Abstract
Optimizing a patients’ condition before surgery to improve the postoperative outcome can be achieved by using prehabilitation; preoperative interventions focusing on modifiable risk factors to improve the physical, nutritional, and mental status of the patient. A multimodal, multidisciplinary approach induces a synergistic effect between the various interventions and affects the outcome postoperatively. While awaiting higher-quality evidence, the worldwide implementation of prehabilitation programs has started, resulting in a true revolution in perioperative care.
Keywords: functional capacity, multimodal, perioperative care, prehabilitation, surgical complications
Introduction
“Prehabilitation”, “Fit 4 Surgery”, “Fit 2 Fight”, “pre-rehabilitation”, and “better in – better out” are all expressions of an identical revolutionary thought to optimize the patients’ condition before surgery. Prehabilitation is defined to include assessment of physical, nutritional, and psychological status to determine baseline functional capacity, identify impairments, and intervene in order to improve the patients’ preoperative functional reserve prior to treatment [1], [2]. The interventions address modifiable risk factors with the intention to improve the outcomes of (cancer) treatment [2]. Both short-term outcomes of treatment as well as long-term behavioral changes can be altered [3]. It leads to an improved functional capacity [4], [5], [6], [7], improved nutritional [8], [9] and mental status [9], [10], reduction of complications [11], [12], [13], faster recovery [7], [14], [15], a reduced length of stay in the hospital [16], an improved quality of life [10], [17], and potentially a reduction in costs. While the evidence for improvement of clinical outcomes is growing, prehabilitation is at the threshold of worldwide implementation and already included in guidelines [18], [19], [20].
A growing interest in the postoperative outcome (e.g. quality of life) is the result of rising life expectancy and increasing survival rates of cancer [21], [22]. The fact that metabolic and behavioral risk factors are rising and will continue to do so [23] makes it a necessity for healthcare professionals to raise awareness and emphasize the role of the patient. Lifestyle changes are complex; however, patients are more willing to change behavior positively when facing major surgery [3].
Innovations and technologies in the past 50 years have contributed to the reduction in death rates [22]. Perioperative care is one of the aspects of healthcare, where many developments significantly improved the outcome. Laparoscopy, for instance, has become the golden standard for various surgical procedures due to the positive results. It is also considered as the integral core of Enhanced Recovery After Surgery (ERAS) programs [24], [25]. ERAS programs were introduced in the late 1990s. The principles of ERAS are based on the knowledge that many of the negative effects of major surgery (e.g. loss of muscle mass and body weight as well as a reduced resistance to infection) can be reduced by attenuating the surgical stress response with perioperative interventions [26]. Interventions such as anemia correction, assessment of the risk of postoperative delirium, perioperative pain management with a multimodal opioid-sparing analgesia regime, and anti-ileus management are essential components of an ERAS program [27]. The ultimate purpose is to minimize organ dysfunction postoperatively and enhance rehabilitation [28].
However, despite all of the above, surgery is still related with postoperative morbidity with complications rates varying from 18.4% in non-small cell lung cancer surgery to 65% in esophageal cancer surgery in The Netherlands in 2017 [29]. Postoperative complications result in an increased length of stay in the hospital, readmissions, and elevated healthcare costs, impact patient functioning and quality of life, and have possible implications on mortality [30]. The number of preoperative modifiable risk factors is associated with the risk of severe complications [31]. Furthermore, there is a strong correlation between the number of postoperative complications and the patients’ preoperative functional capacity and nutritional and mental status as determined by lifestyle and behavioral habits such as smoking and drinking [32], [33], [34], [35]. Therefore, further progress in improving perioperative care is desirable.
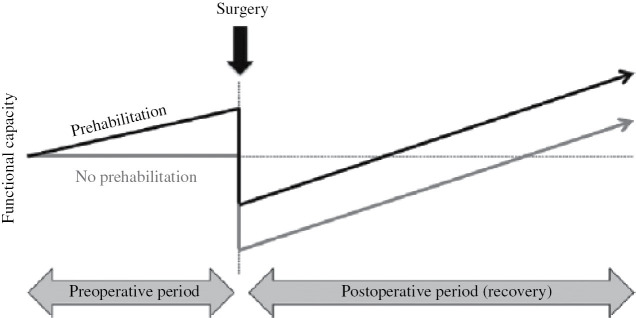
Until recently, efforts to improve recovery after major surgery have primarily focused on intra- and postoperative care [19]. The postoperative period is associated with anxiety and depression, fatigue and weakness, lack of sleep, and anorexia. It is therefore not the optimal period to introduce interventions to accelerate recovery [36], [37]. Furthermore, by addressing the functional capacity preoperatively, the postoperative risk is attenuated [36]. The preoperative period, although limited to several weeks, may be the window of opportunity to improve the patients’ condition and facilitate persistent lifestyle changes [5], [38], [39]. That is where prehabilitation comes in place (Figure 1).
Figure 1:
Concept of prehabilitation.
Theoretic model of surgical prehabilitation based on the concept of increasing functional capacity before surgery. Adapted with permission from Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005; 8: 25.
Preparing a patient for surgery can have different aspects and goals. In orthopedics, specific joints might be trained in strength and range of motion before, for example, hip replacement. Preoperative training will facilitate the recovery of joint function, leading to a shorter hospital stay [40]. In breast cancer surgery, preoperative exercise will have a positive effect on the recovery of the upper extremity functioning after mastectomy [41]. Preparing a patient for abdominal or thoracic surgery, however, is meant to improve the patients’ resilience against the impact of surgery, as even without complications there is a postoperative reduction of functional capacity [42], [43].
Prehabilitation should be multimodal. All pillars on its own affect the surgical outcome, both physically and psychologically. However, there is a synergistic effect of improving a patients’ physical condition, balancing and supplementing nutritional needs, altering mental status through psychological support, and cessation of smoking [44]. This multimodal approach seems unconditional, as exercise and dietary protein intake produce independent and additional effects on anabolism and muscle protein synthesis [45]. A positive mental status will enforce the motivation for training and eating, whereas studies with rodents imply that exercise recruits midbrain dopamine and striatal circuits involved in reward and stress resistance [46], thus improving mental status. Cessation of smoking is included because stopping, even shortly before an operation, will reduce the detrimental effect of the consequences of smoking [47], [48]. One could consider an extra pillar in prehabilitation: correction of other risk factors related to the disease such as anemia, which is present in about half of colorectal cancer patients. Preoperative anemia is associated with increased postoperative complications and decreased overall and disease-free survival in rectal cancer [33], [49]. The correction of the preoperative value is described in the context of ERAS [27] and additionally suggested to increase adherence to prehabilitation [36]. Polypharmacy could be addressed preoperatively, but at Máxima MC we consider both mentioned interventions as part of standard care and suggest, for example, iron supplementation when hemoglobin levels are <7 mmol/L (11.3 g/dL) as well as the involvement of the anesthetist as early in the oncological pathway as possible.
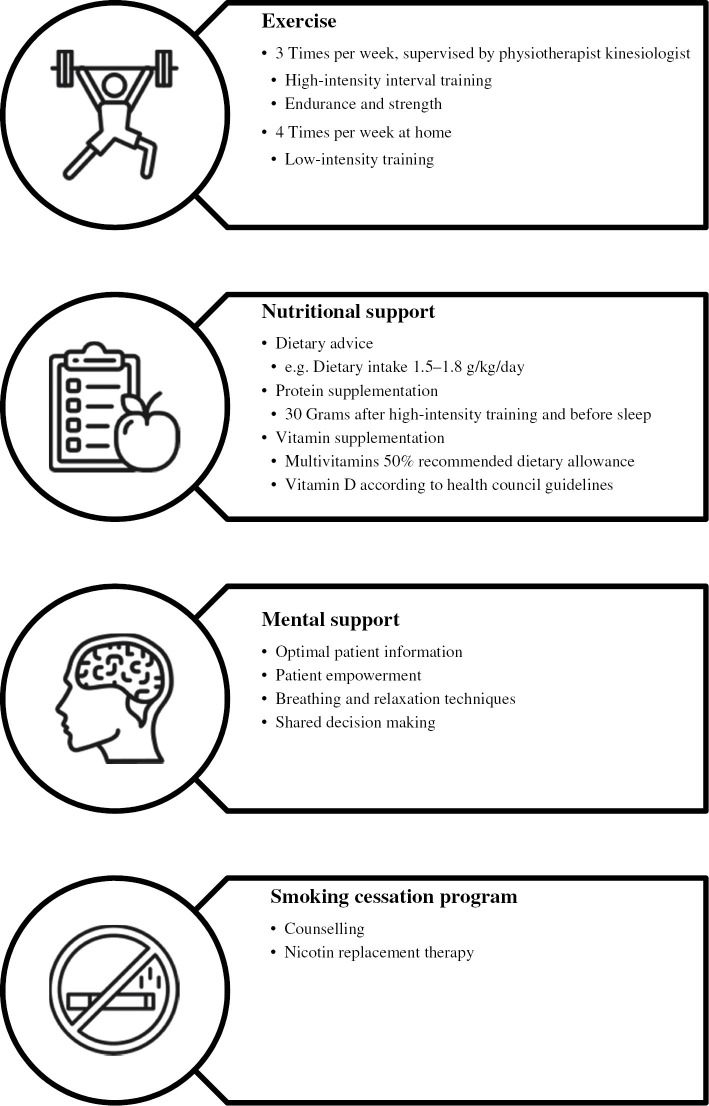
An international research consortium on prehabilitation, under guidance of professor Dr. Francesco Carli from McGill University Montréal, has created “best practice” for multimodal prehabilitation in colorectal cancer surgery in 2016. A four-pillar program consists of high-intensity interval training on endurance and strength, nutritional support with protein and vitamin supplementation, mental support, and a smoking cessation program (Figure 2). This program has been adapted in many clinical studies and subsidiary requests, also for diseases other than colorectal cancer populations.
Figure 2:
International prehabilitation protocol.
The research consortium is conducting the international PREHAB trial (NL58281.015.16) [50], which started in 2017. Some 714 participants undergoing resection for colorectal carcinoma are being randomized in either the control or the four-pillar multimodal prehabilitation program. Perioperative care is given according to ERAS guidelines. Eight hospitals in five different countries contribute to the study, but the final inclusion is not to be expected before the end of 2020. A nonrandomized feasibility study of the protocol was conducted at Máxima MC. Program evaluation revealed a high attendance rate (90%) and a high level of patient satisfaction. There were no adverse effects, and both strength and endurance were improved preoperatively. Figure 3 illustrates the increase in functional capacity [6-min walk test (6MWT)] after 4 weeks of prehabilitation. Four weeks after surgery, there was a significant difference compared to control patients. Forty percent of control patients were at their baseline functional capacity 4 weeks after surgery, whereas in the prehabilitation group 86% were at or higher than their baseline level (p<0.01) [15].
Figure 3:
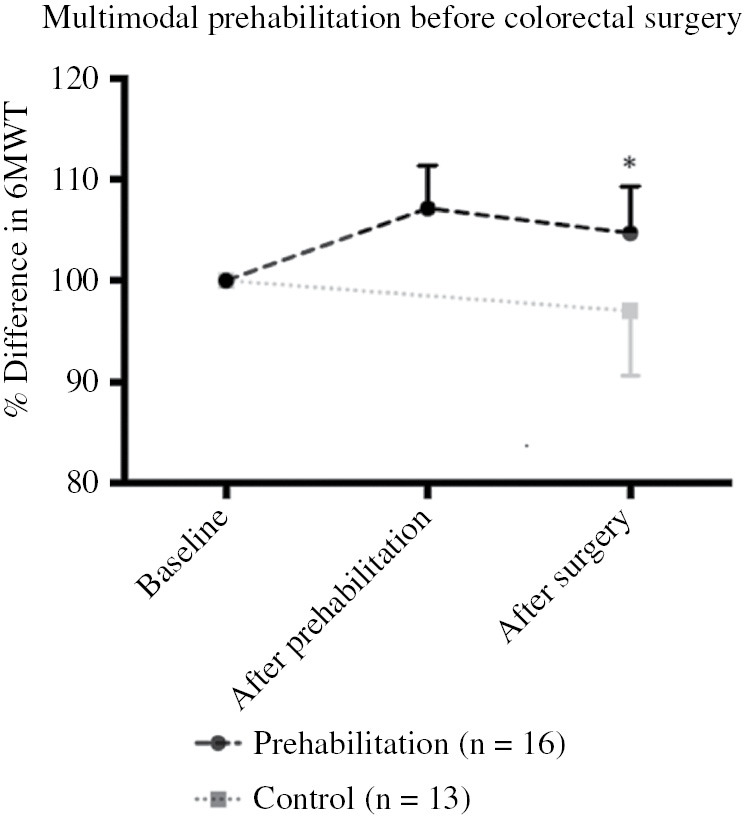
Pilot study on multimodal prehabilitation before colorectal cancer surgery.
Patients who underwent a prehabilitation program of 4-weeks before colorectal cancer surgery showed progress after training and performed better 4-weeks postoperatively (p<0.05, analysis of variance) on functional capacity (6MWT) compared to controls who received standard preoperative care.
It might sound logical to improve modifiable risk factors in the period before surgery to reduce complications, but the implementation of prehabilitation needs true courage and management changes within each hospital [51]. Even believers will face managers only looking at the cost of prehabilitation and do not take into account the potential benefits of the reduction of complications and hospital stay. In a sense, they might be right as evidence is not strong yet and recommendations are still weak [19].
High-level evidence that multimodal prehabilitation will reduce complications and improve outcome must come from randomized studies. The research group of Dr. G. Martínez-Palli from the Hospital Clínic de Barcelona published a study on 144 randomized patients undergoing major abdominal surgery. Prehabilitation contained a motivational interview, high-intensity training, and promotion of lifestyle and physical activity. Sixty-five patients were evaluated. In the intervention group, aerobic capacity was enhanced and the number of patients with postoperative complications was reduced by 51%. Also, the rate of complications was reduced significantly [11]. The latest systematic reviews on prehabilitation in abdominal surgery described similar beneficial effects of prehabilitation but also stated the need for a well-designed randomized controlled trial to evaluate the positive effects in more detail and to identify suitable target populations [12], [13]. Several studies using hospital- or home-based prehabilitation for various surgical procedures have been or will be initiated [50], [52], [53], [54], [55]. We hope that more evidence will be available before the publication of the results of the international PREHAB study in colorectal cancer at the end of 2020, as we do not want to wait with broad implementation.
A potential negative aspect of prehabilitation could be a delay of surgery. The interval between referral and treatment, the so called “window of opportunity”, is limited. However, at least some extra time can be bought by optimizing the diagnostic pathway. Our personal experience is that the interval between referral and diagnosis as determined during a multidisciplinary team meeting can be as short as 1 week. At Máxima MC, the delay caused by the prehabilitation program in patients with colorectal surgery was limited to 1 week. The literature, however, states that a treatment delay of 56–62 days (>8 weeks) should not be detrimental for the patient with respect to long-term outcomes [56], [57]. A recent publication might be very persuasive, demonstrating improved disease-free survival after prehabilitation for stage III colorectal cancer surgery [58].
International cooperation
In 2017, Professor Carli brought together a limited number of international experts on perioperative care and prehabilitation in Montréal, Canada. All aspects of multimodal prehabilitation were discussed and definitions were determined. The next convention, called the Second Prehabilitation World Conference, was held in Eindhoven, The Netherlands, in 2018, hosting more than 450 delegates from 22 countries. During the 2018 conference, the International Prehabilitation Society was initiated (www.prehabsociety.com). The society, together with the Royal College of Anaesthetists, organized the Third World Conference at the British Museum in London. The program was sold out and hosted more than 700 surgeons, anesthetists, (specialized) nurses, physical therapists, nutritionists, sports physicians, psychologists, urologists, orthopedists, and representative from other disciplines. A guideline on prehabilitation for cancer patients developed on behalf of Macmillan cancer support together with the Royal College of Anaesthetists and the National Institute of Health Research was presented at this congress. This document proposes a framework for implementation [59].
The 2020 event will be held in Barcelona, Spain, and the 2021 event is scheduled in Melbourne, Australia.
The International Prehabilitation Society wants to bring together all stakeholders involved in making patients fit for surgery. Although we believe in high-intensity, multimodal prehabilitation, any initiative to improve a patients’ physical, nutritional, and mental status before surgical or medical therapy is considered of value. We want to share knowledge and protocols while gathering the evidence that is needed for the broad implementation of prehabilitation. The International Prehabilitation Society has initiated a communication platform for members and nonmembers and encourages every country to create a national chapter and website to facilitate national cooperation. Healthcare professionals who want to contribute to the German Chapter are kindly asked to contact Dr. Florian Herrle (florian.herrle@umm.de).
Conclusion
Multimodal prehabilitation before surgery increases the patients’ physical, nutritional, and mental status before surgery. There is growing evidence that prehabilitation leads to a reduction of complications and a faster recovery after surgery. Although it has already been adopted in several new guidelines, we are still at the threshold of implementation. The question remains: Should we offer our patients the chance to get fit for surgery now or do we need to wait for more evidence?
Supporting Information
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/iss-2019-0017).
Author statement
Research funding: The international PREHAB study was partially funded by The Dutch Cancer Society (KWFKankerbestrijding) and by an Investigator Initiated Study grant from Johnson & Johnson Medical. Protein in the study was donated by FrieslandCampina. Conflict of interest: The authors stated no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research was not related to either human or animal use.
Author contributions
Charlotte J.L. Molenaar: conceptualization; visualization; writing – original draft. Nicole E. Papen-Botterhuis: writing – original draft; writing – review and editing. Florian Herrle: writing – original draft; writing – review and editing. Gerrit D. Slooter: conceptualization; visualization; writing – original draft.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1]. Silver JK, Baima J Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013;92:715–27. [DOI] [PubMed] [Google Scholar]
- [2]. Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am 2017;28:49–64. [DOI] [PubMed] [Google Scholar]
- [3]. West MA, Wischmeyer PE, Grocott MP Prehabilitation and nutritional support to improve perioperative outcomes. Curr Anesthesiol Rep 2017;7:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [5]. Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol 2017;56:295–300. [DOI] [PubMed] [Google Scholar]
- [6]. Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg 2018;153:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121:937–47. [DOI] [PubMed] [Google Scholar]
- [8]. Gillis C, Fenton TR, Sajobi TT, Minnella EM, Awasthi R, Loiselle SÈ, et al. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: a pooled analysis of randomized controlled trials. Clin Nutr 2019;38:1053–60. [DOI] [PubMed] [Google Scholar]
- [9]. Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SMH, et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 2018;27:289–98. [DOI] [PubMed] [Google Scholar]
- [10]. Lindback Y, Tropp H, Enthoven P, Abbott A, Oberg B PREPARE: presurgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial. Spine J 2018;18:1347–55. [DOI] [PubMed] [Google Scholar]
- [11]. Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–6. [DOI] [PubMed] [Google Scholar]
- [12]. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [13]. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg 2019;43:1661–8. [DOI] [PubMed] [Google Scholar]
- [14]. Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [15]. van Rooijen SJ, Molenaar CJL, Schep G, van Lieshout RHMA, Beijer S, Dubbers R, et al. Making patients fit for surgery: introducing a four pillar multimodal prehabilitation program in colorectal cancer. Am J Phys Med Rehabil 2019;98:888–96. [DOI] [PubMed] [Google Scholar]
- [16]. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology 2018;155:391–410.e4. [DOI] [PubMed] [Google Scholar]
- [17]. Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504–12. [DOI] [PubMed] [Google Scholar]
- [18]. Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:285–305. [DOI] [PubMed] [Google Scholar]
- [19]. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg 2019;43:659–95. [DOI] [PubMed] [Google Scholar]
- [20]. Tew GA, Ayyash R, Durrand J, Danjoux GR Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery. Anaesthesia 2018;73:750–68. [DOI] [PubMed] [Google Scholar]
- [21]. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1684–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018;392:2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Buia A, Stockhausen F, Hanisch E Laparoscopic surgery: a qualified systematic review. World J Methodol 2015;5:238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Abeles A, Kwasnicki RM, Darzi A Enhanced recovery after surgery: current research insights and future direction. World J Gastrointest Surg 2017;9:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kehlet H Manipulation of the metabolic response in clinical practice. World J Surg 2000;24:690–5. [DOI] [PubMed] [Google Scholar]
- [27]. Kehlet H, Joshi GP Enhanced recovery after surgery: current controversies and concerns. Anesth Analg 2017;125:2154–5. [DOI] [PubMed] [Google Scholar]
- [28]. Kehlet H, Wilmore DW Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98. [DOI] [PubMed] [Google Scholar]
- [29]. Yearly report Dutch Institute Clinical Auditing (DICA). 2017 [Internet] cited 2019 Aug 12 Available at: https://dica.nl/media/1583/DICA_Jaarrapportage_2017_-_Registraties.pdf. [Google Scholar]
- [30]. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:32–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. van Rooijen S, Carli F, Dalton SO, Johansen C, Dieleman J, Roumen R, et al. Preoperative modifiable risk factors in colorectal surgery: an observational cohort study identifying the possible value of prehabilitation. Acta Oncol 2017;56:329–34. [DOI] [PubMed] [Google Scholar]
- [32]. West MA, Asher R, Browning M, Minto G, Swart M, Richardson K, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 2016;103:744–52. [DOI] [PubMed] [Google Scholar]
- [33]. Wilson RJ, Davies S, Yates D, Redman J, Stone M Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth 2010;105:297–303. [DOI] [PubMed] [Google Scholar]
- [34]. Sorensen LT, Jorgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jorgensen P Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg 1999;86:927–31. [DOI] [PubMed] [Google Scholar]
- [35]. Burden ST, Hill J, Shaffer JL, Todd C Nutritional status of preoperative colorectal cancer patients. J Hum Nutr Diet 2010;23:402–7. [DOI] [PubMed] [Google Scholar]
- [36]. Baldini G, Ferreira V, Carli F Preoperative preparations for enhanced recovery after surgery programs: a role for prehabilitation. Surg Clin North Am 2018;98:1149–69. [DOI] [PubMed] [Google Scholar]
- [37]. Carli F, Ferreira V Prehabilitation: a new area of integration between geriatricians, anesthesiologists, and exercise therapists. Aging Clin Exp Res 2018;30:241–4. [DOI] [PubMed] [Google Scholar]
- [38]. Mayo NE, Feldman L, Scott S, Zavorsky G, Kim DJ, Charlebois P, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505–14. [DOI] [PubMed] [Google Scholar]
- [39]. O’Doherty AF, West M, Jack S, Grocott MP Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. Br J Anaesth 2013;110:679–89. [DOI] [PubMed] [Google Scholar]
- [40]. Calatayud J, Casana J, Ezzatvar Y, Jakobsen MD, Sundstrup E, Andersen LL High-intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:2864–72. [DOI] [PubMed] [Google Scholar]
- [41]. Yang A, Sokolof J, Gulati A The effect of preoperative exercise on upper extremity recovery following breast cancer surgery: a systematic review. Int J Rehabil Res 2018;41:189–96. [DOI] [PubMed] [Google Scholar]
- [42]. Poon JT, Fan JK, Lo OS, Law WL Enhanced recovery program in laparoscopic colectomy for cancer. Int J Colorectal Dis 2011;26:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Lawrence VA, Hazuda HP, Cornell JE, Pederson T, Bradshaw PT, Mulrow CD, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg 2004;199:762–72. [DOI] [PubMed] [Google Scholar]
- [44]. Scheede-Bergdahl C, Minnella EM, Carli F Multi-modal prehabilitation: addressing the why, when, what, how, who and where next?. Anaesthesia 2019;74:20–6. [DOI] [PubMed] [Google Scholar]
- [45]. Gillis C, Wischmeyer PE Pre-operative nutrition and the elective surgical patient: why, how and what?. Anaesthesia 2019;74 Suppl 1 27–35. [DOI] [PubMed] [Google Scholar]
- [46]. Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, et al. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci 2016;43:1190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Fukui M, Suzuki K, Matsunaga T, Oh S, Takamochi K Importance of smoking cessation on surgical outcome in primary lung cancer. Ann Thorac Surg 2019;107:1005–9. [DOI] [PubMed] [Google Scholar]
- [48]. Pierre S, Rivera C, Le Maitre B, Ruppert AM, Bouaziz H, Wirth N, et al. Guidelines on smoking management during the perioperative period. Anaesth Crit Care Pain Med 2017;36:195–200. [DOI] [PubMed] [Google Scholar]
- [49]. van Rooijen SJ, Huisman D, Stuijvenberg M, Stens J, Roumen RMH, Daams F, et al. Intraoperative modifiable risk factors of colorectal anastomotic leakage: why surgeons and anesthesiologists should act together. Int J Surg 2016;36:183–200. [DOI] [PubMed] [Google Scholar]
- [50]. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. van Rooijen SJ Colorectal cancer surgery: clinical improvements, introducing prehabilitation [dissertation] 2018. [Google Scholar]
- [52]. Yau DKW, Wong MKH, Wong WT, Gin T, Underwood MJ, Joynt GM, et al. PREhabilitation for improving QUality of recovery after ELective cardiac surgery (PREQUEL) study: protocol of a randomised controlled trial. BMJ Open 2019;9:e02797–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Allen S, Brown V, Prabhu P, Scott M, Rockall T, Preston S, et al. A randomised controlled trial to assess whether prehabilitation improves fitness in patients undergoing neoadjuvant treatment prior to oesophagogastric cancer surgery: study protocol. BMJ Open 2018;8:e023190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Woodfield J, Zacharias M, Wilson G, Munro F, Thomas K, Gray A, et al. Protocol, and practical challenges, for a randomised controlled trial comparing the impact of high intensity interval training against standard care before major abdominal surgery: study protocol for a randomised controlled trial. Trials 2018;19:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Berkel AEM, Bongers BC, van Kamp MS, Kotte H, Weltevreden P, de Jongh FHC, et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: study protocol of a randomized controlled trial. BMC Gastroenterol 2018;18:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Strous MTA, Janssen-Heijnen MLG, Vogelaar FJ Impact of therapeutic delay in colorectal cancer on overall survival and cancer recurrence – is there a safe timeframe for prehabilitation?. Eur J Surg Oncol 2019;45:2295–301. [DOI] [PubMed] [Google Scholar]
- [57]. Hangaard Hansen C, Gogenur M, Tvilling Madsen M, Gogenur I The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol 2018;44:1479–85. [DOI] [PubMed] [Google Scholar]
- [58]. Trepanier M, Minnella EM, Paradis T, Awasthi R, Kaneva P, Schwartzman K, et al. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Ann Surg 2019;270:493–501. [DOI] [PubMed] [Google Scholar]
- [59]. Macmillan Cancer Support Prehabilitation for people with cancer: principles and guidance for prehabilitation within the management and support of people with cancer [Internet]. London; 2019. cited 2019 Aug 14 Available at: www.macmillan.org.uk/assets/prehabilitation-guidance-for-people-with-cancer.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.