Abstract
Relapse is the main cause of treatment failure after allogeneic stem cell transplant (alloSCT) in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). Injectable azacitidine can improve post-transplant outcomes but presents challenges with exposure and compliance. Oral CC-486 allows extended dosing to prolong azacitidine activity. We investigated use of CC-486 maintenance therapy after alloSCT.
Adults with MDS or AML in morphologic complete remission at CC-486 initiation (42 to 84 days after alloSCT) were included. Patients received 1 of 4 CC-486 dosing schedules per 28-day cycle for up to 12 cycles. Endpoints included safety, pharmacokinetics, graft-versus-host disease (GVHD) incidence, relapse/progression rate, and survival.
Of 30 patients, 7 received CC-486 once daily for 7 days per cycle (200 mg, n = 3; 300 mg, n = 4) and 23 for 14 days per cycle (150 mg, n = 4; 200 mg, n = 19 [expansion cohort]). Grades 3 to 4 adverse events were infrequent and occurred with similar frequency across regimens. Standard concomitant medications did not alter CC-486 pharmacokinetic parameters. Three patients (10%) experienced grade III acute GVHD and 9 experienced chronic GVHD. Of 28 evaluable patients, 6 (21%) relapsed or had progressive disease: 3 of 7 patients (43%) who had received 7-day dosing and 3 of 23 (13%) who had received 14-day dosing. Transplant-related mortality was 3%. At 19 months of follow-up, median overall survival was not reached. Estimated 1-year survival rates were 86% and 81% in the 7-day and 14-day dosing cohorts, respectively.
CC-486 maintenance was generally well tolerated, with low rates of relapse, disease progression, and GVHD. CC-486 maintenance may permit epigenetic manipulation of the alloreactive response postallograft. Findings require confirmation in randomized trials. (ClinicalTrials.gov NCT01835587.)
Keywords: CC-486, Acute myeloid leukemia, Myelodysplastic syndromes, Allogeneic stem cell, transplantation, Maintenance therapy
INTRODUCTION
Allogeneic stem cell transplantation (alloSCT) is a potentially curative therapeutic option for patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML). Disease relapse occurs in 35% to 45% of patients after alloSCT and is the most frequent cause of treatment failure and mortality [1–4]. Moreover, relapse after alloSCT is associated with poor prognosis despite salvage chemotherapy, donor lymphocyte infusions, and/or second transplants [4].
Duration of remission is a key determinant of patient outcomes after alloSCT [5]. A longer interval from transplant to relapse is associated with reduced risk of death [5]. Therefore, maximizing the duration of remission is an important treatment goal [6], and novel therapeutic strategies are needed to provide long-term disease control and extend remission in the post-transplant setting.
Acute and chronic graft-versus-host disease (GVHD) are major causes of non-relapse mortality after alloSCT [7]. Post-transplant maintenance therapy should be well tolerated, with acceptable myelotoxicity and limited drug–drug interactions, and should reduce the incidence or severity of GVHD without impeding graft-versus-leukemia effects [8].
The DNA methyltransferase inhibitor, azacitidine, is a well-established treatment option for higher-risk MDS and AML [9–11], shown to increase expression of epigenetically silenced leukemia antigens and to induce a CD8+ T cell response to tumor antigens post-transplant, potentially augmenting a graft-versus-leukemia effect [12–14]. Studies further suggest azacitidine may accelerate reconstitution of immunomodulatory regulatory T cells, which may reduce GVHD risk [12,15,16]. The dual activity of azacitidine as an antileukemic agent and inhibitor of GVHD makes it a promising agent for post-transplant therapy. Encouraging preliminary data have been reported for s.c. azacitidine maintenance therapy after alloSCT in patients with MDS and AML [14,17,18], although challenges with exposure and compliance are limitations of s.c. administration. AML and MDS are associated with hypermethylation and subsequent silencing of tumor suppressor genes [19]. With the recommended dosing schedule of s.c. azacitidine (75 mg/m2/day given on days 1 to 7 in 28-day treatment cycles), global DNA reduction is maximal at mid-cycle, whereupon remethylation begins and methylation returns to pre-treatment levels by cycle end [20].
CC-486 is a novel oral formulation of azacitidine that allows for prolonged azacitidine exposure and sustained DNA hypomethylation over the entire 28-day treatment cycle by using extended dosing schedules [21,22]. Here, we report final results of a prospective phase I/II dose-finding study of CC-486 maintenance treatment after alloSCT in patients with AML or MDS.
METHODS
Study Design
This multicenter, open-label study was conducted in accordance with Good Clinical Practice, per the International Conference on Harmonization Guideline E6, and with ethical principles outlined in the Declaration of Helsinki. The protocol was approved by the institutional review boards of all participating centers. All patients provided written informed consent. This study is registered at ClinicalTrials.gov (NCT01835587).
Patients
Patients aged ≥18 years with a diagnosis of MDS or AML according to World Health Organization criteria [23] who had undergone alloSCT with myeloablative or reduced-intensity conditioning regimens were eligible. Related and unrelated donors were permitted. Stem cells could be from peripheral blood or bone marrow. Donors could have a single mismatch at the HLA-A, -B, -C, -DRB1, or -DQB1 loci. Patients must have had Eastern Cooperative Oncology Group performance status score ≤2 and were to be in morphologic complete remission (CR; ie, ≤5% bone marrow blasts) with absolute neutrophil counts ≥1.0 × 109/L and platelets ≥ 75 × 109/L before CC-486 treatment initiation, which was to occur 42 to 84 days after alloSCT. This post-alloSCT interval was to allow for adequate marrow recovery before starting CC-486 treatment, based on our previous experience with parenteral azacitidine [17].
Key exclusion criteria were use of hypomethylating agents, lenalidomide, thalidomide, pomalidomide, chemotherapy, or any other investigational agent after alloSCT; grade ≥II acute GVHD or evidence of gastrointestinal GVHD at screening; or malignancies other than MDS or AML, unless diseasefree for ≥1 year.
Endpoints
The primary objectives were to determine a safe and effective CC-486 dosing regimen and the maximum tolerated dose (MTD) of CC-486 in patients with MDS or AML in the post-alloSCT setting. Secondary endpoints included overall survival (OS), cumulative 1-year relapse- and progression-free survival (RPFS), time to relapse, relapse rate, incidence of acute and chronic GVHD, time to treatment discontinuation, and pharmacokinetic parameters. The safety population included all patients who received ≥ 1 CC-486 dose. The efficacy population included all patients who received ≥ 1 CC-486 dose and had ≥ 1 post-baseline efficacy assessment. The pharmacokinetic population comprised a subset of study patients.
Determination of CC-486 Dose
A standard 3+3 dose-escalation design was followed to evaluate 4 CC-486 dosing schedules in repeated 28-day cycles: CC-486 200 mg (Cohort 1) or 300 mg (Cohort 2) once daily (QD) for 7 days per cycle or CC-486 150 mg (Cohort 3) or 200 mg (Cohort 4) QD for 14 days per cycle. Patients received enough CC-486 doses at a site visit on day 1 of each cycle to complete dosing for that cycle. The MTD was established if 2 dose-limiting toxicities (DLTs) occurred in a cohort during the first 2 treatment cycles. At the MTD, or if the MTD was not reached, a cohort could be expanded with an additional 10 to 12 patients to further evaluate that dosing regimen.
A DLT was defined as any of the following treatment-emergent adverse events (TEAEs), considered by the investigator to be related to CC-486: a clinically significant grade ≥3 nonhematologic toxicity, including nausea, diarrhea, or vomiting despite adequate medical intervention; absolute neutrophil counts < .5×109/L lasting >1 week despite myeloid growth factor support; platelets < 10× 109/L lasting > 1 week despite transfusion support; failure to reach absolute neutrophil counts ≥ 1.0×109/L and/or platelets ≥ 25×109/L in the presence of a hypocellular bone marrow (< 10%) within 56 days after the start of a treatment cycle; inability to initiate a subsequent cycle of CC-486 within 28 days of the anticipated start because of any treatment-related, non-hematologic TEAEs; and any toxic effect requiring dose reduction or treatment interruption.
CC-486 treatment continued until unacceptable toxicity, disease relapse or progression, development of grades III to IV acute or severe chronic GVHD, consent withdrawal, death, or until a maximum of 12 CC-486 cycles had been administered.
Efficacy and Safety
Efficacy and safety measurements were based on complete blood counts monitored weekly for the first 2 cycles (8 weeks) and then on days 1, 15, and 22 of each cycle thereafter. Bone marrow aspirates and cytogenetic studies were performed every 6 months or more frequently if clinically indicated.
OS was defined as the time from transplantation to death by any cause. RPFS was the time from transplantation to relapse, progressive disease, or death, whichever occurred first. Relapse and progressive disease were defined as the reappearance of > 5% or > 10% bone marrow blasts, respectively, lasting more than 4 weeks. Patients without a documented relapse were censored at the date of their last assessment or study completion. All patients were followed for survival until death, loss to follow-up, withdrawal of consent, or study closure. Progression to AML was collected during follow-up for patients with MDS.
Safety was assessed by TEAE reporting, graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Patients were followed for TEAEs for 28 days after their last CC-486 dose.
Pharmacokinetic Analysis
Blood samples for CC-486 pharmacokinetic analyses were collected pre- and post-dose at .5, 1, 1.5, 2, 2.5, 3, 4, and 6 hours on day 1 of cycles 1 and 2. Plasma samples were analyzed using a validated proprietary HPLC/tandem mass spectrometric method. Pharmacokinetic parameters included maximum observed plasma concentration, time of maximum observed plasma concentration, area under the plasma concentration-time curve from zero to infinity, terminal elimination half-life, apparent total clearance, and apparent volume of distribution. Pharmacokinetic parameters were calculated using noncompartmental methods with Phoenix WinNonlin software (Pharsight Corp, Mountain View, CA). To evaluate potential drug–drug interactions, patients were alternately assigned to take their regular concomitant medications before the visit on day 1 of cycle 1 or 2 and to not to take their regular concomitant medications before the day 1 visit in the other cycle.
Statistical Methods
Demographic, efficacy, and safety outcomes are reported descriptively. No formal comparisons among the CC-486 dosing regimens were planned. OS was estimated using the Kaplan-Meier method. One-year cumulative RPFS rate was based on a competing risk method, in which death without documented progression or relapse is considered a competing risk for progression or relapse. Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Overall, 31 patients were enrolled between July 2013 and November 2015 at 5 study sites. Thirty patients received ≥ 1 dose of CC-486 and comprised the intention-to-treat population. In the combined 7-day dosing cohorts, 3 patients received CC-486 200 mg QD (Cohort 1) and 4 received 300 mg QD (Cohort 2). In the 14-day dosing cohorts, 4 patients received CC-486 150 mg QD (Cohort 3) and 19 patients received CC-486 200 mg QD in an expansion cohort (Cohort 4).
Baseline characteristics were generally comparable between the 7-day and 14-day dosing groups (Table 1). Twenty-six patients (87%) had AML and 4 (13%) had MDS, including 3 patients with International Prognostic Scoring System [24] higher-risk MDS. Patients were generally older (median age, 64.5 years [range, 28 to 80]). All patients had Eastern Cooperative Oncology Group performance status scores ≤ 1. At time of transplantation, 27 patients (90%) were in CR, 25 in first remission and 2 in second remission, and 3 patients (10%) had ≥ 5% bone marrow blasts. Eighteen patients had received a myeloablative conditioning regimen (busulfan and fludarabine, n = 14; busulfan and cyclophosphamide, n = 3; total body irradiation, cyclophosphamide, and thiotepa, n = 1) and 12 patients received a reduced-intensity conditioning regimen with fludarabine and melphalan. Twenty patients had unrelated donors, and 10 received stem cells from a sibling. Eight patients received stem cells from bone marrow and 22 patients from peripheral blood. Median time from alloSCT to start of CC-486 therapy was 81.5 days (range, 45 to 85). The median follow-up for patients in this study was 19.0 months (range, 1.0 to 41.3).
Table 1.
Baseline Characteristics and CC-486 Treatment Exposure
| 200 mg QD for 7 Days (n = 3) | 300 mg QD for 7 Days (n = 4) | 150 mg QD for 14 Days (n = 4) | 200 mg QD for 14 Days (n = 19) | Total (N = 30) | |
|---|---|---|---|---|---|
| Diagnosis | |||||
| AML | 2 (67) | 4 (100) | 4 (100) | 16 (84) | 26 (87) |
| MDS | 1 (33) | 0 | 0 | 3 (16) | 4 (13) |
| Median time from MDS/AML diagnosis to alloSCT, mo (range) | 6.2 (3.3–16.2) | 4.6 (3.4–12.8) | 3.5 (1.4–5.6) | 4.8 (2.7–75.0) | 4.8 (1.4–75.0) |
| Median time from initial diagnosis to CC-486, mo (range) | 8.2 (5.8–17.7) | 6.8 (6.1–15.4) | 6.2 (4.1–8.3) | 12.9 (5.3–77.7) | 7.0 (4.1–77.7) |
| Median time from alloSCT to CC-486, days (range) | 63.0 (45–78) | 81.5 (55–85) | 85.0 (84–85) | 81.0 (45–85) | 81.0 (45–85) |
| Median BM blasts before alloSCT, % (range) | 0 (0–2) | 4.0 (2–8) | 0 (0–1) | 2.0 (0–6) | 2.0 (0–8) |
| Median BM blasts after alloSCT, % (range) | 1.0 (1–1) | .5 (0–2) | 1.0 (0–1) | 1.0 (0–2) | 1.0 (0–2) |
| Median HCT-CI score (range) | 1.0 (0–1) | 0 (0–1) | 2.0 (0–3) | 0 (0–3) | .5 (0–3) |
| Disease status at study entry | |||||
| CR1 | 2 (67) | 2 (50) | 3 (75) | 18 (95) | 25 (83) |
| CR2 | 1 (33) | 1 (25) | 0 | 0 | 2 (7) |
| Active disease | 0 | 1 (25) | 1 (25) | 1 (5) | 3 (10) |
| AML WHO classification | n = 2 | n = 4 | n = 4 | n = 16 | n = 26 |
| Recurrent genetic abnormalities | 0 | 2 (50) | 0 | 7 (44) | 9 (35) |
| Myelodysplasia-related changes | 0 | 0 | 1 (25) | 2 (13) | 3 (12) |
| Therapy-related myeloid neoplasms | 0 | 0 | 1 (25) | 0 | 1 (4) |
| Not otherwise specified | 2 (100) | 2 (50) | 2 (50) | 7 (44) | 13 (50) |
| MDS WHO classification | n = 1 | n = 0 | n = 0 | n = 3 | n = 4 |
| RA / RCMD | 0 | 0 | 0 | 2 (67) | 2 (50) |
| MDS-U | 0 | 0 | 0 | 1 (33) | 1 (25) |
| del(5q) | 1 (100) | 0 | 0 | 0 | 1 (25) |
| MDS IPSS risk classification | n = 1 | n = 0 | n = 0 | n = 3 | n = 4 |
| Low / Intermediate-1 | 0 | 0 | 0 | 1 (33) | 1 (25) |
| Intermediate-2 / High | 1 (100) | 0 | 0 | 2 (67) | 3 (75) |
| NCCN cytogenetic risk at AML diagnosis | n = 2 | n = 4 | n = 4 | n = 16 | n = 26 |
| Favorable | 0 | 2 (50) | 0 | 1 (6) | 3 (12) |
| Intermediate | 2 (100) | 1 (25) | 3 (75) | 12 (75) | 18 (69) |
| Poor | 0 | 0 | 0 | 1 (6) | 1 (4) |
| Missing | 0 | 1 (25) | 1 (25) | 2 (13) | 4 (15) |
| MDS cytogenetic risk at diagnosis | n = 1 | n = 0 | n = 0 | n = 3 | n = 4 |
| Good | 0 | 0 | 0 | 1 (33) | 1 (25) |
| Intermediate | 0 | 0 | 0 | 1 (33) | 1 (25) |
| Poor | 0 | 0 | 0 | 1 (33) | 1 (25) |
| Missing | 1 (100) | 0 | 0 | 0 | 1 (25) |
| Molecular abnormalities | |||||
| NPM1 | 0 | 0 | 0 | 2 (11) | 2 (7) |
| CEBPA | 0 | 0 | 1 (25) | 3 (16) | 4 (13) |
| FLT3-ITD | 1 (33) | 1 (25) | 0 | 3 (16) | 5 (17) |
| ECOG performance status | |||||
| 0 | 1 (33) | 1 (25) | 1 (25) | 8 (42) | 11 (37) |
| 1 | 2 (67) | 3 (75) | 3 (75) | 11 (58) | 19 (63) |
| ≥2 | 0 | 0 | 0 | 0 | 0 |
| Prior injectable HMA use | 1 (33) | 0 | 1 (25) | 7 (37) | 9 (30) |
| Median CC-486 treatment cycles (range) | 7 (6–12) | 5.5 (1–7) | 11.5 (4–12) | 12 (1–12) | 9 (1–12) |
Values are n (%) unless otherwise defined. BM indicates bone marrow; CR, complete remission; CR1, first CR; CR2, second CR; ECOG, Eastern Cooperative Oncology Group; HCT-CI, hematopoietic cell transplantation–specific comorbidity index; HMA, hypomethylating agent; IPSS, International Prognostic Scoring System; MDS-U, MDS-undefined; NCCN, National Comprehensive Cancer Network; RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; WHO, World Health Organization.
The MTD of CC-486 was not reached at doses up to 200 mg/day for 14 days per cycle. No DLT was observed in Cohorts 1 to 3. In Cohort 4 (200 mg QD × 14 days) 1 patient experienced a DLT during the first 2 treatment cycles (grade 4 neutropenia, grade 3 pneumonia), but no additional DLTs occurred and the criteria for MTD were not met. Based on observed efficacy and tolerability of the CC-486 200 mg 14-day dosing regimen, Cohort 4 was subsequently expanded to a total of 19 patients to further assess the clinical activity, safety, and tolerability of this regimen. Based on patient safety considerations with regenerating bone marrows and concern for the development of significant neutropenias or thrombocytopenia post-transplant, no higher dosing regimen was evaluated.
CC-486 Exposure
The median number of CC-486 treatment cycles for all patients was 9.0 (range, 1 to 12) (Table 1). Median duration of treatment was 252.5 days (range, 3 to 371). Thirteen patients (43%) completed all 12 treatment cycles, including 1 of 7 patients (14%) in the combined 7-day dosing group and 12 of 23 patients (52%) in the combined 14-day group. Among the 17 patients (57%) who discontinued treatment before completing 12 cycles, median time to discontinuation was 283.5 days (range, 21 to 401). Reasons for discontinuation included MDS or AML relapse (n = 6, 20% of all patients), withdrawal of consent (n = 5, 17%), GVHD (n = 2, 7%), non-GVHD TEAEs (n = 2, 7%), death (n = 1, 3%), or “other” (n = 1, 3%). “Other” involved a patient in the CC-486 300-mg 7-day dosing arm who had a history of central nervous system leukemia at study entry and was receiving intrathecal methotrexate before and during CC-486 treatment. Because of presentation of central nervous system features characteristic of a transient ischemic attack and suspected central nervous system relapse at cycle 4, as well as administration of radiation therapy, the patient was discontinued because of risk of bleeding. The patient was in CR at all evaluations after discontinuing therapy, was not included in an on-study relapse rate, and was alive and in CR at the end of the study according to bone marrow aspirate samples.
Disease Relapse and Survival
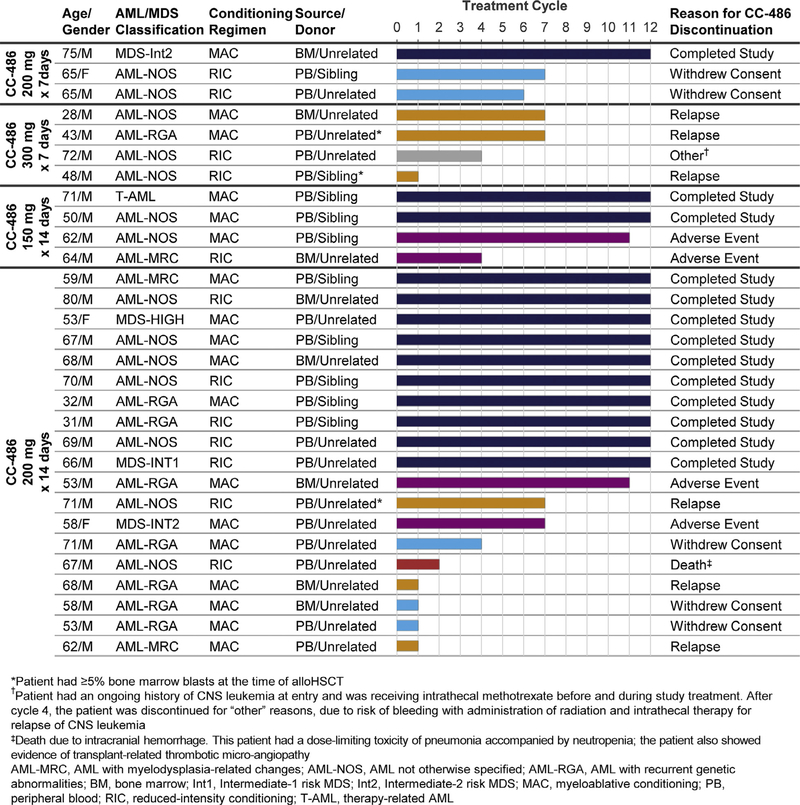
Two patients discontinued in the first treatment cycle and withdrew consent for further follow-up. For the 28 remaining patients, the 1-year rate of relapse or progressive disease during treatment was 21% (n = 6). Three of these 6 patients relapsed during the first treatment cycle (Figure 1). The 3 patients who had ≥ 5% bone marrow blasts at the time of transplant all relapsed on-study, 1 of whom relapsed during treatment cycle 1. The 1-year cumulative incidence of relapse was 3 of 7 (43%) in the combined 7-day dosing group and 3 of 23 (13%) in the combined 14-day dosing group. One-year RPFS rates were 54% and 72% in the 7-day and 14-day CC-486 dosing groups, respectively.
Figure 1.
Patient profiles and duration of CC-486 treatment.
Median OS was not reached in any dosing cohort (range for all patients was 86 to 1324 days) (Figure 2), and estimated 1-year survival rates in the 7-day and 14-day dosing cohorts were 86% and 81%, respectively.
Figure 2.
OS from time of alloSCT.
Acute and Chronic GVHD
One-year cumulative incidence of acute or chronic GVHD was 50% (n = 15). Grade III acute GVHD was reported in 1 patient (3%) in the CC-486 200-mg 14-day dosing cohort. No grade IV acute GVHD was observed. Chronic GVHD was reported in 9 patients (30%) with similar frequency within 3 dosing groups (no chronic GVHD was reported in the 300-mg 7-day dosing cohort). Three of 9 patients had severe chronic GVHD, and the remaining 6 patients had mild or moderate chronic GVHD. Among patients with any GVHD, organ involvement included skin in 8 patients, the lower intestinal tract in 7 patients, and the liver in 2 patients.
Safety and Tolerability
The most frequent TEAEs were gastrointestinal and hematologic events. Twenty-two patients (73%) experienced at least 1 grade 3-to-4 TEAE (Table 2; Supplementary Table 1 shows common TEAEs by CD34+ cell dose threshold at transplant). The most common (≥ 5% of patients) grades 3-to-4 TEAEs occurred at similar frequencies across all 4 dosing cohorts: diarrhea (20%), lymphopenia (20%), vomiting (17%), neutropenia (17%), nausea (13%), anemia (13%), thrombocytopenia (10%), and abdominal pain (7%). Treatment-related serious TEAEs were reported in 4 patients and included hemolysis, thrombocytopenia, neutropenia, diarrhea, nausea, vomiting, asthenia, pyrexia, pneumonia, and intracranial hemorrhage. One TEAE-related death occurred on-study (intracranial hemorrhage) in the patient who experienced the DLT at cycle 2. This patient had raised lactate dehydrogenase at baseline and subsequently developed hemolysis, progressive thrombocytopenia, and a progressive rise in lactate dehydrogenase, and was considered to have had tacrolimus-associated thrombotic thrombocytopenic purpura.
Table 2.
Most Common (≥5% of All Patients) Grades 3–4 TEAEs
| AE | CC-486 200 mg QD for 7 Days (n = 3) | CC-486 300 mg QD for 7 Days (n = 4) | CC-486 150 mg QD for 14 Days (n = 4) | CC-486 200 mg QD for 14 Days (n = 19) | Total (N = 30) |
|---|---|---|---|---|---|
| Patients with ≥1 grade 3–4 TEAE | 2 (67) | 3 (75) | 3 (75) | 14 (74) | 22 (73) |
| Hematologic | |||||
| Lymphopenia | 0 | 0 | 3 (75) | 3 (16) | 6 (20) |
| Neutropenia | 0 | 0 | 1 (25) | 4 (21) | 5 (17) |
| Anemia | 0 | 0 | 1 (25) | 3 (16) | 4 (13) |
| Thrombocytopenia | 1 (33) | 0 | 0 | 2 (11) | 3 (10) |
| gastrointestinal | |||||
| Diarrhea | 1 (33) | 0 | 2 (50) | 3 (16) | 6 (20) |
| Vomiting | 1 (33) | 1 (25) | 1 (25) | 2 (11) | 5 (17) |
| Nausea | 1 (33) | 0 | 0 | 3 (16) | 4 (13) |
| GI GVHD* | 0 | 0 | 0 | 3 (16) | 3 (10) |
| Abdominal pain | 0 | 0 | 0 | 2 (11) | 2 (7) |
| Other | |||||
| Device-related infection | 0 | 1 (25) | 1 (25) | 0 | 2 (7) |
| Dehydration | 0 | 0 | 1 (25) | 1 (5) | 2 (7) |
| Pneumonia | 0 | 0 | 1 (25) | 1 (5) | 2 (7) |
Values are n (%). GI indicates gastrointestinal.
Acute or chronic.
Pharmacokinetics
Pharmacokinetic data were available for patients receiving 200 mg CC-486 doses, including 4 patients with and without concomitant medications after CC-486 dose administration on day 1 of cycles 1 and 2 (per protocol), 2 patients after CC-486 administration who had not taken concomitant medications, and 9 patients after CC-486 administration who had taken concomitant medications. Thus, azacitidine pharmacokinetic data were available at the CC-486 200-mg dose for a total of 6 patients without concomitant medications and 13 patients with concomitant medications. There were too few patients in the 150-mg/day CC-486 dosing group with meaningful pharmacokinetic data to report (pharmacokinetic outcomes with 300 mg QD CC-486 have been reported elsewhere [22]).
Azacitidine was rapidly absorbed, reaching mean maximum observed plasma concentration within approximately 1 hour post-dose and then decreasing in a multiphasic manner to a nonquantifiable level by the 6-hour time point (Supplementary Figure 1). After CC-486 200-mg dose administration, azacitidine plasma concentration profiles and other pharmacokinetic parameters (Figure 3) were not significantly different when taken with or without standard concomitant medications. Concomitant medications included (but were not limited to) prophylactic antibiotics, calcineurin inhibitors, antifungals, and antiviral agents; red blood cell and platelet transfusions; myeloid growth factors; antiemetics; and drugs to manage gastrointestinal complications. Moreover, pharmacokinetic parameters were within range of those reported for nontransplant patients treated with CC-486 in a different study [22].
Figure 3.
Azacitidine pharmacokinetic parameters with and without concomitant medications after 200 mg CC-486.
DISCUSSION
Disease recurrence is a major therapeutic challenge in patients with MDS or AML undergoing alloSCT, and treatment options are limited [4,5]. Risk of disease relapse after alloSCT is a composite of many factors, including age, cytogenetic and molecular status at diagnosis, and remission status at the time of transplantation [4,25–27]. Remission duration is one of the strongest predictors of post-transplant survival [5,28,29]. This is the first prospective trial to evaluate post-transplant CC-486 therapy as a strategy to prevent or delay relapse in patients with AML or MDS. Therapy with CC-486 for 1 year was associated with a relatively low (21%) overall rate of disease relapse during treatment.
In the current study the RPFS rate was higher in the combined 14-day dosing cohort than in the 7-day dosing group, supporting the rationale for extended CC-486 dosing. The 1-year cumulative rate of relapse/disease progression with CC-486 maintenance administered for 14 days per cycle (13%) compares favorably with rates reported in studies of with 5-day dosing of low-dose s.c. azacitidine maintenance after alloSCT [14,17], although meaningful conclusions are elusive when comparing results of different studies with different patient populations and endpoints. For example, a phase I study evaluating low-dose s.c. azacitidine 8 to 40 mg/m2/day administered for 5 days per cycle after alloHSCT in patients with high-risk MDS or AML showed a 53% relapse rate at a median follow-up of 20.5 months. However, that study included a high proportion of patients with advanced disease characteristics, and most patients were not in CR at the time of transplant [17]. In any case, at-home administration of oral maintenance therapy may be more convenient for patients than making multiple daily clinic visits for parenteral drug administration. In the current study patients received enough CC-486 at the clinic on day 1 of each cycle to complete CC-486 dosing for that cycle at home.
Survival outcomes associated with CC-486 maintenance were also relatively favorable. Median OS was not reached in any dosing cohort at a median follow-up of 19 months, and estimated 1-year survival rates were above 80%.
Expected rates of post-transplant serious chronic GVHD range from approximately 25% to 30% [30,31] The incidence of severe chronic GVHD in our study was low (10%), and only 2 patients discontinued the study due to a GVHD event. The generally mild presentation and low incidence of GVHD in this study support the hypothesis that CC-486 maintenance may permit epigenetic manipulation of the alloreactive response after transplantation. Two mechanisms have been proposed by which azacitidine is believed to induce tolerance and reduce the risk of GVHD: conversion of alloreactive donor T cells into suppressive regulatory T cells via hypomethylation of the FOXP3 promoter and suppression of alloreactive T cell proliferation [12,15,16,32].
Once-daily CC-486 was generally well tolerated; the MTD was not reached in this study, and there was no meaningful difference in the frequency or severity of AEs among dosing regimens. The most common TEAEs were gastrointestinal and hematologic, consistent with previous reports of low-dose s.c. azacitidine post-transplant and of front-line CC-486 in MDS and AML [17,21]. Rate of discontinuation due to TEAEs was low, with most discontinuations due to MDS or AML relapse (20% of all patients). Patients undergoing alloSCT are particularly vulnerable to myelosuppression and other toxicities [33,34]. Rates of hematologic TEAEs with CC-486 in this and other studies are lower than those seen with injectable hypomethylating agents [21,35–37]. Despite the pharmacokinetic testing protocol, investigators and patients may have been reluctant to forego the patients’ prescribed concomitant medications in the post-transplant setting. Nevertheless, these data, albeit in a small patient sample, suggest a lack of significant drug–drug interactions with CC-486 and standard concomitant medications such as antibiotics or drugs to manage gastrointestinal events.
Use of maintenance therapy in hematologic disorders remains controversial [38], and whether and when to initiate maintenance treatment and how long to continue it are unresolved issues. The increasing use of next-generation sequencing may allow detection of measurable residual disease (MRD), which can be a harbinger of relapse [39], to inform whether maintenance might benefit some patients, and sustained measurable residual disease negativity may suggest maintenance therapy is unnecessary or could be discontinued. The extent of donor chimerism may also suggest whether maintenance therapy might prolong remission post-transplant [18]. Here, we somewhat arbitrarily planned for 12 CC-486 treatment cycles, with the goal of offering therapy during the period of time with higher risk of AML or MDS relapse, based on historic data [4]. One cannot underestimate the logistic challenges of prolonged maintenance therapy after allogeneic transplantation, which frequently include monitoring by different physicians and hospitals, patient and caregiver fatigue, and need for more intensive monitoring.
Among limitations of these data are that this is a phase I dose-finding study, followed by a small phase II expansion, with no placebo-control group. It is unknown whether the benefit of CC-486 maintenance correlated with improvement in quality of life, because it was not evaluated. Additionally, no information regarding the presence of MRD before or after transplant was collected, and correlations between relapse status and changes in methylation levels during CC-486 study and extent of immune reconstitution were not assessed. Nonetheless, these data support the clinical benefits and acceptable safety profile of CC-486 as maintenance treatment after alloSCT in patients with MDS or AML. Based on these data, the recommended CC-486 post-transplant maintenance dosing regimen is 200-mg daily for 14 days per 28-day cycle. Our findings warrant further study in a larger patient population.
Supplementary Material
ACKNOWLEDGMENTS
Financial disclosure: This study was funded by Celgene Corporation, Summit, NJ. The authors received editorial support during manuscript development from Sheila Truten and Kelly Dittmore of Medical Communication Company, Inc. (Wynnewood, PA) who were funded by Celgene Corporation. Analyses were performed by Celgene Corporation. The authors are fully responsible for all content and editorial decisions and had access to all study data.
Conflict of interest statement: M.d.L.: Celgene Corporation, consultancy, research funding; Pfizer, board of directors or advisory committees; Incyte, consultancy; Amgen, consultancy; Spectrum, consultancy. B.O.: Celgene Corporation, AROG, and Astex, research funding. E.B.P.: spouse has leadership roles and stock ownership at Exelixis, Regulus, and Biogen. S.A.G.: Amgen, Celgene Corporation, Jazz Pharmaceuticals, Kite Pharma, Novartis, and Sanofi, consultancy; Spectrum, consultancy, research funding. B.L.S.: Celgene Corporation, honoraria, consultancy, research funding, speakers bureau; Novartis, research funding, speakers bureau; Alexion, honoraria, speakers bureau; Incyte, honoraria, speakers bureau; Acceleron, data and safety monitoring board; Agios Pharmaceuticals, honoraria, consultancy. B.M.W.: Miragen, consultancy, honoraria. J.H., E.L., B.H., and B.S.S.: Celgene Corporation, employment, equity ownership. C.C.: Celgene Corporation, honoraria, research funding; Jazz Pharmaceuticals, Pfizer, and Janssen, honoraria.
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2018.06.016.
REFERENCES
- 1.Hahn T, McCarthy PL Jr., Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paczesny S, Choi SW, Ferrara JL. Acute graft-versus-host disease: new treatment strategies. Curr Opin Hematol. 2009;16:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer T, Silling G, Groth C, et al. Treatment strategies in patients with AML or high-risk myelodysplastic syndrome relapsed after Allo-SCT. Bone Marrow Transplant. 2015;50:485–492. [DOI] [PubMed] [Google Scholar]
- 4.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010;3:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2015;21:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrovic A, Hale G Clinical options after failure of allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies. Expert Rev Clin. Immunol. 2011;7:515–525. quiz, 526–517. [DOI] [PubMed] [Google Scholar]
- 7.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute’s second international workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation. Part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant. 2014;20:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. [DOI] [PubMed] [Google Scholar]
- 12.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012;119:3361–3369. [DOI] [PubMed] [Google Scholar]
- 13.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–1918. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–121. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder T, Frobel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. 2013;27:1910–1913. [DOI] [PubMed] [Google Scholar]
- 17.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Dunbar A, Gondek LP, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29:2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Manero G, Gore SD, Kambhampati S, et al. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia. 2016;30:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laille E, Shi T, Garcia-Manero G, et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PLoS One. 2015;10 e0135520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89: 2079–2088. [PubMed] [Google Scholar]
- 25.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giralt SA, Champlin RE. Leukemia relapse after allogeneic bone marrow transplantation: a review. Blood. 1994;84:3603–3612. [PubMed] [Google Scholar]
- 27.Ossenkoppele GJ, Janssen JJ, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biol Blood Marrow Transplant. 2007;13:116–123. [DOI] [PubMed] [Google Scholar]
- 29.Ganguly S, Singh J, Divine CL, et al. Is there a plateau in the survival curve after autologous transplantation in patients with intermediate and high-risk acute myeloid leukemia? A 20-year single institution experience. Leuk Res. 2007;31:1253–1257. [DOI] [PubMed] [Google Scholar]
- 30.Flowers ME, Traina F, Storer B, et al. Serious graft-versus-host disease after hematopoietic cell transplantation following nonmyeloablative conditioning. Bone Marrow Transplant. 2005;35:277–282. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J. Rare Dis. 2007;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehx G, Fransolet G, de Leval L, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2016;6 e1314425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamae H, Storer B, Sandmaier BM, et al. Cytopenias after day 28 in allogeneic hematopoietic cell transplantation: impact of recipient/donor factors, transplant conditions and myelotoxic drugs. Haematologica. 2011;96:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292:832–843. [DOI] [PubMed] [Google Scholar]
- 35.Savona MR, Kolibaba K, Conkling P, et al. CC-486 in patients with myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), or chronic myelomonocytic leukemia (CMML): safety, tolerability, and response. Blood (ASH Annual Meeting Abstracts). 2014;124. Abstract 4638. [Google Scholar]
- 36.Santini V, Fenaux P, Mufti GJ, et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol. 2010;85:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashidi A, Walter RB, Tallman MS, Appelbaum FR, DiPersio JF. Maintenance therapy in acute myeloid leukemia: an evidence-based review of randomized trials. Blood. 2016;128:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: consensus document from ELN MRD Working Party. Blood. 2018;131:1275–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.