Abstract
Background
Subjective feeling of social isolation, as can be measured by perceived burdensomeness (PB), is a major risk factor for alcohol misuse. Heightened PB is associated with elevated stress response and diminished cognitive control, both of which contribute to problem drinking. Here, we sought to identify the neural substrates underlying the relationship between PB and alcohol misuse.
Methods
We employed resting-state functional magnetic resonance imaging data collected from 61 problem drinkers to characterize the functional connectivity of the hypothalamus and ventral striatum (VS) in relation to PB. We specifically examined whether the connectivities of the hypothalamus and VS were differentially influenced by PB to produce contrasting effects on alcohol use. Finally, we evaluated how individual differences in social support modulate the inter-relationships of social isolation, neural connectivity, and the severity of problem drinking.
Results
Whole-brain multiple regressions show a positive relationship between PB and hypothalamic connectivity with the hippocampus and an inverse pattern for VS connectivity with the middle frontal gyrus. Difference in strength between the 2 connectivities predicted the severity of problem drinking, suggesting an imbalance involving elevated hypothalamic and diminished prefrontal cortical modulation in socially isolated problem drinkers. A path analysis further revealed that the lack of social support was associated with a bias toward low prefrontal connectivity, which in turn increased PB and facilitated problem drinking.
Conclusions
Altered hypothalamus and VS connectivity may underlie problem drinking induced by social isolation. The current findings also highlight the important role of social support as a potential protective factor against alcohol misuse.
Keywords: Perceived burdensomeness, problem drinking, hypothalamus, ventral striatum, social support
Significance Statement.
Social isolation represents a major risk factor for alcohol misuse. Yet, the neural processes linking social isolation to problem drinking remain poorly understood. Here, by characterizing resting-state functional connectivity of the hypothalamus and ventral striatum (VS) in relation to perceived burdensomeness (PB), we found evidence of elevated hypothalamic hippocampal and diminished prefrontal cortical-VS connectivities in problem drinkers. The net strength of the 2 circuit connectivities predicted drinking severity, thus suggesting deficient control of drinking motivation. Further, the lack of social support was associated with a bias toward lower prefrontal cortical-VS connectivity, which in turn increased PB and facilitated problem drinking. The findings support a mechanism of isolation-induced drinking and highlight the importance of social support as a potential protective factor against alcohol misuse.
Introduction
Social isolation due to perceived burdensomeness (PB), defined as the belief that one is a burden or liability to others (Van Orden et al., 2006), represents a major risk factor of alcohol use disorders (Gauthier et al., 2017). Alcohol can serve to alleviate stress and negative mood resulting from social isolation, perpetuating drinking through negative reinforcement (Gonzalez and Skewes, 2013). Specifically, individuals with high PB frequently withdraw from social interactions (Van Orden et al., 2012), lack in social support (Hollingsworth et al., 2018), and exhibit heightened stress response (Buitron et al., 2016), all of which can promote drinking as a maladaptive coping strategy. PB is also associated with reduced cognitive control (Silva et al., 2015), which plays a role in the enhanced reward response to alcohol in problem drinkers (Fleming and Bartholow, 2014). Heavy drinking alienates family and friends, resulting in further deterioration of social support and worsening sense of isolation, driving individuals to even heavier alcohol use (Peirce et al., 2000). In contrast, social support has been shown to predict lower risk of alcohol misuse in nondependent drinkers (Wills and Ainette, 2012) as well as sustained abstinence in alcoholics following treatment (Marlatt and Witkiewitz, 2004). Despite PB’s significant role in problem drinking, the neural processes inter-relating PB and alcohol misuse remain poorly understood.
Hypothalamic dysfunction may contribute to alcohol misuse elicited by social isolation. The hypothalamus is connected with a network of cortical and subcortical structures, including the hippocampus, amygdala, and prefrontal cortex (PFC) (Jacobson and Sapolsky, 1991; Dedovic et al., 2009a). As a neural hub of motivated behavior, the hypothalamus is integral to the psychophysiological responses to social isolation (Cacioppo et al., 2015). Indeed, social exclusion in humans elicited activation in the hypothalamus (Karremans et al., 2011) along with decreases in oxytocin (Pournajafi-Nazarloo et al., 2013) and increases in cortisol (Cacioppo et al., 2015) levels. Social isolation also raised the hypothalamic neuropeptide Y level (Thorsell et al., 2006) and activated the central preproenkephalin opioid system (Iglesias et al., 1992), likely as a response to reduce social stress (Morales-Medina et al., 2010). These cellular and molecular changes have been found to facilitate ethanol intake as a stress-coping mechanism in animal models (Ehlers et al., 1998; Valdez and Koob, 2004). Additionally, the hypothalamus is thought to modulate alcohol craving through the involvement of both orexigenic and anorexigenic peptides (Leggio, 2009). Stimulation of the lateral hypothalamus elicited craving and reinforced alcohol seeking in rodents (Wayner, 2002; Marchant et al., 2009). Emerging evidence associates alcohol use with reduced levels of oxytocin, a hypothalamic hormone central to social behaviors, in both the hypothalamus and hippocampus (King et al., 2020). It is plausible that deficits in social rewards facilitate alcohol misuse. Indeed, the administration of oxytocin reduced alcohol consumption in rodents (Stevenson et al., 2017) and attenuated activations to alcohol cues in the insula, occipital cortex, and hippocampus in humans (Hansson et al., 2018). Together, both clinical and preclinical studies have suggested that social isolation may enhance hypothalamic stress response and facilitate alcohol consumption.
As a component of the frontostriatal system that regulates motivated behaviors, the ventral striatum (VS) represents another brain region that may support isolation-related substance and alcohol misuse. Alcohol consumption induces VS dopamine release in both social (Boileau et al., 2003) and dependent human drinkers (Boileau et al., 2003). In mice following social defeat, ethanol administration elevated VS dopamine release, enhancing the reinforcing properties of alcohol (Yavich and Tiihonen, 2000). Furthermore, socially deprived rats exhibited reduction in VS norepinephrine, increases in anxiety-like symptoms (Brenes et al., 2008), and greater vulnerability to heavy alcohol consumption (Butler et al., 2016). These preclinical findings implicate the VS in motivating drinking via alcohol’s rewarding and anxiolytic properties. Human studies have also revealed the role of the VS circuits in alcohol craving and consumption (Nikolova et al., 2016; Zhang and Li, 2018). In particular, the VS receives top-down regulatory signals from the PFC (Cohen et al., 2012), and the PFC-VS connectivity may play a role in restraining drinking (Forbes et al., 2014). Disruption of the PFC-VS circuit along with deficits in cognitive control, especially during emotional stress, is widely reported of alcohol and drug users (Li and Sinha, 2008). Thus, the PFC may limit the extent to which the VS facilitates alcohol use in response to social isolation.
In the current study, we sought to identify the neural substrates that support the relationships between social isolation, social support, and problem alcohol use. Using resting-state functional magnetic resonance imaging (fMRI) data in problem drinkers, we examined the patterns of hypothalamus and VS connectivity in relation to PB and drinking severity. We specifically tested the hypothesis that problem drinking increases with social isolation via enhanced hypothalamic connectivity with regions of the stress circuit, including the insula, amygdala, and hippocampus. In contrast, social isolation and alcohol use were expected to be negatively associated with VS connectivity with the PFC, reflecting the reduced cognitive control in drinking to cope with emotional distress. Finally, we employed path analysis to examine the role of social support in moderating the inter-relationships between PB, brain connectivity, and alcohol misuse.
Methods
Participants and Assessments
Sixty-one problem drinkers (24 females; 17 smokers; age = 34.4 ± 11.9 years; education = 14.9 ± 2.71 years) participated in the study. Participants underwent clinical screening and received urine toxicology tests at intake and prior to imaging. All participants were current drinkers with an Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al., 1993) score >6 (see below). All were required to be otherwise physically healthy with no major medical illnesses, current use of prescription medications, history of head injury or neurological illness, current or history of axis I disorders as defined by the DSM-IV, or positive urine test results for illicit substance use. Participants provided written informed consent after details of the study were explained in accordance with institute guidelines and a research protocol approved by the Yale Human Investigation Committee.
To assess the degree of social isolation, participants completed the 15-item version of the Interpersonal Need Questionnaire (Van Orden et al., 2008, 2012). The questionnaire assesses perceived burdensomeness (PB; 6 items) and thwarted belongingness (9 items). We focused on the subscale PB, which refers to the belief that “others would be better off without me.” Individual items were rated on a 7-point scale ranging from 1 (not at all true) to 7 (very true), with some items reversely coded and higher scores indicating greater severity. The current sample showed an average PB score of 10.9 ± 7.3 (mean ± SD), suggesting a relatively higher degree of loneliness compared with healthy adults as reported in previous work (Vanyukov et al., 2017; Chu et al., 2018). To assess the level of social support the participants received, we administered the Multidimensional Scale of Perceived Social Support (MSPSS), which quantifies perceived social support from family, friends, and significant others (Zimet et al., 1988). The 12 items were rated on a 7-point scale ranging from 1 (very strongly disagree) to 7 (very strongly agree), with higher scores indicating greater perceived support. The current sample showed an average MSPSS score of 59.8 ± 18.6.
Participants showed an average of 17.1 ± 12.2 years of drinking and consumed an average of 1.7 ± 1.2 drinks per occasion over the prior month. Participants also completed the AUDIT, with higher scores suggesting greater risk for having or developing an alcohol use disorder. Problem drinking is defined by an AUDIT score of 7 or higher (Donovan et al., 2006). The group’s average AUDIT score was 15.0 ± 8.8. Participants further completed a self-assessment of out-of-control drinking on a scale from 0 to 10 (0 = completely in control, 10 = completely out of control), reporting an average score of 2.8 ± 2.7. Additionally, a subsample of participants (n = 27) was evaluated with the State-Trait Anxiety Inventory and Beck Depression Inventory-II, which yielded an average score of 87.2 ± 24.9 and 14.8 ± 13.6, respectively.
Imaging Protocol and Data Processing
All participants completed one 10-minute run of resting-state fMRI. Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio, Erlangen, Germany). Anatomical 3D MPRAGE images were obtained with spin echo imaging in the axial plane parallel to the anterior commissure–posterior commissure line with repetition time (TR) = 1900 ms, echo time (TE) = 2.52 ms, bandwidth = 170 Hz/pixel, FOV = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm, and no gap. Functional blood oxygenation level-dependent (BOLD) signals were acquired using multiband imaging (multiband acceleration factor = 3) with a single-shot gradient echo-planar imaging sequence. Fifty-one axial slices parallel to the anterior commissure–posterior commissure line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, FOV = 210 × 210 mm, matrix = 84 × 84, voxel size = 2.5 mm isotropic, and no gap.
Imaging data were preprocessed using SPM12 (Wellcome Trust Centre for Neuroimaging). Images from the first 5 TRs at the beginning of the run were discarded to ensure only BOLD signals at steady-state equilibrium between RF pulsing and relaxation were included in analyses. Images of individual participants were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each participant per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each participant. Voxel size after normalization was 2.5 mm isotropic. Finally, the images were smoothed with a Gaussian kernel of 4-mm full width half maximum.
Analysis of Resting-State Functional Connectivity (rsFC)
In the analysis of rsFC, we reduced spurious BOLD variances with additional preprocessing. Signals from the ventricles, white matter, and whole brain were removed through a linear regression in addition to the 6 parameters obtained by rigid body head-motion correction. As BOLD fluctuations below a frequency of 0.1 Hz may contribute to regionally specific BOLD correlations, we applied a temporal band-pass filter (0.009 Hz < ƒ < 0.08 Hz) to the time course to obtain low-frequency fluctuations.
To minimize the effects of micro head motion (>0.1 mm), which represents a significant source of spurious correlations in rsFC analysis, we implemented the “scrubbing” method (Power et al., 2012) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD (t) = | ∆dx (t) | + | ∆dy (t) | + | ∆dz (t) | + | ∆α (t) | + | ∆β (t) | + | ∆γ (t) |, where ∆dx (t) = dx(1 − t) − dx (t), and similarly for the 3 translational parameters (dx, dy, dz) and the 3 rotational parameters (α, β, γ). The 3 rotational parameters were computed as the displacement of millimeters, which were transformed from degrees by calculating displacement on the surface of a sphere with a radius from the cortex to the center of the head of 50 mm. The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across voxels, computed as follows: DVARS (t) = , where the brackets indicate the mean across voxels. Finally, to compute each participant’s correlation map, we removed time points that exceeded the head motion limit FD (t) > 0.5 mm or DVARS (t) > 0.5% (Power et al., 2012). On average, 1% of the time points were removed across participants.
To examine seed-based rsFC, we obtained the hypothalamus mask (k = 10 voxels) from the WFU PickAtlas (Maldjian et al., 2003) and the VS mask (k = 276 voxels) as defined from our previous work using both cytoarchitectonic and topographical criteria (Li et al., 2014). The correlation coefficient between the averaged time course of the seed region and that of every other voxel of the brain was computed then Fisher’s z transformed for each participant. The Z maps were used in group, random-effect analyses in which we conducted a whole-brain multiple regression for each seed (i.e., hypothalamus and VS) against the PB scores with age and sex as the covariates. All imaging results were examined with the threshold of voxel-level P < .001 (uncorrected) in combination with cluster-level P < .05 (corrected for family-wise error of multiple comparisons) according to current reporting standards (Poldrack et al., 2008; Woo et al., 2014).
Path Analysis
We evaluated the differential effects of social support (MSPSS score) on the rsFC of the hippocampus and hypothalamus (Hipp/Hypo) as well as the middle frontal gyrus and VS (MFG/VS), both modulating problem drinking (AUDIT score) via social isolation (PB score) (see Results). Path analysis involves a set of exogenous variables with variance not accounted for by the model and endogenous variables with variance explained in part by other variables in the model (Wuensch, 2016; Le et al., 2020). Path analysis is conducted with regression analysis, which predicts the effects of all other variables on the endogenous variables. The beta weights (β) from these multiple regressions are the path coefficients. Standardized path coefficients convey assumptions about the directionality of interactions between variables. Model fit is typically assessed with fit indices that include the root mean square estimation of approximation (≤ 0.08 for an acceptable fit), chi-square (χ 2/df, ≤ 3), comparative fit index (≥.9), and standardized root mean square residual (≤.08) (Hu and Bentler, 1995; Chen et al., 2008).
Specifically, we included the MSPSS score as the exogenous variable, whereas the Hipp/Hypo rsFC, MFG/VS rsFC, PB, and AUDIT scores served as the endogenous variables. In this model, MSPSS score impacted Hipp/Hypo and MFG/VS connectivities, which in turn indirectly influenced AUDIT scores via their effects on PB. Bootstrapping was employed to evaluate both direct and indirect effects statistically.
Results
Drinking Behavior and Social Isolation Assessments
Across participants, greater severity of problem drinking was associated with greater social isolation and less social support. Table 1 details the linear relationships across drinking and social isolation variables.
Table 1.
Relationships Across Drinking and Social Isolation Characteristics
| PB | MSPSS | AUDIT | Years of drinking | OOC drinking | No. drinks/occasion | |
|---|---|---|---|---|---|---|
| PB | 1 | −.59*** | .46*** | 0.25 | .40** | 0.06 |
| MSPSS | 1 | −.40** | −.29* | −.34* | −.35** | |
| AUDIT | 1 | 0.22 | .78*** | .56*** | ||
| Years of drinking | 1 | .36* | .36** | |||
| OOC drinking | 1 | .52*** | ||||
| No. drinks/occasion | 1 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; MSPSS, Multidimensional Scale of Perceived Social Support; OOC, out-of-control; PB, perceived burdensomeness.
*P < .05, ** P < .01, *** P < .001. No. drinks refers to the average number of drinks per drinking occasion in the past months. All correlations controlled for age and sex.
Perceived Burdensomeness and Resting-State Functional Connectivity
First, we examined the relationship between resting-state connectivity of the hypothalamic seed and PB. A whole-brain multiple regression showed that greater PB was associated with increased hypothalamic connectivity with the right hippocampus and right middle temporal gyrus (Fig. 1A; Table 2). In contrast, lower PB was associated with increased hypothalamic connectivity with the bilateral precentral gyri, paracentral lobule, and right superior parietal lobule.
Figure 1.
Whole-brain multiple regressions of resting-state functional connectivity against perceived burdensomeness (PB) scores. (A) PB showed a significant positive correlation with hypothalamic connectivity (hypo; right inset, in green) with the right hippocampus (Hipp; left inset) and middle temporal gyrus (MTG) and a negative correlation with hypothalamic connectivity with bilateral precentral gyri (PrCG), paracentral lobule (PCL), and right superior parietal lobule (SPL). (B) PB showed a significant positive correlation with ventral striatum (VS) connectivity (right inset, in purple) with bilateral MTG and right putamen (Pu) and a negative correlation with VS connectivity with the right middle frontal gyrus (MFG).
Table 2.
Perceived Burdensomeness and Resting-State Functional Connectivity
| MNI coordinates (mm) | Voxel | Cluster | ||||
|---|---|---|---|---|---|---|
| Seed region | Region | x | y | z | T | k |
| Hypothalamus | Hippocampus | 35 | −26 | −4 | 5.59 | 37 |
| Middle temporal gyrus | 55 | −16 | −12 | 4.52 | 36 | |
| 62 | −18 | −14 | 4.05 | |||
| Precentral gyrus | −35 | −4 | 58 | −4.95 | 53 | |
| −25 | −11 | 58 | −4.43 | |||
| 35 | −4 | 60 | −4.78 | 40 | ||
| 40 | −11 | 60 | −3.75 | |||
| Superior parietal lobule | 20 | −51 | 58 | −4.59 | 66 | |
| Paracentral lobule | 0 | −28 | 63 | −4.31 | 43 | |
| 5 | −18 | 58 | −4.25 | |||
| Ventral striatum | Middle temporal gyrus | 55 | −21 | −10 | 5.02 | 88 |
| 50 | −16 | −4 | 4.68 | |||
| −50 | −34 | −10 | 4.90 | 83 | ||
| −62 | −24 | −14 | 4.18 | |||
| Putamen | 32 | −11 | −7 | 4.20 | 69 | |
| 28 | −1 | −4 | 4.00 | |||
| Middle frontal gyrus | 25 | 44 | 13 | −5.27 | 100 | |
| 30 | 46 | 23 | −3.77 | |||
| 28 | 44 | 30 | −4.21 | 42 | ||
| 20 | 46 | 28 | −4.14 | |||
Positive and negative T values indicate positive and negative correlations, respectively.
MNI, Montreal Imaging Institute.
Similarly, we assessed the VS connectivity in relation to PB, which was positively correlated with VS connectivity with bilateral middle temporal gyri and right posterior putamen (Fig. 1B; Table 2). PB scores were also negatively correlated with VS connectivity with the right MFG.
Differential Role of Hipp/Hypo and MFG/VS rsFC in Drinking
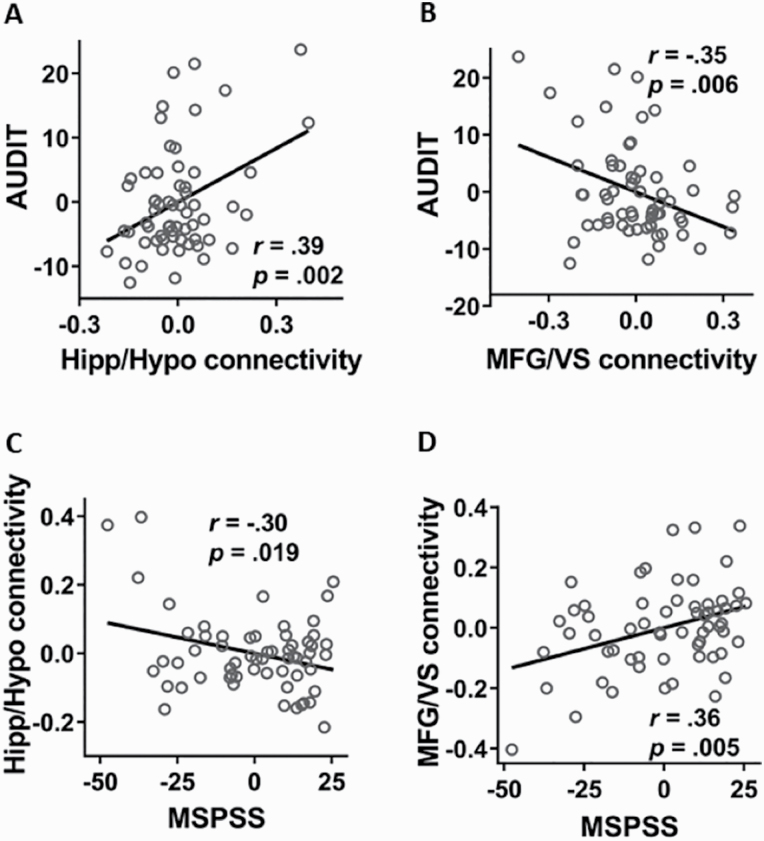
As the hippocampus and MFG have both been demonstrated to be involved in the regulation of social stress and drinking behavior (George et al., 2001; Dager et al., 2013; Vaisvaser et al., 2013), we focused on these regions to better characterize the rsFC of the hypothalamus and VS in relation to PB. We extracted the parameter estimates of the Hipp/Hypo as well as the MFG/VS rsFC. Controlling for age and sex, AUDIT scores showed a positive relationship with the Hipp/Hypo rsFC (r = .39, P = .002; Fig. 2A) and a negative relationship with the MFG/VS rsFC (r = −.35, P = .006; Fig. 2B). In contrast, MSPSS scores were negatively correlated with the Hipp/Hypo rsFC (r = −.30, P = .019; Fig. 2C) and positively correlated with the MFG/VS rsFC (r = .36, P = .005; Fig. 2D). All correlations remained significant with FDR correction for multiple comparisons. To rule out the effects of head motion in these findings, we also included FD (mean ± SD = .15 ± .11 mm) as a covariate in the correlations. The results were virtually unchanged, suggesting that head motion did not represent a confound.
Figure 2.
AUDIT scores showed (A) a positive relationship with Hipp/Hypo rsFC and (B) a negative relationship with MFG/VS rsFC. MSPSS scores showed (C) a negative relationship with Hipp/Hypo rsFC and (D) a positive relationship with MFG/VS rsFC. Note that the scatterplots showed results of partial correlations after the effects of sex and age were removed (i.e., residuals were shown for each variable).
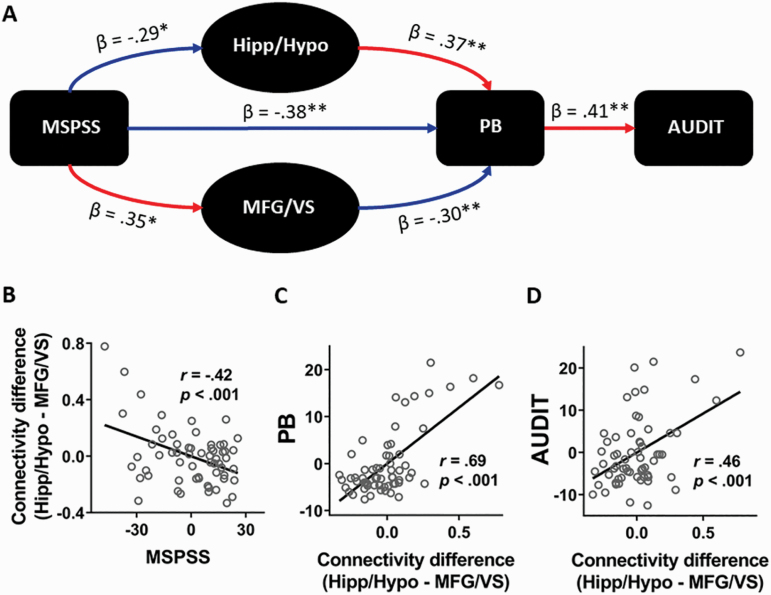
To determine the differential effects of the 2 connectivities in relation to PB and AUDIT scores, we conducted a path analysis, controlling for age, sex, and smoking status. We tested the scenario in which the level of social support (i.e., MSPSS score) influenced the rsFC of Hipp/Hypo as well as MFG/VS, which in turn differentially modulated problem drinking via social isolation, as measured by AUDIT and PB scores, respectively (Fig. 3A). The model showed a good fit (fit indices: root mean square estimation of approximation = .08 [90% CI: 0.00 0.226], χ 2/df = 1.45, standardized root mean square residual = .067, and comparative fit index = .975). Specifically, MSPSS showed significant negative modulation on Hipp/Hypo connectivity (β = −.29, P = .02), which elevated PB (β = .37, P < .001). PB then facilitated problem drinking (β = .41, P = .001). In contrast, MSPSS increased MFG/VS connectivity (β = .35, P = .004), which suppressed PB (β = −.30, P = .001). Taken together, social support exerted opposing effects on the rsFC of Hipp/Hypo and MFG/VS, and the 2 connectivities in turn influenced PB in opposite directions to regulate alcohol use.
Figure 3.
Relationships between social support, social isolation, problem drinking, and the rsFC of the Hipp/Hypo and MFG/VS. (A) Path analysis showed that social support (MSPSS) decreased Hipp/Hypo connectivity and increased MFG/VS connectivity. Hippo/Hypo connectivity was associated with heightened PB and drinking severity, whereas MFG/VS connectivity exhibited the opposite effects. Red and blue arrows indicate positive and negative relationships, respectively. Solid and dotted lines indicate significant and near-significant path coefficients, respectively. (B–D) Greater strength of Hipp/Hypo relative to MFG/VS connectivity was associated with lower social support, higher sense of isolation, and greater degree of problem drinking. *P < .01, **P < .001.
As the rsFC of Hipp/Hypo and VS/MFG showed opposite effects on drinking severity, we next examined how their net impact may determine PB-induced problem drinking. We computed the difference in strength of the 2 connectivities (i.e., Hipp/Hypo rsFC minus VS/MFG rsFC). This connectivity strength difference was negatively correlated with MSPSS (r = −.42, P < .001; Fig. 3B) but positively correlated with PB (r = .69, P < .001; Fig. 3C) and AUDIT (r = .46, P < .001; Fig. 3D) scores. Thus, greater Hippo/Hypo connectivity relative to the MFG/VS connectivity was associated with lower social support, higher sense of isolation, and more severe drinking.
Discussion
The current study examined the neural substrates underlying the relationship between social isolation, social support, and alcohol use in problem drinkers. We found that lack of social support may have induced PB, which in turn facilitated problem drinking via enhanced rsFC between the hypothalamus and hippocampus and reduced rsFC between the MFG and VS. The difference in strength between the 2 connectivities predicted the degree of problem drinking, suggesting enhanced hypothalamic stress response and poor prefrontal cortical control in drinking to cope with social isolation. Taken together, the current study highlighted the neural processes underlying alcohol misuse induced by low social support and heightened sense of burdensomeness.
Hypothalamic Connectivity With the Hippocampus and PB-Induced Drinking
The involvement of the hippocampus in social isolation has been supported with reports of enhanced hippocampal activation during ostracism (Bolling et al., 2011), negative social evaluation (Dedovic et al., 2009b), and social stress (Vaisvaser et al., 2013). Activity of the hippocampus during social exclusion was also positively related to subjective rating of social pain (Bach et al., 2019). The hippocampus shows abundant corticosteroid receptors (Joëls, 2008), which are likely critical for stress-related learning and memory (Lupien and Lepage, 2001). It is thus highly plausible that the hippocampus influences drinking behavior through its functional connectivity with the hypothalamus in reactivity to stress. Indeed, alcohol administration in rodents reduced stress-induced c-Fos expression in the hippocampus (Ryabinin et al., 1995). Alcohol consumption following stress exposure upregulated hippocampal gamma-amino-butyric acid α4 receptors and reduced stress-elicited anxiety (Gomez et al., 2013). Thus, individuals may seek alcohol to relieve isolation-related stress via hippocampal processes.
Social isolation appears to produce similar biochemical changes in the Hipp/Hypo, suggesting that they may be part of the same stress reactivity pathway. For instance, previous rodent work reported elevated corticotropin-releasing hormone (CRH) mRNA (Pournajafi-Nazarloo et al., 2009), reduced serotonin (Brenes and Fornaguera, 2009), and increased pain signal-related nitric oxide (Zlatković et al., 2014) levels in the hippocampus following social deprivation. Similarly in the hypothalamus, CRH increases with stress and likely helps drive the hypothalamic-pituitary-adrenal axis reactivity to chronic stress (Makino et al., 1995). Hypothalamic serotonin immunoreactivity was found to decrease in social isolation, potentially inducing aggression and depression-like behaviors in rats (Veenema et al., 2006). Hypothalamic nitric oxide is generated following stress exposure and thought to facilitate stress response, stress-related learning, and depressive symptoms (Gadek-Michalska et al., 2013). Importantly, CRH, serotonin, and nitric oxide have all been implicated in alcohol seeking and consumption. Drinking severity was associated with genetic polymorphisms of the CRH-receptor 1 (Treutlein et al., 2006). Moreover, pharmacological blockade of CRH-receptor 1 reduced alcohol drinking in rats (Cippitelli et al., 2012). Serotonin deficits have long been linked to alcohol abuse (LeMarquand et al., 1994) and were recently confirmed in a postmortem study of alcoholics (Underwood et al., 2018). Chronic alcohol use was also found to be linked with increased nitric oxide in the brain (Lancaster, 1992). As the Hipp/Hypo have direct anatomical connections (Mesulam et al., 1983), it is likely that social isolation modulates hippocampal activity and hypothalamic stress response, leading to drinking as a coping strategy.
MFG Connectivity With the VS and PB-Induced Drinking
The negative correlation between MFG/VS connectivity strength with PB and with AUDIT scores suggests a role of weakened prefrontal cortical control of striatal response in isolation-induced drinking. Past studies have suggested that stress, including that from social isolation, compromised prefrontal cortical functions (Pibiri et al., 2008; Eiland et al., 2012), including altered connectivity between the MFG and VS (Tottenham and Galván, 2016). In human drinkers, prefrontal cortical deficits have been associated with loss of self-control and compulsive alcohol seeking (Pahng et al., 2017). In rodents, glutamate receptors in the medial PFC were upregulated following social isolation (Zhao et al., 2009). Importantly, increases in medial prefrontal cortical glutamatergic inputs to the VS were subsequently reported to be necessary to overcome aversion to foot shocks and bitter taste paired with alcohol consumption (Seif et al., 2013). These findings demonstrate the importance of prefrontal cortical modulation of VS activity in controlling drinking behavior. Corroborating evidence in dependent human drinkers also shows attenuated MFG/VS connectivity during reward processing, suggesting increased impulsivity (Forbes et al., 2014). Additionally, alcoholics exhibited reduced alcohol cue-elicited prefrontal cortical connectivity with the VS in relation to greater reward sensitivity and attenuated cognitive control (Ray et al., 2014). Such investigations, along with the current work, point to a relationship between social isolation, reduced prefrontal cortical connectivity with the VS, and problem drinking.
Our results suggest that the Hipp/Hypo and MFG/VS resting-state connectivities work in opposite directions in their modulation of PB and problem drinking. Elevated hypothalamic connectivity with the hippocampus was associated with heightened PB and increased drinking severity, whereas the prefrontal cortical connectivity with the VS showed the inverse effects. Furthermore, the difference in their connectivity strength was predictive of the degree of alcohol misuse, suggesting opposing processes in regulating alcohol consumption in response to social isolation. Earlier research has proposed that the hypothalamus, hippocampus, and VS are part of a large system supporting instinctive behaviors in response to both internal needs and motivational stimuli such as reward and threats (Risold et al., 1997). The MFG, in contrast, is an important structure in the PFC commonly implicated in inhibitory control (Munakata et al., 2011). It stands to reason that the balance between these 2 systems determines motivated behaviors. As such, chronic social isolation can create an imbalanced or biased state toward over-reactive stress response and deficiency in inhibitory control, leading to excessive alcohol consumption as a maladaptive behavior.
The Role of Social Support in Alcohol Misuse
As individuals seek alcohol to cope with their negative emotional experiences resulting from social isolation, it is intuitively appealing that social support would serve as a protective factor against alcohol misuse. Indeed, there is ample evidence that those with higher support from family and friends engage less in problem drinking (Piko, 2000; Groh et al., 2007; Hamdan-Mansour et al., 2007). Social support also facilitates recovery from alcohol dependence (Rumpf et al., 2002) and maintains abstinence (Bond et al., 2003) in treatment-seeking individuals. In the treatment of substance and alcohol use disorders, social support has been incorporated as one of the core components of behavioral activation intervention that emphasizes substance-free positive reinforcement (Daughters et al., 2018). Enhancement of social skills, support, and rewards can facilitate cognitive control and reduce avoidance (Hopko et al., 2011), both of which have been found to disengage individuals from drug use (Martínez-Vispo et al., 2018) and sustain abstinence (Daughters et al., 2018). In contrast, lack of social support is associated with increased impulsivity and alcohol consumption in both light (Pauley and Hesse, 2009) and dependent (Jakubczyk et al., 2013) drinkers. In recent months, the COVID-19 pandemic has further highlighted the detrimental effects of reduced social interaction on problem drinking. Studies have reported increased alcohol consumption in those experiencing loneliness and isolation (Wardell et al., 2020). Vulnerable populations such as the elderly (Satre et al., 2020) and individuals with psychiatric disorders (Tso and Park, 2020) who typically receive less social support are particularly at risk. Thus, the current findings are consistent with this body of work showing that social support plays a key role in preventing alcohol misuse. Moreover, by demonstrating the interacting roles of the brain regions implicated in social stress response and inhibitory control, we characterized the neural correlates for this relationship.
Limitations and Conclusions
A number of limitations should be considered. Due to the modest sample size, especially of female drinkers, we did not examine sex differences in the neural processes associating PB to problem alcohol use. As men and women may engage in alcohol misuse via different psychological and neural processes (Ide et al., 2017; Hu et al., 2018), more research is warranted to investigate how social isolation may contribute to problem drinking differently between the sexes. Second, we examined rsFC, which is known to reflect individual differences in functional organization of the brain (Cole et al., 2013). Behavioral paradigms, including those that probe reward processing, cognitive control, and social exclusion, are needed to fully characterize how social isolation contributes to problem alcohol use. Third, while the findings of path analysis suggest causal relationship between clinical variables and neural markers, longitudinal research is required to understand how social isolation and social support may perpetuate and ameliorate problem drinking, respectively. Finally, as we did not have complete data for anxiety and depression, both of which can impact alcohol use, for all participants, we were unable to examine their potential roles in modulating the relationship between social isolation and problem drinking.
In conclusion, by combining functional connectivity analyses and assessments of PB and social support in problem drinkers, we were able to reveal the neural substrates underlying the relationship between social isolation and alcohol misuse. This relationship is characterized by 2 opposing processes, one involving regions implicated in stress response and the other involving prefrontal cortical modulation. Our findings suggest that alcohol misuse in socially isolated drinkers likely involves enhanced stress reactivity and diminished cognitive control. The results also highlight the importance of social support as a protective factor against problem drinking, calling attention to the need to enlist families and friends in the prevention and intervention of alcoholism.
Acknowledgments
This study was supported by NIH grants AA021449, DA023248, and AG067024. The NIH is otherwise not involved in the conceptualization of the study, data collection and analysis, or the decision to publish the current results.
Statement of Interest
All authors declare no conflicts of interest in the current work.
References
- Bach P, Frischknecht U, Bungert M, Karl D, Vollmert C, Vollstädt-Klein S, Lis S, Kiefer F, Hermann D (2019) Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. Eur Neuropsychopharmacol 29:291–305. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA (2011) Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage 54:2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J, Kaskutas LA, Weisner C (2003) The persistent influence of social networks and alcoholics anonymous on abstinence. J Stud Alcohol 64:579–588. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J (2009) The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav Brain Res 198:199–205. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Rodríguez O, Fornaguera J (2008) Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav 89:85–93. [DOI] [PubMed] [Google Scholar]
- Buitron V, Hill RM, Pettit JW, Green KL, Hatkevich C, Sharp C (2016) Interpersonal stress and suicidal ideation in adolescence: an indirect association through perceived burdensomeness toward others. J Affect Disord 190:143–149. [DOI] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR, Weiner JL (2016) Adolescent social isolation as a model of heightened vulnerability to comorbid alcoholism and anxiety disorders. Alcohol Clin Exp Res 40:1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW (2015) The neuroendocrinology of social isolation. Annu Rev Psychol 66:733–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Curran PJ, Bollen KA, Kirby J, Paxton P (2008) An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Sociol Methods Res 36:462–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Walker KL, Stanley IH, Hirsch JK, Greenberg JH, Rudd MD, Joiner TE (2018) Perceived problem-solving deficits and suicidal ideation: evidence for the explanatory roles of thwarted belongingness and perceived burdensomeness in five samples. J Pers Soc Psychol 115:137–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M (2012) Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav 100:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Bour L, Mantione M, Figee M, Vink M, Tijssen MA, van Rootselaar AF, van den Munckhof P, Schuurman PR, Denys D (2012) Top-down-directed synchrony from medial frontal cortex to nucleus accumbens during reward anticipation. Hum Brain Mapp 33:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS (2013) Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante JF, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD (2013) Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin Exp Res 37Suppl 1:E161–E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Magidson JF, Anand D, Seitz-Brown CJ, Chen Y, Baker S (2018) The effect of a behavioral activation treatment for substance use on post-treatment abstinence: a randomized controlled trial. Addiction 113:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC (2009a) The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 47:864–871. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, Pruessner JC (2009b) Neural correlates of processing stressful information: an event-related fMRI study. Brain Res 1293:49–60. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Kivlahan DR, Doyle SR, Longabaugh R, Greenfield SF (2006) Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction 101:1696–1704. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathé AA (1998) Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res 22:1778–1782. [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS (2012) Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming KA, Bartholow BD (2014) Alcohol cues, approach bias, and inhibitory control: applying a dual process model of addiction to alcohol sensitivity. Psychol Addict Behav 28:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. Plos One 9:e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J (2013) Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 65:1655–1662. [DOI] [PubMed] [Google Scholar]
- Gauthier JM, Witte TK, Correia CJ (2017) Suicide ideation, alcohol consumption, motives, and related problems: exploring the association in college students. Suicide Life Threat Behav 47:142–154. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58:345–352. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Lewis MJ, Sebastian V, Serrano P, Luine VN (2013) Alcohol administration blocks stress-induced impairments in memory and anxiety, and alters hippocampal neurotransmitter receptor expression in male rats. Horm Behav 63:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez VM, Skewes MC (2013) Solitary heavy drinking, social relationships, and negative mood regulation in college drinkers. Addict Res Theory 21:285–294. [Google Scholar]
- Groh DR, Jason LA, Davis MI, Olson BD, Ferrari JR (2007) Friends, family, and alcohol abuse: an examination of general and alcohol-specific social support. Am J Addict 16:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan-Mansour AM, Puskar K, Sereika SM (2007) Perceived social support, coping strategies and alcohol use among rural adolescents/USA sample. Int J Ment Health Addict 5:53–64. [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Bühler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstädt-Klein S, Spanagel R (2018) Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology 43:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth DW, Slish ML, Wingate LR, Davidson CL, Rasmussen KA, O’Keefe VM, Tucker RP, Grant DM (2018) The indirect effect of perceived burdensomeness on the relationship between indices of social support and suicide ideation in college students. J Am Coll Health 66:9–16. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Armento ME, Robertson SM, Ryba MM, Carvalho JP, Colman LK, Mullane C, Gawrysiak M, Bell JL, McNulty JK, Lejuez CW (2011) Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: randomized trial. J Consult Clin Psychol 79:834–849. [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler PM (1995) Evaluating model fit. In: Structural equation modeling: concepts, issues, and applications (Hoyle RH, ed), pp 76–99. Thousand Oaks, CA, US: Sage Publications, Inc. [Google Scholar]
- Hu S, Ide JS, Chao HH, Zhornitsky S, Fischer KA, Wang W, Zhang S, Li CR (2018) Resting state functional connectivity of the amygdala and problem drinking in non-dependent alcohol drinkers. Drug Alcohol Depend 185:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li CR (2017) Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: a structural brain imaging study. Neuroimage Clin 14:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias T, Montero S, Otero MJ, Parra L, Fuentes JA (1992) Preproenkephalin RNA increases in the hypothalamus of rats stressed by social deprivation. Cell Mol Neurobiol 12:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Klimkiewicz A, Mika K, Bugaj M, Konopa A, Podgórska A, Brower KJ, Wojnar M (2013) Psychosocial predictors of impulsivity in alcohol-dependent patients. J Nerv Ment Dis 201:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M (2008) Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol 583:312–321. [DOI] [PubMed] [Google Scholar]
- Karremans JC, Heslenfeld DJ, van Dillen LF, Van Lange PA (2011) Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. Int J Psychophysiol 81:44–50. [DOI] [PubMed] [Google Scholar]
- King CE, Gano A, Becker HC (2020) The role of oxytocin in alcohol and drug abuse. Brain Res 1736:146761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE (1992) Alcohol, nitric oxide, and neurotoxicity: is there a connection?–a review. Alcohol Clin Exp Res 16:539–541. [DOI] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Zhang S, Li CR (2020) Pain and reward circuits antagonistically modulate alcohol expectancy to regulate drinking. Transl Psychiatry 10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L (2009) Understanding and treating alcohol craving and dependence: recent pharmacological and neuroendocrinological findings. Alcohol 44:341–352. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C (1994) Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry 36:326–337. [DOI] [PubMed] [Google Scholar]
- Li CS, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L (2014) Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage 97:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R (2008) Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev 32:581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M (2001) Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res 127:137–158. [DOI] [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith MA, Pacák K, Palkovits M, Gold PW (1995) Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology 136:4517–4525. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP (2009) Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci 29:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Witkiewitz K (2004) Relapse prevention for alcohol and drug problems. Am Psychol 59:224–235. [DOI] [PubMed] [Google Scholar]
- Martínez-Vispo C, Martínez Ú, López-Durán A, Fernández del Río E, Becoña E (2018) Effects of behavioural activation on substance use and depression: a systematic review. Subst Abus Treat Prev Policy 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Dumont Y, Quirion R (2010) A possible role of neuropeptide Y in depression and stress. Brain Res 1314:194–205. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC (2011) A unified framework for inhibitory control. Trends Cogn Sci 15:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, Hariri AR (2016) Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry 21:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, McGinn MA, Paulsen RI, Edwards S (2017) The prefrontal cortex as a critical gate of negative affect and motivation in alcohol use disorder. Curr Opin Behav Sci 13:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley PM, Hesse C (2009) The effects of social support, depression, and stress on drinking behaviors in a college student sample. Commun Stud 60:493–508. [Google Scholar]
- Peirce RS, Frone MR, Russell M, Cooper ML, Mudar P (2000) A longitudinal model of social contact, social support, depression, and alcohol use. Health Psychol 19:28–38. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G (2008) Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A 105:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piko B (2000) Perceived social support from parents and peers: which is the stronger predictor of adolescent substance use? Subst Use Misuse 35:617–630. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE (2008) Guidelines for reporting an fMRI study. Neuroimage 40:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Sanzenbacher L, Paredes J, Hashimoto K, Azizi F, Sue Carter C (2009) Stress differentially modulates mRNA expression for corticotrophin-releasing hormone receptors in hypothalamus, hippocampus and pituitary of prairie voles. Neuropeptides 43:113–123. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, Yee J, Azizi F, Carter CS (2013) Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides 43:20–26. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG (2014) Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcohol Clin Exp Res 38:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW (1997) The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev 24:197–254. [DOI] [PubMed] [Google Scholar]
- Rumpf HJ, Bischof G, Hapke U, Meyer C, John U (2002) The role of family and partnership in recovery from alcohol dependence: comparison of individuals remitting with and without formal help. Eur Addict Res 8:122–127. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Melia KR, Cole M, Bloom FE, Wilson MC (1995) Alcohol selectively attenuates stress-induced c-fos expression in rat hippocampus. J Neurosci 15:721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Hirschtritt ME, Silverberg MJ, Sterling SA (2020) Addressing problems with alcohol and other substances among older adults during the COVID-19 pandemic. Am J Geriatr Psychiatry 28:780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Ribeiro JD, Joiner TE (2015) Mental disorders and thwarted belongingness, perceived burdensomeness, and acquired capability for suicide. Psychiatry Res 226:316–327. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM (2017) Oxytocin reduces alcohol consumption in prairie voles. Physiol Behav 179:411–421. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL (2006) The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav 83:28–34. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galván A (2016) Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 70:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G (2006) Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry 11:594–602. [DOI] [PubMed] [Google Scholar]
- Tso IF, Park S (2020) Alarming levels of psychiatric symptoms and the role of loneliness during the COVID-19 epidemic: a case study of Hong Kong. Psychiatry Res 293:113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, Arango V (2018) Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatry 8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, Bar-Haim Y, Hendler T (2013) Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci 7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Koob GF (2004) Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav 79:671–689. [DOI] [PubMed] [Google Scholar]
- Van Orden KA, Lynam ME, Hollar D, Joiner TE (2006) Perceived burdensomeness as an indicator of suicidal symptoms. Cognit Ther Res 30:457–467. [Google Scholar]
- Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr (2008) Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol 76:72–83. [DOI] [PubMed] [Google Scholar]
- Van Orden KA, Cukrowicz KC, Witte TK, Joiner TE (2012) Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 24:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov PM, Szanto K, Hallquist M, Moitra M, Dombrovski AY (2017) Perceived burdensomeness is associated with low-lethality suicide attempts, dysfunctional interpersonal style, and younger rather than older age. Int J Geriatr Psychiatry 32:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID (2006) Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci 24:1711–1720. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Kempe T, Rapinda KK, Single A, Bilevicius E, Frohlich JR, Hendershot CS, Keough MT (2020) Drinking to cope during COVID-19 pandemic: the role of external and internal factors in coping motive pathways to alcohol use, solitary drinking, and alcohol problems. Alcohol Clin Exp Res 44: 1–11. [DOI] [PubMed] [Google Scholar]
- Wayner MJ (2002) Craving for alcohol in the rat: adjunctive behavior and the lateral hypothalamus. Pharmacol Biochem Behav 73:27–43. [DOI] [PubMed] [Google Scholar]
- Wills TA, Ainette MC (2012) Social networks and social support. In: Handbook of health psychology (Baum A, Revenson TA, Singer J, eds), pp 465–492. New York: Psychology Press. [Google Scholar]
- Woo CW, Krishnan A, Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuensch KL ed. (2016) Introduction to path analysis. In: Introduction to path analysis, pp 1–18. Available at: http://core.ecu.edu/psyc/wuenschk/MV/SEM/Path.pdf. [Google Scholar]
- Yavich L, Tiihonen J (2000) Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol 401:365–373. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CSR (2018) Ventral striatal dysfunction in cocaine dependence - difference mapping for subregional resting state functional connectivity. Transl Psychiatry 8:119. Available at: 10.1038/s41398-018-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S (2009) Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry 33:1173–1177. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Gordon K, Farley GK (1988) The Multidimensional Scale of Perceived Social Support. J Pers Assess 52:30–41. [Google Scholar]
- Zlatković J, Todorović N, Bošković M, Pajović SB, Demajo M, Filipović D (2014) Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem 393:43–57. [DOI] [PubMed] [Google Scholar]