Summary
The fetal-to-adult hemoglobin switch is regulated in a developmental stage-specific manner and reactivation of fetal hemoglobin (HbF) has therapeutic implications for treatment of β-thalassemia and sickle cell anemia, two major global health problems. Although significant progress has been made in our understanding of the molecular mechanism of the fetal-to-adult hemoglobin switch, the mechanism of epigenetic regulation of HbF silencing remains to be fully defined. Here, we performed whole-genome bisulfite sequencing and RNA sequencing analysis of the bone marrow-derived GYPA+ erythroid cells from β-thalassemia-affected individuals with widely varying levels of HbF groups (HbF ≥ 95th percentile or HbF ≤ 5th percentile) to screen epigenetic modulators of HbF and phenotypic diversity of β-thalassemia. We identified an ETS2 repressor factor encoded by ERF, whose promoter hypermethylation and mRNA downregulation are associated with high HbF levels in β-thalassemia. We further observed that hypermethylation of the ERF promoter mediated by enrichment of DNMT3A leads to demethylation of γ-globin genes and attenuation of binding of ERF on the HBG promoter and eventually re-activation of HbF in β-thalassemia. We demonstrated that ERF depletion markedly increased HbF production in human CD34+ erythroid progenitor cells, HUDEP-2 cell lines, and transplanted NCG-Kit-V831M mice. ERF represses γ-globin expression by directly binding to two consensus motifs regulating γ-globin gene expression. Importantly, ERF depletion did not affect maturation of erythroid cells. Identification of alterations in DNA methylation of ERF as a modulator of HbF synthesis opens up therapeutic targets for β-hemoglobinopathies.
Keywords: β-thalassemia, fetal hemoglobin, whole-genome bisulfite sequencing, ERF, epigenetics, methylation, genome editing, GYPA+ cells, CD34+ HSPCs, engraftment mice
Introduction
The developmental switch from fetal to adult hemoglobin (Hb) in humans is a stage-specific regulatory process.1 Abnormally high levels of fetal hemoglobin (HbF) in adult life, known as hereditary persistence of fetal hemoglobin (HPFH), described 60 years ago, ameliorate the debilitating clinical manifestation of β-globin mutations in β-thalassemia (MIM: 613985) and sickle cell disease.1,2 The molecular machinery involved in human Hb switching has been extensively studied, leading to identification of genetic modifier genes and the defining of the contribution of modifier variants in modulating the phenotypic diversity of β-thalassemia and sickle cell disease by regulating HbF levels.3, 4, 5
Two transcription factors (TFs), BCL11A and ZBTB7A/LRF, have been identified as major erythroid-specific HbF repressors,6, 7, 8, 9 and both have recently been shown to bind cis-acting elements in the γ-globin promoter.10,11 A domain-focused CRISPR screen has identified heme-regulated inhibitor12 as a potentially druggable target involved in HbF silencing through the regulation of BCL11A.13 In addition, a series of non-deleted HPFH mutations have been characterized in association with elevated levels of HbF through disruption of BCL11A or ZBTB7A binding2,10,11 or creation of a de novo binding site for the erythroid master regulators TAL1, KLF1, and GATA1.2,14, 15, 16 The identification of these naturally occurring mutations has provided potential therapeutic targets for genetic treatment of β-hemoglobinopathies, and some of these are currently being explored. Substantial efforts have also been made to introduce these mutations in the human hematopoietic stem and progenitor cells (HSPCs) with high efficiency through a genome-editing strategy to allow for stable and autologous reactivation of HbF.1,2,17, 18, 19, 20
Silencing of the γ-globin genes (HBG1 [MIM: 142200]/HBG2 [MIM: 142250]) is regulated by the combined action of numerous factors, among which BCL11A and ZBTB7A are direct repressors essential for this regulation.1,2,17,18 Epigenetic regulators such as the NuRD chromatin complex, EHMT, DNMT3A, and KDM1A, along with lineage-defining factors such as KLF1 and MYB, play crucial roles in coordinating the expression of BCL11A (MIM: 606557) or ZBTB7A (MIM: 605878).1,21, 22, 23 In addition, a recent study unraveled LIN28B-mediated genetic silencing of HbF through a direct suppression of BCL11A mRNA expression,24 indicating additional post-transcriptional or epigenetic mechanisms in regulation of HbF expression. In this context, we recently performed a next-generation sequencing (NGS)-based screening of variants in globin gene clusters in a large cohort of β-thalassemia-affected individuals and identified that a common regulatory single nucleotide polymorphism (rSNP), rs368698783, in the HBG promoter is a major genetic modifier capable of ameliorating the severity of thalassemia major through the epigenetic-mediated elevation of HbF expression.25 More recently, we identified a missense mutation, c.2633G>A (p.Ser878Phe), in DNA methyltransferase 1 (DNMT1) in β-thalassemia-affected individuals as a modulator of HbF synthesis by attenuating the interactions of DNMT1 with BCL11A, GATA1, and HDAC1/2 and reducing the recruitment of DNMT1 to the HBG promoters.26 These findings suggest that more genetic and epigenetic factors contributing to HbF reactivation involved in ameliorating β-thalassemia severity remain unidentified.
Alternatively, the present study focused on exploring the protective nature of HPFH resulting from intrinsic epigenetically related regulators in addition to genetic alterations.1,27 Clinically, natural variations in HbF levels produce a wide range of expression from no expression to nearly 100% in β-thalassemia-affected individuals,28 thereby providing a valuable tool to identify candidate epigenetic modulators of HbF by genome-wide analysis of samples from β-thalassemia-affected individuals. We designed a case-based strategy by combining whole-genome bisulfite sequencing (WGBS) and RNA sequencing (RNA-seq) analysis of bone marrow (BM)-derived GYPA+ erythroid cells from β0-thalassemia-affected individuals stratified into low- and high-HbF expression groups to screen for potential epigenetic modulators of HbF expression. This strategy led to the identification of ERF as an HbF repressor in the discovery cohort of individuals with hypermethylation of the ERF (MIM: 611888) promoter as a distinguishing feature of individuals with high HbF levels. This finding was further validated in the GYPA+ cells from an independent cohort of 47 β-thalassemia-affected individuals. Furthermore, using a series of in vivo and in vitro assays, we demonstrated that ERF acted as a transcription repressor of γ-globin genes through direct binding on their distant regulatory elements. Our study provided an epigenetic mechanism for the reactivation of fetal γ-globin expression.
Material and methods
Study design
BM-derived GYPA+ erythroblasts from six unrelated Chinese β0/β0-thalassemia-affected individuals with widely varying levels of HbF were stratified into low-HbF (HbFL: 0.1−0.4 g/dL, n = 3) and high-HbF (HbFH: 8.9−9.2 g/dL, n = 3) groups and screened for candidate epigenetic modulators of HbF expression via WGBS and RNA-seq analysis. The clinical features and genetic analysis of the six affected β-thalassemia individuals and their pedigrees are presented in the supplemental information (Tables 1 and S1, Figure S1). All six subjects genotyped as β0/β0 and αα/αα and tested negative for deletional HPFH and δβ-thalassemia. Peripheral blood (PB)-derived GYPA+ erythroblasts from 47 unrelated individuals with β-thalassemia comprising of low- and high-HbF groups (34 versus 13) were used as a validation set for the identified target gene through bisulfite pyrosequencing. To uncover the role of epigenetic mechanisms in regulating the reactivation of γ-globin expression, we analyzed the function of a target gene by using various in vitro assays with primary human CD34+ HSPCs or HUDEP-2 cells and in vivo assays by engrafting of edited human CD34+ HSPCs into immunodeficient NCG-Kit-V831M mice. To investigate the mechanistic role of ERF in repressing γ-globin, we performed chromatin immunoprecipitation sequencing (ChIP-seq), ChIP-quantitative real-time PCR, and dual luciferase reporter assays following genome editing. All studies included at least two to three biological replicates. Written informed consent was obtained from all the participants and/or their parents. Approval for the extended cohort study was obtained as outlined by the protocol #NFEC-2019-039 approved by Medical Ethics Committee of Nanfang Hospital of Southern Medical University. The study was conducted in accordance with the Declaration of Helsinki.
Table 1.
The phenotypic and genotypic data in six subjects
| Characteristics |
HbFLgroupa |
HbFHgroupa |
p valueb | ||||
|---|---|---|---|---|---|---|---|
| YH | DZ | JWc | JS | FN | JQ | ||
| Age (years)/sex | 7/M | 7/M | 9/M | 10/F | 15/M | 26/M | 0.158/– |
| Age of onset (months) | 8 | 6 | 7 | 108 | 72 | 84 | 0.016 |
| No. of transfusions/year | 12 | 17 | 18 | 2d | 1d | 0 | – |
| Hb (g/dL)e | 7.6 | 4.5 | 4.4 | 9.2 | 9.3 | 9.7 | 0.063 |
| HbF (%)f | 1.3 | 8.9 | 2.3 | 98.9 | 95.7 | 94.8 | 0.000 |
| HbA2 (%) | 2.6 | 2.8 | 3.4 | 1.7 | 5.1 | 5.7 | 0.959 |
| MCV (fL) | 67.3 | 63.0 | 67.9 | 78.2 | 74.0 | 69.0 | 0.468 |
| MCH (pg) | 20.4 | 20.1 | 16.3 | 25.9 | 25.6 | 22.0 | 0.589 |
| MCHC (g/dL) | 33.3 | 33.7 | 34.6 | 31.3 | 34.7 | 31.8 | 0.341 |
| HBB genotypeg | c.[126_129delCTTT]; [52A>T] | c.[126_129delCTTT]; [92+1G>T] | c.[126_129delCTTT]; [52A>T] | c.[84_85insC]; [84_85insC] | c.[216_217insA]; [52A>T] | c.[126_129delCTTT]; [92+1G>T] | – |
| HBA genotype | αα/αα | αα/αα | αα/αα | αα/αα | αα/αα | αα/αα | – |
| Clinical diagnosish | TM | TM | TM | TI | TI | TI | – |
| Methylation level (%)i | 35.9 | 40.6 | 44.2 | 53.3 | 55.8 | 61.7 | 0.002 |
| Modifier genes genotype | |||||||
| BCL11A rs766432 | AA | AA | AA | AC | AC | AC | – |
| HBS1L-MYB rs9399137 | TT | TT | TT | TT | TT | TC | – |
| KLF1 mutation | WT | WT | WT | WT | WT | WT | – |
| HBG1 rs368698783 | GG | GA | GG | AA | GG | GA | – |
| HBG2 rs7482144 | CC | CT | CC | TT | CC | CT | – |
| Deletional HPFH | N | N | N | N | N | N | – |
M, male; F, female; No., number; TM, β-thalassemia major; TI, β-thalassemia intermedia; Hb, hemoglobin; HbA2, hemoglobin A2; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WT, normal; N, no deletional HPFH mutation. Dashes in the p value column denote no value. Diagnostic data and clinical details of these six individuals are included in the supplemental information.
Physical examination showed hepatosplenomegaly in all six individuals.
p values were determined by Student’s t test.
The individual died of severe anemia in 2015 and did not regularly receive blood transfusion.
After the illness, they received blood transfusions one or two times.
To more comprehensively reflect the endogenous situation of hematological parameters for our individuals, hematological data were collected before blood transfusion.
HbF expressed in g/dL for individuals YH, DZ, JW, JS, FN, and JQ was as follow: 0.1, 0.4, 0.1, 9.1, 8.9, and 9.2 g/dL, respectively.
HBB: c.126_129delCTTT, HBB: c.52A>T, HBB: c.216_217insA, HBB: c.92+1G>T, and HBB: c.84_85insC genotype categories are defined as β0/β0.
The mean methylation levels of the eight CpG sites on the ERF promoter calculated by bisulfite sequencing of BM-derived GYPA+ cells from individuals.
Genome editing by CRISPR/Cas9
To generate the ERF, ZBTB7A, BCL11A, UEBS, and DEBS knockout (KO) HUDEP-2 cells, we designed sgRNAs as previously described.31 SgRNA oligonucleotides were cloned into the lentiCRISPR vector (Addgene plasmid ID 52961) through restriction site BsmBI. Lentiviral transduction was performed as previously described.32 To generate the ERF, UEBS, and DEBS KO CD34+ HSPCs, we chemically modified sgRNAs as previously described33 to optimize knockout efficiency. CD34+ HSPCs were cultured in StemSpan SFEM medium supplemented with 100 ng/mL hTPO, 100 ng/mL Flt3l, and 100 ng/mL hSCF for 24 h prior to electroporation. Cells were incubated with 20 μg Cas9 (A36496, Thermo, USA) and 0.2 nmol sgRNA complex for 10 min at room temperature and electroporated via Lonza 4D Nucleofector (V4XP3032) according to the manufacturer’s instructions. After the electroporation, CD34+ HSPCs were subjected to differentiation as previously described.34 On day 8, we harvested one-quarter of the cells for genomic DNA extraction to test the KO efficiency. We used the edited cells to perform quantitative real-time PCR and high-performance liquid chromatography (HPLC), as well as flow cytometry to measure γ-globin expression levels. ChIP assay was performed with ERF, UEBS, or DEBS KO HUDEP-2 cells.
We performed targeted CpG site methylation alteration in HUDEP-2 and CD34+ HSPCs with a dCas9/sgRNA system to induce hypermethylation of the target CpG site in the ERF promoter, as previously described.35 More details are described in the supplemental information.
Erythroid differentiation
Granulocyte colony-stimulating factor (G-CSF)-mobilized adult human CD34+ HSPCs were isolated from the PB of unaffected individuals and separated with a CD34 microbead kit (Miltenyi Biotec, Germany). CD34+ HSPCs were cultured according to a two-phase erythroid differential protocol.34 HUDEP-2 cells were cultured as previously described.36 To induce differentiation, we maintained cells in StemSpan SFEM supplemented with FBS (10%), Dox (1 μg/mL), EPO (3 IU/mL), and hSCF (100 ng/mL) for 5 days and then transferred them into StemSpan SFEM supplemented with FBS (30%), Dox (1 μg/mL), EPO (3 IU/mL), and hSCF (100 ng/mL) for 6 days.
Statistical analysis
The data analysis of WGBS and RNA-seq were performed with the BSseq and the NOIseq package, respectively. Details are provided in the supplemental information. A two-tailed Student’s t test and ANOVA from SPSS v.20 software were used for comparisons between the indicated groups studied. Data are shown as mean ± standard deviation (SD). p values of less than 0.05 were considered to be statistically significant.
Results
Epigenetic dysregulation of ERF is associated with HbF levels in β-thalassemia
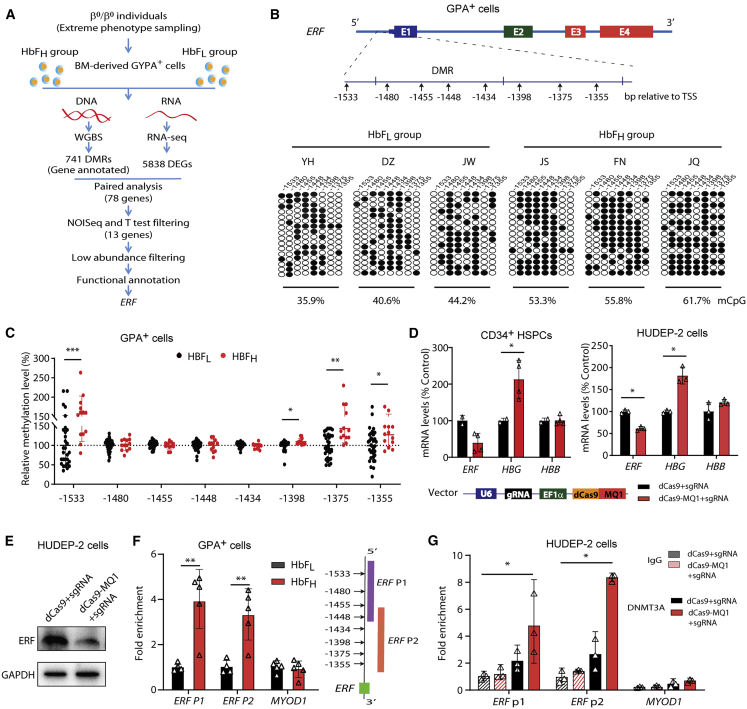
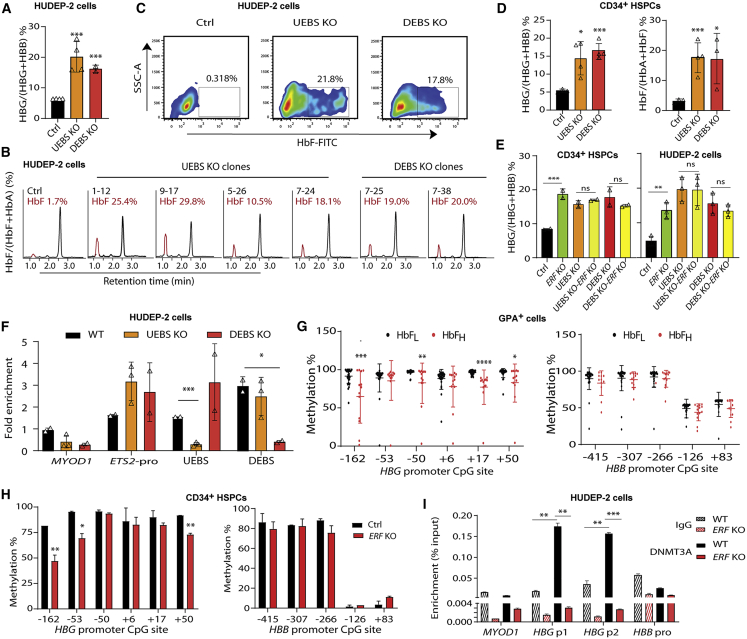
To identify potential epigenetic modulators of HbF in β-thalassemia, we integrated the WGBS and RNA-seq data of the BM-derived GYPA+ erythroid cells from six β-thalassemia-affected individuals with widely varying levels of HbF, designated as HbFH (HbF ≥ 95th percentile) and HbFL (HbF ≤ 5th percentile) groups (three subjects in each group; Tables 1 and S1, Figures 1A and S1). We identified 13 candidate genes among 78 differentially expressed genes (DEGs) with differentially methylated regions (DMRs) in their promoter regions that displayed either hypermethylation and downregulation or hypomethylation and upregulation in the HbFH group compared with the HbFL group (Figures 1A and S2A–S2C). After ruling out seven genes because of their low abundance (FPKM < 1), we focused on ETS2 repressor factor (ERF), which was a transcription factor among the six remaining genes (Table S2). ERF has recently been shown to be required for effective primitive and definitive hematopoiesis in mice.37 Moreover, we noted that most of the eight CpG sites within the ERF promoter were hypermethylated in all three individuals in the HbFH group from WGBS data (Figure S2D). We subsequently validated this result with a bisulfite cloning method (Figure 1B), suggesting a possible association of ERF promoter hypermethylation with an elevated HbF level.
Figure 1.
Identification of ERF as an HbF repressor through transcriptomic and methylation studies in β-thalassemia-affected individuals
(A) A flowchart for the screening of candidate modulators of HbF. The threshold for a positive DMR is defined as differential methylation level ≥ 10% (see the supplemental materials). We screened 741 out of the top 3,000 DMRs (top 3,000 hyper-DMR or hypo-DMR), which are annotated to be located in the promoter regions. 5,835 DEGs were identified according to the following criteria: |log2FC| > 0.8 and divergence probability > 0.8 via NOISeq. 78 genes were enrolled in the candidate list as they presented significantly differential expression in both mRNA and methylation level between HbFL and HbFH groups. We further screened 13 out of the 78 DE-mRNAs (|log2FC| > 0.8 and divergence probability > 0.8 in the NOISeq analysis and p < 0.1 in the further Student’s t test) containing DMRs in their promoter regions, from which we prioritized six candidate genes by filtering out the low abundance with FPKM < 1.0 in either the HbFH or HbFL group. We finally performed functional annotation and identified ERF as the candidate gene with top priority for functional validation.
(B) Top: schematic of ERF gene body. Bottom: analysis of methylation levels at CpG sites (indicated by the distance relative to the transcription start site, TSS) within the DMR in the ERF promoter, evaluated by sequencing. Data were generated with BM-derived GYPA+ cells from individuals with β0-thalassemia (HbFH: n = 3 or HbFL: n = 3) and used in WGBS assays. Each row of eight CpG sites within a group represents a single bisulfite-treated clone with methylated CpGs (•) or unmethylated CpGs (○).
(C) Quantitative measurement of methylation levels of the ERF promoter by bisulfite pyrosequencing in an independent cohort of 47 samples with β-thalassemia (HbFH: n = 13 or HbFL: n = 34). Methylation percentage of each subject relative to the mean level for the subjects with HbFL (the horizontal dashed line) is shown for each CpG site.
(D) Effects of ERF promoter hypermethylation by dCas9-MQ1/sgRNA on ERF, HBG, and HBB expression levels in CD34+ HSPCs (left) and HUDEP-2 cells (right). The schematic of dCas9-MQ1-sgRNA assay is shown in the bottom. MQ1 was methyltransferase that mediated hypermethylation in the target DNA region according to the guide of sgRNA. Two sgRNAs were designed to cover the region of eight CpG sites in the ERF promoter.
(E) Immunoblotting analysis in control and ERF promoter-targeted methylated HUDEP-2 cells. GAPDH served as a loading control.
(F) ChIP-quantitative real-time PCR assays performed with DNMT3A antibody in GYPA+ cells from the HbFH group (n = 5) and HbFL group (n = 5). ERF P1 and P2 covered the region of eight CpG sites in the ERF promoter, as shown in the right panel.
(G) ChIP-quantitative real-time PCR assays of DNMT3A performed in control (dCas9-sgRNA) and methylated (dCas9-MQ1-sgRNA) HUDEP-2 cells. MYOD1 served as the negative control. Data from ≥3 independent experiments are presented as means ± SD (∗p < 0.05; ∗∗p < 0.01).
Furthermore, we performed bisulfite pyrosequencing analysis of DNA from PB GYPA+ cells in an independent cohort of 47 randomly chosen samples of β-thalassemia (Table S3) and noted that four out of the eight CpG sites in the ERF promoter displayed hypermethylation in the HbFH groups compared with the HbFL groups (Figure 1C). Regression analysis showed that methylation levels at three (−1,355, −1,375, and −1,533) of these four CpG sites of the ERF promoter are significantly associated with HbF levels (Figure S3). Moreover, in vitro study confirmed that the ERF promoter hypermethylation is responsible for its low expressed mRNA (Figure 1D) and protein level (Figure 1E) as determined by site-specific methylation through the dCas9-MQ1-sgRNA system,35 which targeted the eight CpG sites within the ERF promoter in CD34+ HSPCs and HUDEP-2 cells (Figures S2E and S2F), leading to elevated γ-globin production (Figure 1D). These findings imply that hypermethylation-mediated ERF downregulation is associated with higher levels of HbF expression in β-thalassemia-affected individuals. Furthermore, enrichment of DNA methyltransferase 3A (DNMT3A) at the ERF promoter was significantly enhanced in the HbFH group compared with the HbFL group (Figure 1F), which was also noted in the ERF promoter site-specific methylated HUDEP-2 cells (Figure 1G). These results imply that the epigenetic silencing of ERF in the HbFH group is associated with DNMT3A-mediated hypermethylation of the ERF promoter.
ERF depletion elevates γ-globin expression in vitro in human CD34+ HSPCs and HUDEP-2 cells
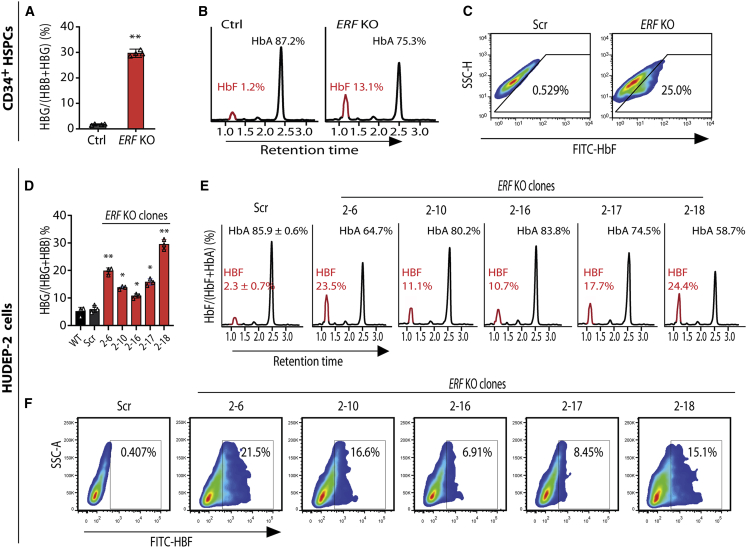
We then used CRISPR/Cas9 editing to generate ERF KO human CD34+ HSPCs for functional assays. Electroporation Cas9/sgRNA RNP into CD34+ HSPCs from a healthy donor19 resulted in a 90% KO efficiency on ERF (Figure S4A). We noted that the proportion of γ-globin mRNA as a percentage of total globin transcripts as assessed by quantitative real-time PCR was significantly increased (increasing from mean of 1.8% to 28.9%) in the ERF-depleted CD34+ HSPCs compared with the controls (Figure 2A), while HbF level was increased from mean 1.2% to 13.1% as assessed by HPLC (Figure 2B) and from mean 0.529% to 25.0% as assessed by flow cytometry assay for measurement of the percentage of HbF-positive cells (Figure 2C) as a consequence of ERF depletion. We further validated the ERF function in ERF KO HUDEP-2 cells (Figures S4B and S4C) and observed that five independent single ERF KO HUDEP-2 clones (Figures 2D and S5) displayed consistent increased HbF levels as assessed by HPLC (Figure 2E) and by flow cytometry (Figure 2F). Intriguingly, HbA level was observed to be decreased in ERF KO CD34+ HSPCs (Figure 2B) and HUDEP-2 cells (Figure 2E) as assessed by HPLC. Moreover, ERF knockdown (KD) and overexpression (OE) in HUDEP-2 cells and CD34+ HSPCs also confirmed the role of ERF as an HbF repressor (Figure S6).
Figure 2.
ERF depletion elevates γ-globin expression in vitro
(A–C) Quantitative measurement of HBG mRNA expression by quantitative real-time PCR (A) and HbF or HbA production by high performance liquid chromatography (HPLC) (B) and by flow cytometry analysis with FITC-conjugated anti-HbF antibody (C) in control (Ctrl or Scr, the non-edited control that has been subject to the same processes as the experimental lines without editing) and ERF KO CD34+ HSPCs.
(D–F) Quantitative measurement of HBG mRNA expression by quantitative real-time PCR (D) and HbF or HbA production by HPLC (E) and by flow cytometry analysis (F) in wild type (WT), Scr, and five independent single ERF KO HUDEP-2 clones. Data from ≥3 independent experiments are presented as means ± SD (∗p < 0.05; ∗∗p < 0.01).
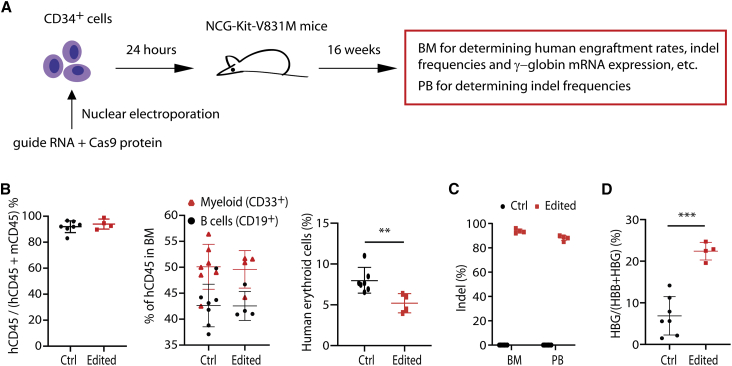
Validation of ERF as an HbF repressor via engraftment assays in immunodeficient mice
To test the impact of ERF editing on CD34+ HSPCs function, we performed in vivo transplantation experiments by engrafting edited ERF-deleted CD34+ HSPCs into immunodeficient NCG-Kit-V831M mice (Figure 3A), an experimental system extensively used to study stem cell function.38 Compared to unedited controls, ERF deletion did not affect either lymphoid (mean 42.3% for unedited versus mean 42.55% for edited) or myeloid (mean 50.37% for unedited versus mean 49.6% for edited) differentiation of the CD34+ in the BM 16 weeks following HSPCs transplantation (Figure 3B). However, a modest decline of erythroid cells was noted for the edited group compared with the control group (Figure 3B). These findings are consistent with previous reports of impaired repopulation ability and quantitative differences in the differentiation of hematopoietic stem cells in the Erf-deficient mice.37
Figure 3.
ERF depletion elevates γ-globin expression in vivo
(A) The experimental design for in vivo functional validations of ERF. ERF-targeted gRNA and Cas9 protein were electroporated into CD34+ HSPCs and after 24 h engrafted into immunodeficient mice by intravenous tail injection. Bone marrow (BM) and peripheral blood (PB) cells were harvested at week 16 after engraftment for further analysis.
(B) Flow cytometry analysis in mouse BM 16 weeks after transplantation for determination of human engraftment rates (left), human cell multilineage reconstitution (myeloid and B cells, middle), and human erythroid cells (right).
(C) Determination of the indel frequencies by Synthego analysis after sequencing of PCR products in PB and BM from engrafted mice.
(D) Measurement of HBG mRNA expression by quantitative real-time PCR in mouse BM 16 weeks after engraftment (n = 4 for edited mice and n = 7 for unedited mouse controls). Data are presented as means ± SD (∗∗p < 0.01; ∗∗∗p < 0.001).
We further noted that the variation of indel frequencies in the engrafted cells from edited mice were modest, 92% to 96% in BM cells and 85% to 90% in PB cells (Figure 3C), and that the indel frequencies in edited cells were consistent with that of input cells (88%), suggesting a high editing efficiency (Figure S7). Most importantly, in BM cells, we observed significant induction of γ-globin in edited cells (mean of 22.4% of total β-like globin in edited cells compared with mean of 6.9% in unedited cells; Figure 3D). These findings provided critical in vivo evidence for the role of ERF in repressing γ-globin synthesis.
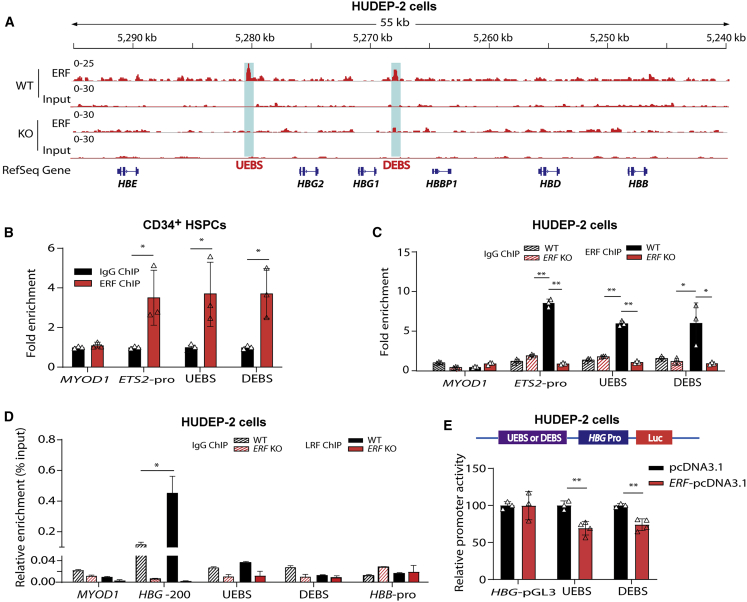
ERF represses γ-globin synthesis by direct binding to the motifs regulating HBG expression
To define the mechanistic basis for ERF in repressing γ-globin, we performed RNA-seq analysis of ERF KO HUDEP-2 cells to evaluate genome-wide gene expression changes induced by ERF depletion. Gene expression profiling and quantitative real-time PCR analysis demonstrated that depletion or overexpression of ERF did not affect the expression of BCL11A, ZBTB7A, KLF1, and MYB (Figures S8A–S8D). Gene Ontology (GO) analysis of differentially expressed genes induced by ERF KO in HUDEP-2 cells was not evident in gene expression signatures relevant to known erythroid transcription factors (Figure S8E). These results suggest that HbF silencing mediated by ERF is independent of previously defined erythroid repressors or complexes. Subsequent, in vitro study indicated that ERF/ZBTB7A or ERF/BCL11A double-knockout (DKO) cells exhibited significantly higher γ-globin expression than ZBTB7A, BCL11A, or ERF single-KO cells (Figures S8F and S8G). This result supports that ERF does not regulate HbF through known HbF modifiers.
We then performed ChIP-seq by using anti-ERF antibody to identify potential ERF-binding sites within the human β-globin gene cluster in HUDEP-2 cells. Intriguingly, we observed two significant ERF-binding signals at 3.6 kb upstream of HBG2 (termed as upstream ERF-binding site, UEBS) and at 1.5 kb downstream (termed as downstream ERF-binding site, DEBS) of HBG1 (Figure 4A, track 1). Intriguingly, UEBS and DEBS are accessible for several known transcription factors and histone modification markers based on public ENCODE ChIP-seq datasets (Figure S9).39,40 Moreover, ChIP-seq results obtained from ERF KO HUDEP-2 cells showed a consistent absence of peaks in UEBS and DEBS (Figure 4A, track 3), which was further confirmed by ChIP-quantitative real-time PCR with anti-ERF antibody in CD34+ HSPCs (Figure 4B) and in ERF KO HUDEP-2 cells (Figure 4C). We also explored whether LRF bound to these two cis-elements to coordinately mediate the silencing of γ-globin genes. ChIP-quantitative real-time PCR results demonstrated LRF showed an independent binding pattern (Figure 4D), consistent with previous studies.11 We further demonstrated that overexpression of ERF inhibited the promoter activity of the luciferase reporter containing UEBS or DEBS in HUDEP-2 cells (Figure 4E).
Figure 4.
Identification of ERF-binding sites in β-globin gene clusters
(A) ChIP-seq binding patterns from HUDEP-2 cells with anti-ERF antibody at the β-globin cluster. Two ERF-binding positive signals 3.6 kb upstream of HBG2 (named UEBS) and 1.5 kb downstream of HBG1 (named DEBS) are marked by the light blue shadows.
(B) Detection of ERF-binding sites by ChIP-quantitative real-time PCR in human CD34+ HSPCs.
(C and D) Detection of ERF-binding (C) or LRF-binding (D) activity in ERF KO HUDEP-2 cells (clone #2–6). IgG served as the negative control for ChIP. MYOD1 and HBB-pro served as the negative control for quantitative real-time PCR. ETS2-pro served as the positive control for quantitative real-time PCR. HBG-200, −200 bp upstream of the TSS in the HBG promoter, served as the positive control for LRF binding.
(E) Top: the schematic of dual luciferase reporter assay. Bottom: effects of UEBS or DEBS as regulatory elements on HBG promoter activity in a pGL3 luciferase reporter in HUDEP-2 cells without (black) or with (bright red) ERF overexpression (OE). The data of each group were normalized to the control (pcDNA3.1). Data are presented as means ± SD (∗p < 0.05; ∗∗p < 0.01).
To evaluate the effects of these two cis-elements on regulation of HbF expression, we disrupted the UEBS or DEBS motifs by using Cas9 system in HUDEP-2 cells and in CD34+ HSPCs (Figure S10A). We carried out copy number analysis on β-globin clusters to rule out the off-target effects that could potentially be caused by the highly duplicated nature of HBG1 and HBG2 and found both HBGs and their intergenic regions to be intact except for the ERF-binding sites (Figures S10A–S10C). We established independent UEBS or DEBS KO HUDEP-2 clones (Figures S10D and S10E) and determined the HBG mRNA level and HbF levels in these cells. We observed significant increases of expressed HBG mRNA (UEBS: mean 20.1% of total β-like globin from four clones; DEBS: mean 16.2% from two clones) and of HbF protein levels (UEBS: mean 21.0% of total hemoglobin; DEBS: mean 19.5%) in edited HUDEP-2 cells (Figures 5A–5C). Similar increases in HBG-expressed mRNA and HbF level were also observed in edited CD34+ HSPCs (expressed mRNA: mean 14.4% and 16.5% in UEBS KO and DEBS KO cells, respectively, and HbF level: mean 17.7% and 16.9% in UEBS KO and DEBS KO cells, respectively) (Figures 5D, S10F, and S10G). The observed changes in HBG mRNA expression in various gene-edited HUDEP-2 and CD34+ HSPCs by disruption of ERF and/or of the two binding sites (Figure 5E), as well as absence of ERF-binding activity in UEBS and DEBS KO HUDEP-2 cells (Figure 5F), confirm an essential role of both ERF and its binding motifs as cis-elements in regulation of HbF expression.
Figure 5.
Knockout of ERF-binding sites led to elevation of HbF in HUDEP-2 and CD34+ HSPCs
(A–C) Quantitative measurement of ERF mRNA expression by quantitative real-time PCR (A) and HbF production by HPLC (B) and by flow cytometry analysis (C) in UEBS/DEBS KO HUDEP-2 clones (n = 4 for UEBS and n = 2 for DEBS, each point indicates the mean value for each clone). The HPLC profiles of HbF from each of the six independent single UEBS/DEBS KO HUDEP-2 clones are shown.
(D) Quantitative measurement of HBG mRNA expression by quantitative real-time PCR (left) and HbF production by HPLC (right) in UEBS/DEBS KO CD34+ HSPCs (editing efficiency: 30% for UEBS, 23% for DEBS).
(E) Quantitative measurement of HBG mRNA expression by quantitative real-time PCR in single or double KO of ERF and/or UEBS (UEBS KO-ERF KO) or DEBS (DEBS KO-ERF KO) CD34+ HSPCs (left) and HUDEP-2 cells (right). The γ-globin expression levels were determined as a percentage of the total β-like globin level (HBG+HBB). One-way ANOVA was used for comparison of the indicated groups. ∗p < 0.05; ∗∗∗p < 0.01. ns, non-significant (p > 0.05).
(F) Detection of ERF-binding sites by ChIP-quantitative real-time PCR in UEBS or DEBS KO HUEDP-2 clones (n = 3 and n = 2, respectively). Data are presented as mean ± SD for each clone.
(G) Quantitative measurement of methylation levels of the HBG (left) and HBB (right) promoter by bisulfite sequencing in the independent cohort of 47 samples with β-thalassemia (HbFH: n = 13 or HbFL: n = 34).
(H) Quantitative measurement of methylation levels of the HBG promoter (left) and HBB promoter (right) by bisulfite sequencing in the ERF KO CD34+ HSPCs.
(I) ChIP-quantitative real-time PCR assay performed with DNMT3A antibody in HBG promoter in WT and ERF KO HUDEP-2 cells. HBG p1 and p2 covered the region of CpG sites from −162 to +50. MYOD1 and HBB pro served as controls.
To uncover the underlying mechanism of the inhibition of γ-globin genes induced by the epigenetic silencing of ERF, we evaluated the methylation levels of HBB and HBG promoters in the GYPA+ cells from the same cohort of 47 β-thalassemia-affected individuals in the above pyrosequencing analysis and found that epigenetic silencing of ERF is significantly associated with general hypomethylation of the HBG promoter (Figure 5G). We validated this finding by knocking out ERF or targeting hypermethylation of ERF in CD34+ HSPCs and found that the ERF depletion and promoter hypermethylation led to reactivation of HbF through hypomethylation of γ-globin genes promoter, typically in −162, −53, −50, +17, and +50 CpG sites (Figures 5H and S2G). In addition, ChIP-quantitative real-time PCR assays showed that the hypomethylation of γ-globin genes promoter induced by the depletion of ERF is likely to be mediated by impaired recruitment of DNMT3A on this CpG island (Figure 5I).
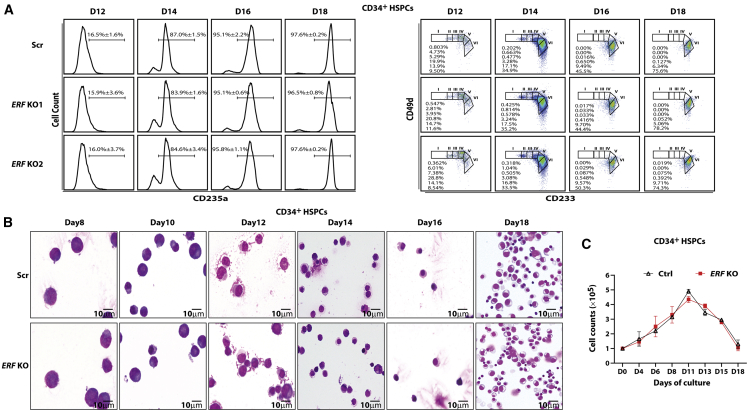
Knockout of ERF has no significant impact on the erythroid cell differentiation
Finally, we examined the effect of ERF on erythroid differentiation. We observed that ERF depletion exerts no significant impact on either erythroid maturation or terminal differentiation of CD34+ HSPCs, as assessed by CD235a, CD49d, and CD233 surface expression (Figure 6A). Furthermore, the morphology, enucleation rate, and growth of the erythroid cells following ERF depletion was comparable to that of wild-type cells (Figures 6B, 6C, and S11A). Similar findings were noted following KO of UEBS or DEBS (Figure S11B). We also observed that depletion of ERF, DEBS, or UEBS exerts no significant impact on erythroid differentiation in HUDEP-2 cells (Figures S11C–S11F). Taken together, these findings suggest that ERF might be a promising therapeutic target for the reactivation of HbF without significantly altering the terminal erythroid differentiation.
Figure 6.
The impact of ERF on the differentiation of CD34+ HSPCs
(A) Left: the expression of CD235a on different time points of culture of CD34+ HSPCs in percentage (mean ± SD, n = 3). Right: terminal erythroid differentiation was examined on indicated days by flow cytometric analysis based on the expression of CD233 and CD49d. Representative plots of CD233 versus CD49d of CD235a+ cells are shown, and the erythroblasts are separated into six populations: proerythroblasts (I), early baso erythroblasts (II), late baso erythroblasts (III), polychromatic (IV), early orthochromatic (V), and late orthochromatic (VI).
(B) Representative images of Wright-Giemsa staining of cytospins in different time points of differentiated CD34+ cells (objective lens, 100×).
(C) Cell growth curves of control and ERF KO CD34+ cells (mean ± SD, n = 2).
Discussion
It is important to identity genome-wide epigenetic modulators that account for phenotypic diversities in a Mendelian disorder. In the present study, we elucidated the epigenetic dysregulation of fetal-to-adult Hb switch in β-thalassemia and discovered epigenetic modulators associated with HbF levels. This strategy based on methylomic and transcriptomic profiling enabled us to identify regulators that cannot be readily detected by conventional GWASs because of the high conservation of their genomic sequences or alterations by epigenetic mechanisms.
Integrative studies of RNA-seq and WGBS led to the identification of candidate genes that were significant in both DEG and DMR analysis. Of the 13 candidate genes identified, ERF is a transcription factor that was able to regulate its target genes through a direct binding on its distant regulatory elements.41,42 ERF was previously reported to be expressed in hematopoietic stem cells and progenitors and displayed a dynamic expression pattern during erythroid differentiation; its expression increased from HSC to proerythroblast stage with subsequent decreased expression at late stages of terminal erythroid differentiation (Figure S12).43,44 Interestingly, previous studies showed that ERF acted as a transcription repressor indispensable for erythroid differentiation.42 Importantly, comprehensive in vitro and in vivo studies enabled us to document that downregulation of ERF in erythroid cells led to significant elevation of HbF, validating the hypothesis that ERF is a transcription repressor of γ-globin genes and its epigenetic downregulation results in reactivation of HbF. By systematically screening methylation states in the promoters of ERF, HBG1, and HBG2 in an independent and extended cohort of 47 β-thalassemia-affected individuals, we defined that hypermethylation of the ERF promoter had led to the haploinsufficiency of ERF, which eventually resulted in the hypomethylation of HBG promoters and re-activation of HbF (Figure 1D). This process of dysregulation in ERF and HBG promoters are mediated by altered recruitment of DNMT3A (Figures 1F, 1G, and 5I). The highly consistent findings from study of two independent cohorts imply that regulating hypermethylation of the ERF promoter represents a protective pathway to decrease ineffective erythropoiesis in β-thalassemia by increased expression of HbF.
In detailed analysis of the methylation states of the DMR, we noted that three of the four CpG sites (−1,533, −1,375, and −1,355) in the cohort of 47 β-thalassemia-affected individuals had statistically significant effects on HbF level. Interestingly, we observed that the differentially methylated sites detected in the extended cohort showed a different pattern compared with that of the six samples subjected to WGBS. This difference is probably due to the considerable dynamics of CpG islands at different developmental stages and different tissues and among different individuals. A single CpG site is also regarded as a methylation variable position (MVP), in which a CpG site contributing to phenotypic alterations is functionally equivalent to a rSNP.45 Therefore, the dysregulated methylation of diverse CpG sites within one single DMR can result in the downregulation of ERF among different individuals with high HbF levels. Future studies using a larger cohort of β-thalassemia-affected individuals should enable more systematic assessment of the heterogeneity of DNA methylation states among these CpG sites within the ERF promoter.
In screening for the known HbF modifier variants in the six β-thalassemia-affected individuals studied, we identified the three individuals in the HbFH group to be carriers of rs766432 in BCL11A, a functional SNP favorable for elevated HbF levels. Our previous studies indicated that the contribution of this variant to the elevation of HbF is relatively mild compared with XmnI (GenBank: NC_000011.9, g.5276169G>A) and KLF1 mutations in the Chinese population,4,25 suggesting the existence of other epigenetic factors responsible for high levels of HbF in this group. To further exclude the potentially confounding effects caused by this variant, we used the same panel for genetic screening relevant to HbF-regulating variants in the extended cohort of 47 β-thalassemia-affected individuals and did not find significant differential distributions in the genotypes of BCL11A, MYB-HBS1L, XmnI, and KLF1 between the HbFL and HbFH group, indicating that DMRs in the ERF promoter are independently associated with the elevation of HbF levels in β-thalassemia-affected individuals.
In terms of mechanistic understanding, we identified two ERF-binding sites, UEBS and DEBS, specifically located upstream of HBG2 and downstream of HBG1, respectively, that are functionally relevant (Figure 1D). A series of in vitro and in vivo studies demonstrate that depletion of ERF led to a considerable increase in γ-globin expression, while the expression of β-globin appeared to decrease proportionally (Figures 2). In addition, RNA-seq data of ERF KO HUDEP-2 cell lines indicated that ERF depletion does not lead to dramatic changes in the known key erythroid genes (Figures S8B–S8E). Moreover, we demonstrated that depletion of ERF attenuated recruitment of DNMT3A to the HBG promoter (Figure 5I), which resulted in demethylation-mediated HBG transcriptional activation. Intriguingly, we also observed that UEBS is associated with recruitments of the active chromatin marker H3K27ac or its deacetylase HDAC based on ENCODE ChIP-seq dataset (Figure S9),39,40 suggesting that ERF binding might interfere with H3K27 acetylation-mediated positive regulation through recruitment of HDAC or DNMT3A. Taken together, ERF acts as a specific repressor of γ-globin genes through an independent pathway of epigenetic regulation.
In summary, we developed a strategy for the identification of epigenetic modulators of HbF expression through extensive phenotype sampling (EPS) followed by transcriptomic and methylomic profiling. Through a series of functional validation studies, we identified an epigenetic pathway for silencing of γ-globin gene expression. This pathway initiated by hypermethylation of the ERF promoter mediated by enrichment of DNMT3A leads to hypomethylation of γ-globin genes and impaired binding of ERF on the HBG promoter and eventually re-activation of HbF in β-thalassemia-affected individuals. Moreover, we found that ERF knockout does not lead to observable changes in either erythroid differentiation or enucleation rate of human primary CD34+ HSPCs and HUDEP-2 cells. Given the high efficiency of site-specific methylation via the current dCas9-MQ1-sgRNA system, the ERF promoter-specific methylation might be a promising target for genetic therapies of β-hemoglobinopathies.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
We thank the individuals for their willingness to participate in this study; Xinping Yang, Shiqi Jia, Xichen Bao, Erwei Song, Yuxuan Wu, and Bingtao Hao for valuable advice and comments regarding this work; and Yi Wu, Feijin Chen, and Dun Liu and colleagues for assistance in collecting samples from individuals and controls. We thank the Central Laboratory of Southern Medical University for providing pyrosequencing reagents and platform. Financial supports from the National Key R&D Program of China (2018YFA0507800 and 2018YFA0507803), the Guangdong Science and Technology Foundation (2019B030316032), and NIH (DK32094) (N.M.) are gratefully acknowledged.
Published: March 17, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.03.005.
Contributor Information
Cunyou Zhao, Email: cyzhao@smu.edu.cn.
Xiangmin Xu, Email: gzxuxm@pub.guangzhou.gd.cn.
Data and code availability
All the data generated in this study were shown in the main text and supplemental information. The data of RNA-seq in ERF KO and ChIP-seq are available in GEO with the number GEO: GSE160603. The source data underlying Figures 1A and S2A–S2D are provided in Table S6. Additional information is also available upon reasonable request to the corresponding authors.
Web resources
ENCODE, https://www.encodeproject.org/
HUGO Gene Nomenclature Committee, https://www.genenames.org/
OMIM, https://omim.org/
Supplemental information
References
- 1.Vinjamur D.S., Bauer D.E., Orkin S.H. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br. J. Haematol. 2018;180:630–643. doi: 10.1111/bjh.15038. [DOI] [PubMed] [Google Scholar]
- 2.Wienert B., Martyn G.E., Funnell A.P.W., Quinlan K.G.R., Crossley M. Wake-up Sleepy Gene: Reactivating Fetal Globin for β-Hemoglobinopathies. Trends Genet. 2018;34:927–940. doi: 10.1016/j.tig.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Mettananda S., Higgs D.R. Molecular Basis and Genetic Modifiers of Thalassemia. Hematol. Oncol. Clin. North Am. 2018;32:177–191. doi: 10.1016/j.hoc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Liu D., Zhang X., Yu L., Cai R., Ma X., Zheng C., Zhou Y., Liu Q., Wei X., Lin L. KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of β-thalassemia. Blood. 2014;124:803–811. doi: 10.1182/blood-2014-03-561779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chondrou V., Kolovos P., Sgourou A., Kourakli A., Pavlidaki A., Kastrinou V., John A., Symeonidis A., Ali B.R., Papachatzopoulou A. Whole transcriptome analysis of human erythropoietic cells during ontogenesis suggests a role of VEGFA gene as modulator of fetal hemoglobin and pharmacogenomic biomarker of treatment response to hydroxyurea in β-type hemoglobinopathy patients. Hum. Genomics. 2017;11:24. doi: 10.1186/s40246-017-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzel S., Garner C., Gut I., Matsuda F., Yamaguchi M., Heath S., Foglio M., Zelenika D., Boland A., Rooks H. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat. Genet. 2007;39:1197–1199. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 7.Sankaran V.G., Menne T.F., Xu J., Akie T.E., Lettre G., Van Handel B., Mikkola H.K., Hirschhorn J.N., Cantor A.B., Orkin S.H. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 8.Uda M., Galanello R., Sanna S., Lettre G., Sankaran V.G., Chen W., Usala G., Busonero F., Maschio A., Albai G. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc. Natl. Acad. Sci. USA. 2008;105:1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda T., Wang X., Maeda M., Canver M.C., Sher F., Funnell A.P., Fisher C., Suciu M., Martyn G.E., Norton L.J. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science. 2016;351:285–289. doi: 10.1126/science.aad3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N., Hargreaves V.V., Zhu Q., Kurland J.V., Hong J., Kim W., Sher F., Macias-Trevino C., Rogers J.M., Kurita R. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell. 2018;173:430–442.e17. doi: 10.1016/j.cell.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martyn G.E., Wienert B., Yang L., Shah M., Norton L.J., Burdach J., Kurita R., Nakamura Y., Pearson R.C.M., Funnell A.P.W. Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat. Genet. 2018;50:498–503. doi: 10.1038/s41588-018-0085-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen J.J. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grevet J.D., Lan X., Hamagami N., Edwards C.R., Sankaranarayanan L., Ji X., Bhardwaj S.K., Face C.J., Posocco D.F., Abdulmalik O. Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells. Science. 2018;361:285–290. doi: 10.1126/science.aao0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wienert B., Funnell A.P.W., Norton L.J., Pearson R.C.M., Wilkinson-White L.E., Lester K., Vadolas J., Porteus M.H., Matthews J.M., Quinlan K.G.R., Crossley M. Editing the genome to introduce a beneficial naturally occurring mutation associated with increased fetal globin. Nat. Commun. 2015;6:7085. doi: 10.1038/ncomms8085. [DOI] [PubMed] [Google Scholar]
- 15.Wienert B., Martyn G.E., Kurita R., Nakamura Y., Quinlan K.G.R., Crossley M. KLF1 drives the expression of fetal hemoglobin in British HPFH. Blood. 2017;130:803–807. doi: 10.1182/blood-2017-02-767400. [DOI] [PubMed] [Google Scholar]
- 16.Martyn G.E., Wienert B., Kurita R., Nakamura Y., Quinlan K.G.R., Crossley M. A natural regulatory mutation in the proximal promoter elevates fetal globin expression by creating a de novo GATA1 site. Blood. 2019;133:852–856. doi: 10.1182/blood-2018-07-863951. [DOI] [PubMed] [Google Scholar]
- 17.Traxler E.A., Yao Y., Wang Y.D., Woodard K.J., Kurita R., Nakamura Y., Hughes J.R., Hardison R.C., Blobel G.A., Li C., Weiss M.J. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016;22:987–990. doi: 10.1038/nm.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Zeng J., Roscoe B.P., Liu P., Yao Q., Lazzarotto C.R., Clement K., Cole M.A., Luk K., Baricordi C. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 2019;25:776–783. doi: 10.1038/s41591-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Li L., Ma Y., Hu H., Li Q., Yang Y., Liu W., Yin S., Li W., Fu B. Reactivation of γ-globin expression through Cas9 or base editor to treat β-hemoglobinopathies. Cell Res. 2020;30:276–278. doi: 10.1038/s41422-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert O., Radtke S., Samuelson C., Carrillo R.R., Perez A.M., Reddy S.S., Lux C., Pattabhi S., Schefter L.E., Negre O. Therapeutically relevant engraftment of a CRISPR-Cas9-edited HSC-enriched population with HbF reactivation in nonhuman primates. Sci. Transl. Med. 2019;11:eaaw3768. doi: 10.1126/scitranslmed.aaw3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnanapragasam M.N., Scarsdale J.N., Amaya M.L., Webb H.D., Desai M.A., Walavalkar N.M., Wang S.Z., Zu Zhu S., Ginder G.D., Williams D.C., Jr. p66Alpha-MBD2 coiled-coil interaction and recruitment of Mi-2 are critical for globin gene silencing by the MBD2-NuRD complex. Proc. Natl. Acad. Sci. USA. 2011;108:7487–7492. doi: 10.1073/pnas.1015341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roosjen M., McColl B., Kao B., Gearing L.J., Blewitt M.E., Vadolas J. Transcriptional regulators Myb and BCL11A interplay with DNA methyltransferase 1 in developmental silencing of embryonic and fetal β-like globin genes. FASEB J. 2014;28:1610–1620. doi: 10.1096/fj.13-242669. [DOI] [PubMed] [Google Scholar]
- 23.Renneville A., Van Galen P., Canver M.C., McConkey M., Krill-Burger J.M., Dorfman D.M., Holson E.B., Bernstein B.E., Orkin S.H., Bauer D.E., Ebert B.L. EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood. 2015;126:1930–1939. doi: 10.1182/blood-2015-06-649087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basak A., Munschauer M., Lareau C.A., Montbleau K.E., Ulirsch J.C., Hartigan C.R., Schenone M., Lian J., Wang Y., Huang Y. Control of human hemoglobin switching by LIN28B-mediated regulation of BCL11A translation. Nat. Genet. 2020;52:138–145. doi: 10.1038/s41588-019-0568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D., Zuo Y., Zhang X., Ye Y., Bao X., Huang H., Tepakhan W., Wang L., Ju J., Chen G. A Genetic Variant Ameliorates β-Thalassemia Severity by Epigenetic-Mediated Elevation of Human Fetal Hemoglobin Expression. Am. J. Hum. Genet. 2017;101:130–138. doi: 10.1016/j.ajhg.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y., Zhang X., Zhang Q., Zhang Y., Ye Y., Yu W., Shao C., Yan T., Huang J., Zhong J. A natural DNMT1 mutation elevates the fetal hemoglobin via epigenetic de-repression of gamma-globin gene in beta-thalassemia. Blood. 2020 doi: 10.1182/blood.2020006425. Published online November 23, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Adelvand P., Hamid M., Sardari S. The intrinsic genetic and epigenetic regulator factors as therapeutic targets, and the effect on fetal globin gene expression. Expert Rev. Hematol. 2018;11:71–81. doi: 10.1080/17474086.2018.1406795. [DOI] [PubMed] [Google Scholar]
- 28.Sripichai O., Fucharoen S. Fetal hemoglobin regulation in β-thalassemia: heterogeneity, modifiers and therapeutic approaches. Expert Rev. Hematol. 2016;9:1129–1137. doi: 10.1080/17474086.2016.1255142. [DOI] [PubMed] [Google Scholar]
- 29.Karimi M., Cohan N., De Sanctis V., Mallat N.S., Taher A. Guidelines for diagnosis and management of Beta-thalassemia intermedia. Pediatr. Hematol. Oncol. 2014;31:583–596. doi: 10.3109/08880018.2014.937884. [DOI] [PubMed] [Google Scholar]
- 30.Galanello R., Origa R. Beta-thalassemia. Orphanet J. Rare Dis. 2010;5:11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa Y., Maeda M., Pasham M., Aguet F., Tacheva-Grigorova S.K., Masuda T., Yi H., Lee S.U., Xu J., Teruya-Feldstein J. Role of the clathrin adaptor PICALM in normal hematopoiesis and polycythemia vera pathophysiology. Haematologica. 2015;100:439–451. doi: 10.3324/haematol.2014.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z., Wang Y., Han X., Zhao X., Peng Y., Li Y., Peng M., Song J., Wu K., Sun S. miR-150 inhibits terminal erythroid proliferation and differentiation. Oncotarget. 2015;6:43033–43047. doi: 10.18632/oncotarget.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei Y., Zhang X., Su J., Jeong M., Gundry M.C., Huang Y.H., Zhou Y., Li W., Goodell M.A. Targeted DNA methylation in vivo using an engineered dCas9-MQ1 fusion protein. Nat. Commun. 2017;8:16026. doi: 10.1038/ncomms16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurita R., Suda N., Sudo K., Miharada K., Hiroyama T., Miyoshi H., Tani K., Nakamura Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peraki I., Palis J., Mavrothalassitis G. The Ets2 Repressor Factor (Erf) Is Required for Effective Primitive and Definitive Hematopoiesis. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00183-17. e00183–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntosh B.E., Brown M.E., Duffin B.M., Maufort J.P., Vereide D.T., Slukvin I.I., Thomson J.A. Nonirradiated NOD,B6.SCID Il2rγ-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consortium E.P., ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis C.A., Hitz B.C., Sloan C.A., Chan E.T., Davidson J.M., Gabdank I., Hilton J.A., Jain K., Baymuradov U.K., Narayanan A.K. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 2018;46(D1):D794–D801. doi: 10.1093/nar/gkx1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bain M., Mendelson M., Sinclair J. Ets-2 Repressor Factor (ERF) mediates repression of the human cytomegalovirus major immediate-early promoter in undifferentiated non-permissive cells. J. Gen. Virol. 2003;84:41–49. doi: 10.1099/vir.0.18633-0. [DOI] [PubMed] [Google Scholar]
- 42.Balasubramanian M., Lord H., Levesque S., Guturu H., Thuriot F., Sillon G., Wenger A.M., Sureka D.L., Lester T., Johnson D.S., DDD Study Chitayat syndrome: hyperphalangism, characteristic facies, hallux valgus and bronchomalacia results from a recurrent c.266A>G p.(Tyr89Cys) variant in the ERF gene. J. Med. Genet. 2017;54:157–165. doi: 10.1136/jmedgenet-2016-104143. [DOI] [PubMed] [Google Scholar]
- 43.Li J., Hale J., Bhagia P., Xue F., Chen L., Jaffray J., Yan H., Lane J., Gallagher P.G., Mohandas N. Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood. 2014;124:3636–3645. doi: 10.1182/blood-2014-07-588806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagger F.O., Kinalis S., Rapin N. BloodSpot: a database of healthy and malignant haematopoiesis updated with purified and single cell mRNA sequencing profiles. Nucleic Acids Res. 2019;47(D1):D881–D885. doi: 10.1093/nar/gky1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakyan V.K., Down T.A., Balding D.J., Beck S. Epigenome-wide association studies for common human diseases. Nat. Rev. Genet. 2011;12:529–541. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated in this study were shown in the main text and supplemental information. The data of RNA-seq in ERF KO and ChIP-seq are available in GEO with the number GEO: GSE160603. The source data underlying Figures 1A and S2A–S2D are provided in Table S6. Additional information is also available upon reasonable request to the corresponding authors.