Abstract
Understanding and predicting responses of ectothermic animals to temperature are essential for decision-making and management. The thermal performance curve (TPC), which quantifies the thermal sensitivity of traits such as metabolism, growth and feeding rates in laboratory conditions, is often used to predict responses of wild populations. However, central assumptions of this approach are that TPCs are relatively static between populations and that curves measured under stable temperature conditions can predict performance under variable conditions. We test these assumptions using two latitudinally matched populations of the ecosystem engineer Mytilus trossulus that differ in their experienced temperature variability regime. We acclimated each population in a range of constant or fluctuating temperatures for six weeks and measured a series of both short term (feeding rate, byssal thread production) and long-term (growth, survival) metrics to test the hypothesis that performance in fluctuating temperatures can be predicted from constant temperatures. We find that this was not true for any metric, and that there were important interactions with the population of origin. Our results emphasize that responses to fluctuating conditions are still poorly understood and suggest caution must be taken in the use of TPCs generated under constant temperature conditions for the prediction of wild population responses.
Keywords: ecosystem engineer, Jensen's inequality, thermal fluctuations, thermal performance curves, Mytilus trossulus
1. Introduction
As anthropogenic climate change rapidly drives the creation of novel climate space [1,2], predicting the responses of organisms becomes imperative for understanding and preparing for novel biomes. The vast majority of organisms are ectotherms, so changes in thermal regimes rapidly impact important biological rates such as whole-organism metabolism, growth rate, locomotor speed and feeding rate. In ectotherms, temperature drives the rates of these variables in a qualitatively predictable fashion called a thermal performance curve (‘TPC’; figure 1; [4,5]). Generally, biological rates are lower at lower temperatures, with an accelerating increasing trajectory as temperature increases until an inflection point is reached. At temperatures higher than the inflection point, increasing temperature increases the rate at a decelerating pace, until a maximum rate (at Topt) is reached. The biological rate then rapidly declines towards zero with any additional increase in temperature, representing the thermal limits of performance. While TPCs often occur in these canonical shapes, many shapes are possible depending both on the particular biological rate being measured and the range of temperatures over which it is measured (see examples in [1]), and therefore TPCs are a strictly empirical phenomenon that only describes the relationship between body temperature and biological rates.
Figure 1.
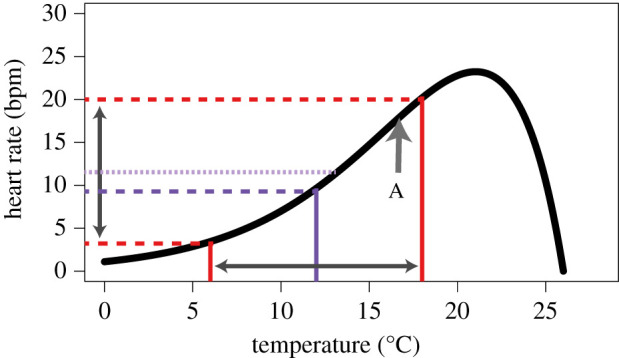
A generalized thermal performance curve (TPC) for Mytilus trossulus relating heart rate to body temperature (redrawn using data from [3]) and illustrating predictions for mean responses over time under fluctuating conditions from Jensen's inequality. The red lines indicate rates derived from the extremes at 6 and 18°C while the purple lines indicate mean rates derived from a constant 12°C (dashed line) as well as variable temperatures between 6 and 18°C (dotted line). Point A indicates the inflection point of the curve (where it switches between acceleration and deceleration). In general, Jensen's inequality calculates that the mean of the response under fluctuating temperatures is elevated relative to the mean under constant temperatures in areas of the curve that are accelerating, while the converse is true in areas of the curve that are decelerating. The magnitude of this effect is directly related to both the amplitude of temperature variation and the degree of curvature of the TPC.
TPCs have been measured for hundreds of species of ectotherms and for dozens of biological rates, in many cases with the goal of predicting population responses to temperature shifts (e.g. [4,6]). Given their generally nonlinear nature, TPCs are also subject to Jensen's inequality (or rate summation effects; [7,8]). This effect states that for nonlinear functions under variable predictors (e.g. variable temperatures), the average value of the response (i.e. the biological rate) is not equal to the response at the average value of the predictor and is predictable from the direction of the second derivative. For example, in the accelerating upwards lower portion of a TPC (figure 1), fluctuating temperatures cause an increase in mean biological rate measured relative to the response under stable temperature conditions. While this is true mathematically for a static nonlinear relationship, whether this holds in a biological system that can adjust thermal dependence of performance in real time is less well-understood [9]. In addition, the vast majority of TPC thermal performance studies have been conducted in the laboratory where organisms only experience test temperatures a single time, and often there is little information about source population history [4,10].
Because fitness is a function of these temperature-sensitive performance metrics, many researchers have used TPCs to predict changes in organism fitness following a change in temperature regime [11,12]. This approach hinges on the assumption that biological rates passively follow along with the TPC relationship when body temperatures fluctuate; in other words, thermal history does not change the shape of the TPC [4]. In particular, this framework assumes that under the variable temperature conditions generally found in the wild, the mean of biological rates should be predictable by rate summation and equal the time corrected average performance at each temperature encountered along with the statically generated TPC for that trait. This is a key assumption for the prediction of ectotherm fitness under climate change: given that habitats are thermally variable, then the TPC framework must be valid under varying climate conditions. While these ideas have received much conceptual attention lately, experimental tests of this hypothesis remain few (reviewed by [4,7,9] but see [10]).
Thermal variability is a key attribute of almost every terrestrial and freshwater system, and even many coastal marine waters. While researchers have recognized the need for further studies of thermal variability, tests remain few and contradictory, and generalizations about the physiological and ecological effects of thermal variation on ectotherms remain elusive (reviewed in [9]). These effects are probably dependent on the relative position of the variation compared with important physiological thresholds like the onset of the heat shock response, cold shock response and mortality (reviewed by [11,12]). Variability close to these thresholds can lead to increases in stress resistance mechanisms, such as investment in cryoprotection following repeated cold stress [13], while simultaneously decreasing mortality by allowing for the opportunity to repair [14,15]. For example, repeated freeze exposures induce a metabolic phenotype associated with increased production of the cryoprotectants glycerol and sorbitol without any increased mortality in the goldenrod gall fly Eurosta solidaginis [16]. By contrast, thermal variation at more permissive temperatures has occasionally led to greater growth rates in other ectothermic vertebrates than would be predicted even by Jensen's inequality and rate summation [17,18], while simultaneously also decreasing metabolic rate [9,19,20]. For example, in striped marsh frogs (Limnodynastes peronii), increased thermal variation significantly increases growth and development rate from egg to adult [18], while in juvenile Atlantic salmon (Salmo salar), increased temperature variation significantly reduces routine and maximal metabolic rate [9]. These changes are accompanied by proteomic [21] and metabolomic [22] shifts, that suggest rapid and significant changes in the biochemistry of metabolism as a result of fluctuating thermal regimes. If this generalization holds, this suggests a major problem with applying TPCs derived from steady-state laboratory experiments to wild populations.
While understanding the ecological and physiological impacts of temperature fluctation is of general importance, by examining these concepts in foundation species and ecosystem engineers, it may also be possible to extend our derived insights to ecosystem-level functions. Such species have larger impacts on the structure and function of ecological communities than many others. For example, on temperate rocky coasts, mussels are a key ecosystem engineer forming massive beds that provide a structural foundation for whole communities [23,24]. In the absence of strong predation, mussels are highly pervasive as the competitively dominant species [24,25]. When present, they form substrate and shelter for species representing almost all phyla of marine animals and exclude the presence of large macrophytes [23,24,26]. They are able to do this in both sheltered and extreme environments owing to their ability to attach themselves to each other and their rocky substrate using byssal threads [27]. Mussels are also direct providers of ecosystem services. As a by-product of their feeding behaviour, mussels filter out any microparticulate matter suspended in the water column, keep the edible parts for themselves and return the rest to the sediment as larger pseudofaeces [28–30]. Mussels are also culturally and economically important food sources to both modern and historic communities worldwide, and current fisheries are valued in the millions in Canada alone [31,32]. The ecological importance of mussels is further observable by their successful invasions on multiple continents, leading to the restructuring of ecological communities in both marine and freshwater systems [23,33].
Here, we contrast the responses of two populations of the ecosystem engineer, the bay mussel Mytilus trossulus (which were matched for latitude but differed in the natural thermal variability they experience), to constant and fluctuating laboratory acclimation, to test three key assumptions of the TPC model. These are: (i) that performance under variable conditions can be predicted from performance under constant conditions via rate summation; (ii) that for a particular population, thermal performance of different traits (e.g. thermal sensitivity of growth and feeding) should have relatively equal thermal sensitivity; and finally (iii) that thermal history predicts responses to fluctuating temperatures. We show that generally the response to fluctuating temperatures cannot be reliably predicted from responses to constant temperatures (via rate summation), that differing measures of thermal performance do not correlate, and that thermal history impacts responses to fluctuating temperatures in unpredictable ways. Taken together, we conclude that better integration of physiological responses to temperature variation is needed to accurately predict ectotherm responses to changing climate.
2. Methods
(a). Mussel collection and acclimation
We collected bay mussels (M. trossulus) between 2.0 and 3.5 cm in length in January 2016 from below the water line on floating docks at the Reed Point Marina (49.291° N, −122.883° W) and the Tofino Marina (49.151° N, −125.897° W), both in British Columbia, Canada. Tofino is on the west coast of Vancouver Island, and because of its proximity to the open Pacific Ocean has relatively stable sea surface temperatures that vary between 7.3 and 9.2°C in January, and between 7 and 14.9°C throughout the entire year (electronic supplementary material, figure S1). Reed Point Marina is deep within the Burrard Inlet of Vancouver, and as such sea surface temperature is significantly more variable on the seasonal scale (between 10.5°C and 20.7°C from January to July; electronic supplementary material, figure S1). After collection, mussels were transported on ice to the University of British Columbia where they were placed in a 30 ppt seawater table at 12°C and laboratory acclimated for two weeks. We then individually labelled mussels using bee tags, measured total length along the longest axis with calipers (to 0.1 mm), and chose 360 size-matched mussels (average wet mass = 3.1 ± 0.07 g) from each population for experimental acclimation. A subset of 23 individuals from Reed Point and 28 from Tofino were sacrificed at the end of experimentation for genetic species identification (electronic supplementary material).
We haphazardly assigned mussels to one of four different temperature treatments: constant temperatures at 6, 12 and 18°C, or treatment that fluctuated between 6 and 18°C every 4 days (average of 12°C). These temperatures were chosen based on local temperature conditions experienced by both populations and with reference to thermal optima for M. trossulus (figure 1; [3]). While the amplitude of our temperature fluctuations was greater than the fluctuations subtidal mussels would normally experience over timescales of hours (tidally driven) to days (upwelling-driven), it was well within what intertidal individuals can experience on a daily basis, and we have recorded low intertidal water temperature fluctuations of up to 10°C on a single rising tide at a Burrard Inlet site with abundant mussels and a strong but shallow thermocline (electronic supplementary material, figure S2). Regardless, our goal was not to perfectly mimic natural conditions, but to explore ecophysiological impacts of temperature variation.
Mussels were held in groups of 10 per population in plastic mesh containers in 260 l independently circulating seawater mesocosms (one container per population per aquarium for a total of 20 mussels aquarium−1), which were stocked with locally obtained natural seawater (25 ppt). We replicated each experimental treatment either four or five times in separate aquaria: five aquaria for the constant 12°C and variable treatments, and four aquaria for the constant 6°C and 18°C treatments, for a total of 18 aquaria. Mussels were acclimated at the experimental temperatures for a total of six weeks. Water chemistry (dissolved inorganic carbon and salinity) was measured at the beginning, middle and end of the experiment, and calcium content was measured at the end (water chemistry methods and results in the electronic supplementary material). Survival to the end of the experiment was defined as the ability to keep valves closed, and the length was again measured to give a total growth rate (in mm shell length increased).
(b). Byssal thread regeneration
Following the end of the experimental acclimation, we examined the effect of temperature treatment and population source on the ability to regenerate byssal threads by measuring both byssal thread strength and number of threads produced during 24 h as in [34]. We placed 7–10 mussels from each treatment per population in individual 250 ml plastic cups filled with Marina aquarium gravel, with their ventral side facing the gravel, isolated from the sides of the cup to ensure that any byssal threads produced by the mussels were attached to the gravel, which allowed us to obtain a measure of byssal thread production. All byssal thread regeneration experiments were performed at their respective acclimation temperatures, while the mussels that were acclimated to fluctuating temperatures were held at 12°C (the average temperature). At the end of the 24 h period, we counted the total byssal threads produced, then carefully removed five threads from each individual. Threads were cut from an attachment point on the gravel and at the valve of the mussel, then glued onto approximately 3 cm squares cut from transparency film using LePage Ultra Gel Super Glue and left to dry for 1 h. Each square had a small slit cut into one side, over which the thread was placed so that the thread ran perpendicular to the slit. Once glued, the initial length (mm) of each thread was recorded. After the glue had dried, threads were stored in 12°C seawater overnight to prevent desiccation prior to testing.
Byssal thread strength was measured using an Instron 5500R system (Illinois Tool Works Inc., Norwood MA). The threads were kept submerged in seawater until they were ready to be tested. The transparency squares were held in place in the Instron using pneumatic clamps. Once installed, each square was cut in half by extending the pre-cut slit, so that the byssal thread could be placed under tension. Each thread was pulled at a constant rate (10 mm min−1) until the threads broke. We then calculated the breaking strain (change in length divided by initial length) of each thread. Byssal threads that broke during the installation process were excluded from the analysis, leaving a total of 281 threads (4.09 ± 0.14 threads mussel−1), and the mean breaking strain was calculated per mussel.
(c). Filtration rate
Mussels from all experimental treatments were transferred from experimental mesocosms to a common recirculating seawater table held at 12°C. Incubators (Panasonic MIR-154) were pre-programmed with stable temperatures at 6°C, 12°C and 18°C (two replicate incubators per temperature, with successive trials performed over several days). Owing to equipment limitations, we contrasted only mussels that had been acclimated to the constant 12°C or the fluctuating temperature. We placed individual mussels in a plastic mesh basket in the top half of a 1 l beaker filled with filtered seawater (at 34 µm) at a starting temperature of 12°C. A stir bar was placed in the bottom half of the beaker for circulation. The beakers were placed, on top of a stir plate, in an incubator set to the given test temperature for 30 min to allow for the mussels to acclimate to the test temperature and to remove any residual food particles that may be caught in mussel gills. After this 30 min cleaning and acclimating period, we transferred each mussel within the incubator to a container with 200 ml of filtered seawater at the desired test temperature. The mussels were then fed 2 ml of concentrated (but well mixed) Thalassiosira sp. (Brightwell Aquatics Phytogold) for an approximate concentration of 20 000 cells ml−1. We took 250 µl water samples from the well-mixed test container at three time points: pre-food addition (T0), immediately post-food addition (T1), and after 30 min of feeding (T30). We quantified feeding rate by estimating the difference in the concentration of algal cells at T1 and T30 in the beakers using a FlowCam (flow rate 0.3 ml min; FlowCAM versus Series, Fluid Imaging Technologies).
(d). Predicting performance in variable conditions based on performance in stable conditions
Based upon rate summation as predicted by Jenson's inequality, we calculated the predicted performance of the mussels under variable conditions from their performance and time at each temperature [7]. In our case, the mussels spent eight 4 day stretches at 6°C and seven 4 day stretches at 18°C. Because we did not measure the thermal performance of each mussel in the variable treatment at these two temperatures, we estimated it by bootstrapping the data from the mussels that were measured at those data points [7]. For this, we created 10 000 resampled datasets from the measurements at 6 and 18°C. Each resampled dataset had the same number of replicates as the original. We then calculated the weighted averages for each of these 10 000 resampled datasets. We used the mean and standard deviation of these 10 000 weighted averages as our new mean predicted performance and standard error (as the standard deviation of a mean of means is equivalent to the standard error). Using this bootstrapped data, we conducted a permutation test: any measured mean that fell above the 95th percentile is considered significantly greater than our prediction. This method does not explicitly model tank effects, as each estimate is based on observations from two randomly selected mussels (from the 6 and 18°C treatments) which must be selected from different tanks. Random assortment of these observations is thus a requirement of these methods.
(e). Statistics
After examining histograms to test for violations of the assumption of normality, mussel performance metrics were generally investigated by linear mixed-effects models performed using the lme4 package [35] in R [36] with experimental treatment and population source as independent and potentially interacting fixed factors and mesocosm as a random factor nested within them. A type III ANOVA implemented with Satterthwaite's method in the lmerTest package [37] was used to test the statistical significance of fixed factors, and if interactions were found to be non-significant, they were dropped from the model. Survival was investigated using generalized linear mixed-effects models with a binomial distribution implemented using lme4 with mesocosm modelled as a random effect nested within the population and acclimation treatment. Seawater chemistry and mussel feeding rate were tested among treatments using ANOVA, as the individual mesocosm was the unit of replication. Byssal thread production was investigated using a generalized linear model with a quasi-Poisson distribution, as mussels were placed in a common garden. Alpha was set to 0.05 in all tests.
3. Results
(a). Seawater chemistry
Calcium content (electronic supplementary material, figure S3) did not vary among experimental acclimations. Salinity gradually rose over the course of the experiment but did not vary significantly among treatments (electronic supplementary material, figure S4). Dissolved inorganic carbon generally rose among the acclimation treatments through the experiment, and was generally lowest in the 18°C group, but the effect size was relatively small (approx. 5%, electronic supplementary material, figure S5). Seawater temperature was initially not quite at the target temperatures (within 2°C) in the 6 and 18°C groups, but reached within 1°C within the first 4 days, while the variable temperature groups were within 0.5°C of the target temperatures (electronic supplementary material, figures S6 and S7).
(b). Survival
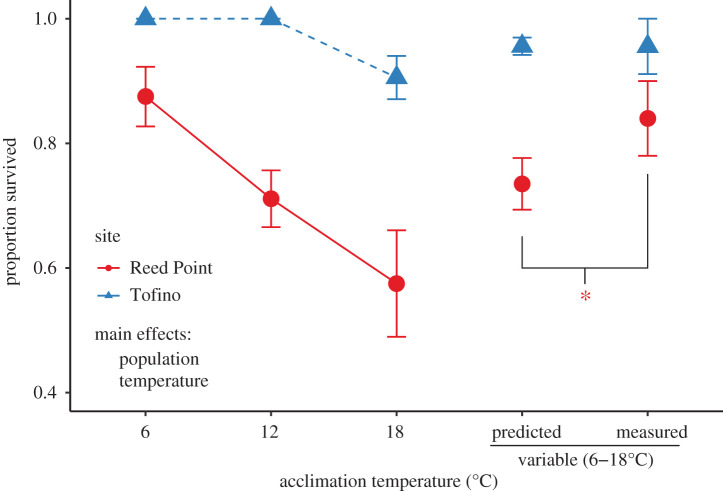
A total of 298 out of the initial 346 mussels survived for the duration of the experiment. All individuals from our subset (23 individuals from Reed Point and 28 from Tofino) were genetically identified as M. trossulus individuals, without any evidence of hybridization with other Mytilus species (electronic supplementary material, table S1). Survivorship was very high in the lowest temperature acclimations (figure 2) but declined at higher temperatures. In particular, we found that survivorship was significantly lower in the Reed Point population (χ21 = 24.41, 345, p < 0.001, binomial generalized linear mixed-effects model) and was significantly lower at higher temperatures (χ23 = 14.12, 342, p = 0.003; figure 2). There was no significant difference in survival of Tofino mussels in the variable temperature treatment as compared to our prediction based on stable temperature treatments (p = 0.519). However, more Reed Point mussels survived in variable temperatures than we would have predicted from the measurements made at stable temperatures (p = 0.007).
Figure 2.
Proportion of mussels alive at the end of the experiment based upon temperature treatment and collection site. Significant main effects are indicated, an asterisk indicates significant difference between measured and predicted values under temperature variation. Survivorship was lower among mussels collected at Reed Point. Survival also decreased at higher stable temperatures. Survival of Reed Point mussels under variable conditions was higher than predicted from survivorship at stable conditions. Predicted values shown for variable conditions are bootstrapped estimates based upon survival rates at stable temperatures. All values shown are means ± s.e. except for predicted values, which are means ± s.d., as is standard for bootstrapped values.
(c). Growth
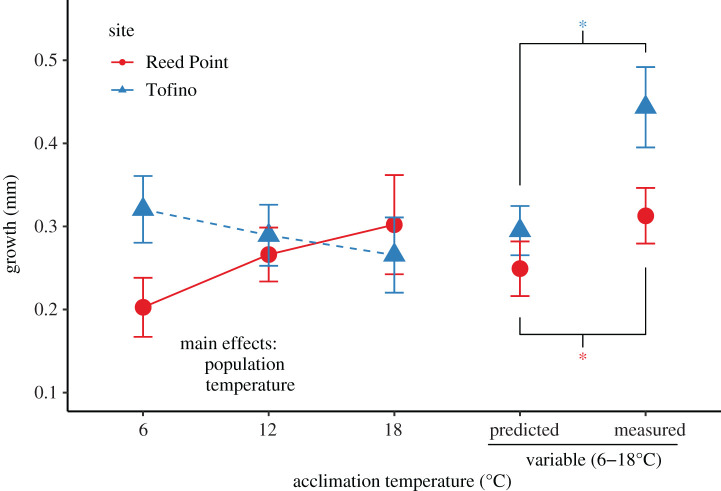
The growth of the 298 mussels that survived the experiment was examined as the difference in shell length from the beginning to the end of the experiment. We first examined whether initial length was a significant predictor of growth, but found no effect and dropped that term from our models. Mussels from Tofino grew more at almost every temperature than mussels from Reed Point (figure 3; F1,32.70 = 4.96, p = 0.033), and mussels under variable temperatures grew more than mussels under all constant temperature regimes (figure 3; F3,33.12 = 3.44, p = 0.028), but there was no interaction between these effects. Mussels from both Tofino and Reed Point grew more in variable conditions than we had predicted based on growth rates at the constant temperatures (figure 3; p < 0.0001 and 0.032, respectively).
Figure 3.
Growth of mussels based upon temperature treatment and collection site. Overall the mussels collected in Tofino grew more than those collected at Reed Point. Significant main effects are indicated, an asterisk indicates significant difference between measured and predicted values under temperature variation. Mussels from both sites grew more under variable temperature conditions than predicted based upon stable temperature conditions. Predicted values for variable conditions are bootstrapped estimates based upon growth at stable temperatures. All values shown are means ± s.e. except for predicted values, which are means ± s.d., as is standard for bootstrapped values.
Mussels were similar in shell mass, wet flesh mass and dry flesh mass across all acclimation treatments (p > 0.2 in all cases), but Tofino mussels had significantly heavier shells than Reed Point mussels (F1,13.63 = 48.85, p < 0.001) and significantly heavier dry flesh mass (F1,31.90 = 0.035). To better understand growth allocation, we examined the proportion of total wet mass that was allocated to tissue versus shell. We found a trend towards an interaction between the population of origin and acclimation temperature (F3,12.45 = 3.08, p = 0.067), with Reed Point mussels acclimated to variable temperatures having a much higher proportion of their body mass allocated to soft tissue than shell compared to mussels from other site by treatment combinations (electronic supplementary material, figure S8; p = 0.0152 for the contrast).
(d). Feeding rate
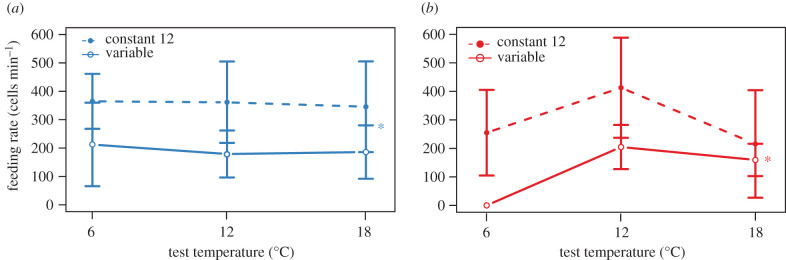
Feeding rate was measured as algal cell drawdown over 30 min in a total of 65 mussels; 34 from Reed Point and 31 from Tofino. There was no statistically significant effect of either test temperature or population source on feeding rate, but in both populations acclimation to variable temperatures led to significantly lowered feeding rate relative to constant temperature acclimation at all test temperatures from an average of 550.14 ± 39.0 cells min−1 to 337.14 ± 7.28 cells min−1 (figure 4; F1,63 = 6.43, p = 0.0137).
Figure 4.
Feeding rate of M. trossulus based on collection location, temperature acclimation and test temperature. Asterisks indicate significant differences between mussels acclimated to constant and variable temperatures. (a) Mussels from Tofino, BC, (b) Mussels from Reed Point, BC. There was no significant effect of test temperature on mussels from either location, but mussels acclimated to variable temperature conditions had significantly lower feeding rates than mussels acclimated to constant 12°C. All values shown are means ± s.e.
(e). Byssal threads
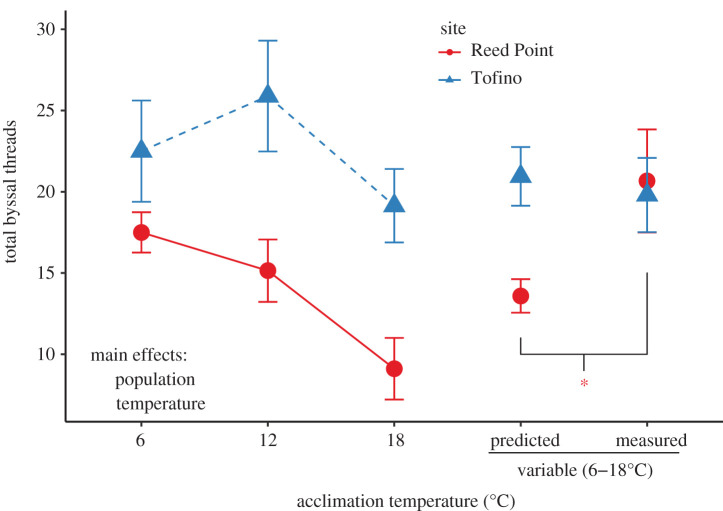
We measured the strength of a total of 281 byssal threads from 68 mussels (4–9 mussels per treatment × population, mean = 4.1 threads mussel−1). We found no effect of population or temperature acclimation or their interaction on the mean extension of byssal threads before snapping (p > 0.26 for all terms). We ln-transformed maximum load measures to improve normality and similarly found no effect of population, temperature acclimation, or their interaction on the mean strength of byssal threads (as measured by the maximum load (N) before snapping; p > 0.4 for each term). We found significant overdispersion in a Poisson generalized linear model predicting the number of byssal threads based on the population and acclimation treatment, so continued with a quasi-poisson generalized linear model. We found that Tofino mussels produced significantly more byssal threads than Reed Point mussels (figure 5; Tofino: 21.84 ± 1.5 threads mussel−1, Reed point: 15.5 ± 1.4 threads mussel−1; deviance explained = 23.5, n = 29–32, p = 0.005), and that acclimation at 18°C lead to significantly fewer byssal threads produced in both populations (figure 5; deviance explained = 41.63, n = 12–17, p = 0.003). There was a near-significant interaction between population and temperature acclimation (p = 0.099), which was owing to the particularly low byssal thread production at 18°C in the Reed Point population (figure 5). There was no significant difference in byssal thread production of Tofino mussels exposed to variable conditions compared to our prediction (p = 0.739). However, Reed Point mussels exposed to variable conditions produced more byssal threads than we would have predicted from the measurements at stable temperatures (p < 0.0001).
Figure 5.
The number of byssal threads produced by mussels varied by temperature treatment and collection site. Significant main effects are indicated, the asterisk indicates significant difference between measured and predicted values under temperature variation. Tofino mussels produced significantly more byssal threads than Reed Point mussels. Mussels produced significantly fewer byssal threads when acclimated to at 18°C than when acclimated to other stable conditions. Reed Point mussels acclimated to variable temperature conditions produced significantly more mussels than predicted based upon acclimation to stable conditions. However, there was no difference between the amount of byssal threads predicted and actually produced by Tofino mussels acclimated to variable temperature treatments. Predicted values for variable conditions are bootstrapped estimates based upon byssal threat production at stable temperatures. All values shown are means ± s.e. except for predicted values, which are means ± s.d., as is standard for bootstrapped values.
4. Discussion
Here we show that, in our study system, performance measured under constant temperature acclimation does not predict performance under fluctuating conditions—a key assumption of the TPC model. Indeed, in certain cases, performance under variable conditions did not even fall within the range of values observed across the three constant temperature treatments. We additionally show that thermal performance has differing plasticity depending on the measure of interest: feeding rate, survival, growth and byssal thread production all have unique thermal dependence; it is not yet clear which one is the best suited for predicting fitness in wild populations. Finally, we show that different populations of the same species from the same latitude can have very different plasticity in the thermal sensitivity of traits. None of these effects was explainable based on rate summation—the measured effects demonstrated differing acclimation to fluctuating and constant temperature conditions that differed occasionally in direction but more often in the magnitude of the effect. In the light of our results, we urge caution when applying TPC data from the laboratory to field conditions.
We designed our experiment to include two broad types of response variables: those that are integrated over the long time period of the acclimation (i.e. growth, survival), and those that can be measured on very short timescales (i.e. byssal thread production, feeding rate) to examine whether timescale of the response variable affected the ability for constant temperature acclimation to predict response to fluctuating temperature acclimation. We broadly found that the timescale of metric did not affect how predictable the fluctuating result was, but rather that population origin regularly interacted with the response to temperature fluctuations. For example, survival over the course of the experiment was predicted well in the fluctuating group by constant temperature acclimation only in the mussels from Tofino, while growth rate under fluctuating conditions was closer to matching the constant temperature predictions in the mussels from Reed Point (figure 3), while fluctuating temperatures caused a significant depression in feeding rate at all tested temperatures in both populations.
Interestingly, we found that acclimation to variable temperatures appears to induce a distinct state characterized by a significantly higher growth rate coupled with lowered filtration rate. The mechanism behind this observed higher growth rate is not immediately apparent from our dataset, but there are a limited number of possibilities in a laboratory environment with controlled food availability. Either filtration rate increases, assimilation efficiency increases, calcification efficiency increases, or maintenance cost of tissue could decrease, all allowing for a higher growth rate. Our filtration rate data indicate the first possibility seems unlikely, as filtration rates were lower in mussels acclimated to variable conditions. Some of this difference may be owing to unquantified stress associated with the initial reallocation of the variable acclimation mussels to a constant 12°C prior to filtration trials at the three test temperatures. Regardless, the lack of compelling evidence for increases in filtration rate in response to variable conditions leaves changes in assimilation efficiency and tissue maintenance costs as reasonable possibilities to consider. In the closely related congener Mytilus edulis, acclimation to differing constant temperatures did not cause changes in assimilation efficiency [38], but it is possible that acclimation to fluctuating temperatures could. In ectothermic vertebrates, decreased metabolic rate has been regularly observed as a response to fluctuating temperature acclimation [19,39]. By contrast, in one study of M. edulis, metabolic rates were not significantly reduced following 30 days of acclimation to a 11–19°C daily fluctuation [40], although this acclimation also resulted in a significant reduction in filtration rate relative to the constant control at intermediate temperatures. This study suggested a significant increase in scope for growth at higher temperatures that traded off with scope for growth at intermediate temperatures, which may be what is occurring in our data. It is also possible that acclimation to fluctuating conditions includes compartmentalization of physiological processes: if one process runs maximally at a low temperature and another is most efficient at a higher temperature, it is possible to have overall higher growth rates under fluctuating conditions. For example, intertidal bivalves (including the closely related congener M. edulis) grow faster than would be predicted by the time available for feeding [41], suggesting that energetic savings during less favourable times could offset reduced opportunities for feeding. Calcification is also temperature dependent but discontinuous in bivalves [42], and may represent a process which proceeds both optimally and sufficiently during discrete intervals at a particular temperature that may be suboptimal for other processes. A final consideration is a reproductive investment, which we did not measure but could tradeoff against growth. It is possible that certain thermal profiles triggered gametogenesis and/or resulted in differential energy allocation to reproductive versus somatic tissue, and the nature of thermally driven resource allocation patterns may have differed between sites. Taken together, it is clear that understanding the relative temperature sensitivities of metabolic rate and assimilation rate, and their plasticities in real-world conditions is a key next step for predicting mussel performance.
We found significant effects of source population on growth rate, survivorship and byssal thread production. Because the animals we used were collected as adults, we cannot distinguish between local adaptation, maternal effects and developmental acclimation to explain this. Local adaptation is relatively common in marine invertebrates [43], even for taxa with long-distance larval dispersal. Similarly, seasonal plasticity in byssal thread production is clear in M. edulis, but developmental plasticity is less well-understood [44]. Broadly speaking it appeared that the Tofino population was much less sensitive to high temperatures, which was unexpected owing to their position on the open coast where such high water temperatures are unlikely to be experienced. We expected the mussels from the relatively sheltered and thermally variable inner coast near Vancouver, where summer water temperatures can exceed 18°C (electronic supplementary material, figures S1 and S2), to be significantly more heat tolerant. This effect might be owing to our collection timing occurring mid-winter, although we note that both populations experience relatively similar temperatures mid-winter (electronic supplementary material, figure S1). In the closely related congener Mytilus californianus, developmental acclimation to wave-protected (and therefore generally warmer) sites increases thermal tolerance permanently, while adult acclimation does not [45]. This suggests that the lower thermal tolerance we observed in the Reed Point individuals was not an effect of seasonal acclimation. While there has been some work on interspecific variation in byssal thread properties in mussels [46], less is known about intraspecific variation. Given that our Tofino collections took place from a sheltered marina dock, we suspect that the increased byssal thread production from this population may represent a local adaptation to the greater wave action of the adjacent open coast; gene flow via larval dispersal is expected to be high between the Tofino marina and nearby wave-exposed shorelines. Taken together, we hesitate to ascribe a particular mechanism to the pattern we observed; however, it is clear that inter-population variation on even relatively small spatial scales can cause significant changes in growth rate, ecological functions; and survival under differing temperatures.
There have been relatively few studies that examine the predictability of TPCs under fluctuating temperatures. One study found that the population growth rates of the green alga Tetraselmis tetrahele in fluctuating conditions is predictable from TPCs generated under constant laboratory conditions [10]. However, other studies that focused on within-generation effects in longer lived species such as marsh frogs [18], rainbow trout [47] and salmon [9] have had little success. Indeed, mathematical modelling suggests that the timescale of environmental change relative to the lifespan of the organism may be an important consideration in the use of TPCs [48]. It may be expected that responses to fluctuating conditions are more predictable when the period of fluctuation is equal to or greater than the lifespan of the individual, but short time-scale fluctuations such as our experiment can induce significant plasticity and therefore understanding the mechanisms underlying plastic responses to temperature variation is key for predicting responses to fluctuation.
5. Conclusion
In this study, we tested three key assumptions of the TPC model as laid out by Sinclair and colleagues [4]: (i) that responses to fluctuating temperatures can be predicted from responses to constant temperatures; (ii) that thermal performance does not vary across a species' geographical range; and (iii) that differing performance traits should have similar thermal sensitivity. We have demonstrated, using a variety of both short and moderate-term performance traits, that these assumptions do not always hold in an ecologically important marine species. In addition, we demonstrated that the effects we observed could not be driven by simple rate summation effects, suggesting that acclimation to thermal variability itself had occurred. While the limited set of experimental conditions we were able to perform necessarily limits the conclusions we can draw from this single experiment, we encourage further studies that both increase the number of fluctuating experimental groups and use ecologically relevant exposure regimes. Understanding the impacts of thermal variation on the physiological functioning and resulting fitness of ectotherms is key for predicting the impacts of climate change on these organisms, but we currently lack a theoretical framework or clear proximate causes that explain observed empirical effects.
We suggest several ways forward from this impasse. First, by making the assumptions of the TPC model explicit, and including rate summation effects as a mathematical null hypothesis in physiological and ecological studies, we can move forward on this complex problem in a more rigorous way. Second, we suggest that progress on understanding the physiological and biochemical mechanisms of responses to temperature fluctuation will accelerate the construction of more mechanistic models of responses to temperature variation that can incorporate a wide range of effects and drive theoretical understanding. Finally, ecological studies conducted in the laboratory should re-evaluate ‘control’ conditions and include natural temperature variation whenever possible.
Supplementary Material
Acknowledgements
We thank the members of UBC Comparative Physiology for the loan of many magnetic stir plates for the feeding rate trials, Mary O'Connor for the use of the FlowCam, Patrick Martone for the use of the Instron, and Michael Sackville for calcium measurements. We thank two anonymous reviewers for their valuable contributions that improved the manuscript.
Ethics
Mussels were collected under Fisheries and Oceans Canada scientific collecting permits XR 245 2015 and XR 278 2015.
Data accessibility
All code and raw data are available on the Open Science Framework at: doi:10.17605/OSF.IO/K4DF5.
Authors' contributions
K.E.M. conceived of the experiment, performed mesocosm measurements, carried out initial analyses, wrote the first draft and produced half of the figures. K.M.A. helped design the experiment, performed mesocosm and mussel mass measurements, carried out the bootstrap analysis and produced the other half of the figures. N.E.M.B. helped design the experiment, performed feeding rate experiments and helped maintain mesocosms. J.K.D. helped design the experiment, performed byssal thread measurements and helped maintain mesocosms. K.L.F. and C.A.K. helped design the experiment, collected mussels and setup and maintained mesocosms. J.R.B. performed feeding rate experiments and collected FlowCam data. H.G.S. performed genetic analyses of mussel species. C.D.G.H. helped design the experiment, helped draft the manuscript and funded laboratory work. All authors were responsible for writing the methods of the data they collected and all contributed to the drafting and editing of the manuscript. All have given their approval for publication and agree to be accountable for the work performed therein.
Competing interests
The authors have no competing interests to report.
Funding
K.E.M. and C.D.G.H. are supported by individual NSERC Discovery grants. The mesocosms and incubators used in this project were funded by Canada Foundation for Innovation Leaders Opportunity Fund awards to C.D.G.H.
References
- 1.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738-5742. ( 10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams J, Jackson S. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475-482. ( 10.1890/1540-9295(2007)[475:NCNCAE]2.0.CO;2) [DOI] [Google Scholar]
- 3.Braby CE, Somero GN. 2006. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J. Exp. Biol. 209, 2554-2566. ( 10.1242/jeb.02259) [DOI] [PubMed] [Google Scholar]
- 4.Sinclair BJ, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372-1385. ( 10.1111/ele.12686) [DOI] [PubMed] [Google Scholar]
- 5.Schulte PM, Healy TM, Fangue NA. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691-702. ( 10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
- 6.Angilletta MJ. 2006. Estimating and comparing thermal performance curves. J. Therm. Biol. 31, 541-545. ( 10.1016/j.jtherbio.2006.06.002) [DOI] [Google Scholar]
- 7.Denny M. 2017. The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen's inequality. J. Exp. Biol. 220, 139-146. ( 10.1242/jeb.140368) [DOI] [PubMed] [Google Scholar]
- 8.Ruel JJ, Ayres MP. 1999. Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361-366. ( 10.1016/S0169-5347(99)01664-X) [DOI] [PubMed] [Google Scholar]
- 9.Morash AJ, Neufeld C, MacCormack TJ, Currie S. 2018. The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J. Exp. Biol. 221, jeb164673. ( 10.1242/jeb.164673) [DOI] [PubMed] [Google Scholar]
- 10.Bernhardt JR, Sunday JM, Thompson PL, O'Connor MI. 2018. Nonlinear averaging of thermal experience predicts population growth rates in a thermally variable environment. Proc. R. Soc. B 285, 20181076. ( 10.1098/rspb.2018.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704-706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 12.Vasseur DA, et al. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612. ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall KE, Sinclair BJ. 2012. The impacts of repeated cold exposure on insects. J. Exp. Biol. 215, 1607-1613. ( 10.1242/jeb.059956) [DOI] [PubMed] [Google Scholar]
- 14.Tollarová-Borovanská M, Lalouette L, Koštál V. 2009. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: role of 70 kda heat shock protein expression. Cryo-Letters 30, 312-319. ( 10.1016/j.cbpa.2006.12.033) [DOI] [PubMed] [Google Scholar]
- 15.Renault D, Nedved O, Hervant F, Vernon P. 2004. The importance of fluctuating thermal regimes for repairing chill injuries in the tropical beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) during exposure to low temperature. Physiol. Entomol. 29, 139-145. ( 10.1111/j.0307-6962.2004.00377.x) [DOI] [Google Scholar]
- 16.Marshall KE, Sinclair BJ. 2018. Repeated freezing induces a trade-off between cryoprotection and egg production in the goldenrod gall fly, Eurosta solidaginis. J. Exp. Biol. 221, jeb177956. ( 10.1242/jeb.177956) [DOI] [PubMed] [Google Scholar]
- 17.Kingsolver JG, Higgins JK, Augustine KE. 2015. Fluctuating temperatures and ectotherm growth: distinguishing non-linear and time-dependent effects. J. Exp. Biol. 218, 2218-2225. ( 10.1242/jeb.120733) [DOI] [PubMed] [Google Scholar]
- 18.Niehaus AC, Angilletta MJ, Sears MW, Franklin CE, Wilson RS. 2012. Predicting the physiological performance of ectotherms in fluctuating thermal environments. J. Exp. Biol. 215, 694-701. ( 10.1242/jeb.058032) [DOI] [PubMed] [Google Scholar]
- 19.Chown SL, Haupt TM, Sinclair BJ. 2016. Similar metabolic rate-temperature relationships after acclimation at constant and fluctuating temperatures in caterpillars of a sub-Antarctic moth. J. Insect Physiol. 85, 10-16. ( 10.1016/j.jinsphys.2015.11.010) [DOI] [PubMed] [Google Scholar]
- 20.Bozinovic F, Catalan TP, Estay SA, Sabat P. 2013. Acclimation to daily thermal variability drives the metabolic performance curve. Evol. Ecol. Res. 15, 579-587. [Google Scholar]
- 21.Colinet H, Nguyen TTA, Cloutier C, Michaud D, Hance T. 2007. Proteomic profiling of a parasitic wasp exposed to constant and fluctuating cold exposure. Insect Biochem. Mol. Biol. 37, 1177-1188. ( 10.1016/j.ibmb.2007.07.004) [DOI] [PubMed] [Google Scholar]
- 22.Colinet H, Renault D, Javal M, Berková P, Šimek P, Koštál V. 2016. Uncovering the benefits of fluctuating thermal regimes on cold tolerance of Drosophila flies by combined metabolomic and lipidomic approach. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1861, 1736-1745. ( 10.1016/J.BBALIP.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 23.Sadchatheeswaran S, Branch GM, Robinson TB. 2015. Changes in habitat complexity resulting from sequential invasions of a rocky shore: implications for community structure. Biol. Invasions 17, 1799-1816. ( 10.1007/s10530-014-0837-4) [DOI] [Google Scholar]
- 24.Harley CDG. 2011. Climate change, keystone predation, and biodiversity loss. Science 334, 1124-1127. ( 10.1126/science.1210199) [DOI] [PubMed] [Google Scholar]
- 25.Paine RT. 1974. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15, 93-120. ( 10.1007/BF00345739) [DOI] [PubMed] [Google Scholar]
- 26.Paine RT, Trimble AC. 2004. Abrupt community change on a rocky shore: biological mechanisms contributing to the potential formation of an alternative state. Ecol. Lett. 7, 441-445. ( 10.1111/j.1461-0248.2004.00601.x) [DOI] [Google Scholar]
- 27.Babarro JMF, Carrington E. 2013. Attachment strength of the mussel Mytilus galloprovincialis: effect of habitat and body size. J. Exp. Mar. Biol. Ecol. 443, 188-196. ( 10.1016/j.jembe.2013.02.035) [DOI] [Google Scholar]
- 28.Mackie GL, Wright CA. 1994. Ability of the zebra mussel, Dreissena polymorpha to biodeposit and remove phosphorus and bod from diluted activated sewage sludge. Water Res. 28, 1123-1130. ( 10.1016/0043-1354(94)90199-6) [DOI] [Google Scholar]
- 29.Al-Mamun A, Khan MA. 2011. Freshwater mussels (Margaritifera margaritifera): bio-filter against water pollution. World Appl. Sci. J. 12, 580-585. [Google Scholar]
- 30.Soto D, Mena G. 1999. Filter feeding by the freshwater mussel, Diplodon chilensis, as a biocontrol of salmon farming eutrophication. Aquaculture 171, 65-81. ( 10.1016/S0044-8486(98)00420-7) [DOI] [Google Scholar]
- 31.Jones TL, Richman JR. 1995. On mussels: Mytilus californianus as a prehistoric resource. North Am. Archaeol. 16, 33-58. ( 10.2190/g5tt-yfhp-je6a-p2tx) [DOI] [Google Scholar]
- 32.Parkington J. 2003. Middens and moderns: shellfishing and the Middle Stone Age of the Western Cape, South Africa. Acad. Sci. South Africa 99, 243-247. [Google Scholar]
- 33.Strayer D, Caraco N, Cole J, Findlay S, Pace M. 1999. Transformation of freshwater ecosystems by bivalves: a case study of zebra mussels in the Hudson River. Bioscience 49, 19-27. ( 10.1525/bisi.1999.49.1.19) [DOI] [Google Scholar]
- 34.Donnell MJO, George MN, Carrington E. 2013. Mussel byssus attachment weakened by ocean acidification. Nat. Clim. Chang. 3, 1-4. ( 10.1038/nclimate1846) [DOI] [Google Scholar]
- 35.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.r-project.org. [Google Scholar]
- 37.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1-26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 38.Widdows J, Bayne BL. 1971. Temperature acclimation of Mytilus edulis with reference to its energy budget. J. Mar. Biol. Assoc. UK 51, 827-843. ( 10.1017/S0025315400018002) [DOI] [Google Scholar]
- 39.Estay SA, Lima M, Bozinovic F. 2014. The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 123, 131-140. ( 10.1111/j.1600-0706.2013.00607.x) [DOI] [Google Scholar]
- 40.Widdows J. 1976. Physiological adaptation of Mytilus edulis to cyclic temperatures. J. Comp. Physiol. 105, 115-128. ( 10.1007/BF00691115) [DOI] [Google Scholar]
- 41.Gillmor RB. 1982. Assessment of intertidal growth and capacity adaptations in suspension-feeding bivalves. Mar. Biol. 68, 277-286. ( 10.1007/BF00409594) [DOI] [Google Scholar]
- 42.Malone PG, Dodd JR. 1967. Temperature and salinity effects on calcifiation rate in Mytilus edulis and its paleoecological implications. Limnol. Oceanogr. 12, 432-436. ( 10.4319/lo.1967.12.3.0432) [DOI] [Google Scholar]
- 43.Sanford E, Kelly MW. 2011. Local adaptation in marine invertebrates. Ann. Rev. Mar. Sci. 3, 509-535. ( 10.1146/annurev-marine-120709-142756) [DOI] [PubMed] [Google Scholar]
- 44.Moeser GM, Carrington E. 2006. Seasonal variation in mussel byssal thread mechanics. J. Exp. Biol. 209, 1996-2003. ( 10.1242/jeb.02234) [DOI] [PubMed] [Google Scholar]
- 45.Gleason LU, Strand EL, Hizon BJ, Dowd WW. 2018. Plasticity of thermal tolerance and its relationship with growth rate in juvenile mussels (Mytilus californianus). Proc. R. Soc. B 285, 20172617. ( 10.1098/rspb.2017.2617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouhlel Z, et al. 2017. Interspecies comparison of the mechanical properties and biochemical composition of byssal threads. J. Exp. Biol. 220, 984-994. ( 10.1242/jeb.141440) [DOI] [PubMed] [Google Scholar]
- 47.Callaghan NI, Tunnah L, Currie S, MacCormack TJ. 2016. Metabolic adjustments to short-term diurnal temperature fluctuation in the rainbow trout (Oncorhynchus mykiss). Physiol. Biochem. Zool. 89, 498-510. ( 10.1086/688680) [DOI] [PubMed] [Google Scholar]
- 48.Williams CM, Buckley LB, Sheldon KS, Vickers M, Pörtner H-O, Dowd WW, Gunderson AR, Marshall KE, Stillman JH. 2016. Biological impacts of thermal extremes: mechanisms and costs of functional responses matter. Integr. Comp. Biol. 56, 73-84. ( 10.1093/icb/icw013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code and raw data are available on the Open Science Framework at: doi:10.17605/OSF.IO/K4DF5.