Abstract
Water exchange (WE) and artificial intelligence (AI) have made critical advances during the past decade. WE significantly increases adenoma detection and AI holds the potential to help endoscopists detect more polyps and adenomas. We performed an electronic literature search on PubMed using the following keywords: water-assisted and water exchange colonoscopy, adenoma and polyp detection, artificial intelligence, deep learning, neural networks, and computer-aided colonoscopy. We reviewed relevant articles published in English from 2010 to May 2020. Additional articles were searched manually from the reference lists of the publications reviewed. We discussed recent advances in both WE and AI, including their advantages and limitations. AI may mitigate operator-dependent factors that limit the potential of WE. By increasing bowel cleanliness and improving visualization, WE may provide the platform to optimize the performance of AI for colonoscopies. The strengths of WE and AI may complement each other in spite of their weaknesses to maximize adenoma detection.
KEYWORDS: Adenoma detection rate, Adenoma miss rate, Artificial intelligence, Computer-aided colonoscopy, Water exchange
INTRODUCTION
Colorectal cancer (CRC) is the second most common cancer and the third leading cause of cancer-related mortality in Taiwan. The rate of CRC has been increasing in Taiwan [1]. Early detection and removal of precancerous polyps is critical for CRC prevention [2]. Adenoma detection rate (ADR), defined as the proportion of patients with at least one adenoma of any size, is an important quality indicator for colonoscopy. Every 1% increase in ADR correlates with 3% reduction in interval CRC and 5% decrease in its related mortality [3]. In a study of 29,969 participants in the Taiwanese Nationwide CRC Screening Program who underwent colonoscopy after positive fecal immunochemical testing, the overall ADR was 39.5%. Of the included hospitals, 5.4% had ADR <15%, 77.5% had ADR between 15% and 30%, and 17.1% had ADR >30% [4]. While the Taiwanese population may have a slightly lower ADR compared to its Western counterpart (14.7% vs. 20.7% in one study), gastroenterology societies in Western countries recommend an ADR ≥30% in males and ≥20% in females [5,6].
Among the strategies to increase ADR, water exchange (WE) has proven to be effective in at least three randomized controlled trials (RCTs) [7,8,9]. In contrast to conventional air insufflation colonoscopy, evacuation of water and debris in WE provides salvage cleaning during insertion and better visualization of polyps during withdrawal, leading to higher adenoma detection. The benefit of WE may be reduced, however, if the endoscopist failed to recognize suspicious lesions. On the other hand, artificial intelligence (AI) shows promising results in improving ADR [10]. Nonetheless, false alarms caused by bubbles and fecal debris may undermine its utility. In this review, we discuss how the two methods may complement each other to optimize polyp and adenoma detection.
WATER EXCHANGE INCREASES ADENOMA DETECTION RATE COMPARED WITH Air Insufflation Colonoscopy
Conventional colonoscopy uses air to insufflate the colon. Air elongates and distends the colon, resulting in patient discomfort and intubation difficulty. To avoid the downsides of air insufflation, WE completely excludes the use of air during insertion by turning the air button off at the beginning of the procedure. Instead of air, scope advancement is guided by infusion of water, which is removed almost simultaneously to keep the colon shortened and the lumen collapsed, thus attenuating insertion pain during colonoscopy. Multiple RCTs and meta-analyses had shown that WE significantly reduced insertion pain as compared with air insufflation. WE also increased cecal intubation rate in potentially difficult, unsedated colonoscopy (92.7% vs. 76.4%; P = 0.033) [11], and proportions of patients completing colonoscopy without sedation during on demand sedation (93.3% vs. 76.0%, P = 0.006) [12]. A meta-analysis found that patients' willingness to repeat a colonoscopy was significantly greater for WE than air insufflation [13]. However, the longer insertion time needed for infusing clean water and suctioning dirty water during WE has impeded adoption by more endoscopists. A recent meta-analysis showed that WE incurred an increase in total procedure time of about 2 min [14].
Further, Leung et al. observed that WE had numerically higher adenoma yield [15]. This serendipitous observation motivated researchers from around the world to perform RCTs to establish the efficacy of WE colonoscopy in improving ADR [7,8,9]. Hsieh et al. found that the overall ADR for WE and air insufflation was 49.8% and 37.8% in a prospective, multicenter RCT, respectively [8]. WE significantly increased ADR when compared to air insufflation (P = 0.016). Cadoni et al. performed a prospective, multicenter double-blinded RCT in patients undergoing screening colonoscopy [7]. Compared to air insufflation, WE significantly increased overall ADR (49.3% vs. 40.4%, P = 0.03) and right colon ADR (24.0% vs. 16.9%, P = 0.04). In a large prospective multicenter RCT in China, Jia et al. found that ADR was 18.3% for WE and 13.4% for air insufflation (P < 0.001) [9]. In all these studies, WE had significantly higher bowel cleanliness scores (Boston Bowel Preparation Scales) compared with air insufflation [Table 1] [16].
Table 1.
Key references which compared colon ADR and AMR between water exchange and air insufflation colonoscopy
| Water exchange | Air insufflation | P | |
|---|---|---|---|
| Jia et al. [9] | 1653 cases | 1650 cases | |
| Overall BBPS (mean±SD): 7.3±1.6 | Overall BBPS: 7.0±2.3 | <0.001 | |
| Right colon BBPS: 2.3±0.7 | Right colon BBPS: 2.2±1.5 | <0.001 | |
| Overall ADR: 18.3% | Overall ADR: 13.4% | <0.001 | |
| Hsieh et al. [8] | 217 cases | 217 cases | |
| Overall BBPS (mean±SD): 7.1±1.3 | Overall BBPS: 6.2±1.1 | <0.001 | |
| Overall ADR: 49.8% | Overall ADR: 37.8% | 0.016 | |
| Cadoni et al. [7] | 408 cases | 408 cases | |
| Overall BBPS, median (IQR): 9.0 (7.0-9.0) | Overall BBPS: 8.0 (6.0-9.0) | <0.001 | |
| Right colon BBPS: 3.0 (2.0-3.0) | Right colon BBPS: 2.0 (2.0-3.0) | <0.001 | |
| Overall ADR: 49.3% | Overall ADR: 43.4% | 0.04 | |
| Right colon ADR: 24.0% | Right colon ADR: 16.9% | 0.03 | |
| Cheng et al. [18] | 86 cases | 86 cases (CO2 insufflation) | |
| Overall BBPS (mean±SD): 7.4±0.7 | Overall BBPS: 7.0±0.5 | <0.001 | |
| Overall ADR: 53.5% | Overall ADR: 58.1% | 0.645 | |
| Overall AMR*: 18.9% | Overall AMR: 28.2% | 0.071 | |
| Right colon AMR*: 17.5% | Right colon AMR: 33.8% | 0.034 |
*Miss rates are based on per adenoma analysis (number of adenomas missed in the first colonoscopy divided by the total number of adenomas detected during both the first and second colonoscopies). ADR: Adenoma detection rate, AMR: Adenoma miss rate, BBPS: Boston bowel preparation scale, SD: Standard deviation
Improvement in bowel cleanliness is a likely reason for the superior ADR with WE. Most of the water infused is suctioned by the time cecal intubation is achieved. Thus, there is less need for cleaning-related activities during the withdrawal phase. With fewer distractions from cleaning the mucosa during withdrawal and the associated colonic contraction, the endoscopist can focus better on inspecting the mucosa and searching for polyps [17].
ADENOMA MISS RATE AND WATER EXCHANGE
Adenoma miss rate (AMR) is of great concern in colonoscopy. In a study of 183 patients undergoing back-to-back colonoscopies, the AMR was 24% [19]. AMR was higher for adenomas ≤0.5 cm than adenomas ≥1 cm (27% vs. 6%). Interestingly, numerically more adenomas were missed in the right colon than the left colon (27% vs. 21%, P > 0.05). This was attributed to the fact that it was more difficult to achieve adequate bowel cleanliness in the right colon.
Since WE was effective in improving ADR, WE was speculated to decrease AMR. In a retrospective observational study comparing WE and CO2 insufflation, Cheng et al. found that WE had significantly lower AMR in the right colon (17.5% vs. 33.8%, P = 0.034) and proximal colon (15.5% vs. 30.4%, P = 0.018) [18]. Notably, the study emphasized near-complete removal of water during insertion. This was in accordance with previous RCTs that showed WE significantly increased ADR where 91%–102% of infused water was removed during insertion [7,8,9]. Proper implementation of WE by aiming for near-complete removal of infused water during insertion appeared to be the key to reducing AMR.
Like most colonoscopy techniques, the extent of the beneficial effects (e.g., increased ADR and reduced AMR) of WE is operator dependent. Therefore, the advantage of WE may be diminished by endoscopist-related factors, such as failure to recognize lesions, physician inattention or fatigue, suboptimal withdrawal time, and inadequate inspection techniques [20].
ARTIFICIAL INTELLIGENCE AND COMPUTER-AIDED COLONOSCOPY
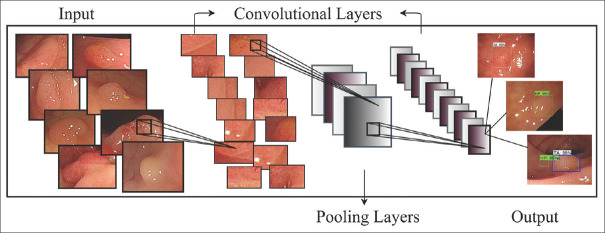
AI refers to computer “self-learning” and “problem-solving.” It is the ability of a computer system or machine to interpret external data, learn from the data set, and use those learning to achieve a specific goal. This machine learning model, or predictive algorithm, is adaptive to new circumstances, and its accuracy improves over time with more data analyzed. Deep learning is a subfield of machine learning that applies algorithms of artificial neural networks. It employs multiple layers of algorithms to progressively extract higher-level features from a raw input. At each layer, the algorithm identifies specific features and classifies them for the next layer until a complex relevant concept is formed at the highest layer. For example, a lower layer may identify edges. A higher layer would identify certain shapes formed by the edges. Finally, at the highest layer, where all these features are incorporated, a particular image may be identified [Figure 1] [21].
Figure 1.
Schematic outline of CNN feature extraction and classification for the polyp images
There are different variations of deep neural networks. The convolutional neural networks (CNNs) are particularly suitable for image and video analysis, including quantification, recognition, and classification [22]. The CNNs have been applied in the medical field to assist in pathologic lesion detection.
One such application is computer-aided colonoscopy, which employs CNNs to improve conventional colonoscopy. Computer-aided detection (CADe) and computer-aided diagnosis (CADx) are the two major fields in computer-aided colonoscopy. Whereas CADx assesses a polyp's likelihood of being neoplastic or hyperplastic to prevent unwarranted polypectomies, CADe aims to detect any polyp regardless of its histology. With the emergence of deep learning algorithms, the performance of CADe has achieved close to expert endoscopist level in terms of polyp detection [23]. CADe has been reported to increase polyp detection and reduce polyp miss rate in retrospective and prospective studies [Table 2].
Table 2.
Summary of studies for computer aided convolutional neural network polyp detection
| Algorithm and method | Image | Dataset | Processing speed | Outcomes | |
|---|---|---|---|---|---|
| Misawa et al. 2018 [24] | 3D CNN (ex vivo) | Video | 73 videos divided into 546 short videos Training: 105 polyps positive, 306 polyps negative; testing: 50 polyps positive, 85 polyps negative |
Sensitivity: 90%; specificity: 63.3%; accuracy: 76.5% | |
| Urban et al. 2018 [25] | CNN VGG19 (ex vivo) | Still and video | Training: 8641 images Testing Set 1: 9 videos Set 2: 11 videos (missed polyp simulation) |
10 ms/frame | Sensitivity at 75% False negative rate: 96.9% Accuracy: 96.4% Frame by frame false positive rate: 5% |
| Yamada et al. 2019 [26] | Faster R-CNN VGG 16 (ex vivo) | Still and video | Training: 4087 images, and 135,874 video frames Testing: 4840 still images, and 77 videos |
21.9 ms/image 30 frames/s |
Sensitivity: 97.3%; specificity: 99.0%; AUC: 0.975 |
| Becq et al. 2020 [27] | SegNet CNN (ex vivo) | Video | 50 prospectively collected videos | PDR: 81% Sensitivity: 98.8% Positive predictive value: 40.6% |
|
| Klare et al. 2019 [28] | CNN | Live | 55 live colonoscopies | 50 ms of latency | Sensitivity: 75.3%; ADR: 29% |
| Wang et al. 2019 [29] | CNN Real time RCT |
Live | Training: 5545 images Test: 536 routine colonoscopy |
25 frames/s 77 ms latency |
ADR: 20.3% (conventional) versus 29.1% (CAD), P<0.001 Mean number of polyps per procedure: 0.51 versus 0.97, P<0.001 |
| Liu et al. 2020 [30] | 3D CNN Real time RCT |
Live | Training: 101 polyp positive; 300 polyp negative Testing: 46 polyp positive; 88 polyp negative |
PDR: Control (28%) versus CAD (44%), P<0.001 ADR: Control (24%) versus CAD (39%), P<0.001 |
3D: Three-dimensional, ADR: Adenoma detection rate, CAD; CADe: Computer-aided detection, CNN: Convolutional neural network, PDR: Polyp detection rate, R-CNN: Region-convoluted neural networks, VGG: Visual geometry group
Fernández-Esparrach et al. designed a model that used energy maps to inform the likelihood of the presence of a polyp [31]. The model achieved a sensitivity of 70.4% (95% confidence interval [CI] 60.3%–80.8%) and a specificity of 72.4% (95% CI 61.6%–84.6%) for polyp detection. While the model's sensitivity and specificity were suboptimal, the study demonstrated AI's potential applicability in colonoscopy. To improve AI's performance and easy accessibility, Urban et al. trained a CADe system using over 8000 images from screening colonoscopies [25]. Their CADe system was able to achieve a high accuracy of 96% for polyp detection. However, it also had a false positive rate of 5%. Misawa et al. [24] developed a similar CADe system that achieved a high sensitivity of 90% but only had a specificity of 63.3% and an accuracy of 76.5%. However, 64.5% of the lesions the system detected were flat polyps, suggesting that CADe may enhance detection of these difficult-to-detect flat lesions. Yamada et al. confirmed this finding [26]. Their AI system had a high sensitivity of 92.2% for non-polypoid lesions. Moreover, it had higher sensitivity (97.3% vs. 87.4%) and specificity (99.0% vs. 96.4%) in detecting lesions than the endoscopists. Becq et al. also found that CADe could augment endoscopists' polyp detection in patients with different preparation qualities [27]. In 50 colonoscopies where 55 polyps were removed, Becq's CADe system identified additional 47 “definite polyps” and 63 “possible polyps” that were missed by the endoscopist. The sensitivity and positive predictive values of the system were 98.8% and 40.6%, respectively. PDR was higher for CADe compared with the endoscopist (81% vs. 62%). However, the system also had a high false-positive rate of 59.1%. These studies showed promising results for CADe, but they were performed in vitro under controlled settings using either still images or videos.
The feasibility of CADe in clinical practices was tested by Klare et al., who compared polyp detection rate (PDR) and ADR between expert endoscopists and a CADe system during real-time colonoscopies [28]. The endoscopists had a PDR of 56.4% (95% CI, 42.3%–69.7%) and an ADR 30.9% (95% CI, 19.1%–44.8%), whereas the CADe system achieved a PDR of 50.9% (95% CI, 37.1%–64.4%) and an ADR of 29.1% (95% CI, 17.6%–42.9%). Although the CADe system did not significantly impact PDR or ADR, the study demonstrated that real-time AI application in colonoscopy was possible. Wang et al. conducted the first prospective RCT assessing the efficacy of a real-time CADe system on ADR [29]. The CADe significantly increased ADR by nearly 50% (29.1% vs. 20.3%, P < 0.001). The CADe detected more diminutive adenomas (185 vs. 102, P < 0.001) and hyperplastic polyps (114 vs. 52, P < 0.001). Enhancing hyperplastic PDR could be useful for diagnose-and-leave strategy in future application. In a large prospective RCT, Liu et al. compared the efficacy of a CADe with conventional colonoscopy in 1,026 patients [30]. The system significantly improved PDR (0.44 vs. 0.28, P < 0.001) and ADR (0.39 vs. 0.23, P < 0.001). The CADe also detected significantly more small adenomas and significantly more sessile and flat adenomas. Thirty-six false alarms were reported, mostly from bubbles (33.3%), feces (16.7%), and residual fecal matter (13.9%). These RCTs provided strong evidence that AI could significantly enhance ADR in real time.
In addition to increasing polyp detection with CADe, CADx is able to differentiate neoplastic from nonneoplastic diminutive (≤5 mm) polyps, achieving a negative predictive value of 90% [32]. This fulfills the Preservation and Incorporation of Valuable Endoscopic Innovations-2 criteria for diagnose-and-leave strategy. AI's high hyperplastic PDR also supports the diagnose-and-leave strategy. A recent study showed that compared to the traditional resect-all-polyps strategy, a diagnose-and-leave strategy supported by the AI prediction could reduce the average annual colonoscopy cost by 18.9% ($149.2 million) [33]. This result certainly encourages the wider use of AI in colonoscopy. On the other hand, a recent meta-analysis showed that AI slightly increased the withdrawal time (usually < 1 min) [34].
WATER EXCHANGE AND ARTIFICIAL INTELLIGENCE COMPLEMENT EACH OTHER
How water exchange might help artificial intelligence
AI has demonstrated high sensitivity of 90%–98.8% for polyp and adenoma detection, but its use may be limited by high false-positive rate and “false alarms,” which are usually triggered by bubbles, debris, and fecal matters [24,26,27]. False alarms may distract endoscopists and negatively impact ADR. False positive rate up to about 60% has been reported [27]. This is especially concerning as many studies on AI and CADe utilized selected “ideal” images or video recordings that may not be representative of real-life conditions with variable bowel cleanliness.
Even with split-dose bowel regimen, there are still about 14% of patients with inadequate bowel preparation [35]. Another way to improve bowel cleanliness is intraprocedural cleaning. With air insufflation, endoscopists usually focus on reaching the cecum as quickly as possible during insertion and clean the mucosa during withdrawal phase. Cleaning is an integral part of the withdrawal technique in air insufflation colonoscopy, and poor cleaning technique is correlated with lower ADR [36]. In contrast, WE provides salvage cleansing by infusing clean water and suctioning out dirty water during insertion. Head-to-head comparisons of WE and air insufflation have consistently shown better bowel cleanliness scores with WE [Table 1] [7,8,9,17], suggesting that insertion salvage cleansing in WE is more effective than withdrawal cleaning in air insufflation and provides a better platform for AI.
One potential quality indicator of colonoscopy that WE might help with is clarity of the colonoscopy lens. When the lens is blurred by fecal materials, it obscures the views of the endoscopist and surely would interfere with the scan of AI. Because of the difficulty to objectively evaluate the duration and degree of clarity during colonoscopy, Hsieh et al. chose the frequency of lens cleaning as a surrogate marker. In a blinded analysis of withdrawal phase videos from RCTs comparing WE and air insufflation, WE significantly reduced the frequency of lens cleaning compared with air insufflation (mean [standard deviation], 2.4 [3.2] vs. 5.5 [5.0], P < 0.001) [18]. Recent advances in the use of AI to assess the quality of colonoscopies make objective scoring of lens clarity possible [37]. Future studies using AI to compare the lens clarity and its impact on ADR during WE and air insufflation will be able to further address this issue.
How artificial intelligence might help water exchange
The role of CADe is similar to that of a “second observer” who points out the presence of a polyp to the endoscopist in real time. In a retrospective cohort study, inexperienced endoscopists detected significantly less diminutive polyps (≤3 mm) and flat polyps compared to expert endoscopists (461 vs. 97 and 422 vs. 28, respectively, both P < 0.001) [38]. Moreover, the study showed detection rate of histological advanced adenomas increased with the experience of the endoscopist, implying that the inexperienced endoscopists were missing small polyps with advanced histology. The inclusion of a second observer, either a trainee or an experienced nurse, during colonoscopy has been shown to significantly increase ADR [39,40,41]. Of note, the increase in ADR seems to be directly proportional to the experience of the second observer. Compared with colonoscopies performed by an attending gastroenterologist alone, the involvement of fellows increased ADR with each year of training [39]. CADe has achieved expert level in terms of polyp detection and would be a very helpful “second observer” [25]. Moreover, CADe has been shown to significantly increase detection of diminutive adenomas and nonpolypoid polyps that could have otherwise been missed by inexperienced endoscopists [26,29]. With all of its merits, WE still had a 17.5% AMR (per adenoma) in the right colon [17]. With the addition of CADe, WE could be expected to achieve a lower AMR and higher PDR.
Negative impacts of fatigue on outcomes have been well documented in both medical fields, such as resident trainees, and surgeons, as well as non-medical professions, such as pilots and truck drivers [42,43,44,45]. In endoscopists who worked a full-day shift, the ADR of the afternoon colonoscopy was significantly lower than that of the morning (RR: 1.18; 95% CI, 1.07–1.29) [46]. A retrospective study reported a significant decline in ADR for each subsequent hour of the day [47]. Compared to air insufflation, WE usually requires longer insertion time and total procedure time [48], which might aggravate endoscopist fatigue. AI has been used to help surgeons reduce fatigue in surgery [49]. Using sound alarms and visible signals to indicate the finding of a polyp, CADe might draw the attention of a fatigued endoscopist back to the endoscopic view and prevent missing of polyps.
CADe could serve as an additional observer to mitigate some of the operator-dependent factors that may limit the efficacy of WE. On the other hand, WE may provide an optimal platform for AI by improving bowel cleanliness and clarity of the endoscopic lens, thereby reducing false alarms. Thus, WE and AI may have synergistic effects on improving ADR.
CONCLUSION
Both AI and WE hold the promise to improve adenoma detection. WE can enhance the performance of AI by improving bowel cleanliness and reducing false alarms. AI can maximize the efficacy of WE by promoting recognition of polyps. They may complement each other to optimally improve ADR.
Financial support and sponsorship
The study was supported by research fund from the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation. Dr. Leung's research and publication effort is supported by VA Clinical Merit and ASGE Clinical Research Funds.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ministry of Health and Welfare, Cancer Registry Annual Report. Taiwan: 2016. [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, O'brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The national polyp study workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu SY, Chuang SL, Chen SL, Yen AM, Fann JC, Chang DC, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: Analysis of the Taiwanese nationwide colorectal cancer screening program. GUT. 2017;66:293–300. doi: 10.1136/gutjnl-2015-310256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soon MS, Kozarek RA, Ayub K, Soon A, Lin TY, Lin OS. Screening colonoscopy in Chinese and Western patients: A comparative study. Am J Gastroenterol. 2005;100:2749–55. doi: 10.1111/j.1572-0241.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 7.Cadoni S, Falt P, Rondonotti E, Radaelli F, Fojtik P, Gallittu P, et al. Water exchange for screening colonoscopy increases adenoma detection rate: A multicenter, double-blinded, randomized controlled trial. Endoscopy. 2017;49:456–67. doi: 10.1055/s-0043-101229. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh YH, Tseng CW, Hu CT, Koo M, Leung FW. Prospective multicenter randomized controlled trial comparing adenoma detection rate in colonoscopy using water exchange, water immersion, and air insufflation. Gastrointest Endosc. 2017;86:192–201. doi: 10.1016/j.gie.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Jia H, Pan Y, Guo X, Zhao L, Wang X, Zhang L, et al. Water exchange method significantly improves adenoma detection rate: A multicenter, randomized controlled trial. Am J Gastroenterol. 2017;112:568–76. doi: 10.1038/ajg.2016.501. [DOI] [PubMed] [Google Scholar]
- 10.Alagappan M, Brown JR, Mori Y, Berzin TM. Artificial intelligence in gastrointestinal endoscopy: The future is almost here. World J Gastrointest Endosc. 2018;10:239–49. doi: 10.4253/wjge.v10.i10.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo H, Zhang L, Liu X, Leung FW, Liu Z, Wang X, et al. Water exchange enhanced cecal intubation in potentially difficult colonoscopy. Unsedated patients with prior abdominal or pelvic surgery: A prospective, randomized, controlled trial. Gastrointest Endosc. 2013;77:767–73. doi: 10.1016/j.gie.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh YH, Tseng CW, Koo M, Leung FW. Feasibility of sedation on demand in Taiwan using water exchange and air insufflation: A randomized controlled trial. J Gastroenterol Hepatol. 2020;35:256–62. doi: 10.1111/jgh.14839. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Huang QK, Dong XL, Jin PP. Water exchange versus air insufflation for colonoscopy: A meta-analysis. Saudi J Gastroenterol. 2018;24:311–6. doi: 10.4103/sjg.SJG_118_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadoni S, Hassan C, Frazzoni L, Ishaq S, Leung FW. Impact of water exchange colonoscopy on endoscopy room efficiency: A systematic review and meta-analysis. Gastrointest Endosc. 2019;89:159–67E. doi: 10.1016/j.gie.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Leung FW, Harker JO, Jackson G, Okamoto KE, Behbahani OM, Jamgotchian NJ, et al. A proof-of-principle, prospective, randomized, controlled trial demonstrating improved outcomes in scheduled unsedated colonoscopy by the water method. Gastrointest Endosc. 2010;72:693–700. doi: 10.1016/j.gie.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–5. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh YH, Koo M, Tseng CW, Yang HW, Leung FW. Reduction of multitasking distractions underlies the higher adenoma detection rate of water exchange compared to air insufflation Blinded analysis of withdrawal phase videos. United European Gastroenterol J. 2019;7:230–8. doi: 10.1177/2050640618817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CL, Kuo YL, Hsieh YH, Tang JH, Leung FW. Water exchange colonoscopy decreased adenoma miss rates compared with literature data and local data with CO 2 insufflation: An observational study. BMC Gastroenterol. 2019;19:143. doi: 10.1186/s12876-019-1065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 21.Esteva A, Robicquet A, Ramsundar B, Kuleshov V, Depristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25:24–9. doi: 10.1038/s41591-018-0316-z. [DOI] [PubMed] [Google Scholar]
- 22.Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 23.Mori Y, Kudo SE, Berzin TM, Misawa M, Takeda K. Computer-aided diagnosis for colonoscopy. Endoscopy. 2017;49:813–9. doi: 10.1055/s-0043-109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misawa M, Kudo SE, Mori Y, Cho T, Kataoka S, Yamauchi A, et al. Artificial intelligence-assisted polyp detection for colonoscopy: Initial experience. Gastroenterology. 2018;154:2027–9000. doi: 10.1053/j.gastro.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Urban G, Tripathi P, Alkayali T, Mittal M, Jalali F, Karnes W, et al. Deep learning localizes and identifies polyps in real time with 96% accuracy in screening colonoscopy. Gastroenterology. 2018;155:1069–7800. doi: 10.1053/j.gastro.2018.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada M, Saito Y, Imaoka H, Saiko M, Yamada S, Kondo H, et al. Development of a real-time endoscopic image diagnosis support system using deep learning technology in colonoscopy. Sci Rep. 2019;9:14465. doi: 10.1038/s41598-019-50567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becq A, Chandnani M, Bharadwaj S, Baran B, Ernest-Suarez K, Gabr M, et al. Effectiveness of a deep-learning polyp detection system in prospectively collected colonoscopy videos with variable bowel preparation quality. J Clin Gastroenterol. 2020;54:554–7. doi: 10.1097/MCG.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 28.Klare P, Sander C, Prinzen M, Haller B, Nowack S, Abdelhafez M, et al. Automated polyp detection in the colorectum: A prospective study (with videos) Gastrointest Endosc. 2019;89:576–820. doi: 10.1016/j.gie.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut. 2019;68:1813–9. doi: 10.1136/gutjnl-2018-317500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu WN, Zhang YY, Bian XQ, Wang LJ, Yang Q, Zhang XD, et al. Study on detection rate of polyps and adenomas in artificial-intelligence-aided colonoscopy. Saudi J Gastroenterol. 2020;26:13–9. doi: 10.4103/sjg.SJG_377_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Esparrach G, Bernal J, López-Cerón M, Córdova H, Sánchez-Montes C, Rodríguez de Miguel C, et al. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy. 2016;48:837–42. doi: 10.1055/s-0042-108434. [DOI] [PubMed] [Google Scholar]
- 32.Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. 2018;169:357–66. doi: 10.7326/M18-0249. [DOI] [PubMed] [Google Scholar]
- 33.Mori Y, Kudo SE, East JE, Rastogi A, Bretthauer M, Misawa M, et al. Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: An add-on analysis of a clinical trial (with video) Gastrointest Endosc. 2020;92:905–11.e1. doi: 10.1016/j.gie.2020.03.3759. [DOI] [PubMed] [Google Scholar]
- 34.Aziz M, Fatima R, Dong C, Lee-Smith W, Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35:1676–83. doi: 10.1111/jgh.15070. [DOI] [PubMed] [Google Scholar]
- 35.Spadaccini M, Frazzoni L, Vanella G, East J, Radaelli F, Spada C, et al. Efficacy and tolerability of high- vs.low-volume split-dose bowel cleansing regimens for colonoscopy: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1454–65.e14. doi: 10.1016/j.cgh.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 36.Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointest Endosc. 2011;74:128–34. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Thakkar S, Carleton NM, Rao B, Syed A. Use of artificial intelligence-based analytics from live colonoscopies to optimize the quality of the colonoscopy exam in real-time: Proof of concept. Gastroenterology. 2020;158:1219–21.e2. doi: 10.1053/j.gastro.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solís-Muñoz P, Solís-Herruzo JA, Rodríguez-Muñoz S. Experience of the endoscopist increases detection rates of smaller size and higher histological grade polyps. J Gastroenterol Hepatol. 2014;29:1237–41. doi: 10.1111/jgh.12537. [DOI] [PubMed] [Google Scholar]
- 39.Peters SL, Hasan AG, Jacobson NB, Austin GL. Level of Fellowship Training Increases Adenoma Detection Rates. Clin Gastroenterol Hepatol. 2010;8:439–42. doi: 10.1016/j.cgh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Lee CK, Park DI, Lee SH, Hwangbo Y, Eun CS, Han DS, et al. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: A multicenter, prospective, randomized study. Gastrointest Endosc. 2011;74:1094–102. doi: 10.1016/j.gie.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Aslanian HR, Shieh FK, Chan FW, Ciarleglio MM, Deng Y, Rogart JN, et al. Nurse observation during colonoscopy increases polyp detection: A randomized prospective study. Am J Gastroenterol. 2013;108:166–72. doi: 10.1038/ajg.2012.237. [DOI] [PubMed] [Google Scholar]
- 42.Gaba DM, Howard SK. Patient safety: Fatigue among clinicians and the safety of patients. N Engl J Med. 2002;347:1249–55. doi: 10.1056/NEJMsa020846. [DOI] [PubMed] [Google Scholar]
- 43.Eastridge BJ, Hamilton EC, O'keefe GE, Rege RV, Valentine RJ, Jones DJ, et al. Effect of sleep deprivation on the performance of simulated laparoscopic surgical skill. Am J Surg. 2003;186:169–74. doi: 10.1016/s0002-9610(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell JA. Fatigue in aviation. Travel Med Infect Dis. 2005;3:85–96. doi: 10.1016/j.tmaid.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Philip P, Taillard J, Moore N, Delord S, Valtat C, Sagaspe P, et al. The effects of coffee and napping on nighttime highway driving: A randomized trial. Ann Intern Med. 2006;144:785–91. doi: 10.7326/0003-4819-144-11-200606060-00004. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Zhao SB, Wang SL, Fang J, Xia T, Su XJ, et al. Comparison of efficacy of colonoscopy between the morning and afternoon: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:661–7. doi: 10.1016/j.dld.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 47.Sanaka MR, Deepinder F, Thota PN, Lopez R, Burke CA. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104:1659–64. doi: 10.1038/ajg.2009.249. [DOI] [PubMed] [Google Scholar]
- 48.Fuccio L, Frazzoni L, Hassan C, La Marca M, Paci V, Smania V, et al. Water exchange colonoscopy increases adenoma detection rate: A systematic review with network meta-analysis of randomized controlled studies. Gastrointest Endosc. 2018;88:589–970. doi: 10.1016/j.gie.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Ogiwara T, Goto T, Nagm A, Hongo K. Endoscopic endonasal transsphenoidal surgery using the Iarms operation support robot: Initial experience in 43 patients. Neurosurg Focus. 2017;42:E10. doi: 10.3171/2017.3.FOCUS16498. [DOI] [PubMed] [Google Scholar]