Abstract
Objectives
To document medication abortion clinical practice changes adopted by providers in response to the COVID-19 pandemic.
Study design
Longitudinal descriptive study, comprised of three online surveys conducted between April to December, 2020. We recruited sites from email lists of national abortion and family planning organizations.
Results
Seventy-four sites opted to participate. We analyzed 55/74 sites (74%) that provided medication abortion and completed all three surveys. The total number of abortion encounters reported by the sites remained consistent throughout the study period, though medication abortion encounters increased while first-trimester aspiration abortion encounters decreased. In response to the COVID-19 pandemic, sites reduced the number of in-person visits associated with medication abortion and confirmation of successful termination. In February 2020, considered prepandemic, 39/55 sites (71%) required 2 or more patient visits for a medication abortion. By April 2020, 19/55 sites (35%) reported reducing the total number of in-person visits associated with a medication abortion. As of October 2020, 37 sites indicated newly adopting a practice of offering medication abortion follow-up with no in-person visits.
Conclusions
Sites quickly adopted protocols incorporating practices that are well-supported in the literature, including forgoing Rh-testing and pre-abortion ultrasound in some circumstances and relying on patient report of symptoms or home pregnancy tests to confirm successful completion of medication abortion. Importantly, these practices reduce face-to-face interactions and the opportunity for virus transmission. Sustaining these changes even after the public health crisis is over may increase patient access to abortion, and these impacts should be evaluated in future research.
Implications statement
Medication abortion serves a critical function in maintaining access to abortion when there are limitations to in-person clinic visits. Sites throughout the country successfully and quickly adopted protocols that reduced visits associated with the abortion, reducing in-person screenings, relying on telehealth, and implementing remote follow-up.
Keywords: COVID-19, Medication abortion, Practice change, Rh-factor, Telemedicine, Ultrasound
1. Introduction
Abortion providers have used mifepristone and misoprostol safely and effectively to end pregnancies for 20 years in the United States. During this period, clinicians and researchers have refined and improved protocols for medication abortion, including modification of dosing, route of administration, and standards for pre- and post-abortion screening [1], [2], [3], [4], [5]. A substantial body of evidence confirms that medication abortion protocols can safely be modified to require fewer interactions with clinicians, including omission of pre-abortion screenings, postabortion follow-up without in-person clinic visits, and use of telehealth for peri-abortion counseling [1,2,[6], [7], [8], [9], [10], [11]]. Recent research also demonstrated the safety and efficacy of coupling telemedicine with mailing medication abortion drugs directly to patients [12].
The Federal Drug Administration has a set of regulations, known as Risk Evaluation Mitigation Strategy (REMS), that require mifepristone be dispensed directly by a clinician to the patient in a healthcare setting [13]. These regulations, along with abortion-specific regulations at the state-level, have limited the capacity of many abortion providers to adopt protocols that reduce the patient's in-person visits to a clinic [14], [15], [16].
The COVID-19 pandemic prompted rapid reconfiguration of healthcare delivery to reduce face-to-face interactions between patients and clinicians. Providers of essential and time-sensitive services, such as abortion, had to pivot quickly to new practices [17]. Early in the pandemic, one study documented that nearly 90% of independent abortion clinics adjusted at least one of their clinical practices in response to the pandemic [18]. The most common changes reported were transitioning to phone- or video-based follow-up after an abortion and using more telehealth visits for initial abortion consultation visits, practices well-supported by the evidence but not previously widely adopted [18,19].
Because medication abortion requires no hands-on examination or intervention from the provider, it is one option for providing safe and timely abortion services while also limiting exposure to COVID-19 for patients, staff, and clinicians. State-based directives that reduced access to surgical abortion or clinic-level policies that promoted medication abortion over surgical may have resulted in increasing the overall proportion of patients choosing medication abortion to end their pregnancies during the pandemic [17,20,21].
In July 2020, a federal court enjoined the FDA from enforcing the REMS on mifepristone during the COVID-19 public health emergency. This decision was in response to a lawsuit filed by the American Civil Liberties Union on behalf of a coalition of medical experts and advocates, including the American College of Obstetricians and Gynecologists (ACOG) and SisterSong Women of Color Reproductive Justice Collective. This ruling permitted clinicians to mail medication directly to patients or to send the medications through a mail-order pharmacy where permitted by state law [22], however the Supreme Court overturned this injunction in January 2021, reinstating the REMS and halting the use of direct-to-patient or mail-order pharmacy for administration of mifepristone.
Before and during the pandemic, professional societies and organizations have issued recommendations for adoption of modified medication abortion protocols (sometimes called “low- or no- test” protocols) that reduce the number of in-person visits required for the abortion [5,19,23,24]. We sought to document if and how clinicians adapted their medication abortion practices in response to the COVID-19 pandemic, and if these changes reflected the guidance issued by these professional organizations.
2. Materials and Methods
2.1. Study design
We conducted three surveys between April and October 2020. The surveys focused on pandemic-responsive changes in service delivery, with special attention paid to clinical innovations in abortion and contraception care that may ultimately reduce barriers to family planning care. We invited sites to participate in March and April of 2020, and closed enrollment prior to distribution of the first survey. All sites that opted-in to participate in the study received all three surveys during the 3 designated data collection windows: 4/16/2020 to 5/22/2020; 8/4/2020 to 8/14/2020; and 11/2/2020 to 11/13/2020, respectively. The Advarra Institutional Review Board (IRB) deemed the study exempt from IRB oversight. Sites reported all data at the clinic level and reported no information about patient outcomes or personal health information.
2.2. Sample
We recruited a convenience sample derived from the Society of Family Planning (SFP) Abortion Clinical Research Network (the Network), a collaborative research enterprise comprised of approximately 78 abortion clinics throughout the United States. The Network sites reflect the types and locations of abortion providers in the country, with representation from all regions and all practice settings, including independent clinics, Planned Parenthood affiliates, and hospital-based medical centers.
We invited all Network sites to participate through a private listserv in March and April 2020. We also invited new sites to become Network sites and join the study via announcements to the SFP membership mailing list, Abortion Care Network (ACN) listserv, and the National Abortion Federation membership.
Respondent sites included organizations with one clinical location and organizations with multiple affiliated clinical locations. If a respondent site had more than one clinical location, we requested information regarding site characteristics and abortion volume for only their highest volume site, and asked them to reference all clinical locations when answering all other questions about services offered and adoption or modification of practices.
2.3. Survey design
Sites completed surveys online via Qualtrics. Survey instruments are available online here: https://societyfp.org/research-support/abortion-clinical-research-network/network-study-family-planning-visits-during-the-covid-19-pandemic/. Questions in the first survey included pre-pandemic clinical practices (services routinely offered, routine screenings, and standard visit structure), practice changes made in response to COVID-19 during the first several months of the pandemic, and whether these practice changes were ongoing at the time of the survey. In the second and third surveys, respondents indicated if they had changed or adopted practices specifically and exclusively in response to COVID-19 at any point since the start of the pandemic and if those changes were ongoing at the time of the survey. In the second and third survey, we also asked sites to indicate their current practices for Rh-factor testing and ultrasound before medication abortion, regardless of whether the policy was new or had changed due to the pandemic. We reviewed findings from each survey period and iteratively modified questions for the next survey period to ensure we captured innovations as thoroughly and accurately as possible.
Sites reported number of encounters for each abortion service and total abortion encounters in the months of February (considered pre-pandemic), March or April (dependent on the date the first survey was completed), and May, June, July, August, September, and October 2020.
In order to preserve confidentiality of respondents, each site used an assigned ID number when completing surveys. Study staff maintained a secure spreadsheet linking sites to study IDs. Given the marked geographic variability of abortion providers in the United States, we considered state to be an identifiable characteristic of clinics. We therefore re-categorized respondents into the four 2010 US Census Regions (West, Midwest, South, Northeast) based on the site's address [25].
For the purposes of this analysis, we limited the sample to sites that indicated medication abortion as a service they routinely provided before the pandemic and that completed all 3 surveys.
3. Results
Seventy-four sites across the United States (n = 72) and Canada (n = 2) opted in to receive the survey. Of the total respondent sites, 52/74 (70%) were existing Network member sites and 22/74 (30%) were not existing Network member sites. Of the 74 total participating sites in the study, 55/74 (74%) reported routinely providing medication abortion before the pandemic and completed all three surveys. These are the only sites included in our analysis presented here. As seen in Table 1 , the vast majority of sites are urban and approximately half are located in academic- or hospital-affiliated settings.
Table 1.
Characteristics of survey respondent sites providing medication abortion in the US February 2020 (N = 55)
| n (%) | |
|---|---|
| Regiona | |
| Northeast | 20 (36%) |
| West | 15 (27%) |
| Midwest | 8 (15%) |
| South | 12 (22%) |
| Clinic type | |
| Academic/hospital-based | 26 (47%) |
| Independent clinic | 14 (26%) |
| Planned Parenthood affiliate | 15 (27%) |
| Urbanicityb | |
| Urban county | 53 (96%) |
| Rural county | 2 (4%) |
| Family planning services | |
| Contraception | 53 (96%) |
| Medication abortion | 55 (100%) |
| First trimester procedural abortion | 54 (98%) |
| Second trimester procedural abortion | 50 (91%) |
| Total abortion encounters per month | |
| 1–25 | 11 (20%) |
| 26–100 | 15 (27%) |
| 101–150 | 8 (14%) |
| 151–250 | 9 (16%) |
| 251–500 | 3 (6%) |
| 501–1000 | 4 (7%) |
| More than 1000 | 3 (6%) |
| Volume data not reported | 2 (4%) |
Regions are reflective of the Census Bureau regions.
Urban is defined as an areas of 50,000 or more people or urban clusters of at least 2500 and less than 50,000 people; rural is any other county that does not meet this definition.
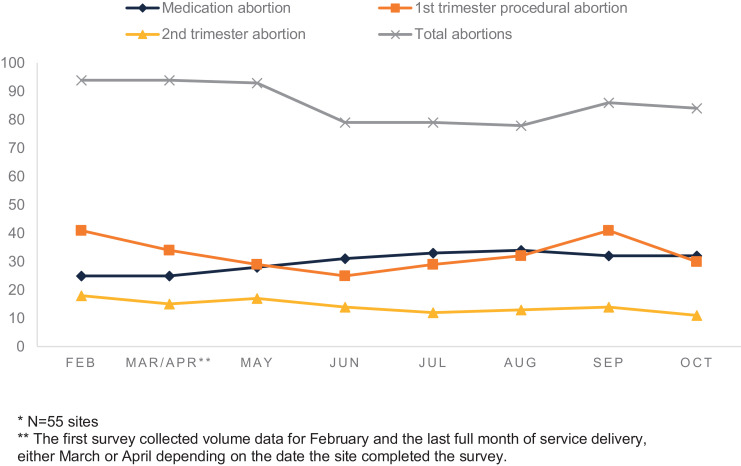
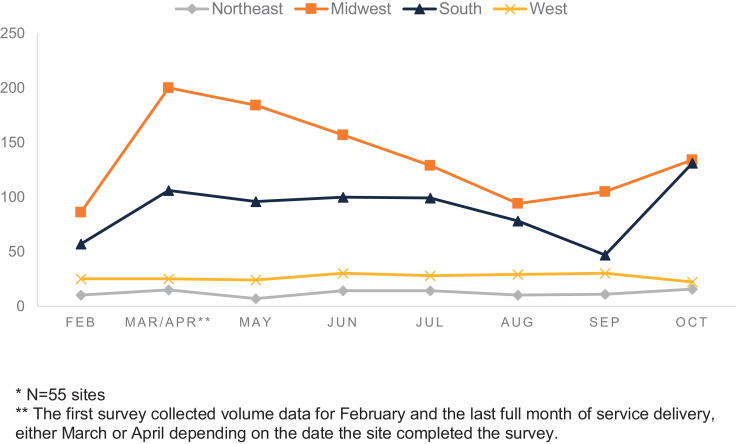
The total number of encounters for abortion reported by each site throughout the study period varied widely with a small number of sites reporting very high volumes and a small number of sites reporting very low volumes (including 0 encounters in some months). We report medians instead of means due to this wide, skewed distribution of encounters per site. Among sites that reported volumes for each time period, overall abortion encounters dropped from the beginning to the end of the reporting period with a median of 94 per site (range 0-1237) prepandemic and 84 per site (range 2-1404) in October 2020 (Fig. 1 ). Medication abortion encounters increased throughout the reporting period, with a median of 25 per site (range 0-294) in the prepandemic period and 32 per site (range 1-767) by the end of October 2020. First trimester procedural or aspiration abortion encounters slightly decreased, from a median of 37 per site (range 0-526) prepandemic to 35 per site (range 2-508) at the end of October 2020. Medication abortion encounters reported at each site varied by region throughout the pandemic (Fig. 2 ), with the Midwest and South reporting more medication abortion than the Northeast or West throughout the study period.
Fig. 1.
Median monthly abortion encounters per site by abortion type in the US February–October 2020*. *N = 55 sites. **The first survey collected volume data for February and the last full month of service delivery, either March or April depending on the date the site completed the survey.
Fig. 2.
Median monthly medication abortion encounters per site by US geographic region, February–October 2020*. *N = 55 sites. **The first survey collected volume data for February and the last full month of service delivery, either March or April depending on the date the site completed the survey.
In response to the COVID-19 pandemic, sites made swift changes to their medication abortion practice, most broadly and rapidly adopting protocols that reduced the number of in-person visits associated with medication abortion and confirmation of successful termination. In February 2020, considered the prepandemic period for all respondent sites, 39/55 sites (71%) required 2 or more visits per patient for a medication abortion. By April 2020, 19/55 sites (34%) reported reducing the total number of in-person visits associated with a medication abortion.
Responsive practices that could reduce the number of in-person visits required for a medication abortion included telemedicine and new modalities for dispensing medication abortion drugs. Before the pandemic, 10/55 sites (18%) reported using telehealth (either video or telephone encounters) as a standard component of their medication abortion practice. As of October 2020, 39 sites indicated they had newly adopted telehealth for medication abortion in response to the pandemic. Additionally, in October 2020, 5/55 sites (9%) offered curbside pickup of mifepristone, 4/55 sites (7%) mailed medication abortion medicines to patients, and 39/55 sites (71%) used telehealth for some component of medication abortion follow-up in response to the pandemic. Sites also reported changes in Rh-factor testing and ultrasound practices before medication abortion as a response to the pandemic. These changes included forgoing tests in certain circumstances, as well as modifications to acceptable documentation of screening (such as accepting ultrasound from outside providers or documentation of Rh status from blood donor cards).
Prior to the pandemic, 54/55 sites (98%) reported routine use of ultrasound to confirm successful medication abortion termination, while 11/55 sites (20%) routinely offered patients the option of at- home high-sensitivity pregnancy tests. As of April 2020, 34 sites reported newly accepting at home high-sensitivity pregnancy tests for confirmation of successful medication abortion in response to the pandemic. As of October 2020, 37 sites indicated they had newly adopted a practice of offering medication abortion follow-up with no in-person visits due to COVID-19.
General practices for Rh-factor testing and ultrasound, irrespective of the pandemic, varied over the study period. When reporting on February 2020 policies, 51/54 sites (94%) indicated that they required all patients to have Rh-factor testing before a medication abortion (one site did not respond to this question). When reporting on October 2020 policies, 15/54 sites (27%) indicated that they required all patients to have Rh-factor testing before a medication abortion. Similarly, general policies for ultrasound prior to a medication abortion varied over time. Reporting on February 2020 policies, 55/55 sites (100%) required an ultrasound before medication abortion. When reporting on October 2020 policies, 40/55 sites (73%) required an ultrasound before a medication abortion. We observed these decreases in the number of sites requiring Rh-factor testing and preabortion ultrasound across all clinic types and geographic locations (Table 2 ).
Table 2.
General Rh-factor testing and ultrasound policies in February and October 2020 as reported by survey respondent sites in the US, by region and site typea
| Northeastbn = 19 | Westn = 15 | Southn = 12 | Midwestn = 8 | |
|---|---|---|---|---|
| Rh-factor testing required for all patients prior to medication abortion-February | 16 (84%) | 14(93%) | 12 (100%) | 8 (100%) |
| Rh-factor testing required for all patients prior to medication abortion-October | 5 (26%) | 1 (7%) | 5 (42%) | 4 (50%) |
| Northeastn = 20 | Westn = 15 | Southn = 12 | Midwestn = 8 | |
| Ultrasound testing required for all patients prior to medication abortion-February | 20(100%) | 15 (100%) | 12 (100%) | 8 (100%) |
| Ultrasound required for all patients prior to medication abortion-October | 15 (74%) | 8 (53%) | 10 (83%) | 7 (88%) |
| Academic/hospital-based*n=25 | Independentn=14 | Planned Parenthood affiliaten=15 | ||
| Rh-factor testing required for all patients prior to medication abortion-February | 23 (92%) | 12 (86%) | 15 (100%) | |
| Rh-factor testing required for all patients prior to medication abortion-October | 7 (28%) | 6 (43%) | 2 (13%) | |
| Academic/hospital-basedn = 26 | Independentn = 14 | Planned Parenthood affiliaten = 15 | ||
| Ultrasound testing required for all patients prior to medication abortion-February | 26 (100%) | 14 (100%) | 15 (100%) | |
| Ultrasound testing required for all patients prior to medication abortion-October | 20 (76%) | 12 (86%) | 8 (53%) | |
Reported as n(%).
One academic/hospital-based site in the Northeast did not report Rh-factor testing in October and is omitted from this table for Rh-factor policies.
4. Discussion
Sites participating in our surveys quickly transitioned to protocols that reduced face-to-face interactions and risk for virus transmission between patients, staff, and clinicians. These modified protocols included telehealth and use of at-home pregnancy tests or patient symptom checklists for medication abortion follow-up, both of which have a considerable body of literature supporting such practices. However, sites did not widely adopt fully remote medication abortion care, but instead used a patchwork of telehealth for periabortion counseling, remote dispensing of medication abortion pills, and home-based follow-up. Sites may have had state- or organizational-level policies that restricted their ability to implement fully remote models. It may be that sites adopted as many innovations as their specific setting allowed. In so doing, these providers ensured that abortion remained available despite the uncertainty of the public health crisis.
The increasing volume of medication abortions provided during this study period underscores the critical role of medication abortion to overall abortion availability when access is constrained, whether due to local restrictions or global health crises. Additionally, a patient primarily drives the timing of when a medication abortion begins, as compared to the clinic and procedural constraints that may dictate the time of day or day of the week when an aspiration procedure occurs. During a period when social and healthcare safety nets are threatened or destroyed, patients may be drawn to a process that they can tailor to their specific needs.
Our study is limited by the nature of using a convenience sample. The respondent clinics over-represent academic/hospital-affiliated practices and urban clinics. Due to iterative modifications, each survey contained some unique questions not included in the other surveys; we cannot track trends in responses to these questions over time. As we predominately asked sites to report changes made specifically in response to COVID-19, our data may not accurately capture all practice changes that occurred during this timeframe.
Our findings show the breadth and speed of clinical practice changes adopted by family planning providers during the pandemic. These innovations may reduce barriers to abortion and could be embraced by patients even after the current public health crisis is over. Ongoing monitoring and documentation of the persistent use of these modified protocols and research regarding their acceptability among patients is warranted as the pandemic evolves.
Conflict of Interest
None.
Funding
This work was supported by the Tara Health Foundation and an anonymous donor.
Role of funding source
The authors are solely responsible for the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
References
- 1.Raymond EG, Grossman D, Wiebe E, Winikoff B. Reaching women where they are: eliminating the initial in-person medical abortion visit. Contraception. 2015;92:190–193. doi: 10.1016/j.contraception.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Harper C, Ellertson C, Winikoff B. Could American women use mifepristone-misoprostol pills safely with less medical supervision? Contraception. 2002;65:133–142. doi: 10.1016/s0010-7824(01)00300-6. [DOI] [PubMed] [Google Scholar]
- 3.Beal MW. Update on medication abortion. J Midwifery Womens Health. 2007;52:23–30. doi: 10.1016/j.jmwh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Bartz D, Goldberg A. Medication abortion. Clin Obstet Gynecol. 2009;52 doi: 10.1097/GRF.0b013e3181a2b026. [DOI] [PubMed] [Google Scholar]
- 5.Creinin MD, Grossman D. Medication abortion up to 70 days of gestation. Contraception. 2020;102:225–236. doi: 10.1016/j.contraception.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Horvath S, Tsao P, Huang Z-Y, Zhao L, Du Y, Sammel MD, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1–6. doi: 10.1016/j.contraception.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiebe ER, Campbell M, Aiken ARA, Albert A. Can we safely stop testing for Rh status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contracept X. 2019;1 [Google Scholar]
- 8.Perriera LK, Reeves MF, Chen BA, Hohmann HL, Hayes J, Creinin MD. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143–149. doi: 10.1016/j.contraception.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30–35. doi: 10.1016/j.contraception.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Endler M, Lavelanet A, Cleeve A, Ganatra B, Gomperts R, Gemzell-Danielsson K. Telemedicine for medical abortion: a systematic review. BJOG. 2019;126:1094–1102. doi: 10.1111/1471-0528.15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman D, Grindlay K. Alternatives to ultrasound for follow-up after medication abortion: a systematic review. Contraception. 2011;83:504–510. doi: 10.1016/j.contraception.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Raymond E, Chong E, Winikoff B, Platais I, Mary M, Lotarevich T, et al. TelAbortion: evaluation of a direct to patient telemedicine abortion service in the United States. Contraception. 2019;100:173–177. doi: 10.1016/j.contraception.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Mifeprex risk evaluation and mitigation strategy (REMS) program 2016. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifeprex_2016-03-29_REMS_document.pdf (accessed September 1, 2020).
- 14.Mifeprex REMS Study Group Sixteen years of overregulation: time to unburden Mifeprex. N Engl J Med. 2017;376:790–794. doi: 10.1056/NEJMsb1612526. [DOI] [PubMed] [Google Scholar]
- 15.Fok WK, Mark A. Abortion through telemedicine. Curr Opin Obstet Gynecol. 2018;30 doi: 10.1097/GCO.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications: position statement 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications (accessed January 12, 2021).
- 17.Roberts SCM, Schroeder R, Joffe C. COVID-19 and independent abortion providers: findings from a rapid-response survey. Perspect Sex Reprod Health. 2020;52:217–225. doi: 10.1363/psrh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upadhyay UD, Schroeder R, Roberts SCM. Adoption of no-test and telehealth medication abortion care among independent abortion providers in response to COVID-19. Contracept X. 2020;2 doi: 10.1016/j.conx.2020.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond EG, Grossman D, Mark A, Upadhyay UD, Dean G, Creinin MD, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361–366. doi: 10.1016/j.contraception.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramaswamy A, Weigel G, Sobel L, Salganicoff A. Kaiser Family Foundation Policy Watch; 2020. Medication abortion and telemedicine: innovations and barriers during the COVID-19 emergency. https://www.kff.org/policy-watch/medication-abortion-telemedicine-innovations-and-barriers-during-the-covid-19-emergency/ (accessed January 12, 2021) [Google Scholar]
- 21.Jones RK, Lindberg L, Witwer E. COVID-19 abortion bans and their implications for public health. Perspect Sex Reprod Health. 2020;52:65–68. doi: 10.1363/psrh.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Civil Liberties Union . ACLUOrg; 2020. Federal court blocks FDA restriction that unnecessarily imposes COVID-19 risks on patients seeking abortion care. https://www.aclu.org/press-releases/federal-court-blocks-fda-restriction-unnecessarily-imposes-covid-19-risks-patients (accessed January 12, 2021) [Google Scholar]
- 23.Reproductive Health Access Project. No touch medication abortion protocol 2020. https://www.reproductiveaccess.org/resource/no-touch-mab-protocol/ (accessed January 12, 2021).
- 24.Mark A, Foster AM, Grossman D, Prager SW, Reeves M, Velásquez CV, et al. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation's Clinical Policies Committee. Contraception. 2019;99:265–266. doi: 10.1016/j.contraception.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 25.2010 Census Regions and Divisions of the United States 2018. https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html (accessed January 12, 2021).