Charcoal (water-based solution) | – | 60 |
| 22 Treatment group | NaHCO3+ KMNO4 + Charcoal (water-based solution) | NAC | 36 | |
| El-Ebiary and aboelfadl [21] | 15 Control group | NaHCO3+ Charcoal (water-based solution) | – | 80 |
| 15 Treatment group | NaHCO3+ Charcoal | NAC | 33 | |
| Halvae et al. [8] | 16 Control group | NaHCO3+ KMNO4 + Charcoal (water-based solution) | – | 50 |
| 20 Treatment group | NaHCO3+ KMNO4 + Charcoal (water-based solution) | Vitamin E | 15 | |
| Elgazzar et al. [34] | 25 Control group | Charcoal (water-based solution) | – | 80 |
| 25 Treatment group | Charcoal (water-based solution) | L-carnitine | 60 | |
| Current study (2020) | 30 Group I | KMNO4 (water-based solution) | – | 73.33 |
| 30 Group II | Paraffin oil (oily solution) | – | 36.67 | |
| 30 Group III | Paraffin oil (oily solution) | Coenzyme Q10 | 23.33 |
Conclusion
The conventional gastric lavage that was used in the management of AlP poisoning in group I was associated with poor prognosis. Therefore, GIT decontamination with aqueous solutions should be avoided whenever AlP poisoning is suspected. There was a significant improvement in the prognosis of the patients belonging to group II who received liquid paraffin oil as an alternative GIT decontamination method. The addition of Co Q10 to paraffin oil in group III further improves acute AlP poisoning outcome. Thus, rapid oral intake of any available oil as a prehospital treatment or immediately on hospital admission could critically improve the outcome. Besides, the addition of Co Q10 to the oil further improve patients’ prognosis.
Supplementary Material
Acknowledgments
No acknowledgments.
Conflict of interest statement
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1. Farzaneh E, Ghobadi H, Akbarifard Met al. Prognostic factors in acute aluminium phosphide poisoning: a risk-prediction nomogram approach. Basic Clin Pharmacol Toxicol 2018;123:347–55. doi: 10.1111/bcpt.13005. [DOI] [PubMed] [Google Scholar]
- 2. Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol 2012;63:61–73. doi: 10.2478/10004-1254-63-2012-2182. [DOI] [PubMed] [Google Scholar]
- 3. Berry A, Singh G, Kaur Set al. Aluminium phosphide: toxicity mechanism and credible treatments. World J Pharm Pharmaceutical Sci 2015;4:2277–93. doi: 10.4103/0974-2700.83868. [DOI] [Google Scholar]
- 4. Mehrpour O, Amouzeshi A, Dadpour Bet al. Successful treatment of cardiogenic shock with an intraaortic balloon pump following aluminium phosphide poisoning. Arch Ind Hyg Toxicol 2014;65:121–7. doi: 10.2478/10004-1254-65-2014-2393. [DOI] [PubMed] [Google Scholar]
- 5. Singh Y, Joshi SC, Satyawali Vet al. Acute aluminium phosphide poisoning, what is new? Egypt J Intern Med 2014;26:99–103. doi: 10.4103/1110-7782.145298. [DOI] [Google Scholar]
- 6. Nakhaee S, Mehrpour O, Balali-Mood M. Does N-acetyl cysteine have protective effects in acute aluminum phosphide poisoning? Indian J Crit Care Med 2017;21:539–40. doi: 10.4103/ijccm.IJCCM_223_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gheshlaghi F, Lavasanijou MR, Moghaddam NAet al. N-acetylcysteine, ascorbic acid, and methylene blue for the treatment of aluminium phosphide poisoning: still beneficial? Toxicol Int 2015;22:40–4. doi: 10.4103/0971-6580.172255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halvaei Z, Tehrani H, Soltaninejad Ket al. Vitamin E as a novel therapy in the treatment of acute aluminum phosphide poisoning. Turk J Med Sci 2017;47:795–800. doi: 10.3906/sag-1512-6. [DOI] [PubMed] [Google Scholar]
- 9. Marashi SM, Majidi M, Sadeghian Met al. Protective role of coenzyme Q10 as a means of alleviating the toxicity of aluminum phosphide: an evidence-based review. Tzu Chi Med J 2015;27:7–9. doi: 10.1016/j.tcmj.2014.12.002. [DOI] [Google Scholar]
- 10. Sheta A, El-Banna A, Abd-Elmeguid Ret al. A study of the predictive factors of mortality in acute poisoning with aluminum phosphide with special reference to echocardiography and SOFA score. ESPR 2019;26:33135–45. doi: 10.1007/s11356-019-06457-4. [DOI] [PubMed] [Google Scholar]
- 11. Sanaei-Zadeh H, Marashi SM. Gastric decontamination in aluminium phosphide poisoning: a case against the use of water-based solutions. Arh Hig Rada Toksikol 2016;67:339–40. doi: 10.1515/aiht-2016-67-2900. [DOI] [PubMed] [Google Scholar]
- 12. Shadnia S, Rahimi M, Pajoumand Aet al. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol 2005;24:215–8. doi: 10.4103/0972-5229.151019. [DOI] [PubMed] [Google Scholar]
- 13. Farahani MV, Saroosh D, Marashi SM. Thoughts on the current management of acute aluminum phosphide toxicity and proposals for therapy: an evidence based review. Indian J Crit Care Med 2016;20:724–30. doi: 10.4103/0972-5229.195712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agrawal VK, Bansal A, Singh RKet al. Aluminum phosphide poisoning: possible role of supportive measures in the absence of specific antidote. Indian J Crit Care Med 2015;19:109–12. doi: 10.4103/0972-5229.151019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abd Elghany SA, Heshmat MM, Oreby Met al. Evaluation of various scoring systems in prediction of acute aluminum phosphide (ALP) poisoning outcome. Ain Shams J Forensic Med Clin Toxicol 2018;30:117–27. doi: 10.21608/AJFM.2018.18202. [DOI] [Google Scholar]
- 16. Lia H, Chen F. Preparation and quality evaluation of coenzyme Q10 long-circulating liposomes. Saudi J Biol Sci 2017;24:797–802. doi: 10.1016/j.sjbs.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viele K, Berry S, Neuenschwander Bet al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat 2014;13:41–54. doi: 10.1002/pst.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz K, Grimes D. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359:515–9. doi: 10.1016/S0140-6736(02)07683-3 [DOI] [PubMed] [Google Scholar]
- 19. Wahid Z, Latiff A, Ahmad K. Application of one-way ANOVA in completely randomized experiments. J Phys: Conf Series 2017. doi: 10.1088/1742-6596/949/1/012017. [DOI] [Google Scholar]
- 20. Ranstam J. Hypothesis-generating and confirmatory studies, Bonferroni correction, and pre-specification of trial endpoints. Acta Orthop 2019;90:297. doi: 10.1080/17453674.2019.1612624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El-Ebiary A, Abuelfad A. N-acetylcysteine as an adjuvant in the treatment of acute aluminum phosphide poisoning: a randomized clinical trial. Ain Shams J Forensic Med Clin Toxicol 2017;28:38–46. doi: 10.21608/AJFM.2017.18278. [DOI] [Google Scholar]
- 22. Pocock S. The combination of randomized and historical controls in clinical trials. J Chronic Dis 1976;29:175–88. doi: 10.1016/0021-9681(76)90044-8. [DOI] [PubMed] [Google Scholar]
- 23. Saha J, Azad K, Hossain Met al. Aluminium phosphide poisoning cases in a tertiary care hospital. J Dhaka Med Coll 2014;23:3–6. [Google Scholar]
- 24. Khan M, Khurram M, Raza S. Gender based differences in patients of poisoning managed at a medical unit. J Pak Med Assoc 2019;69:1025–8. [PubMed] [Google Scholar]
- 25. Soltaninejad K, Beyranvand MR, Momenzadeh Set al. Electrocardiographic findings and cardiac manifestations in acute aluminum phosphide poisoning. J Forensic Leg Med 2012;19:291–3. doi: 10.1016/j.jflm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 26. Louriz M, Dendane T, Abidi Ket al. Prognostic factors of acute aluminum phosphide poisoning. Indian J Med Sci 2009;63:227–34. doi: 10.4103/0019-5359.53386. [DOI] [PubMed] [Google Scholar]
- 27. Ghassemi Toussi A. Successful treatment of aluminum phosphide; is it possible? Asia Pac J Med Toxicol 2017;6:92–8. [Google Scholar]
- 28. Kalawat S, Thakur V, Thakur Aet al. Cardiovascular profile of aluminium phosphide poisoning and its clinical significance. Int J Adv Med 2016;3:859–64. doi: 10.18203/2349-3933.ijam20163505. [DOI] [Google Scholar]
- 29. Eshraghi A, Rajaei N, Mood MBet al. Changes of QT dispersion in patients suffering from aluminium phosphide poisoning (rice pill). Open Access Maced J Med Sci 2019;7:2251–5. doi: 10.3889/oamjms.2019.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaiswal S, Verma R, Tewari N. Aluminum phosphide poisoning: effect of correction of severe metabolic acidosis on patient outcome. Indian J Crit Care Med 2009;13:21–4. doi: 10.4103/0972-5229.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehrpour O, Gurjar M. Cardiogenic shock: the main cause of mortality in acute aluminum phosphide poisoning. Indian J Crit Care Med 2017;4:246–7. doi: 10.4103/ijccm.IJCCM_97_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdel-Hady R, Mohamed A, Mohammed M. Supportive measures in the treatment of aluminum phosphide poisoning as a trial to reduce mortality at Assiut university hospital, Egypt. AJFMFS 2019;1:1210–22. doi: 10.26735/16586794.2019.008. [DOI] [Google Scholar]
- 33. Tehrani H, Halvaie Z, Shadnia Set al. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clin Toxicol 2013;51:23–8. doi: 10.3109/15563650.2012.743029. [DOI] [PubMed] [Google Scholar]
- 34. Elgazzar F, Keshk W, Khalifa H. Early L-carnitine therapy in severe acute aluminum phosphide poisoning: a randomized controlled clinical trial. Egypt J Forensic Sci Appli Toxicol 2019;19:147–64. doi: 10.21608/EJFSAT.2020.17184.1097. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


