Abstract
Background:
Marine seaweeds are a famous type of traditional food containing various kinds of secondary metabolites. These organisms have different biological activities such as antitumor, antiviral, and antioxidant. The aim of this study was to investigate the effects of total extract of Sargassum glaucescens on neuropathy pain induced by paclitaxel in mice.
Materials and Methods:
S. glaucescens was collected from the Persian Gulf. The seaweed was extracted by maceration with methanol-ethyl acetate (1:1) solvent. The effect of the extract on neuropathy pain induced by paclitaxel was analyzed. All results were analyzed by one-way analysis of variance.
Results:
Acute administration of S. glaucescens extract (100 and 200 mg/kg intraperitoneally [i.p.]) 30 min before the test on the 11th day significantly reduced the duration of paw licking (P < 0.001).
Conclusions:
Chronic treatment with S. glaucescens extract (100, 150, and 200 mg/kg i.p.) from the 6th day until the 10th day reduced the duration of paw licking. Therefore, S. glaucescens should be considered for further treatment of neuropathy.
Keywords: Neuropathy, paclitaxel, sargassum, seaweed
Introduction
Cancer is a leading cause of death worldwide, however, with new chemotherapy agents, it is more curable nowadays.[1] Chemotherapeutic agents may cause toxicity on peripheral nerves lead to mild Paresthesia's, loss of sensory function, neuropathy pain, severe ataxia, weakness, and pronounced disability. Hence, the quality of life may further reduced because of the engagement of nerve fibers with orthostatic hypotension, disability, and also incontinence. Although many compounds with cytotoxic activity are reported to be neurotoxic, only a few of them lead to side effects such as peripheral neuropathy.[2]
Paclitaxel (Taxol®) is a novel cytotoxic agent isolated from the bark of the plant Taxus brevifolia, with pivotal activity against different tumors such as the breast, ovarian, lung, and head-and-neck cancers. However, this drug stimulates microtubule assembly leading to neuropathy as one of its side effects.[3] One of the most important limiting factors of paclitaxel is peripheral sensory neuropathy, especially with increasing doses of the drug even in combination form with other neurotoxic cytotoxic agents such as cisplatin.[4] Therefore, it is necessary to look for agents that effectively reduce neuropathy pain.[5] Different mechanisms are involved in paclitaxel-induced neuropathy pain, such as the involvement of opioids and adrenergic receptors, the release of inflammatory factors, and the stress-oxidative system.[6,7]
Seaweeds are attractive marine organisms in the biomedical area, especially because of their bioactive metabolites with antimicrobial, anti-inflammatory, antioxidant, antiviral, anti-Alzheimer, and antitumor activities.[8,9,10] One of the most interesting compounds extracted from these organisms that have currently received high attention from academic researchers and pharmaceutical companies for drug design includes fucoidans, a type of sulfated polysaccharides isolated from brown algae, showing different pharmacologic activities such as anti-inflammatory and anticancer.[9] Other metabolites biosynthesized by seaweeds with economic effects in food and in human health include natural pigments and carotenoids antioxidant agents reducing the incidence of many disorders, especially those mediated by light.[10]
Genus of Sargassum is widely distributed in tropical oceans of the world. There are various reports on their secondary compounds and also their biological activities.[11] They usually contain terpenoids that exhibit biological activities such as cell antioxidant activity, toxicity, vasodilatory effects, and inhibition of acetylcholinesterase.[12,13]
Iran, in the South part, has more than that of 1260 km coastal lines. Moreover, >250 species of different seaweeds have been reported from this area.[14] There have been only a few studies on the pharmacological effects of these marine organisms in this region of Iran. Because of anti-inflammation of Sargassum glaucescens, a seaweed from the Persian Gulf, in this study, we investigated if this herb can prevent or reduce paclitaxel-induced neuropathy pain. The role of opioid receptor and alpha-2 adrenergic receptor have been evaluated as possible mechanisms.
Materials and Methods
Authentication of plant material
The seaweed was collected from the Persian Gulf coasts of Iran, Bushehr Province. Voucher specimen was deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences and was identified by the Agricultural and Natural Resources Research Center of Bushehr (Code: 2662).
Preparation of the extracts
Collected and identified plant sample was cut into small pieces, air-dried, and stored in glass containers until the time of extraction. About 500 g of the dried plant material was macerated for 6 days with the solvents methanol: Ethyl acetate (1:1). The extracts were filtered and evaporated at room temperature by rotary evaporator to the dry residue (6.7 g) and stored in low temperature pending biological analysis.
Animals
NMRI (Naval Medical Research Institute) male mice weighing 20–30 g were used. They were housed under a 12-h light/dark cycle and had enough access to water and food. The animals were assigned to different treatment groups randomly (n = 6–8 in each group) and all procedures were conducted in agreement with the guidelines for the care of laboratory animals of the Ethics Committee of Isfahan University of Medical Sciences and followed the European Commission Directive (86/609/EEC) for animal experiments.
Chemicals
Paclitaxel was purchased from the Sigma-Aldrich company, Inc., (St. Louis, MO, USA) and dissolved in the solvent ethanol-cremophor-saline (5:5:90, v/v/v). To understanding the role of opioids receptors, naloxone was obtained from Touliddarou (Tehran, Iran). Yohimbine from Touliddarou, alpha-2 adrenergic antagonist, was used to evaluate the role of adrenergic receptors. They all dissolved in normal saline 0.9%.
General procedures for drug treatments
To induce the neuropathy pain, paclitaxel was injected on the 1st day and given daily for 4 additional days (2 mg/kg) intraperitoneally (i.p.) with a cumulative dose of 10 mg/kg. Sham group received vehicle.
To assess the effects of S. glaucescens extract on the development (chronic effect) of neuropathy pain, S. glaucescens extract (25, 50, 100, 150, and 200 mg/kg i.p.) were administered once daily from the 6th day until the 10th day. Cold allodynia test was assessed on the 11th day after the first paclitaxel injections [Figure 1].[15]
Figure 1.
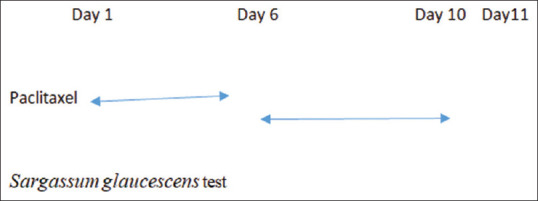
Schematic representing of chronic protocol
To determine the effect of S. glaucescens extract on the expression (acute effect) of paclitaxel-induced neuropathy pain, it was injected 30 min before pain assessment on the 11th day in another group of mice.
Behavioral tests of neuropathy pain
Animals were placed on the top of an aluminum mesh table. They were permitted to adapt for 15 min. Acetone drop was applied to the plantar surface of each hind paw by a needle for determination of cold allodynia and repeated within 30 s interval for three times. The time spent for licking the paw was checked. The following formula was used to calculate and express the percentage of licking frequency (number of trials attending the hind-paw/total number of trials) ×100. The total time was calculated if the percentage was > 66 (s).
Evaluation of the role of other receptors (opioid and alpha-2 adrenergic receptors)
To identify the role of the opioid system in the anti-nociceptive effects of S. glaucescens extract, animals were treated by naloxone (1 mg/kg i.p.) on the 11th day. Yohimbine (10 mg/kg) was also applied.
Data analysis
Data are presented as mean ± standard deviation. Data were compared by one-way analysis of variance (ANOVA) followed by Fisher's least significant difference post hoc test for multiple comparisons. Two-way ANOVA was used to compare the antagonistic effect of naloxone. Time-course analysis of behavioral data was compared by repeated-measures ANOVA for each experimental group.
Results
Chronic Effect of S. glaucescens extract on the development of cold allodynia.
Chronic treatment with S. glaucescens extract (100, 150, and 200 mg/kg i.p.) from the 6th day until the 10th day reduced the duration of paw licking [Figure 2].
Figure 2.
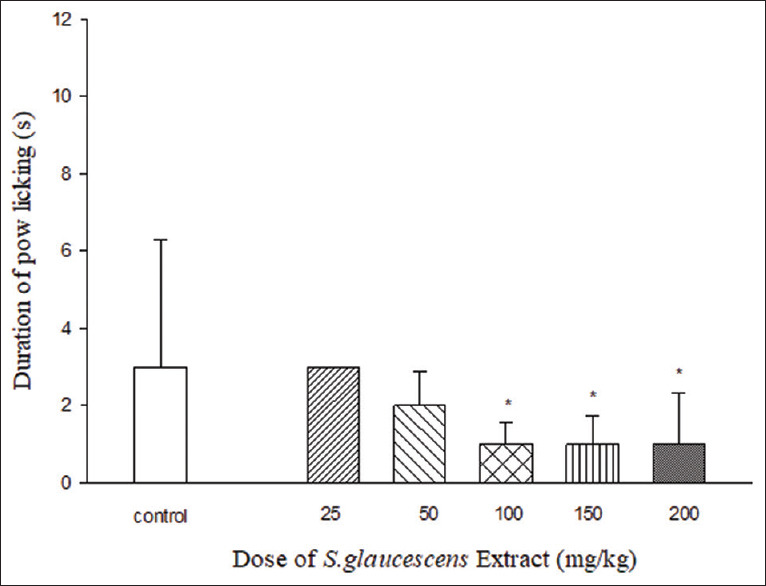
Chronic effect of Sargassum glaucescens extract on the development of cold allodynia. *P < 0.05 in compare with control
Acute Effect of S. glaucescens extract on the expression of cold allodynia.
Acute administration of S. glaucescens extract (100 and 200 mg/kg i.p.) 30 min before the acetone test on the 11 significantly reduced paw licking [Figure 3]. Naloxone and yohimbine could not reverse the result.
Figure 3.
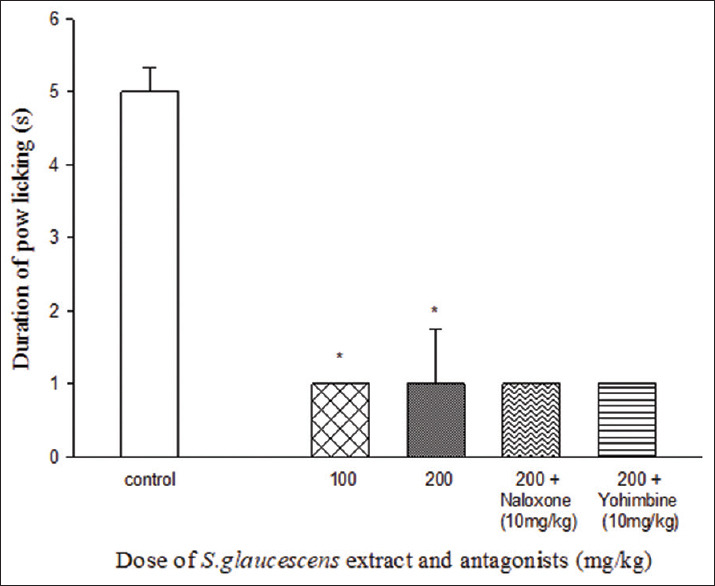
Acute effect of Sargassum glaucescens extract on the development of cold allodynia. *P < 0.05 in compare with control
Discussion
In this study, we observed that of S. glaucescens significantly reduced neuropathy pain induced with paclitaxel. In both acute and chronic form, however, blocking of adrenergic and opioids did not reverse the results. Several mechanisms have been proposed about the possible mechanism of paclitaxel-induced neuropathy pain. It has been shown that inhibition of complex III, a mitochondrial reactive oxygen species producing site, increased antinociceptive effects on the development and maintenance of paclitaxel-induced pain.[2]
Sargassum species are brown seaweed from tropical regions. These organisms are rich sources of bioactive compounds such as proteins, vitamins, dietary fibers, carotenoids, and minerals and also many secondary metabolites such as flavonoids, terpenoids, polyphenols, sterols, sulfated polysaccharides, sargaquinoic acids and also sargachromenol, and pheophytine. Hence, Sargassum species have great potential as pharmaceutical and nutraceutical sources.[13,16,17,18,19,20]
Similar studies are now looking to find a substance to control the side effects of paclitaxel and other chemotherapeutic agents. Yang et al., showed that dextromethorphan and oxycodone can be used to treat neuropathy pain in mice. Their results demonstrated that acute administration of oxycodone (1–5 mg/kg) suppressed SNL-induced mechanical allodynia. Cold allodynia is a kind of Nociception assay to assess the ability of an animal like mice to the feeling of pain.[21]
Considering our results, S. glaucescens extract at concentrations of 100,150, and 200 mg/kg were more effective on improving paclitaxel side effects treatment. Chronic treatment reduced the duration of paw licking.
Numerous studies showed different properties of seaweeds including suppression of inflammation.[21] It should be noted that a similar study that was conducted by Dang Diem Hong and Partners, demonstrated that extracts of the Sargassum swartzii have potent analgesic and anti-inflammatory effects, without any serious toxic effect at the highest possible doses.[22] In addition, we investigated the effects of naloxone and Yohimbine on S. glaucescens extract-induced. It was shown that there is no significant paw withdrawal frequency and paw licking duration amongst mice which were administrated by naloxone or yohimbine compared to mice that were not administrated by those, it can be concluded that opioid and alpha-2 adrenergic receptor may be involved in this process. Therefore, acute and chronic administration of S. glaucescens extract can be used to reduce the side effects of paclitaxel on feeling pain and nociceptive sensitivity.
Conclusions
These results indicated that S. glaucescens extract, when administered during chemotherapy or ever after that can prevent the expression and development of neuropathy pain. This effect is not inhibited by naloxone. Further researches are necessary to clarify the possible mechanisms of S. glaucescens extract in the attenuation of neuropathy pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to the Vice Chancellor of Research, Isfahan University of Medical Sciences and Iran National Science Foundation (INSF) for financial support.
References
- 1.S Wild, C P. Wild. International Agency for Research on Cancer. Vol. 2014. France: WHO Press, IARC Publisher; 2014. World Cancer Report. [Google Scholar]
- 2.Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol. 2015;75:659–70. doi: 10.1007/s00280-014-2607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: Recent insights and future perspectives. Curr Neuropharmacol. 2006;4:165–72. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimnas JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–94. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- 5.Ghannadi A, Shabani L, Yegdaneh A. Cytotoxic, antioxidant and phytochemical analysis of Gracilaria species from Persian Gulf. Adv Biomed Res. 2016;5:139. doi: 10.4103/2277-9175.187373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf CJ, Mannion RJ. Neuropathy pain: Aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 7.Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathy pain. Eur J Pharmacol. 2001;429:1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- 8.Hoang SN, Dinch KC, Hoang MH, Dang DH. Initial Study of Screening of Anti-Inflammatory Compounds from Some Species of Vietnamese Seaweeds.Proceeding of National Conference on Life Sciences. Quy Nhon City: Quy Nhon University, Science and Technics Publishing House; 2007. pp. 773–6. [Google Scholar]
- 9.Esfahani HN, Vaseghi G, Safaiean L, Pilehvaran A, Abed A, Rfiiean M. Gender differences in a mouse model of chemotherapy-induced neuropathy pain. Lab Anim. 2016;50:15–20. doi: 10.1177/0023677215575863. [DOI] [PubMed] [Google Scholar]
- 10.Kang JY, Khan MN, Park NH, Cho JY, Lee MC, Fujii H, et al. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–90. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Itoh H, Noda H, Amano H, Zhuaug C, Mizuno T, Ito H. Antitumor activity and immunological properties of marine algal polysaccharides, especially fucoidan, prepared from Sargassum thunbergii of Phaeophyceae. Anticancer Res. 1993;13:2045–52. [PubMed] [Google Scholar]
- 12.Cho SH, Kang SE, Cho JY, Kim AR, Park SM, Hong YK, et al. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J Med Food. 2007;10:479–85. doi: 10.1089/jmf.2006.099. [DOI] [PubMed] [Google Scholar]
- 13.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res Pharm Sci. 2013;8:113–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Sohrabipour J, Rabiei R. The Checklist of Green Algae of the Iranian Coastal Lines of the Persian Gulf and Gulf of Oman; Iran. J. Bot. 2007;13:146–9. [Google Scholar]
- 15.Mori T, Kanbara T, Harumiya M, Iwase Y, Masumoto A, Komiya S, et al. Establishment of opioid-induced rewarding effects under oxaliplatin- and paclitaxel-induced neuropathy in rats. J Pharmacol Sci. 2014;126:47–55. doi: 10.1254/jphs.14134fp. [DOI] [PubMed] [Google Scholar]
- 16.Vaseghi G, Sharifi M, Dana N, Ghasemi A, Yegdaneh A. Cytotoxicity of sargassum angustifolium partitions against breast and cervical cancer cell lines. Adv Biomed Res. 2018;7:43. doi: 10.4103/abr.abr_259_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbari V, Zafari S, Yegdaneh A. Anti-tuberculosis and cytotoxic evaluation of the seaweed Sargassum boveanum. Res Pharm Sci. 2018;13:30–7. doi: 10.4103/1735-5362.220965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yegdaneh A, Putchakarn S, Yuenyongsawad S, Ghannadi A, Plubrukarn A. 3-oxoabolene and 1-oxocurcuphenol, aromatic bisabolanes from the sponge Myrmekioderma sp. Nat Prod Commun. 2013;8:1355–7. [PubMed] [Google Scholar]
- 19.Mehdinezhad N, Ghannadi A, Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res Pharm Sci. 2016;11:243–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Gu W, Chen J, Patra P, Yang X, Gu Q, Wei L, et al. Nanoformulated water-soluble paclitaxel to enhance drug efficacy and reduce hemolysis side effect. J Biomater Appl. 2017;32:66–73. doi: 10.1177/0885328217708458. [DOI] [PubMed] [Google Scholar]
- 21.Yang PP, Yeh GC, Huang EY, Law PY, Loh HH, Tao PL. Effects of dextromethorphan and oxycodone on treatment of neuropathy pain in mice. J Biomed Sci. 2015;22:81. doi: 10.1186/s12929-015-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong DD, Hien HM, Anh HT. Studies on the analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulata Forsskal (Chlorophyta) in experiment animal models. Afr J Biotechnol. 2011;10:2308–13. [Google Scholar]


