Abstract
Background:
Gestational diabetes mellitus (GDM) is one of the commonly occurring high-risk obstetric complications that accounts for 4%–9% of total pregnancies. The present study was an attempt to assess the effect of GDM on composition of the neonatal oral microbiota.
Materials and Methods:
In this study, oral samples from 155 full-term vaginally delivered newborns were collected with sterile swabs. Seventy-five mothers diagnosed with GDM group and 80 were nondiabetic mothers (control). The oral microbiota was evaluated and analyzed by SPSS software.
Results:
The mean gestational age in Group I was 38.1 weeks and in Group II was 39.6 weeks. Firmicutes was present in 38.1% in Group I versus 77.6% in Group II patients, Actinobacteria was seen in 15.2% in Group I and 7.4% in Group II, Bacteroidetes in 27.6% in Group I and 7.9% in Group II, Proteobacteria in 9.5% in Group I and 3.8% in Group II, and Tenericutes in 9.6% in Group I and 3.3% in Group II. There was a significant difference in major genera Prevotella, Bacteroidetes, Bifidobacterium, Corynebacterium, Ureaplasma, and Weissella in both groups (P < 0.05).
Conclusion:
There was increased bacterial microbiota in neonates born to mothers with GDM as compared to neonates born to nondiabetic mothers. Assessment of initial oral microbiota of neonates could help in assessing the early effect of GDM on neonatal oral microbial flora.
Keywords: Gestational diabetes mellitus, neonates, oral microbiota
Introduction
The state of hyperglycemia during pregnancy was regarded as gestational diabetes mellitus (GDM) irrespective whether it was present before pregnancy and continued after pregnancy. GDM is one of the commonly occurring high-risk obstetric complications that accounts for 4%–9% of total pregnancies.[1] It is different from Type I and Type II diabetes. It is more prevalent during the second and third trimester of pregnancy. As reported by the International Diabetes Federation (2017), GDM affected that one-seventh of live births all over the world. Considering the great impact of GDM on both mothers and infants, it has gained attention among gynecologists worldwide.[2] It is not only the state of carbohydrate intolerance but also it found to be associated with other risks such as caesarian section, preeclampsia, shoulder dystocia, preterm birth, and neonatal hypoglycemia.[3] All these are adverse short-term pregnancy outcomes. Long-term pregnancy outcomes included Type II diabetes mellitus (DM), cardiovascular diseases, obesity, and neonatal malformations. Apart from this, the incidence of attention deficit, linguistic competence, and lower level of cognition is also common in infants born to mothers with GDM.[4]
It has been demonstrated in studies that microorganisms are present in the gut before birth. Hence, alteration in microbiota at various sites such as oral cavity, skin, and gut can result into numerous diseases. Early life is the period for development and colonization of gut microbiota which alters the maturation of the newborn's immune system.[5] Recent studies showed that alteration of intestinal microflora has deleterious effects on metabolic status and immune system. There is an occurrence of intestinal microflora in infants of mothers with GDM.[6]
Oral microbiota plays an important role in shaping human health. Alteration of oral microflora in early life can lead to dental caries, periodontal diseases, and oral mucosal diseases. Initial colonizers of oral cavity in the newborn have an impact on the growth of the newborn. It has been established that various factors including endogenous and mother status affect composition of the neonatal oral microbiome.[7] The aim of the present study was to analyze the effect of GDM on the composition of the neonatal oral microbiota.
Materials and Methods
In the present study, enrollment of 155 term neonates, which were delivered vaginally was done. Inclusion criteria were infants with gestational age 37–42 weeks, infants with birth weight > 2500 g, and infants without any significant congenital and fetal chromosomal abnormalities. The exclusion criteria were mothers without preeclampsia, eclampsia, pregnancy-induced hypertension (PIH), maternal obesity and infections, and patients with a negative history of any antibiotic therapy in the past 1 month.[8] All patients were well informed regarding the study and their consent was taken. Data such as name, age of mother, gestational age, birth weight (grams), prepregnancy body mass index (BMI), and antepartum BMI were recorded. The diagnosis of GDM was based on the findings such as fasting plasma glucose ≥5.1 mmol/L or 1 h postoral glucose tolerance test (OGTT) glycemia ≥10 mmol/L or 2 h postglucose tolerance test (OGTT) glycemia ≥8.5 mmol/L. Twenty-five mothers found to be have GDM and forty were nondiabetic mothers, so we divided them in Group I (GDM) and Group II (Control). Mothers were managed with exercise (a 30-min daily moderate exercise) and diet control. All samples from mothers were taken. For collection of neonatal samples, sterile swabs were collected 1 min after birth. After collection of sample, the entire sample was transferred to the laboratory (two laboratories were used: Omega Microbiology and Diagnostic Lab, Patna and Dr. Jain's Microbiology and Pathology lab, Ludhiana) and was used for identification and isolation of aerobic and anaerobic bacteria. All different colonies should be isolate and plated on an anaerobic and aerobic blood agar plate and chocolate agar plate. These plates are incubated for 1–6 days at 37°C. Using a strong magnifying glass and employing Gram staining, an initial examination of the colonies was done. Furthermore, identification of anaerobes was done using organism-specific anaerobic agar media (Rogosa agar/Lactobacillus selection agar, Columbia anaerobic agar, Bacteroides Bile Esculin, cooked meat broth, Thioglycollate, brain–heart infusion agar, MacConkey agar, and Tellurite blood agar). Further analysis was assisted by conducting a series of biochemical tests (indole, catalase, nitrate, and urease test) with different sugar and variable substrates. Incubation was done for 1–6 days, depending on the growth rate of the isolate. Anaerobic condition was maintained by chemical and anaerobic gas pack jar. Bacterial isolates were subcultured on agar plates at regular intervals to maintain viability and metabolic activities [Figure 1]. All the agar plates were stored at a temperature of 4°C preservation and maintenance. Results were entered in MS Excel sheet for statistical analysis using SPSS software version 20.0 (IBM, Armonk, New York). Unpaired t-tests and Fisher's exact tests were used to study differences between GDM and Non diabetic mellitus group (NDM) groups. The level of significance was set at 0.05.
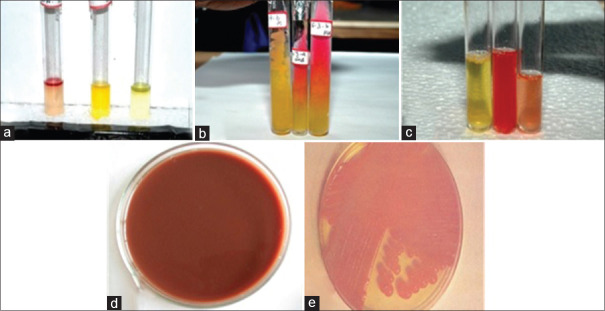
Figure 1.
(a) Indole test is negative for Lactobacillus and Acinetobacter, (b) sugar fermentation test for Lactobacillus, (c) magnetic resonance test is negative and Voges–Proskauer test is positive for Bifidobacterium, (d) Potassium tellurite agar for Corynebacterium, and (e) growth of Acinetobacter on MacConkey agar
Results
Table 1 shows that Group I comprised GDM (75) and Group II nondiabetic group (80) (Control). Table 2 shows that mean gestational age in Group I was 38.1 weeks and in Group II was 39.6 weeks and birth weight was 3059.1 g in Group I and 3255.3 g in Group II. The difference was significant (P < 0.05). There were 43 males and 32 females in Group I and 45 males and 35 females in Group II. The difference was nonsignificant (P > 0.05). Table 3 shows that the mean value of Shannon index for the assessment of oral phyla in Group I was 3.38 and in Group II was 2.91. The difference found to be significant (P < 0.05).
Table 1.
Distribution of patients
| Groups | Group I | Group II |
|---|---|---|
| Status | Gestational diabetes mellitus | Nondiabetic group (control) |
| Number | 75 | 80 |
Table 2.
Assessment of neonatal parameters in both groups
| Parameters | Group I | Group II | P |
|---|---|---|---|
| Gestational age (weeks) | 38.1 | 39.6 | 0.04 |
| Birth weight (g) | 3059.1 | 3255.3 | 0.01 |
| Male | 43 | 45 | 0.31 |
| Female | 32 | 35 |
Table 3.
Assessment of oral microbial diversity (Phyla) with Shannon index in both groups
| Shannon index | Group I | Group II | P |
|---|---|---|---|
| Mean±SD | 3.38±1.21 | 2.91±0.91 | 0.02 |
SD: Standard deviation
Firmicutes was present in 38.1% in Group I versus 77.6% in Group II patients, Actinobacteria was seen in 15.2% in Group I and 7.4% in Group II, Bacteroidetes in 27.6% in Group I and 7.9% in Group II, Proteobacteria in 9.5% in Group I and 3.8% in Group II, and Tenericutes in 9.6% in Group I and 3.3% in Group II [Graph 1]. The difference was found to be significant (P < 0.05).
Graph 1.
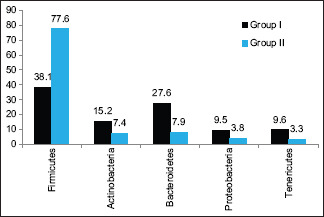
Assessment of oral microbiota in both groups
Graph 2 shows that major genera were Prevotella seen 16.5% in Group I and 6.7% in Group II, Bacteroidetes 7.8% in Group I and 3.02% in Group II, Bifidobacterium 5.62% in Group I and 2.64% in Group II, Corynebacterium 7.02% in Group I and 2.84% in Group II, Ureaplasma seen 6.78% in Group I and 0.25% in Group II, and Weissella seen 8.45% in Group I and 0.05% in Group II. The difference was found to be significant (P < 0.05).
Graph 2.
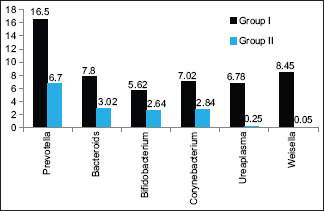
Assessment of major genera in both groups
Table 4 shows that positive Pearson's correlation of gestational age was found with Firmicutes (r = 0.319, P < 0.05) in Group II and Bacteroidetes (r = 0.683, P < 0.05) and Prevotella (r = 217, P < 0.05) in Group I.
Table 4.
Pearson’s correlation of gestational age with microbiota
| Microbiota | Group I | Group II | ||
|---|---|---|---|---|
| Pearson correlation (r) | Significant (two-tailed) | Pearson correlation (r) | Significant (two-tailed) | |
| Firmicutes | 0.319 | 0.612 | 0.241 | 0.016 |
| Bacteroidetes | 0.683 | 0.002 | 0.115 | 0.256 |
| Prevotella | 0.217 | 0.018 | 0.124 | 0.238 |
Discussion
In the present study, we included 155 term neonates delivered vaginally. Seventy-five mothers were found to have GDM and eighty were nondiabetic mothers, so we divided them into Group I (GDM) and Group II (Control). It was observed that nondiabetic mothers had significantly higher birth weight, gestational age, and gestational weight gain. In neonates, oral microbiome consisted of Actinobacteria, Firmicutes, Proteobacteria, Bacteroidetes, and Tenericutes in neonatal oral microbiome. While analyzing statistically, it was seen that, in the GDM group, there was a significantly higher incidence of Genus Alistipes, Streptococcus, and Faecalibacterium. Furthermore, the mean Shannon index (oral phyla) in Group I and Group II was 3.36 and 2.95, respectively. Our results were in concordance with the results obtained by previous authors who also reported similar findings in their respective studies. Su et al.[9] extracted meconium DNA from 34 full-term newborns. They reported a significant difference in relation to gut microbiota among GDM newborns and controls. In GDM group, they observed an increase in Proteobacteria and Actinobacteria phyla and a decline in Bacteroidetes. However, there was a significant reduction in the Prevotella and Lactobacillus in GDM neonates. They also observed a significant positive correlation in between phylum Actinobacteria and genus Acinetobacter with maternal fasting glucose levels and negatively correlation between fasting blood glucose with phylum Bacteroidetes and genus Prevotella. In the present study, Firmicutes was found to be in higher amount among controls (Group II), while the incidence of Actinobacteria, Bacteroidetes, Proteobacteria, and Tenericutes was significantly higher in GDM group. Our results were in concordance with the results obtained by He et al., who also reported similar findings.[8]
In the present research, while comparing the major genera in between the two study groups, significant results were obtained. Incidence of Prevotella, Bacteroidetes, Bifidobacterium, Corynebacterium, Ureaplasma, and Weissella was significantly higher among GDM groups. In a previous study conducted by Wang et al., authors collected oral, intestinal, and vaginal samples from 581 GDM mothers and oral, pharyngeal, meconium, and amniotic fluid samples from 248 neonates. Their results also demonstrated altered microbiota of neonates and GDM pregnant women. They observed that microbes with variations in the maternal and neonatal microbiota showed the intergenerational concordance of microbial variation associated with GDM.[10]
We also observed a positive correlation of gestational age with Firmicutes in Group I and Bacteroidetes and Prevotella in Group I. Factors such as maternal status, type of feeding, and environment greatly affect neonatal oral microbiota. Under physiologic conditions, the gastrointestinal tract of the fetus is said to be sterile with the initial acquaintance of the immune system to commensals happening during the way through the birth canal. These primordial alterations on a long-term basis are considered the settling phase for mucosal and systemic immune system. The procedure by which neonate organ systems acclimatize to the intimidating environment of microbial colonization remains partly understood. However, parameters contained in maternal milk are said to define some of these early responses to commensals.[11,12,13] GDM is a significant risk factor for general health of both neonatal and maternal health.[11] Women are more prone to develop preeclampsia, PIH, and in neonates, there can be respiratory distress syndrome, fetal macrosomia, and Type II DM in offspring. There are chances of microbiota dysbiosis in the meconium of newborns due to maternal diabetes status.[12,13,14]
In GDM patients, carbohydrate deficiency can affect the postprandial glycemic response. Lipopolysaccharides are a significant component of cell wall of Gram-negative bacteria, and it plays a substantial pathogenetic role of certain bacterial infections.[8] Its enhancement in GDM patients may have significant effects on the health of neonates, and hence further exploration of results with higher parameters is necessary.
Conclusion
Authors found that there was increased bacterial microbiota in neonates born to mothers with GDM as compared to neonates born to nondiabetic mothers. However, large-scale studies are necessary to substantiate the result obtained in our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35((Suppl 1)):64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–8. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36:617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29:743–54. doi: 10.1016/j.tem.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Cheng YW, Caughey AB. Gestational diabetes: Diagnosis and management. J Perinatol. 2008;28:657–64. doi: 10.1038/jp.2008.62. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Mclntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: Association of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: Perinatal outcomes. Obstet Gynecol. 2008;112:1015–22. doi: 10.1097/AOG.0b013e31818b5dd9. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Wu J, Xiao B, Xiao S, Li H, Wu K. The initial oral microbiota of neonates among subjects with gestational diabetes mellitus. Front Pediatr. 2019;7:513. doi: 10.3389/fped.2019.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su M, Nie Y, Shao R, Duan S, Jiang Y, Wang M, et al. Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS One. 2018;13:e0205695. doi: 10.1371/journal.pone.0205695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67:1614–25. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–6. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 12.Blod C, Schlichting N, Schülin S, Suttkus A, Peukert N, Stingu CS, et al. The oral microbiome – The relevant reservoir for acute pediatric appendicitis? Int J Colorectal Dis. 2018;33:209–18. doi: 10.1007/s00384-017-2948-8. [DOI] [PubMed] [Google Scholar]
- 13.Reddy RM, Weir WB, Barnett S, Heiden BT, Orringer MB, Lin J, et al. Increased variance in oral and gastric microbiome correlates with esophagectomy anastomotic leak. Ann Thorac Surg. 2018;105:865–70. doi: 10.1016/j.athoracsur.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]