Abstract
The decline in species richness at higher latitudes is among the most fundamental patterns in ecology. Whether changes in species composition across space (beta-diversity) contribute to this gradient of overall species richness (gamma-diversity) remains hotly debated. Previous studies that failed to resolve the issue suffered from a well-known tendency for small samples in areas with high gamma-diversity to have inflated measures of beta-diversity. Here, we provide a novel analytical test, using beta-diversity metrics that correct the gamma-diversity and sampling biases, to compare beta-diversity and species packing across a latitudinal gradient in tree species richness of 21 large forest plots along a large environmental gradient in East Asia. We demonstrate that after accounting for topography and correcting the gamma-diversity bias, tropical forests still have higher beta-diversity than temperate analogues. This suggests that beta-diversity contributes to the latitudinal species richness gradient as a component of gamma-diversity. Moreover, both niche specialization and niche marginality (a measure of niche spacing along an environmental gradient) also increase towards the equator, after controlling for the effect of topographical heterogeneity. This supports the joint importance of tighter species packing and larger niche space in tropical forests while also demonstrating the importance of local processes in controlling beta-diversity.
Keywords: beta-diversity, gamma-diversity, sampling bias, latitude, species packing, niche specialization
1. Introduction
Beta-diversity is the variation of species composition across space, and it is a key element of conservation planning because it indicates whether diversity is concentrated within a few sites or spread across many sites [1–3]. One factor enhancing beta-diversity should be large niche space, i.e. more species sharing more available niches, perhaps associated with abiotic habitat heterogeneity [4–10]. Another feature elevating beta-diversity would be dense species packing, i.e. many narrow niches result from stable climate and high productivity [5,7–11]. Both stable climate and greater productivity would then lead to higher beta-diversity at low latitudes [9,12–14]. On the other hand, if beta-diversity is driven mostly by abiotic heterogeneity, we would not expect a latitudinal gradient in beta-diversity, because the abiotic heterogeneity should not vary with latitude. These alternatives remain unresolved, and studies on the causes of the latitudinal gradient in beta-diversity appear to reach opposing conclusions [15–21]. Underlying the debate has been controversy about statistical biases in tools for measuring beta-diversity.
The bias in beta-diversity metrics arises from dependence on a sample size that interacts with gamma-diversity [20,22–24], a bias that is easy to illustrate using simple measures of species overlap. Small samples rarely (if ever) capture all local species. Two small samples from two sites that have exactly the same composition will appear to differ by randomly capturing different subsets of the local communities. The fewer species sampled, the greater this artefactual beta-diversity will appear [22,24]. A crucial aspect of the sample size bias is the dependence on gamma-diversity it engenders, since small samples underestimate diversity more severely in species-rich sites than in species-poor sites [20–22,24,25]. This bias has led authors to develop metrics that correct beta-diversity for sample size [22,25,26] or tools based on comparisons with null models [20,23]. Crucial in the sample size bias is the dependence on gamma-diversity it engenders, because larger samples are needed in species-rich sites [21,22,24,25]. Once correcting for sample size bias, gamma-diversity dependence should be removed, and it should be straightforward to compare beta-diversity across a gradient of species diversity in order to evaluate the importance of species packing and total niche space.
We carry out this comparison using a steep latitudinal gradient in tree species richness, as documented in our census of 3 million trees at 21 sites spanning 50° of latitude in East Asia [27,28]. We define beta-diversity within each plot, so it is a measure of how tree species partition local niche space, then we compare the local estimates of beta-diversity across the latitudinal gradient. In a previous simulation study, Cao et al. [26] identified that the corrected beta-Shannon diversity index is highly effective at removing the bias arising from beta-diversity metrics in small samples of high gamma-diversity communities [26]. With this corrected index, we can answer two fundamental questions about variation in beta-diversity and its impact on the overall species richness: (i) is there a latitudinal gradient in within-plot beta-diversity? and (ii) do local environmental heterogeneity, niche marginality (the distance between the species optima relative to the overall mean habitat), and niche specialization contribute to the latitudinal patterns of beta-diversity? By simultaneously testing the importance of local heterogeneity and latitude, we can establish whether species packing and total niche space contributes to a higher richness in tropical relative to temperate forests.
2. Material and methods
(a). Forest dynamics plots
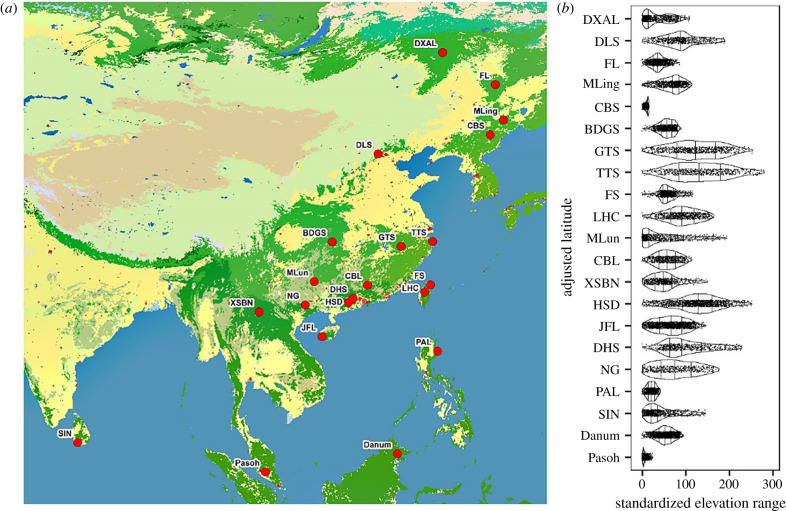
We used data from 21 forest dynamics plots (15–52 ha) that are part of the ForestGEO and Chinese Forest Biodiversity Monitoring Networks [27,28] (figure 1a; electronic supplementary material, table S1). All stems with a diameter at breast height of equal to or greater than 1 cm were spatially mapped, tagged, measured and identified to species [29]. The plots range from tropical rain forest at 2.98° N latitude to a boreal forest at 51.82° N latitude (electronic supplementary material, table S1), from sea level to more than 1400 m elevation, and local topographical variation is as low as 17.7 m and as high as 298.6 m (figure 1b; electronic supplementary material, table S1).
Figure 1.
The spatial distribution of forest dynamics plots (a), and their elevational ranges (b); (b) shows the latitudinal pattern of elevation range, which was transformed by subtracting the minimum elevation of each plot. The width of each violin plot reflects probability density distribution of mean elevation for 20 × 20 m subplots in each forest dynamics plot. Full plot names are listed in the electronic supplementary material, table S1. (Online version in colour.)
We divided plots into non-overlapping quadrats of different scales (grain sizes) (10 × 10 m, 20 × 20 m, and 50 × 50 m) in order to assess the effect of grain size on beta-diversity [19,21]. We define alpha-diversity as the quadrat level diversity, and gamma-diversity as plot level diversity. In the main results, we present only the results at a grain size of 20 × 20 m, and details of results at a grain size of 10 × 10 m and at a grain size of 50 × 50 m can be found in the electronic supplementary material (electronic supplementary material, table S2 and figure S2).
Plot latitudes were adjusted for mean elevation: adding 100 km of latitude per 100 m increase in elevation. Local environmental heterogeneity was quantified in terms of topography, which was the only environmental factor consistently available across all plots. Specifically, we used the ratio of surface area to planimetric as a metric of topographical heterogeneity, calculating at grain sizes of 10 × 10 m, 20 × 20 m and 50 × 50 m, which provided a useful measure of the range and roughness of the overall plot, based on digital elevation models [6,30]. Local habitat and species niches were defined using six topographical factors as environmental variables: mean elevation, convexity, slope, aspect, topographical wetness index and altitude above channel [31–33].
(b). Measurement of beta-diversity
To remove gamma-diversity dependence caused by the sample-size bias of beta-diversity metrics, we used the correction method designed for the Shannon diversity index based on the relationship between cumulative diversity and sample size [34]. The beta-Shannon diversity index measures the heterogeneity of pooled communities and is calculated as the effective number of compositionally distinct and equally abundant communities [35,36]:
| 2.1 |
| 2.2 |
| 2.3 |
where , and are alpha-, beta- and gamma-Shannon diversity, respectively; pi is the proportional abundance of species i; S and N are the total number of species and the total number of local communities (or plots), respectively, in the pooled communities. Alpha- and gamma-Shannon diversity are mathematically independent (i.e. gamma-diversity does not contain information of alpha-diversity) [35]. Beta-Shannon diversity weights all species by their abundance. We then used a sample-size dependence correction method to reduce the bias in beta-Shannon diversity for comparing beta-diversity among regions [25,34]. As in a species accumulation curve, the expected cumulative alpha- or gamma-diversity was depicted as a function of sample size, while sample completeness was estimated from community structures of samples [25,34]. Beta-diversity was then estimated from asymptotically approximated alpha- and gamma-diversity based on the diversity sample-size curve. Details of the undersampling correction method for the beta-Shannon diversity can be found in the electronic supplementary material, S1. Simulation work conducted by Cao et al. [26] confirmed that beta-metrics that incorporate an undersampling correction method were more effective at removing the dependence on gamma-diversity and inferring casual mechanisms compared to other uncorrected beta-diversity metrics or null models [26].
(c). Community-level niche differentiation
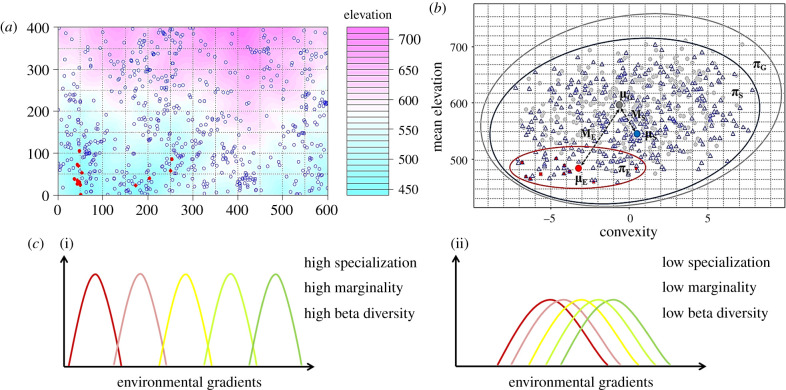
Niche differentiation was described using attributes of specialization and marginality. Niche specialization was defined as SD(available habitat)/SD(habitat used), in which SD(available habitat) represented the standard deviation of environmental conditions for a community and SD(habitat used) represented the standard deviation of environmental conditions occupied by a species (illustrated in figure 2). Niche marginality was defined as the distance between a species' optimum and the mean environmental conditions within the plot (figure 2) [37,38]. Both specialization and marginality were calculated from multivariate measures of habitat, known as ecological niche factor analysis [37]. To better meet the assumption of normality of residual in a regression model and approximate the linear relationship between niche specialization and explanatory variables (electronic supplementary material, figure S1a,c,e), the log- and Box-Cox transformations [39] were applied for niche specialization across grain sizes (electronic supplementary material, figure S1b,d,f). Based on the precise mapping of all individuals in these plots, the community-level niche marginality and specialization were respectively quantified as species-level niche marginality and specialization weighted by relative species abundance. Higher community-level niche specialization indicates the fine partitioning of available niche space, while higher community-level niche marginality indicates a larger deviation from mean environmental conditions of a community and thus suggesting a larger niche space. Topographical variables are typically strongly correlated with the variation in resources such as water availability and soil conditions [40,41], thus can capture potentially important axes of niche differentiation. The aspect was computed as sin(aspect) and cos(aspect), and other topographical variables were Box-Cox transformed before being included in analyses [39].
Figure 2.
Illustration of niche specialization and marginality of Euonymus oblongifolius and Symplocos stellaris in the Gutianshan forest dynamics plot (600 × 400 m). (a) Red solid points represent the spatial distribution of E. oblongifolius, and blue circles represent the spatial distribution of S. stellaris. (b) Illustration of niche specialization and marginality of E. oblongifolius and S. stellaris in two-dimensional niche space based on mean elevation and convexity of distributed 20 × 20 m quadrats. Niche marginality is the distance from the mean habitat of the focal species to the mean habitat of community habitats. μE, μS and μG represent centroids of environmental conditions for E. oblongifolius, S. stellaris and the entire community, and distances ME and MS are niche marginalities of two species. Likewise, niche specialization is the ratio of the entire habitat range of a community to habitat range of the focal species. πE, πS and πG stand for the distributional range of for E. oblongifolius, S. stellaris and the entire community in two-dimensional niche space, respectively, the ratio of πG /πE and πG /πS are niche specialization of two species. Grey points indicate the topographical variation of the entire community, red squares show higher niche specialization and marginality of E. oblongifolius, whereas blue triangles indicate lower specialization and marginality of S. stellaris. (c) Hypothetical relationships between beta-diversity and niche. Higher community-level niche specialization indicates the fine partitioning of available niche space, while higher community-level niche marginality suggests a larger niche space. Therefore, higher specialization and marginality lead to a higher beta-diversity (i), while lower specialization and marginality lead to a lower beta-diversity (ii). (Online version in colour.)
(d). Statistical analysis
To examine the significance of latitudinal gradients in local beta-diversity, niche specialization and niche marginality, we first modelled beta-diversity, community-level niche specialization and niche marginality against topographical heterogeneity and adjusted latitude separately using simple linear regression models. Subsequently, to determine the relative effect sizes of adjusted latitude and topography, we performed multiple linear regression models with beta-diversity, niche specialization, and niche marginality as response variables, respectively, and all variables were scaled using (x – mean(x))/SD(x) before being included.
All statistical analyses were performed with R software, v. 3.6.4 [42]. The corrected beta-Shannon diversity was calculated using R package ‘entropart’ and ‘vegan' [43,44]. The topographical variables were computed using the ‘RSAGA’ package [45] and the SAGA GIS software [46]. Ecological niche factor analysis was implemented to calculate niche metrics using R package ‘adehabitatHS' [47].
3. Results
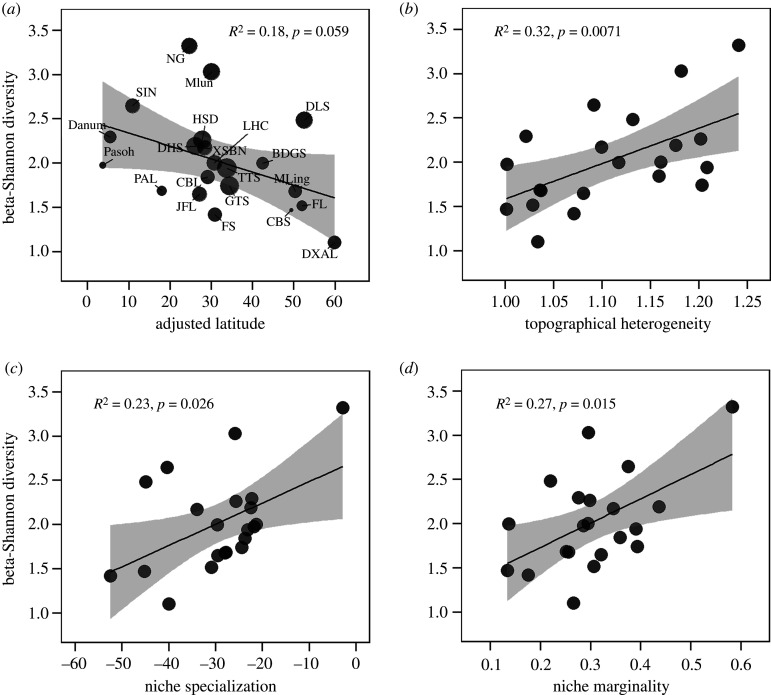
Gamma-diversity declined by more than 40-fold from tropical to temperate latitudes, from 818 species at Pasoh to 18 at Daxinganling (electronic supplementary material, table S1). Beta-diversity measured by the corrected beta-Shannon diversity also declined with latitude, although this pattern was not significant (figure 3a). However, the corrected beta-Shannon diversity was significantly correlated with latitude (e.g. 20 × 20 m, standardized effect size = −0.39, p = 0.033) in multiple regression models, after controlling for the effect of local topographical heterogeneity (electronic supplementary material, figure S2c). We also found that beta-diversity was positively correlated with community-level niche specialization, niche marginality and local topographical heterogeneity (figures 3b–d, electronic supplementary material, figure S3). We obtained similar results across three grain sizes although the effect size of topographical heterogeneity and latitude varied with grain sizes (electronic supplementary material, figures S2, S3).
Figure 3.
Relationships of beta-diversity (measured by corrected beta-Shannon diversity) with adjusted latitude (a), local topographical heterogeneity (b), community-level niche specialization (c), and niche marginality (d) at grain size of 20 × 20 m. In each panel, R2 and p-value of the linear regression models are shown, the shaded areas represent the 95% confidence intervals of the predictions (electronic supplementary material, table S2). Full plot names in (a) are listed in the electronic supplementary material, table S1. Community-level niche specialization was Box-Cox transformed in (c).
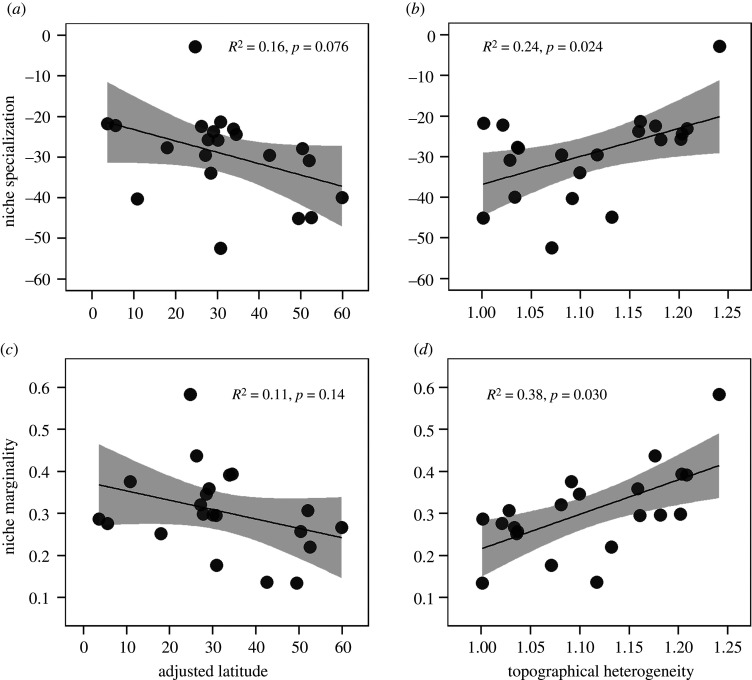
Various predictors of beta-diversity were also associated with latitude. Both community-level niche specialization and niche marginality significantly decreased from tropical to temperate forests at some grain sizes (figure 4a,c; electronic supplementary material, figure S6a,c). However, topographical heterogeneity did not have a significant relationship with latitude (electronic supplementary material, figure S5). Both niche specialization and niche marginality were positively correlated with each other (electronic supplementary material, figure S4g–i), and both were also positively associated with local topographical heterogeneity (figure 4b,d; electronic supplementary material, figure S4a–f). Multiple linear regression models confirmed these results, showing that specialization and marginality both significantly declined with latitude after controlling for topographical heterogeneity at most grain sizes. In the multiple regression models, the effect sizes of topographical heterogeneity were larger than those of adjusted latitude in predicting specialization and marginality (electronic supplementary material, table S3, figure S6b,d).
Figure 4.
The relationships of community-level niche specialization (a,b) and marginality (c,d) with adjusted latitude and local topographical heterogeneity at grain size of 20 × 20 m. Community-level niche specialization was Box-Cox transformed. R2 and p-value of the linear regression models are shown in each panel, and shaded areas represent the 95% confidence intervals of the predictions (electronic supplementary material, table S4).
4. Discussion
Whether beta-diversity contributes to the latitudinal diversity gradient has been intensely debated in recent years, largely because of the bias in beta-diversity metrics in small samples of high gamma-diversity communities [17,20–24]. To move this debate forward, we first examined the latitudinal gradient in beta-diversity by removing the gamma-diversity and sample-size bias with a correction for undersampling [25,34], while also accounting for the effect of topographical heterogeneity statistically. Our results showed that beta-diversity increased from high to lower latitudes, in line with a number of previous studies also finding higher beta-diversity in the tropics [2,13,18,48]. This supports the hypothesis that beta-diversity contributes to the latitudinal gradient in species richness. Because topographical heterogeneity did not systematically vary with latitude, it appears that local topographical heterogeneity does not contribute to the latitudinal gradient in beta-diversity, in line with previous findings [49,50].
High beta-diversity in the tropics reveals higher species turnover at lower latitudes, meaning tighter species packing and expanded niche space in tropical relative to temperate forests [5,9,12,48,51]. These hypotheses have been investigated for decades, with dense species packing in large niche space attributed to a stable climate and higher productivity in the tropics [5,9,51–53]. We found increasing niche marginality and specialization towards lower latitudes, supporting this hypothesis. Perhaps larger niche space enables more species to use more variable resources, while higher niche specialization allows species to specialize on narrower subsets of the resources available [5,9,51–53]. These consequently reduce niche overlap and competition between co-occurring species and facilitate species coexistence [54]. Tighter species packing and larger niche space in the tropics could be related to other mechanisms as well, such as higher diversification rate [55] and stronger conspecific negative density dependence [56,57] at lower latitudes.
We also conclude that beta-diversity at the extent of 15–52 ha is largely driven by local processes—specifically, topographical heterogeneity and the niche differentiation it fosters. However, topographical heterogeneity did not contribute to the latitudinal gradient in beta-diversity (figures 3 and 4). This may seem an unsurprising result, but the roles of local ecological processes have been questioned given the broad latitudinal gradient of gamma-diversity [12,20]. We suggest that the effect of local processes has been obscured by the biases in beta-diversity metrics of small samples from high gamma-diversity communities in previous studies [23]. Moreover, our large samples over 55 degrees of latitude provide comparable measures of niche differentiation, topographical heterogeneity and beta-diversity, well beyond what was available in early studies [6,58]. Our results could be refined by considering the influence of additional factors that contribute to local environmental heterogeneity and niche differentiation, such as soil types and soil nutrients [59], which could also contribute to beta-diversity. The biases in beta-diversity metrics in a small sample from high gamma-diversity communities are also associated with other attributes of communities such as the species abundance distributions [60], and tests of the alternative techniques in other systems are warranted.
In conclusion, our results support that a latitudinal gradient in beta-diversity contributes to the latitudinal gradient in tree species richness after separately controlling for local topographical heterogeneity and the bias in beta-diversity metrics in small samples of high gamma-diversity areas. Our results further suggest tighter species packing and larger niche space in tropical forests [12,51,53], but also confirmed environmental heterogeneity as a determinant of beta-diversity. Our findings help resolve the ongoing debates on the contribution of local beta-diversity to the latitudinal gradient of species richness.
Supplementary Material
Acknowledgements
We thank Dingliang Xing, Tak Fung, Fangliang He and Gabriel Arellano for comments on the earlier draft. We thank Alex Karolus for leading the census in the Danum Valley forest plot, and we are grateful to Mike Bernados and Bill McDonald for species identifications, to Fangliang He, Stuart Davies and Shameema Esufali for advice and training, to Qianjiangyuan National Park, the Center for Forest Science at Morton Arboretum, Fushan Research Center, Lienhuachih Research Center and Sri Lankan Forest Department for logistical support and the hundreds of fieldworkers and students who measured and mapped the trees analysed in this study.
Data accessibility
The data supporting figures 1–4 and code for data analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.tht76hdww [61]. Full census data are available upon reasonable request from the data portal of ForestGEO (http://ctfs.si.edu/datarequest/).
Authors' contributions
K.C., R.C., X.M., K.M. and J.C.S. designed research, K.C. and X.M. compiled and analysed data; K.C., R.C., X.M., K.M. and J.C.S. wrote the draft with substantial input from L.C., W.X., D.F.R.P.B. and M.J.B. Many authors contributed to the data collection of forest censuses and all authors contributed to revisions of the manuscript.
Competing interests
We declare we have no competing financial interests.
Funding
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000) and National Natural Science Foundation of China (NSFC 31770478). Data collection was funded by many organizations, principally, NSFC 31470490, 31470487, 41475123, 31570426, 31570432, 31570486, 31622014, 31660130, 31670441, 31670628, 31700356, 31760141, 31870404 and 32061123003, the Southeast Asia Rain Forest Research Programme (SEARRP), National Key Basic Research Program of China (Grant No. 2014CB954100), SEARRP partners especially Yayasan Sabah, HSBC Malaysia, financial project of Heilongjiang Province (XKLY2018ZR01), National Key R&D Program of China (2016YFC1201102 and 2016YFC0502405), the Central Public-interest Scientific Institution Basal Research Fund (CAFYBB2017ZE001), CTFS Forest GEO for funding for Sinharaja forest plot, the Taiwan Forestry Bureau (92-00-2-06 and tfbm960226), the Taiwan Forestry Research Institute (93AS-2.4.2-FI-G1, 94AS-11.1.2-FI-G1, and 97AS-7.1.1.F1-G1) and the Ministry of Science and Technology of Taiwan (NSC92-3114-B002-009) for funding the Fushan and Lienhuachih plots, Scientific Research Funds of Heilongjiang Provincial Research Institutes (CZKYF2021B006). J.C.S. considers this work a contribution to his VILLUM Investigator project ‘Biodiversity Dynamics in a Changing World' funded by VILLUM FONDEN (grant no. 16549).
References
- 1.Socolar JB, Gilroy JJ, Kunin WE, Edwards DP. 2016. How should beta-diversity inform biodiversity conservation? Trends Ecol. Evol. 31, 67-80. ( 10.1016/j.tree.2015.11.005) [DOI] [PubMed] [Google Scholar]
- 2.Koleff P, Lennon JJ, Gaston KJ. 2003. Are there latitudinal gradients in species turnover? Glob. Ecol. Biogeogr. 12, 483-498. ( 10.1046/j.1466-822X.2003.00056.x) [DOI] [Google Scholar]
- 3.Anderson MJ, et al. 2011. Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19-28. ( 10.1111/j.1461-0248.2010.01552.x) [DOI] [PubMed] [Google Scholar]
- 4.Alahuhta J, et al. 2017. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J. Biogeogr. 44, 1758-1769. ( 10.1111/jbi.12978) [DOI] [Google Scholar]
- 5.Brown JH. 2014. Why are there so many species in the tropics? J. Biogeogr. 41, 8-22. ( 10.1111/jbi.12228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown C, et al. 2013. Multispecies coexistence of trees in tropical forests: spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proc. R. Soc. B 280, 20130502. ( 10.1098/rspb.2013.0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracewell SA, Clark GF, Johnston EL. 2018. Habitat complexity effects on diversity and abundance differ with latitude: an experimental study over 20 degrees. Ecology 99, 1964-1974. ( 10.1002/ecy.2408) [DOI] [PubMed] [Google Scholar]
- 8.MacArthur R. 1972. Geographical ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Pontarp M, et al. 2019. The latitudinal diversity gradient: novel understanding through mechanistic eco-evolutionary models. Trends Ecol. Evol. 34, 211-223. ( 10.1016/j.tree.2018.11.009) [DOI] [PubMed] [Google Scholar]
- 10.Storch D, Okie JG. 2019. The carrying capacity for species richness. Glob. Ecol. Biogeogr. 28, 1519-1532. ( 10.1111/geb.12987) [DOI] [Google Scholar]
- 11.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233-249. ( 10.1086/282487) [DOI] [Google Scholar]
- 12.Gaston KJ. 2000. Global patterns in biodiversity. Nature 405, 220-227. ( 10.1038/35012228) [DOI] [PubMed] [Google Scholar]
- 13.Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273-309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 14.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192-211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 15.Lenoir J, et al. 2010. Cross-scale analysis of the region effect on vascular plant species diversity in southern and northern European mountain ranges. PLoS ONE 5, e15734. ( 10.1371/journal.pone.0015734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori AS, Shiono T, Koide D, Kitagawa R, Ota AT, Mizumachi E. 2013. Community assembly processes shape an altitudinal gradient of forest biodiversity. Glob. Ecol. Biogeogr. 22, 878-888. ( 10.1111/geb.12058) [DOI] [Google Scholar]
- 17.Qian H, Chen S, Mao L, Ouyang Z. 2013. Drivers of β-diversity along latitudinal gradients revisited. Glob. Ecol. Biogeogr. 22, 659-670. ( 10.1111/geb.12020) [DOI] [Google Scholar]
- 18.Myers JA, Chase JM, Jimenez I, Jorgensen PM, Araujo-Murakami A, Paniagua-Zambrana N, Seidel R. 2013. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 16, 151-157. ( 10.1111/ele.12021) [DOI] [PubMed] [Google Scholar]
- 19.De Cáceres M, et al. 2012. The variation of tree beta diversity across a global network of forest plots. Glob. Ecol. Biogeogr. 21, 1191-1202. ( 10.1111/j.1466-8238.2012.00770.x) [DOI] [Google Scholar]
- 20.Kraft NJB, et al. 2011. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 333, 1755-1758. ( 10.1126/science.1208584) [DOI] [PubMed] [Google Scholar]
- 21.Sreekar R, et al. 2018. Spatial scale changes the relationship between beta diversity, species richness and latitude. R. Soc. Open Sci. 5, 181168. ( 10.1098/rsos.181168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condit R, Perez R, Lao S, Aguilar S, Somoza A. 2005. Geographic ranges and b-diversity: discovering how many tree species there are where. Biologiske Skrifter 55, 57-71. [Google Scholar]
- 23.Myers JA, LaManna JA. 2016. The promise and pitfalls of β-diversity in ecology and conservation. J. Veg. Sci. 27, 1081-1083. ( 10.1111/jvs.12482) [DOI] [Google Scholar]
- 24.Tuomisto H, Ruokolainen K. 2012. Comment on ‘Disentangling the drivers of β diversity along latitudinal and elevational gradients'. Science 335, 1573. ( 10.1126/science.1216393) [DOI] [PubMed] [Google Scholar]
- 25.Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM. 2014. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45-67. ( 10.1890/13-0133.1) [DOI] [Google Scholar]
- 26.Cao K, Svenning J-C, Yan C, Zhang J, Mi X, Ma K. 2021. Undersampling correction methods to control γ-dependence for comparing β-diversity between regions. BioRxiv 2021.01.24.427952. ( 10.1101/2021.01.24.427952) [DOI]
- 27.Anderson-Teixeira KJ, et al. 2015. CTFS-ForestGEO: a worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 21, 528-549. ( 10.1111/gcb.12712) [DOI] [PubMed] [Google Scholar]
- 28.Feng G, Mi X, Yan H, Li FY, Svenning J-C, Ma K. 2016. CForBio: a network monitoring Chinese forest biodiversity. Sci. Bull. 61, 1163-1170. ( 10.1007/s11434-016-1132-9) [DOI] [Google Scholar]
- 29.Condit R. 1998. Tropical forest census plots: methods and results from Barro Colorado Island, Panama and comparison with other plots. Berlin, Germany: Springer. [Google Scholar]
- 30.Jenness JS. 2004. Calculating landscape surface area from digital elevation models. Wildl. Soc. Bull. 32, 829-839. ( 10.2193/0091-7648(2004)032[0829:CLSAFD]2.0.CO;2) [DOI] [Google Scholar]
- 31.Legendre P, Mi X, Ren H, Ma K, Yu M, Sun IF, He F. 2009. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 90, 663-674. ( 10.1890/07-1880.1) [DOI] [PubMed] [Google Scholar]
- 32.Kanagaraj R, Wiegand T, Comita LS, Huth A. 2011. Tropical tree species assemblages in topographical habitats change in time and with life stage. J. Ecol. 99, 1441-1452. ( 10.1111/j.1365-2745.2011.01878.x) [DOI] [Google Scholar]
- 33.Punchi-Manage R, Getzin S, Wiegand T, Kanagaraj R, Savitri Gunatilleke CV, Nimal Gunatilleke IAU, Wiegand K, Huth A, Zuidema P. 2013. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 101, 149-160. ( 10.1111/1365-2745.12017) [DOI] [Google Scholar]
- 34.Chao A, Wang YT, Jost L. 2013. Entropy and the species accumulation curve: a novel entropy estimator via discovery rates of new species. Methods Ecol. Evol. 4, 1091-1100. ( 10.1111/2041-210x.12108) [DOI] [Google Scholar]
- 35.Jost L. 2007. Partitioning diversity into independent alpha and beta components. Ecology 88, 2427-2439. ( 10.1890/06-1736.1) [DOI] [PubMed] [Google Scholar]
- 36.Tuomisto H. 2010. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33, 2-22. ( 10.1111/j.1600-0587.2009.05880.x) [DOI] [Google Scholar]
- 37.Hirzel AH, Hausser J, Chessel D, Perrin N. 2002. Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83, 2027-2036. ( 10.1890/0012-9658(2002)083[2027:Enfaht]2.0.Co;2) [DOI] [Google Scholar]
- 38.Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N. 2010. Defining and measuring ecological specialization. J. Appl. Ecol. 47, 15-25. ( 10.1111/j.1365-2664.2009.01744.x) [DOI] [Google Scholar]
- 39.Box GEP, Cox DR. 1964. An analysis of transformations. J. R. Stat. Soc. B 26, 211-252. ( 10.1111/j.2517-6161.1964.tb00553.x) [DOI] [Google Scholar]
- 40.Wright JS. 2002. Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130, 1-14. ( 10.1007/s004420100809) [DOI] [PubMed] [Google Scholar]
- 41.Fortunel C, Lasky JR, Uriarte M, Valencia R, Wright SJ, Garwood NC, Kraft NJB. 2018. Topography and neighborhood crowding can interact to shape species growth and distribution in a diverse Amazonian forest. Ecology 99, 2272-2283. ( 10.1002/ecy.2441) [DOI] [PubMed] [Google Scholar]
- 42.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 43.Marcon E, Hérault B. 2015. entropart: an R package to measure and partition diversity. J. Stat. Softw. 67, 1-26. ( 10.18637/jss.v067.i08) [DOI] [Google Scholar]
- 44.Oksanen J, et al. 2018. vegan: community ecology package. R package version 2.5-1. See https://CRAN.R-project.org/package=vegan.
- 45.Brenning A. 2008. Statistical geocomputing combining R and SAGA: the example of landslide susceptibility analysis with generalized additive models. Hamburger Beiträge zur Physischen Geographie und Landschaftsökologie 19, 410. [Google Scholar]
- 46.Conrad O, Bechtel B, Bock M, Dietrich H, Fischer E, Gerlitz L, Wehberg J, Wichmann V, Böhner J. 2015. System for automated geoscientific analyses (SAGA) v. 2.1.4. Geosci. Model Dev. 8, 1991-2007. ( 10.5194/gmd-8-1991-2015) [DOI] [Google Scholar]
- 47.Calenge C. 2006. The package ‘adehabitat’ for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197, 516-519. ( 10.1016/j.ecolmodel.2006.03.017) [DOI] [Google Scholar]
- 48.Vazquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1-19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 49.Chu C, et al. 2019. Direct and indirect effects of climate on richness drive the latitudinal diversity gradient in forest trees. Ecol. Lett. 22, 245-255. ( 10.1111/ele.13175) [DOI] [PubMed] [Google Scholar]
- 50.Alstad AO, Damschen EI, Givnish TJ, Harrington JA, Leach MK, Rogers DA, Waller DM. 2016. The pace of plant community change is accelerating in remnant prairies. Sci. Adv. 2, e1500975. ( 10.1126/sciadv.1500975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ricklefs R, Schluter D. 1993. Species diversity: regional and historical influences. In Species diversity in ecological communities: historical and geographical perspectives (eds Ricklefs R, Schluter D), pp. 350-359. Chicago, IL: University of Chicago Press. [Google Scholar]
- 52.Evans KL, Greenwood JJ, Gaston KJ. 2005. Dissecting the species-energy relationship. Proc. R. Soc. B 272, 2155-2163. ( 10.1098/rspb.2005.3209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacArthur RH. 1965. Patterns of species diversity. Biol. Rev. 40, 510-533. ( 10.1111/j.1469-185X.1965.tb00815.x) [DOI] [Google Scholar]
- 54.Arellano G, Umaña MN, Macía MJ, Loza MI, Fuentes A, Cala V, Jørgensen PM. 2017. The role of niche overlap, environmental heterogeneity, landscape roughness and productivity in shaping species abundance distributions along the Amazon–Andes gradient. Glob. Ecol. Biogeogr. 26, 191-202. [Google Scholar]
- 55.Fine PVA. 2015. Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Evol. Syst. 46, 369-392. ( 10.1146/annurev-ecolsys-112414-054102) [DOI] [Google Scholar]
- 56.Umaña MN, et al. 2017. The role of functional uniqueness and spatial aggregation in explaining rarity in trees. Glob. Ecol. Biogeogr. 26, 777-786. ( 10.1111/geb.12583) [DOI] [Google Scholar]
- 57.Fine PVA, Mesones I, Coley PD. 2004. Herbivores promote habitat specialization by trees in Amazonian forests. Science 305, 663-665. ( 10.1126/science.1098982) [DOI] [PubMed] [Google Scholar]
- 58.Shen G, He F, Waagepetersen R, Sun IF, Hao Z, Chen ZS, Yu M. 2013. Quantifying effects of habitat heterogeneity and other clustering processes on spatial distributions of tree species. Ecology 94, 2436-2443. ( 10.1890/12-1983.1) [DOI] [PubMed] [Google Scholar]
- 59.Baldeck CA, et al. 2013. Soil resources and topography shape local tree community structure in tropical forests. Proc. R. Soc. B 280, 20122532. ( 10.1098/rspb.2012.2532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chao A, Jost L. 2012. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93, 2533-2547. ( 10.1890/11-1952.1) [DOI] [PubMed] [Google Scholar]
- 61.Cao K, et al. 2021. Species packing and the latitudinal gradient in beta-diversity. Dryad Digital Repository. ( 10.5061/dryad.tht76hdww) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cao K, et al. 2021. Species packing and the latitudinal gradient in beta-diversity. Dryad Digital Repository. ( 10.5061/dryad.tht76hdww) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting figures 1–4 and code for data analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.tht76hdww [61]. Full census data are available upon reasonable request from the data portal of ForestGEO (http://ctfs.si.edu/datarequest/).