Heart failure with preserved ejection fraction (HFpEF) is increasingly common and presents challenges for accurate diagnosis. Two recent publications proposed HFpEF diagnostic tools: the HFA-PEFF algorithm1 and the H2FPEF score.2 Efforts to validate these scores in external populations have been limited by lack of a “gold-standard” phenotypic definition of HFpEF, and no prior validation has compared these scores against invasive hemodynamic criteria. We sought to evaluate these diagnostic approaches against a hemodynamic definition of HFpEF in individuals undergoing cardiopulmonary exercise testing (CPET).
We studied patients with chronic dyspnea and preserved left ventricular ejection fraction (≥50%) who underwent clinically-indicated CPET with invasive hemodynamic monitoring3 between 2006 and 2017. After exclusion criteria,3 there were 156 individuals with data required for both scores and available echocardiogram within one year of CPET date. All subjects underwent comprehensive testing including resting and exercise hemodynamic measurements, CPET gas exchange, and NT-proBNP. We defined our reference standard (invasive HFpEF, HFpEFinv) using the following criteria: elevated pulmonary capillary wedge pressure (PCWP) at rest (rPCWP ≥15 mmHg) OR during exercise (exPCWP ≥15 mmHg) coupled with an abnormally steep change in PCWP relative to cardiac output (CO) (ΔPCWP/ΔCO>2.0 mmHg·L−1·min−1), based on the established physiologic and prognostic significance of this threshold from our prior work.3, 4 Echocardiograms were reviewed per society guidelines.5 Risk stratification by each score was compared against HFpEFinv. HFA-PEFF analysis included evaluation of non-invasive score alone along with the algorithm’s invasive criteria. The study was approved by Massachusetts General Hospital’s institutional review board, and all participants provided informed consent. Study data will be shared upon reasonable request to corresponding author.
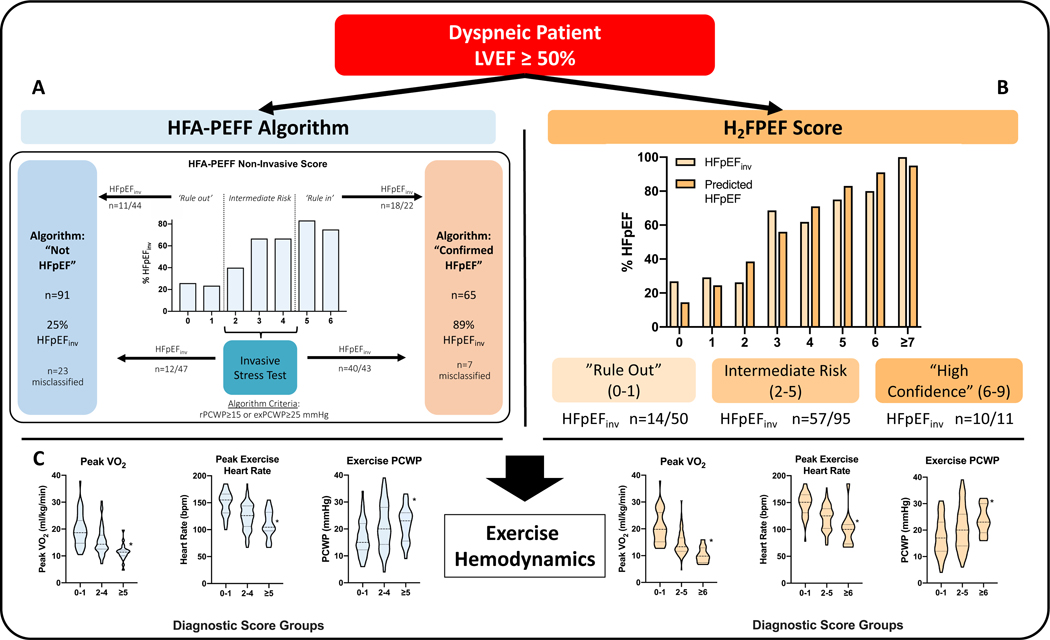
A total of 156 participants (59±16 years, 67% women) were included. Across both tools, individuals with higher scores were older and had more comorbidities including diabetes, hypertension, and hyperlipemia (p<0.01 for all). For the HFA-PEFF algorithm, 44 (28%) participants had ‘rule out’ scores (0–1 points) (Figure A). Within this low-risk group, 11 (25%) were found to have HFpEFinv. Conversely, 22 (14%) had ‘rule in’ scores (≥5 points), of whom 4 (18%) did not have HFpEFinv, bringing the number misclassified by the non-invasive portion to 15/66 (23%). After addition of the algorithm’s invasive criteria, there was agreement in classification when compared with HFpEFinv in 126/156 (81%), with total misclassification of 30 patients (19%).
Figure |.
Classification of the total sample (n=156) according to the HFA-PEFF algorithm** (A) and the H2FPEF score (B), along with corresponding rates of invasively-defined HFpEFinv. For the H2FPEF score, predicted HFpEF prevalence was derived from the original score publication.2 In (C), important metrics of exercise performance are shown stratified by HFA-PEFF and H2FPEF scores.
* Denotes P<0.05 in pairwise comparison of low vs high groups using two-tailed T-tests or Wilcoxon rank-sum test, as appropriate for the data distribution, when PANOVA or PKruskal-Wallis is significant across groups.
** Global longitudinal strain was not performed and thus was excluded from HFA-PEFF non-invasive score; left atrial linear dimensions were used in place of left atrial volumes. Diastolic stress test was not performed.
For the non-invasive score, the negative predictive value of scores of 0–1 was 75% [95% CI 62–88%], while positive predictive value of scores of 5–6 was 82% [66–98%]. The area under the receiver operating characteristics (ROC) curve was 0.73 [0.65–0.81]. Sensitivity and specificity of the full algorithm were 72% [61–81%] / 91% [82–96%], while positive and negative predictive value were 89% [81–97%] / 75% [66–84%].
Figure (B) compares predicted vs. actual HFpEF prevalence across H2FPEF scores. Among the low score group (0–1 points, n=50), 14 individuals (28%) had confirmed HFpEFinv. Among 95 individuals with intermediate and 11 with high H2FPEF scores, 60% and 91% met HFpEFinv criteria, respectively. Using a cut point of ≥5, sensitivity and specificity were 31% [95% CI 21–42%] and 92% [83–97%], and the positive and negative predictive values were 81% [67–95%] and 55% [46–64%]. The area under the ROC curve was 0.74 [0.66–0.81].
Exercise capacity was markedly impaired across the entire cohort (peak oxygen consumption 16.1±5.7 ml/kg/min) and was worse in the high score groups (Figure C). Higher score groups also had more impaired chronotropic response and higher filling pressures.
We assessed diagnostic performance of two contemporary HFpEF diagnostic tools against an invasive hemodynamic definition. While both tools performed well overall and track closely with exercise performance, our findings highlight potential misclassification among individuals with low scores. These findings raise a note of caution about the use of these clinical approaches to rule out HFpEF in dyspneic patients, which appears to be the most salient limitation of both tools. Further, this underdiagnosis at low scores underscores the difficulty of using resting parameters to define a phenotype that is fundamentally exertional in nature and highlights the potential of exercise to serve as a diagnostic probe to unmask abnormal physiology.
While some argue for a fixed cutoff for exPCWP, we have chosen to use a criterion indexed to CO, given a) the incremental predictive ability of this definition above and beyond PCWP4 for clinical events and b) the strong physiologic rationale for incorporating measures of flow into pressure assessment, given the obligatory rise in filling pressures with increasing CO.4 We acknowledge several limitations, including a patient cohort that was relatively young compared to many HFpEF patients and the absence of a non-invasive ‘diastolic stress test’ and two important echocardiographic parameters (global longitudinal strain and left atrial volumes). In conclusion, we demonstrate good overall performance of the HFA-PEFF algorithm and the H2FPEF score, but highlight potential misclassification, particularly at low scores. Further validation of these tools in varied populations and clinical settings is required.
Acknowledgements:
Drs Churchill and Ho had full access to all the data in this study and take responsibility for its integrity and the data analysis.
Sources of Funding: This work was supported by grants from the National Institutes of Health [R01-HL134893 to JEH, R01-HL140224 to JEH, K24 HL153669 to JEH, R01-HL142809 to RM, K23-HL138260 to MN, and R01-HL131029 to GDL] and the American Heart Association [15GPSGC24800006 to GDL].
Disclosures: Dr. Ho has received research supplies from EcoNugenics, Inc and research grants from Bayer and Gilead Sciences.
References
- 1.Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 2.Reddy YNV, Carter RE, Obokata M, Redfield MM and Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Zampierollo GA, Jarolim P, Pappagianopoulos PP, et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation. 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]