Clinical Implications.
-
•
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive patients with inborn errors of immunity (IEI) showed a similar infection-fatality rate, a lower incidence in pediatric age, and a younger age at death than the SARS-CoV-2–positive Italian population. The fatality rate was lower than previously reported from other IEI cohorts. Antibody deficiencies showed a long-lasting SARS-CoV-2 positivity.
Early reports described an unexpected low number of patients affected by inborn errors of immunity (IEI) with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, the incidence and mortality rates in IEI are still a matter of speculation, and a detailed figure is lacking because cohorts of patients with IEI were not compared with the general population in a given country.1 , 2 Because of the high burden of coronavirus disease 2019 (COVID-19) in Italy, we evaluated the impact of the pandemic on patients with IEI enrolled by 21 centers in the IPINet national registry (www.ipinet.org)3 with the aim to assess SARS-CoV-2 incidence and infection-fatality rate in different IEI entities in a cohort of 3263 adult and pediatric patients for which we have the exact figure available thanks to the Italian registry for each nosological entity, to quantify the length of time of SARS-CoV-2 positivity, and to verify whether a condition of lymphopenia might be a possible predictor of COVID-19 outcome. All data were compared with the data of the SARS-CoV-2–positive Italian population.
Patients with IEI diagnosed according to the European Society for Primary Immune Deficiencies criteria were considered SARS-CoV-2 positive if confirmed by PCR. The PCR test was repeatedly administered in each patient, according to the rule to test for SARS-CoV-2 every time a patient is attending a hospital site. In SARS-CoV-2–positive patients, PCR test was administered every 10 days until the result was negative. The cumulative incidence, and infection-fatality rate, was calculated by age and by diagnosis. We used the Italian National Istutute of Health report on the SARS-CoV-2 pandemic in Italy to obtain national estimates, and we compared data by Student t test for continuous variables by STATA 10 (Stata-Corp, College Station, Tex). A P value of less than .05 indicates statistical significance.
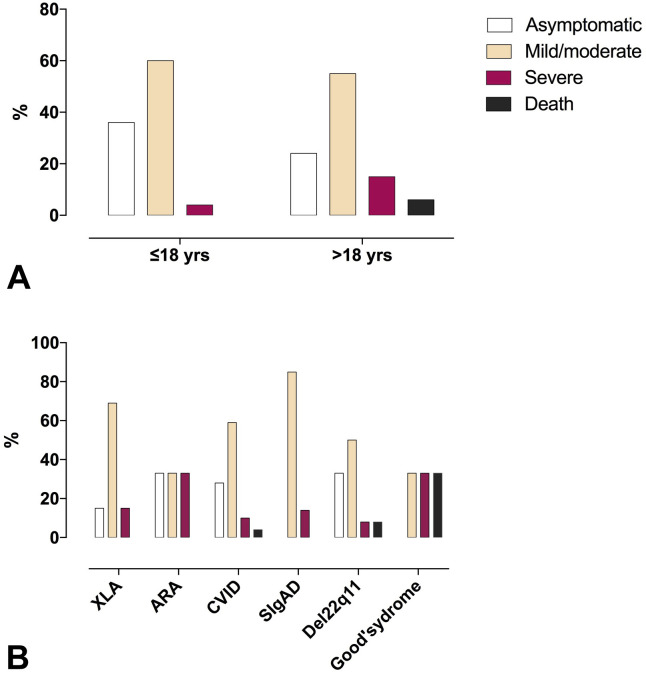
In the 1-year study period, 131 cases of SARS-CoV-2 infection were notified among 3263 patients with IEI, 33 of them 18 years or younger. According to World Health Organization criteria 2020,4 patients might be stratified in asymptomatic, mild, moderate, and severe COVID-19. The asymptomatic condition, revealed by the screening of patients attending the hospital sites, and of household contacts, was reported in 36.3% of patients 18 years or younger, and 24.5% of patients older than 18 years. Mean age was similar in asymptomatic, mild/moderate, or severe COVID-19 patients, and in patients who died from COVID-19, with the exception of asymptomatic adult patients who were younger than adult patients with severe COVID-19 (P < .003) (Table I ). Patients with IEI with severe COVID-19 and patients who later died from COVID-19 had a limited spectrum of IEI diagnosis: Common Variable Immune Deficiency (CVID), Del 22q11, and Good’s syndrome.
Table I.
Demographic data, and disease severity of SARS-CoV-2–positive patients with IEI
| SARS-CoV-2 positive | % | Mean age |
|---|---|---|
| ≤18 y | 25.1 | 9.6 ± 5.7 |
| Male | 60.6 | |
| Asymptomatic | 36.3 | 6.2 ± 2.9 |
| Mild/moderate | 60.6 | 5.6 ± 4.2 |
| Severe | 3.03 | 1 |
| Death | 0 | |
| >18 y | 74.8 | 43.9 ± 15.8 |
| Male | 58.2 | |
| Asymptomatic | 24.5 | 38.0 ± 17.0∗ |
| Mild/moderate | 55.1 | 41.6 ± 16.8 |
| Severe | 15.8 | 50.9 ± 14.8 |
| Death | 5.1 | 48.5 ± 13.0 |
Mean age asymptomatic vs severe COVID-19 >18 y: P < .03.
At the end of February 2021, the cumulative incidence per 100,000 of confirmed infections was 4.01 in patients with IEI and 5.22 in the general population (Table II ). Only the incidence in pediatric age was significantly lower in patients with IEI (2.36) in comparison to that in the Italian pediatric population (4.11; P < .001), a finding possibly due to the continuous patients' education on protection procedures our patients have been following since diagnosis. The highest number of SARS-CoV-2–infected subjects was in the group 19 to 49 years for IEIs and the general population. The overall infection-fatality rate was 3.81% in IEIs, compared with 3.28% in the Italian population (P = .61) and 5.10% in adult patients with IEI compared with 3.68% in the adult general population (P = .5). Nonetheless, the fatality rate among Italian patients with IEI is lower than previously reported from other IEI cohorts, ranging from 9.571 to 25.2 Patients with IEI showed a younger age at death (median age, 52 years, range, 30-59, vs 83 years, range, 0-109), and did not have those comorbidities predisposing to a severe COVID-19 in the nonimmunocompromised population.5 Preexisting comorbidities associated with COVID-19 severity were described in only 6 of 11 patients with IEI with severe COVID-19 (1 hypertension, 2 cardiomyopathy, 3 chronic lung diseases) and in only 2 of 5 patients with IEI who died from SARS-CoV-2 infection (hypertension and obesity).
Table II.
Cumulative incidence per 100,000, and infection-fatality percent for IEI by diagnosis: Comparison of IEI (total, pediatric, and adult age) to data (total, pediatric, and adult) of the Italian population
| IEI entity | No. of SARS-CoV-2–positive patients | No. of patients with IEI enrolled | Cumulative incidence (per 100,000) | Infection-fatality rate (%) |
|---|---|---|---|---|
| CVID | 74 | 1161∗ | 6.4 | 4.05 |
| XLA | 13 | 148 | 8.8 | 0 |
| ARA | 3 | 17 | 17.6 | 0 |
| SIgAD | 7 | 961 | 0.7 | 0 |
| Good’s syndrome | 3 | 24 | 12.5 | 33.3 |
| Del 22q11 | 12 | 527 | 2.3 | 8.3 |
| WAS | 0 | 5 | 0 | 0 |
| CGD | 0 | 66 | 0 | 0 |
| AT | 2 | 54 | 3.7 | 0 |
| HIE syndrome | 0 | 50 | 0 | 0 |
| ALPS | 1 | 12 | 8.3 | 0 |
| CD4 lymphopenia | 2 | 26 | 7.7 | 0 |
| APDS | 2 | 2 | † | 0 |
| Aicardi-Goutiers | 1 | 1 | † | 0 |
| Prolidase deficiency | 1 | 1 | † | 0 |
| MyD88 deficiency | 1 | 1 | † | 0 |
| NBAS deficiency | 1 | 1 | † | 0 |
| XIAP | 0 | 1 | † | 0 |
| Neutropenia | 2 | 39 | 5.1 | 0 |
| Post-HSCT, post–gene therapy, and postthymic transplant | 6 | 162 | 3.70 | 0 |
| IEI (total number) | 131 | 3,263 | 4.01 | 3.81 |
| <18 y | 33 | 1,396‡ | 2.36 | 0 |
| >18 y | 98 | 1,867 | 5.25 | 5.10 |
| Italian population (total number) | 3,123,368 | 59,816,655 | 5.22 | 3.28 |
| <18 y | 417,752 | 10,160,000 | 4.11 | 0.005 |
| >18 y | 2,705,616 | 49,656,655 | 5.45 | 3.68 |
APDS = activated phosphoinositide 3-kinase δ syndrome; ARA = autosomal recessive agammaglobulinemia; AT = ataxia telangiectasia; CGD = chronic granulomatous disease; CVID = Common Variable Immune Deficiency; HIE = hyper IgE; HSCT = hematopoietic stem cell transplantation; MyD88 = myeloid differentiation factor 88; SIgAD = selective IgA deficiency; WAS = Wiskott Aldrich Syndrome; XIAP = X-linked inhibitor of apoptosis; XLA = X-linked agammaglobulinemia.
SARS-CoV-2–positive CVID vs SARS-CoV-2–positive SIgAD: P = .04.
This figure cannot be calculated because we do not have a disease register for these rare IEI and we do not know the possible number of affected patients in Italy.
IEI <18 y vs Italian population <18 y: P < .001.
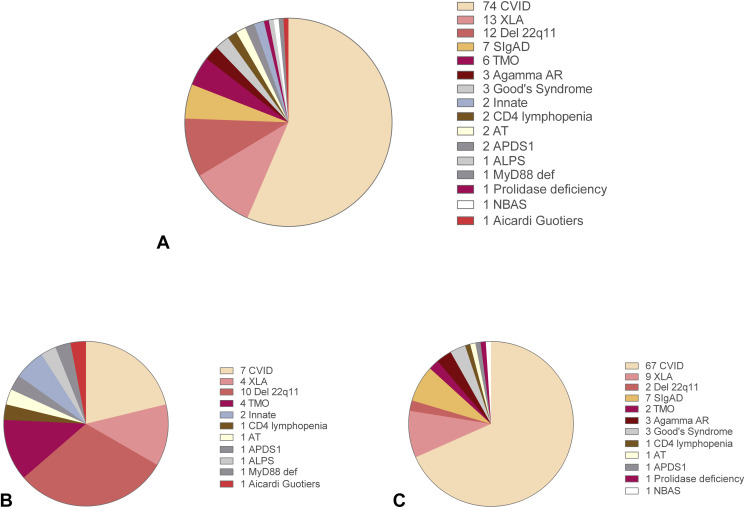
Distribution of SARS-CoV-2–infected patients by IEI entities and by children and adult populations is shown in Figure E1 (A-C) in this article's Online Repository at www.jaci-inpractice.org. Del 22q11 and CVID accounted for the most affected IEI in the pediatric and adult age, respectively. The cumulative incidence, and infection-fatality rate by type of IEI and by age, is presented in Table II. Given the low numbers among different IEI entities, a higher SARS-CoV-2 incidence was found only by comparing CVID to Selective IgA Deficiency (SIgAD) (P = .04). The fatality rate was high in Good’s syndrome and in Del 22q11, both conditions associated with a T-cell defect. A condition of lymphopenia and CD4 lymphopenia was detected in the pre–SARS-CoV-2 period in about 10% and 20% of IEI, respectively, mainly in patients with Del22q11 and CVID. However, this was not a risk factor for the subsequent COVID-19 severity. As reported in nonimmunocompromised adult patients,6 neutrophil/lymphocyte ratio was higher in patients with severe COVID-19 than in asymptomatic patients (7.3 ± 7.4 vs 2.0 ± 0.9; P = .008), and in patients with mild/moderate disease (3.3 ± 3.9; P = .04).
Figure E1.
Distribution of SARS-CoV-2–infected patients by (A) IEI entities and by (B) children and (C) adult populations. ALPS = autoimmune lymphoproliferative synìdrome; APDS1 = activated phosphoinositide 3-kinase δ syndrome; ARA = autosomal recessive agammaglobulinemia; AT = ataxia telangectasia; CVID = Common Variable Immune Deficiency; SIgAD = selective IgA deficiency; TMO = post-hematopoietic stem cell transplantation; XLA = X-linked agammaglobulinemia.
Because patients with IEI might struggle with clearing the infection, we calculated the time from the first SARS-CoV-2–positive PCR test result to the first SARS-CoV-2–negative PCR test result. One-third of patients with antibody deficiencies were SARS-CoV-2 positive for more than 3 weeks, representing a possible risk factor for viral spreading.7 A similar length was observed in patients with agammaglobulinemia (56.4 ± 38.1 days), CVID (47.6 ± 20.9 days), and SIgAD (52.5 ± 71.2 days). Shorter times were described in patients with Del 22q11 (29.1 ± 33.9 days; P < .01) (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org).
Figure E2.
COVID-19 severity by (A) age and by (B) IEI entity in the Italian IEI cohort. ARA = autosomal recessive agammaglobulinemia; CVID = Common Variable Immune Deficiency; XLA = X-linked agammaglobulinemia.
The long time of observation might have helped correct some initial conclusions also from our group,8 because patients with agammaglobulinemia and autosomal recessive agammaglobulinemia might also show a severe COVID-19, even if none died. Our study has a major limitation of possible underestimation, but less relevant than that described in the general population,9 because we started our study at the early stages of the pandemic, and we followed our patient rigorously. The purely descriptive data set on patients with IEI might be the basis for a comparison over time of the trend of SARS-CoV-2 infection in this population as is for data on the trend of SARS-CoV-2 infection in the general population.
Acknowledgments
We thank our patients and their families.
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository.
References
- 1.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho H.E., Mathew S., Peluso M.J., Cunningham-Rundles C.E. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract. 2021;9:490–493.e2. doi: 10.1016/j.jaip.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lougaris V., Pession A., Baronio M., Soresina A., Rondelli R., Gazzurelli L. The Italian Registry for Primary Immunodeficiencies (Italian Primary Immunodeficiency Network; IPINet): twenty years of experience (1999-2019) J Clin Immunol. 2020;40:1026–1037. doi: 10.1007/s10875-020-00844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Country & technical guidance—coronavirus disease 2019. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance Available from:
- 5.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 6.Song L., Liang E.-Y., Wang H.-M., Shen Y., Kang C.-M., Xiong Y.-J. Differential diagnosis and prospective grading of COVID-19 at the early stage with simple hematological and biochemical variables. Diagn Microbiol Infect Dis. 2021;99:115169. doi: 10.1016/j.diagmicrobio.2020.115169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S.L., Mertens A.N., Crider Y.S., Nguyen A., Pokpongkiat N.N., Djajadi S. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11:4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]