Abstract
Apart from the result of multiple diseases as well as aging, arterial stiffness (AS) predicts cardiovascular disease (CVD), especially in patients with chronic kidney disease (CKD). Patients with CKD have high CVD prevalence, and an extraordinarily high risk for CVD might be related to nontraditional risk factors, including AS. The mechanism of AS development could be attributed to oxidative stress, inflammation, uremic milieu (e.g., uremic toxins), vascular calcification, and cumulative effects of traditional cardiovascular risk factors on arteries such as diabetes mellitus or hypertension. There were a variety of non-invasive techniques to measure AS. One of these techniques is carotid–femoral pulse wave velocity, which is the reference measurement of AS and is related to long-term CVD outcomes. AS progression has corresponding medical treatments with modest beneficial results. This review briefly discusses the risk factors, measurements, and treatments associated with AS.
KEYWORDS: Arterial stiffness, Cardiovascular disease, Chronic kidney disease, Pulse wave velocity
INTRODUCTION
Patients with chronic kidney disease (CKD) have increased adverse long-term outcomes largely associated with cardiovascular diseases (CVDs), resulting from traditional risk factors such as diabetes mellitus (DM) and hypertension (HTN), as well as CKD-specific risk factors [1]. Stiffening of the vascular wall known as arterial stiffness (AS), which is caused by deregulation of elastin fibers and collagen, oxidative stress, disordered mineral metabolisms, and low-grade inflammation, could cause increased myocardial preload and decreased perfusion pressure of the coronary artery, predicting future CVD in patients with CKD [2,3,4]. AS has two proposed mechanisms that causing blood pressure (BP) variation from the aorta to peripheral arteries, possibly resulting in future adverse events [5]. One is wave reflection, which occurs at all levels of the arterial tree where the arterial caliber dies or branches and leads to a backward wave during a systolic period in the arterial system [5]. The other mechanism is pressure amplification, which is a physiological phenomenon involving flexible and elastic arteries in young people [6]. Normally, this phenomenon occurs along the aortic length as the pulse wave propagates with low central systolic BP (SBP) compared with peripheral SBP [6]. As the arteries stiffen, the reflection wave increases, and an earlier backward wave ascending to the aorta during systole occurs; these events result in high central SBP and more stress on the left ventricle as well as exposing a more increased pulse pressure (PP) to those feeding arteries or low-impedance vasculatures, such as kidneys or brains, with organ parenchyma exposed to high BP levels and mechanical strain along with future CV events [2,5,7]. The noninvasive technique carotid–femoral pulse wave velocity (cfPWV), which is calculated with the distance of segments from the carotid to the femoral artery and the elapsed time that the propagation wave travelled, indicates vascular function and strongly predicts CVD and mortality in patients with CKD and end-stage renal disease (ESRD) independent of traditional CV risk factors [2,8]. Furthermore, patients with CKD who have high cfPWV levels are more likely to have adverse renal outcomes including halving renal function, ESRD, and even mortality [9]. According to a meta-analysis, AS, which is measured by pulse wave velocity (PWV), is an independent predictor of total CV events, CV mortality, and all-cause mortality [2].
RISK FACTORS OF ARTERIAL STIFFNESS
Cumulative damage of vascular wall properties can develop AS, thereby increasing the transmitting PP to low-impedance circulation as well as exposing to high BP and mechanical strain, further resulting in CVD, renal dysfunction, and mortality [Figure 1]. Some risk factors contribute to the development and progression of AS.
Figure 1.
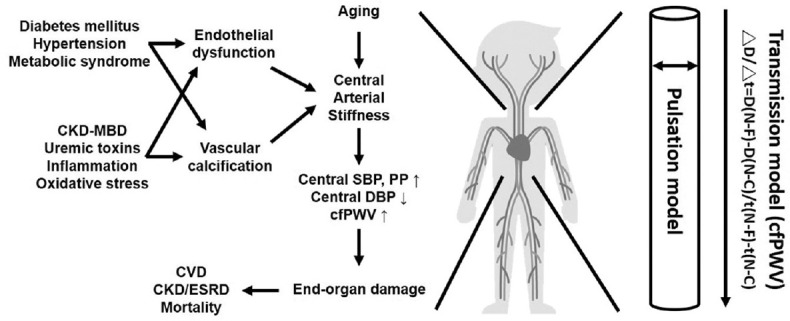
Simplified pathogenesis and outcomes of arterial stiffness (left). Models of measuring arterial stiffness (right). cfPWV: Carotid-femoral pulse wave velocity; CKD-MBD: Chronic kidney disease related mineral bone disease, CVD: Cardiovascular disease, ESRD: End-stage renal disease, ΔD/Δt: Distance divided by transit time of propagation wave between (sternal notch [N] and femoral artery [F]) and (sternal notch [N] and carotid artery [C])
Age
Age-related vascular function impairment has been likely related to endothelial dysfunction with vasodilator and vasoconstrictor imbalance as well as the dysregulated remodeling of elastin and collagen, consequently losing arterial elasticity and thickening the vascular wall [10,11,12]. An in vivo study revealed that impaired endothelium-dependent dilation associated with age is modulated by nitric oxide (NO) reduction along with increased reactive oxygen species (ROS) production [11]. Aging also contributes to a higher expression of vasoconstrictors, not vasodilators such as endothelial NO synthase, impairing the endothelial function [12]. In cohort studies, aging is an independent predictor of carotid intima-media thickness and negatively correlates with vascular resistance index [13,14]. In addition to causing functional changes, aging might additionally contribute to structural vascular changes, such as elastin fragmentation, and especially, medial-layer calcification by disordered mineral metabolism in CKD [2,15,16]. Therefore, in patients with ESRD, a marked aging-related AS with a more increased aortic PWV occurs [15]. Furthermore, AS measured by cfPWV has a significantly positive correlation with aging in ESRD [17,18], metabolic syndrome (MetS) [19], and DM [20,21].
Blood pressure
The pathophysiological correlation between AS and HTN is that increased AS reduces the lumen diameter, causing a premature return of the reflected wave in late systole and further resulting in increased PP and SBP and decreased DBP [1]. In addition, cfPWV positively correlates with SBP, as well as MetS and waist circumference, in patients with HTN and DM [21,22]. In a systematic review involving 26,970 subjects, other than traditional risk factors such as gender, dyslipidemia, smoking, and body mass index (BMI), BP elevation, as well as aging, is independently associated with cfPWV [23].
Vascular calcification
VC is caused by an imbalance between the inhibitors and promoters of vascular osteogenesis; AS resulting from VC increases progressively as the renal function declines, and VC prevalence increases from 37% in CKD stage 1–2 to 77% in stage [24,25,26]. As renal function decreases, a dysregulated complex interplay of mineral metabolism occurs, considering that both hyperphosphatemia and hypercalcemia could induce the vascular smooth muscle cells to transform into a calcifying phenotype and accelerate VC [27,28]. In patients with CKD, hyperphosphatemia and hypercalcemia, as well as calcium x phosphate product, is related to increased VC [29]. Therefore, together with the reduction of anticalcifying factors, the abnormal regulation of calcium and phosphate is one of the mechanisms initiating both phenotypic change and vascular wall mineralization. Furthermore, as the arterial elasticity is impaired, the PP transmitting to distal arterial beds increases, leading to vascular insufficiency and organ damage, as well as high CV morbidity and mortality [30,31].
Renal function and uremic toxins
The presence of CKD could be both a cause and an effect of increased AS incidence, as evidenced by a higher cfPWV in patients with eGFR less than 60 ml/min/1.73 m2 [32] and a negative association between eGFR and PWV in advanced CKD and coronary artery disease (CAD) cases without renal disease [3,33]. Furthermore, an increased aortic PWV is an independent predictor of CV events and mortality, and it improves the prediction of the risks to such events in patients with advanced CKD [4,34].
Moreover, the process of vascular damages as the renal function declines is mediated by gut-derived protein-bound uremic toxins, which include p-cresyl sulfate (PCS) and indoxyl sulfate (IS) [24,35]. Both PCS and IS can inhibit endothelial proliferation and induce ROS through NADPH oxidase induction to prevent NO production [36,37]. In addition to inducing endothelial dysfunction, PCS and IS can worsen AS by oxidative stress, impairing endothelial cell repair and inducing vascular smooth muscle cell proliferation [35]. Value of IS associated with the severity of aortic calcification scores and cfPWV and predicting the overall status and CV mortality in patients with CKD [38]. In addition, serum PCS is associated with PWV mediated by inflammation along with higher CV events and mortality in patients with CKD and HD [39,40]. According to these studies, IS and PCS individually correlate with endothelial dysfunction and cfPWV in patients suffering from ESRD [41,42].
Inflammation
As chronic inflammation plays a role in arterial aging, inflammation has been associated with endothelial dysfunction and AS in CKD and ESRD cases [17,41,43,44]. London reported that age-related aortic stiffening worsens as C-reactive protein increases [45]. However, in the Chronic Renal Insufficiency Cohort Study, baseline inflammatory markers correlate with AS but cannot predict long-term AS changes, highlighting that inflammatory markers might be useful AS biomarkers and that other factors, such as traditional and CKD-related factors, are more important than inflammation to cause AS progression in patients with CKD [46].
Metabolic syndrome and diabetes mellitus
Being described as central obesity along with dyslipidemia, hyperglycemia, and HTN, MetS together with DM and chronic inflammation is a risk factor for AS development [10]. In a longitudinal study, BMI and waist circumference positively correlates with cfPWV, indicating that adiposity is an AS predictor [47]. Additionally, together with MetS, each component of hyperglycemia, high SBP, and increased waist circumference is associated with PWV in the middle-age population [21,44], and the PWV values significantly increase as the number of MetS component increases in patients suffering from HTN and DM without CAD or nephropathy [19,48]. Similarly, our previous study showed that patients with CAD who have concurrent MetS can have a higher risk of developing AS [49]. Therefore, overall, MetS is a risk factor of AS development.
Meanwhile, glucose tolerance deterioration is independently associated with central AS, with decreasing arterial compliance and carotid–femoral transit time and increased aortic augmentation index [50]. In DM, the production of advanced glycation end products increases, showing a significant association with cfPWV independent of age, gender, BP, or fasting sugar in a community-dwelling population [51]. According to a population-based study, patients with impaired glucose metabolism and DM have a lesser carotid–femoral transit time, which indicates an increased central AS compared with those with normal glucose metabolism [50]. In patients with DM, increased cfPWV is associated with longer DM duration and macrovascular complication irrespective of age, gender, BP, or renal function [52]. Likewise, our study revealed that patients with CAD who have high AS have higher percentages of DM with higher levels of fasting sugar, insulin, and HOMA-IR than those with low AS [49]. In one meta-analysis, which enrolled 1222 patients with DM, DM incidence was significantly associated with AS measured by cfPWV [53]. Furthermore, a systematic review demonstrated that age and BP, as well as DM, are consistently independently associated with cfPWV [23,53].
ARTERIAL STIFFNESS DIAGNOSIS
Considering that AS is an independent risk factor for CVD, measurements of the vascular elasticity are crucial for future health management. AS can be measured through several techniques with proper inter- and intraoperator reproducibility; these techniques are categorized into transmission model (measuring cfPWV) and pulsation model (measuring carotid artery compliance and distensibility by ultrasonography) [Figure 1]. Device selection depends on the way AS is being measured, the time and space available, any required training, the technical expertise of personnel conducting the examination, and the cost of the device. Lim et al. had conducted a study comparing the different AS measures, such as carotid–ankle vascular index, PWV (cfPWV and brachial–ankle PWV [baPWV]), and carotid artery compliance, in healthy subjects varying in age; they found that the strength of correlations between these indices are notably different because of the various properties of methodology used and the location of arteries measured [54]. More specifically, cfPWV is more associated with other methods that measure the transmission model, including the baPWV and carotid–ankle vascular index; meanwhile, its association with pulsation model is extremely mild [54]. Methods of measuring the transmission model including cfPWV and baPWV are associated with CV events, CV mortality, and all-cause mortality [2,55]. One meta-analysis showed that cfPWV, as well as baPWV, is significantly associated with DM incidence irrespective of ethnicity [53]. However, although both cfPWV and baPWV are associated with target organ damage according to a longitudinal study conducted in 1599 community-dwelling elderly Asians, cfPWV is more independently associated with carotid intima–media thickness and creatinine clearance rate than baPWV [56]. Furthermore, cfPWV has been proposed as the “reference standard” measure of AS because of it is linked to increased mortality according to a multitude of clinical evidence [57,58,59,60] and it is more predictive of CV mortality than carotid–radial PWV or femorotibial PWV in a longitudinal study of patients with ESRD [60]. Therefore, we diagnosed AS by measuring the cfPWV using applanation tonometry, which is noninvasive, easy to learn, and practicable in an outpatient setting [17,18,41]. For daily practice, a fixed reference value of 10 m/s was proposed to define AS on published epidemiological studies taking into account the influences of age, BP and measuring distance between common carotid artery and femoral artery [61,62].
Several longitudinal studies indicated the importance of cfPWV as a surrogate to predict future CV events [57,58,59,60]. According to a study involving community-dwelling adults aged 74 ± 3 years, who were observed for an average of 4.6 years, cfPWV is independently associated with CV mortality, coronary heart disease, and stroke at each quartile of an increasing velocity [57]. Another study conducted in patients with HTN aged 50 ± 13 years, who were followed up by approximately 9.3 years, showed that with every 5 m/s increment of cfPWV, a higher risk by 2.14 and 2.35 times is observed for the development of all-cause and CV mortality, respectively [58]. Patients with DM who were followed up for roughly 10 years showed that each 1 m/s increment of PWV independently increased their risk for all-cause and CV mortality by approximately 1.08 times [59]. In patients with CKD and ESRD, cfPWV could predict the incidence of congestive heart failure hospitalization and CV mortality; the cutoff value and the area under the curve for predicting CV mortality were 10.75 m/s and 83.4 ± 2.3, with 84% sensitivity, 73% specificity, 87.3% negative predictive value, and 72% positive predictive value [60,63]. Furthermore, a meta-analysis concluded that cfPWV is not only a simple clinical method to evaluate AS but also the most informative vessel for predicting CV outcomes [2].
ARTERIAL STIFFNESS TREATMENTS
Given that cfPWV is the most validated index of AS, trials with various interventions have been designed to investigate the effects on this surrogate index and hopefully to improve the CVD outcomes. Herein, we reviewed several meta-analyses discussing the effects of medications and body weight loss on AS and abbreviated the treatments as “ABCDE,” which stands for Antihypertensive agents, Body weight loss, Cholesterol lowering agents, and DM and ESRD/CKD treatments.
Antihypertensive medications
Antihypertensive medication could passively reduce AS through BP-dependent mechanism and improve AS by exerting its pleiotropic effects on vascular wall modification [64,65]. According to an observational cohort study that took approximately 5.4 years, sustained cfPWV reduction can be obtained by routine clinical practice of antihypertensive medications [66]. Angiotensin receptor blocker (ARB) and angiotensin-converting enzyme inhibitor (ACEi), which block the renin–angiotensin system (RAS), can improve BP and vascular elasticity and reduce AS better than calcium-channel blockers (CCB), beta-blockers, and diuretics independent of BP changes [64]. Mallareddy et al. showed that ACEi can reduce cfPWV, with average absolute and relative values of −1.15 m/s and −9.74%, respectively, in patients with HTN [67]. However, in a longitudinal study by London et al., ACEi (perindopril), as well as CCB (nitrendipine), induced a similar reduction in cfPWV [68]. Asmar et al. found that the combination therapy of indapamide and perindopril results in a similar reduction of aortic PWV to atenolol, indicating that diuretics have a rather neutral effect on AS beyond brachial artery BP reduction [69]. Another meta-analysis showed that treatment with ACEi results in a pooled mean cfPWV change of −1.69 m/s compared with placebo independent of BP reduction, but not superior to ARB, CCB, beta-blocker, and diuretics [70]. According to Peng et al., ARB exhibited better effects on cfPWV than placebo with regard to AS, with a significant overall cfPWV reduction of −0.425 m/s but not superior to CCB, diuretics, ACEi, or beta-blocker [71]. Moreover, Wenquan et al. found that beta-blocker exerted better effects than placebo on PWV (−1.15 m/s; 95% CI, −1.561 to −0.669), but is less favorable than ACEi or ARB on all indices except heart rate [72]. Taken together, AS could be controlled by most antihypertensive medications; RAS-blocking agents displayed effects not inferior to others; diuretics could serve as a monotherapy or as an add-on agent to decrease BP, with neutral effects on AS; and these BP-independent changes are amplified with long-term treatments. Therefore, an optimal antihypertensive agent to lower BP and improve AS should be selected individually.
Body weight loss
Weight loss reduces cfPWV by mitigating vascular remodeling and inflammation [73]. Petersen et al. conducted a meta-analysis, which included studies on patients with energy-restricted diet with or without exercise, antiobesity agents, and bariatric surgery followed up for 8–52 weeks; through diet and lifestyle intervention, the cfPWV improved by an average weight loss of 8% of the initial body weight [73].
Statin as a cholesterol-lowering agent
HMG-CoA reductase inhibitors (statins) are the most potent agents in reducing low-density lipoprotein cholesterol and in improving CV events including AS, possibly through the pleiotropic effects, such as anti-inflammation [74]. D'elia et al. recently reported that statin therapy had significant and favorable effects, with −0.68% reduction in cfPWV independent of the changes of BP, lipid profiles, and statin types [75]. In patients with CKD, statin can induce an insignificant 41% slower rate of PWV increment compared with placebo-treated patients, possibly because of the distinct arteriosclerosis of vascular walls [76]. As mentioned, AS plays an important role in predicting future CV events, and each 1 m/s increment in PWV increases 15% of CV and all-cause mortality risks; hence, a nearly 7% reduction of PWV by statin treatment might substantially reduce CV events, but this consideration remains questionable in patients with CKD/ESRD [2].
DM treatments
Considering that DM is associated with AS occurrence according to multifactorial factors, interventions for AS improvement include targeting BP and glucose by administering RAS-blocking and oral hypoglycemic agents, respectively [77,78,79]. Newly developed antidiabetic agents such as dipeptidyl peptidase-4 inhibitor (DDP-4i), glucagon-like peptide-1 receptor agonist (GLP-1 RA), and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) not only lower the glucose level but also exhibit effects on the vascular wall [80,81,82]. Batzias et al. conducted a meta-analysis and reported that both DDP-4i and GLP-1 RA can induce a significant reduction in PWV, possibly related to their glucose-lowering effects [80]. In addition to the beneficial effects on CVD [81], Solini et al. reported that patients with DM who use dapagliflozin, which is an SGLT-2i, manifest PWV reduction, independent of BP level decrease, through the possible mechanism of mitigating the oxidative stress [82]. However, the conclusion that the new antidiabetic agents can improve AS, should be interpreted with caution, given the modest quality of evidence along with significant heterogeneity between studies and the existing controversy on their use in advanced CKD.
End-stage renal disease and chronic kidney disease treatments
Various medications can ameliorate AS progression in patients with ESRD [83]. An oral adsorbent that is known to decrease serum protein-bound uremic toxins, can increase flow-mediated vasodilatation with a decrease in the IS levels as well as oxidized/reduced glutathione ratio and decreased PWV in patients with CKD [37,84]. Moreover, managing disordered bone-and-mineral metabolism by antagonizing hyperphosphatemia and hypocalcemia without resulting in hypercalcemia with phosphate binders, vitamin D, and calcimimetics reportedly affects vascular properties [85]. Using vitamin D analogs and phosphate binder with sevelamer or cinacalcet does not significantly improve the cfPWV compared with using placebo; a similar finding was found in using recombinant human erythropoietin and folic acid to lower homocysteine [83,86]. Vitamin D deficiency was associate with endothelial dysfunction and a recently published meta-analysis showed that nutritional vitamin D supplements resulted in significant pooled difference of cfPWV (standardized mean difference: −0.29; 95% confidence interval: −0.51–−0.06), which indicated improved AS, especially in those with vitamin D deficiency [87], but the correlation with long-term CVD needed to be elucidated. London et al. found that RAS blockade, as well as CCB, can reduce cfPWV [68]. Nonetheless, according to the meta-analysis conducted by Rodriguez et al., CCB might show an advantage over RAS inhibitors in decreasing AS, but such studies are limited by study designs [83]. These controversial results might relate to arteriosclerosis and vascular calcification refractory to pharmacologic treatments when ESRD is reached; thus, early intervention against the risk factors of AS is necessary.
CONCLUSION
Given that AS is strongly associated with the occurrence of CV events and AS is one of the earliest detectable indices of vascular diseases, an easy, precise, and reliable technique or device for monitoring needs to be established. In improving the long-term outcomes of CVD, AS has been considered as a potential modifiable risk factor and as a biomarker for the risk stratification for long-term CV events. For cfPWV measurement, several devices with different indications and limitations are available. Based on multiple mechanisms for AS development, multifactorial strategies toward these mechanisms are also available, with promising results. In particular, we proposed the strategy “ABCDE” to alleviate AS progression. Finally, well-designed clinical trials for AS treatment and the long-term impacts on CV events in daily clinical practice are required to validate these interventions.
Financial support and sponsorship
This study was supported by a grant from the Ministryof Science and Technology, Taiwan (MOST-106-2314-B-303-019-MY3), and Buddhist Tzu Chi Medical Foundation, Taiwan (TCMF-MP 107-01-01).
Conflicts of interest
Dr. Bang-Gee Hsu, the section editor at Tzu Chi Med J, had no role in the peer review process and the decision to publish this article. The other author declared that he has no conflicts of interest.
Acknowledgments
The authors would like to thank Enago (www.enago.tw) for the English language review.
REFERENCES
- 1.Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20(Suppl 1):S45–50. [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Ilyas B, Dhaun N, Markie D, Stansell P, Goddard J, Newby DE, et al. Renal function is associated with arterial stiffness and predicts outcome in patients with coronary artery disease. QJM. 2009;102:183–91. doi: 10.1093/qjmed/hcn171. [DOI] [PubMed] [Google Scholar]
- 4.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–7. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 5.Sharman JE, Davies JE, Jenkins C, Marwick TH. Augmentation index, left ventricular contractility, and wave reflection. Hypertension. 2009;54:1099–105. doi: 10.1161/HYPERTENSIONAHA.109.133066. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38:1461–6. doi: 10.1161/hy1201.097723. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JM, Ogilvie RI, et al. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–11. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 8.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 9.Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study (chronic renal insufficiency cohort) Hypertension. 2018;71:1101–7. doi: 10.1161/HYPERTENSIONAHA.117.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083. [DOI] [PubMed] [Google Scholar]
- 11.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, et al. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–32. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Matsumoto M, Tanaka M, et al. Circulating fatty acid-binding protein 4 concentration predicts the progression of carotid atherosclerosis in a general population without medication. Circ J. 2018;82:1121–9. doi: 10.1253/circj.CJ-17-1295. [DOI] [PubMed] [Google Scholar]
- 14.Naghavi M, Yen AA, Lin AW, Tanaka H, Kleis S. New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: Data from 6084 patients registry. Int J Vasc Med. 2016;2016:1348028. doi: 10.1155/2016/1348028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London GM, Safar ME, Pannier B. Aortic aging in ESRD: Structural, hemodynamic, and mortality implications. J Am Soc Nephrol. 2016;27:1837–46. doi: 10.1681/ASN.2015060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean G, Chazot C, Bresson E, Zaoui E, Cavalier E. High serum sclerostin levels are associated with a better outcome in haemodialysis patients. Nephron. 2016;132:181–90. doi: 10.1159/000443845. [DOI] [PubMed] [Google Scholar]
- 17.Wu CF, Hou JS, Wang CH, Lin YL, Lai YH, Kuo CH, et al. Serum sclerostin but not DKK-1 correlated with central arterial stiffness in end stage renal disease patients. Int J Environ Res Public Health. 2020;17:E1230. doi: 10.3390/ijerph17041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou JS, Lin YL, Wang CH, Lai YH, Kuo CH, Subeq YM, et al. Serum osteoprotegerin is an independent marker of central arterial stiffness as assessed using carotid-femoral pulse wave velocity in hemodialysis patients: A cross sectional study. BMC Nephrol. 2019;20:184. doi: 10.1186/s12882-019-1374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J, Xie Q, Han Y, Chen B, Li L, Zhou G, et al. Relationship between components of metabolic syndrome and arterial stiffness in Chinese hypertensives. Clin Exp Hypertens. 2020;42:146–52. doi: 10.1080/10641963.2019.1590385. [DOI] [PubMed] [Google Scholar]
- 20.Tseng PW, Hou JS, Wu DA, Hsu BG. High serum adipocyte fatty acid binding protein concentration linked with increased aortic arterial stiffness in patients with type 2 diabetes. Clin Chim Acta. 2019;495:35–9. doi: 10.1016/j.cca.2019.03.1629. [DOI] [PubMed] [Google Scholar]
- 21.Levisianou D, Melidonis A, Adamopoulou E, Skopelitis E, Koutsovasilis A, Protopsaltis I, et al. Impact of the metabolic syndrome and its components combinations on arterial stiffness in Type 2 diabetic men. Int Angiol. 2009;28:490–5. [PubMed] [Google Scholar]
- 22.Ramirez AJ, Christen AI, Sanchez RA. Serum uric acid elevation is associated to arterial stiffness in hypertensive patients with metabolic disturbances. Curr Hypertens Rev. 2018;14:154–60. doi: 10.2174/1573402114666180413143312. [DOI] [PubMed] [Google Scholar]
- 23.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension. 2009;54:1328–36. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 24.Ossareh S. Vascular calcification in chronic kidney disease: Mechanisms and clinical implications. Iran J Kidney Dis. 2011;5:285–99. [PubMed] [Google Scholar]
- 25.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–6. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama M, Kaizu Y, Nagata M, Ura Y, Ikeda H, Shimamoto S, et al. Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: A cross-sectional study. BMC Nephrol. 2013;14:22. doi: 10.1186/1471-2369-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–9. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 28.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, et al. High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48:727–38. doi: 10.1053/j.ajkd.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 32.Yun BH, Chon SJ, Cho SH, Choi YS, Lee BS, Seo SK. Decreased renal function is a risk factor for subclinical coronary atherosclerosis in korean postmenopausal women. J Menopausal Med. 2016;22:167–73. doi: 10.6118/jmm.2016.22.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55:1110–5. doi: 10.1161/HYPERTENSIONAHA.109.143024. [DOI] [PubMed] [Google Scholar]
- 34.Zoungas S, Cameron JD, Kerr PG, Wolfe R, Muske C, McNeil JJ, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis. 2007;50:622–30. doi: 10.1053/j.ajkd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J Am Soc Nephrol. 2014;25:1897–907. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–51. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6:30–9. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–7. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3-4 chronic kidney disease. Arch Med Res. 2014;45:309–17. doi: 10.1016/j.arcmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Lai YH, Wang CH, Kuo CH, Lin YL, Tsai JP, Hsu BG. Serum p-cresyl sulfate is a predictor of central arterial stiffness in patients on maintenance hemodialysis. Toxins (Basel) 2019;12:10. doi: 10.3390/toxins12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CH, Lai YH, Kuo CH, Lin YL, Tsai JP, Hsu BG. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins (Basel) 2019;11:589. doi: 10.3390/toxins11100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ioannou K, Stel VS, Dounousi E, Jager KJ, Papagianni A, Pappas K, et al. Inflammation, endothelial dysfunction and increased left ventricular mass in chronic kidney disease (CKD) patients: A longitudinal study. PLoS One. 2015;10:e0138461. doi: 10.1371/journal.pone.0138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sipilä K, Koivistoinen T, Moilanen L, Nieminen T, Reunanen A, Jula A, et al. Metabolic syndrome and arterial stiffness: The health 2000 Survey. Metabolism. 2007;56:320–6. doi: 10.1016/j.metabol.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.London GM. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018;45:154–8. doi: 10.1159/000485146. [DOI] [PubMed] [Google Scholar]
- 46.Peyster E, Chen J, Feldman HI, Go AS, Gupta J, Mitra N, et al. Inflammation and arterial stiffness in chronic kidney disease: Findings from the CRIC Study. Am J Hypertens. 2017;30:400–8. doi: 10.1093/ajh/hpw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, et al. Adiposity, obesity, and arterial aging: Longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension. 2015;66:294–300. doi: 10.1161/HYPERTENSIONAHA.115.05494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokoyama H, Kuramitsu M, Kanno S, Tada J, Yokota Y, Kamikawa F. Relationship between metabolic syndrome components and vascular properties in Japanese type 2 diabetic patients without cardiovascular disease or nephropathy. Diabetes Res Clin Pract. 2007;75:200–6. doi: 10.1016/j.diabres.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Chen YC, Hsu BG, Wang JH, Lee CJ, Tsai JP. Metabolic syndrome with aortic arterial stiffness and first hospitalization or mortality in coronary artery disease patients. Diabetes Metab Syndr Obes. 2019;12:2065–73. doi: 10.2147/DMSO.S218718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: The Hoorn Study. Hypertension. 2004;43:176–81. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 51.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22:74–9. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agnoletti D, Mansour AS, Zhang Y, Protogerou AD, Ouerdane S, Blacher J, et al. Clinical interaction between diabetes duration and aortic stiffness in type 2 diabetes mellitus. J Hum Hypertens. 2017;31:189–94. doi: 10.1038/jhh.2016.58. [DOI] [PubMed] [Google Scholar]
- 53.Yapei Y, Xiaoyan R, Sha Z, Li P, Xiao M, Shuangfeng C, et al. clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: An up-to-date meta-analysis. Med Sci Monit. 2015;21:2467–75. doi: 10.12659/MSM.894693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships among various measures of central artery stiffness. Am J Hypertens. 2016;29:1024–8. doi: 10.1093/ajh/hpw045. [DOI] [PubMed] [Google Scholar]
- 55.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556–62. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Zhu M, Bai B, Chi C, Yu S, Teliewubai J, et al. Comparison of carotid-femoral and brachial-ankle pulse-wave velocity in association with target organ damage in the community-dwelling elderly Chinese: The Northern Shanghai study. J Am Heart Assoc. 2017;6:e004168. doi: 10.1161/JAHA.116.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 58.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 59.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 60.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–6. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 61.Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'Establishing normal and reference values'. Eur Heart J. 2010;31:2338–50. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 63.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7:709–16. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: A meta-analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–42. doi: 10.1097/HJH.0b013e328346a583. [DOI] [PubMed] [Google Scholar]
- 65.Mahmud A, Feely J. Effect of angiotensin ii receptor blockade on arterial stiffness: Beyond blood pressure reduction. Am J Hypertens. 2002;15:1092–5. doi: 10.1016/s0895-7061(02)02982-5. [DOI] [PubMed] [Google Scholar]
- 66.Ait-Oufella H, Collin C, Bozec E, Laloux B, Ong KT, Dufouil C, et al. Long-term reduction in aortic stiffness: A 5.3-year follow-up in routine clinical practice. J Hypertens. 2010;28:2336–41. doi: 10.1097/HJH.0b013e32833da2b2. [DOI] [PubMed] [Google Scholar]
- 67.Mallareddy M, Parikh CR, Peixoto AJ. Effect of angiotensin-converting enzyme inhibitors on arterial stiffness in hypertension: Systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2006;8:398–403. doi: 10.1111/j.1076-7460.2006.05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–96. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 69.Asmar RG, London GM, O'Rourke ME, Safar ME REASON Project Coordinators and Investigators. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: A comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 70.Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: A meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012;221:18–33. doi: 10.1016/j.atherosclerosis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Peng F, Pan H, Wang B, Lin J, Niu W. The impact of angiotensin receptor blockers on arterial stiffness: A meta-analysis. Hypertens Res. 2015;38:613–20. doi: 10.1038/hr.2015.51. [DOI] [PubMed] [Google Scholar]
- 72.Niu W, Qi Y. A meta-analysis of randomized controlled trials assessing the impact of beta-blockers on arterial stiffness, peripheral blood pressure and heart rate. Int J Cardiol. 2016;218:109–17. doi: 10.1016/j.ijcard.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: Systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:243–52. doi: 10.1161/ATVBAHA.114.304798. [DOI] [PubMed] [Google Scholar]
- 74.Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:1571–5. doi: 10.1136/ard.2003.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D'elia L, La Fata E, Iannuzzi A, Rubba PO. Effect of statin therapy on pulse wave velocity: A meta-analysis of randomized controlled trials. Clin Exp Hypertens. 2018;40:601–8. doi: 10.1080/10641963.2017.1411498. [DOI] [PubMed] [Google Scholar]
- 76.Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Sharman JE, Coombes JS. Effects of atorvastatin on arterial stiffness in chronic kidney disease: A randomised controlled trial. J Atheroscler Thromb. 2010;17:235–41. doi: 10.5551/jat.2683. [DOI] [PubMed] [Google Scholar]
- 77.Karalliedde J, Smith A, DeAngelis L, Mirenda V, Kandra A, Botha J, et al. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension. 2008;51:1617–23. doi: 10.1161/HYPERTENSIONAHA.108.111674. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004;53:1382–6. doi: 10.1016/j.metabol.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: A randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–30. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 80.Batzias K, Antonopoulos AS, Oikonomou E, Siasos G, Bletsa E, Stampouloglou PK, et al. Effects of newer antidiabetic drugs on endothelial function and arterial stiffness: A systematic review and meta-analysis. J Diabetes Res. 2018;2018:1232583. doi: 10.1155/2018/1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Norhammar A, Bodegård J, Nyström T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the declare-TIMI 58 trial: A nationwide observational study. Diabetes Obes Metab. 2019;21:1136–45. doi: 10.1111/dom.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc Diabetol. 2017;16:138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez RA, Spence M, Hae R, Agharazii M, Burns KD. Pharmacologic therapies for aortic stiffness in end-stage renal disease: A systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7:2054358120906974. doi: 10.1177/2054358120906974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakamura T, Kawagoe Y, Matsuda T, Ueda Y, Shimada N, Ebihara I, et al. Oral adsorbent AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27:121–6. doi: 10.1159/000077536. [DOI] [PubMed] [Google Scholar]
- 85.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 86.Rodríguez AJ, Scott D, Srikanth V, Ebeling P. Effect of vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf) 2016;84:645–57. doi: 10.1111/cen.13031. [DOI] [PubMed] [Google Scholar]
- 87.Chen NC, Hsu CY, Mao PC, Dreyer G, Wu FZ, Chen CL. The effects of correction of Vitamin D deficiency on arterial stiffness: A systematic review and updated meta-analysis of randomized controlled trials. J Steroid Biochem Mol Biol. 2020;198:105561. doi: 10.1016/j.jsbmb.2019.105561. [DOI] [PubMed] [Google Scholar]


