Abstract
Nervous systems across Animalia not only share a common blueprint at the biophysical and molecular level, but even between diverse groups of animals the structure and neuronal organization of several brain regions are strikingly conserved. Despite variation in the morphology and complexity of eyes across malacostracan crustaceans, many studies have shown that the organization of malacostracan optic lobes is highly conserved. Here, we report results of divergent evolution to this ‘neural ground pattern’ discovered in hyperiid amphipods, a relatively small group of holopelagic malacostracan crustaceans that possess an unusually wide diversity of compound eyes. We show that the structure and organization of hyperiid optic lobes has not only diverged from the malacostracan ground pattern, but is also highly variable between closely related genera. Our findings demonstrate a variety of trade-offs between sensory systems of hyperiids and even within the visual system alone, thus providing evidence that selection has modified individual components of the central nervous system to generate distinct combinations of visual centres in the hyperiid optic lobes. Our results provide new insights into the patterns of brain evolution among animals that live under extreme conditions.
Keywords: hyperiid amphipods, neuroanatomy, compound eyes, optic lobes, brain evolution
1. Introduction
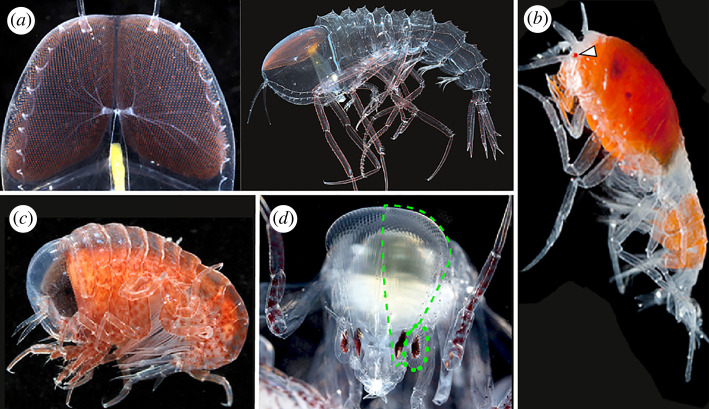
The vast column of water below the ocean's surface and above the deep-sea floor, the midwater, harbours a diverse community of poorly documented animals that display numerous adaptations to survival in this habitat like no other [1]. In the upper reaches of the midwater (100–1000 m), limited solar light penetration, an abundance of bioluminescence and the need to see without being seen have pushed the evolution of visual systems to the extreme [2]. Members of the amphipod suborder Hyperiidea (Arthropoda: Crustacea: Malacostraca) live exclusively in the midwater and exhibit a particularly impressive diversity of eye designs. These include reduced or absent eyes (figure 1b) [3], reflective eye cups [4], dorsally directed eyes covering the entire head (figure 1a) [5], eyes with dorsally and laterally directed zones (figure 1c) [5–7], replicate eye pairs (figure 1d) [5–7], eyes with 360° fields of view [8] and eyes with numerous retinas [9]. Despite the broad variation seen in hyperiid external visual structures, visual circuits and neural organization behind these eyes are largely under-investigated.
Figure 1.
Four hyperiid amphipod eye morphologies. (a) Cystisoma magna, huge dorsally directed compound eyes with a diffuse retinal sheet. Ventral view of the brain and the retinal sheet (left) and whole animal (right). (b) Lanceola sayana, tiny compound eyes (white arrowhead). (c) Hyperia galba, one pair, large, dome-like compound eyes with dorsally and laterally directed regions. (d) Phronima sedentaria, two pairs compound eyes (dashed line indicates dorsal eye, dotted indicates the lateral eye). Body lengths approximately: 8 cm C. magna (a), 1 cm L. sayana (b), 0.8 cm H. galba (c) and 1.5 cm P. sedentaria (d). (Online version in colour.)
In arthropods, visual information is relayed from photoreceptor cells in the eye to the central brain through a series of visual processing neuropils in the optic lobes. In Malacostraca (the largest class of crustaceans with approx. 40 000 extant species including shrimps, crabs, lobsters, krill, isopods and amphipods), the organization of the optic lobes is typified by a distinct ground pattern of three nested optic neuropils connected with two successive optic chiasmata (crossed axons) [10–12]. These optic neuropils are, from distal to proximal (from the eyes in), the lamina, medulla and lobula. A fourth optic neuropil, the satellite lobula plate, which is linked through uncrossed axons from the medulla and lobula, has also been identified in various groups of malacostracans [11–13]. Three putative optic lobe neuropils have also been identified in several stem-group arthropod fossils from the lower and middle Cambrian [14–16], suggesting that this ground pattern arrangement may have been evolutionarily stable for more than 500 Myr [14–16]. It is worth noting that the names used here for the malacostracan optic neuropils are adopted from, but may not be homologous to, those neuropils with the same names in insects.
The functions of malacostracan optic neuropils have been studied with electrophysiology and optical recording in the brachyuran crabs Neohelice granulata and Carcinus maenas. It was shown that neurons in the lobula are essential for computing object features [17], object motion [18–22] and encoding certain flow field information [23], while those in the lobula plate are implicated in computing wide-field motion and processing optic flow information that mediates optomotor responses [12,13]. Although the experimental evidence came only from a few model species, those functional attributes are generally assumed to apply across malacostracans based on the overwhelming structural conservation and anatomical similarity of those constituent neurons in the optic lobes [12,24].
Given the broad diversity of eye morphologies within hyperiids, how are their optic lobes organized to serve those unique eyes? Because the central nervous system is one of the most energetically expensive tissues, is an expansion in the optic lobes accompanied by a reduction in other sensory processing centres, such as the olfactory lobes? In this study, we investigate the brain organization and neural circuits that lie beneath the various hyperiid eye types. Specifically, we address whether or not the malacostracan optic lobe ground pattern remains conserved across hyperiids with different eye forms, and how the various sensory adaptations in the eyes relate to the structure and complexity of the brain.
2. Methods
(a). Animals
Specimens of Cystisoma magna and Lanceola sayana were collected with the Monterey Bay Aquarium Research Institute's remotely operated vehicle Doc Ricketts operated from the Research Vessel Western Flyer between December 2016 and September 2018. Specimens of Hyperia galba and Phronima sedentaria were collected over the same time period, from the R/V Western Flyer using a modified midwater tucker trawl (1.5 m × 1.5 m opening, 1000–200 µm mesh). ROV dives and trawls were completed over the Monterey Submarine Canyon between the surface and 1500 m depth (36° 32′ N, 122° 30′ W) from 06.00 to 00.00 h. Additional specimens of C. magna, P. sedentaria and H. galba were collected in February 2018 (POS520) and 2019 (POS532) from the submersible Jago and multinet hauls using a Hydrobios Maxi multinet (0.5 m2 in aperture, 2 mm mesh size, nine nets) aboard the R/V Poseidon operated by GEOMAR Helmholtz-Zentrum für Ozeanforschung. Specimens of Procambarus clarkii were obtained from Carolina Biological Supply, Burlington, NC. Specimens of Alima pacifica were collected at Lizard Island Research Station near Australia's Great Barrier Reef (Great Barrier Reef Marine Park Authority Permit no. G12/35005.1, Fisheries Act no. 140763), Neogonodactylus oerstedii in the Florida Keys, USA and Gammarus mucronatus, in Gloucester Point, VA, USA.
(b). Osmium-ethyl gallate staining
The staining was described in a previous study [25]. In brief, heads from live animals were detached and fixed in cacodylate fixative (2% glutaraldehyde, 1% paraformaldehyde in 0.16 M sodium cacodylate buffer) with 10% sucrose at 4°C overnight. After several washes in cacodylate buffer, brain tissue was dissected and immersed in 1% osmium tetroxide in the dark with continuous agitation for 2.5 h at 4°C and an additional 1 h at room temperature. After several washes in buffer, tissue was put in a second immersion with supersaturated ethyl gallate (approx. 1% in distilled water) in the dark with continuous agitation for 1.5 h at 4°C and an additional 30 min at room temperature. After several washes in distilled water, tissue was dehydrated, transferred into Durcupan plastic (Sigma, St. Louis, MO) via propylene-oxide and polymerized at 65°C. Blocks were serially sectioned at 12–16 µm, mounted with Permount (Electron Microscopy Science, Hatfield, PA) and covered with a cover slip for light microscopy.
(c). Immunohistochemistry
Five specimens of each of the following species, the hyperiids H. galba and P. sedentaria, the crayfish P. clarkii and the mantis shrimp N. oerstedii, were used for comparative immunolabelling of their optic lobes, following the procedures described previously [26]. In brief, brains were fixed overnight in 4% paraformaldehyde in phosphate buffer (pH 7.4) with 10% sucrose, and then washed in phosphate-buffered saline (PBS), embedded in albumin gelatin and sectioned at 60 µm with a vibratome. After being washed with PBS-TX (0.5% Triton X-100 in PBS), sections were blocked in 5% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 h and then incubated overnight in monoclonal anti-allatostatin antibody (Developmental Studies Hybridoma Bank, University of Iowa, IA) and anti-FMRFamide antibody (Immunostar, Hudson, WI) on a shaker at room temperature. The following day, sections were washed with PBS-TX and incubated overnight in the secondary goat anti-mouse immunoglobulins conjugated to Alexa Fluor 555 (3 : 1000) and goat anti-rabbit immunoglobulins conjugated to Alexa Fluor 633 (3 : 1000; Thermo Fisher Scientific, Waltham, MA). The following day, sections were washed with PBS, mounted with elvanol (25% polyvinyl alcohol, 25% glycerol and 50% PBS) and covered with a cover slip for confocal microscopy.
(d). Imaging and three-dimensional reconstructions
Osmium-ethyl gallate-stained preparations were serially imaged using an Olympus BX 63 microscope with camera, imported into Amira (6.5) and aligned in the z-plane with ‘automatic alignment’ module in Amira. Three-dimensional reconstructions were made by manually tracing the outline of each neuropil at each depth, followed by volume rendering using the ‘generate surface’ module. The size of brain, optic lobe and each optic neuropil was obtained by the ‘material statistics’ modules. Confocal reconstructions of immunolabeled optic lobes were made with a Leica SP5 laser scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL). Images of 1024 × 1024 pixel resolution at 12-bit colour depth were scanned using a 10×/0.4 Plan Apochromat objective or a 20×/0.75 PL APO CS2 objective. Selected images were digitally assembled and adjusted for brightness and contrast, and had a high pass filter uniformly applied using Adobe Photoshop CC 2019 (Adobe Systems, San Jose, CA).
3. Results
(a). Conserved organization in a non-hyperiid amphipod's optic lobes
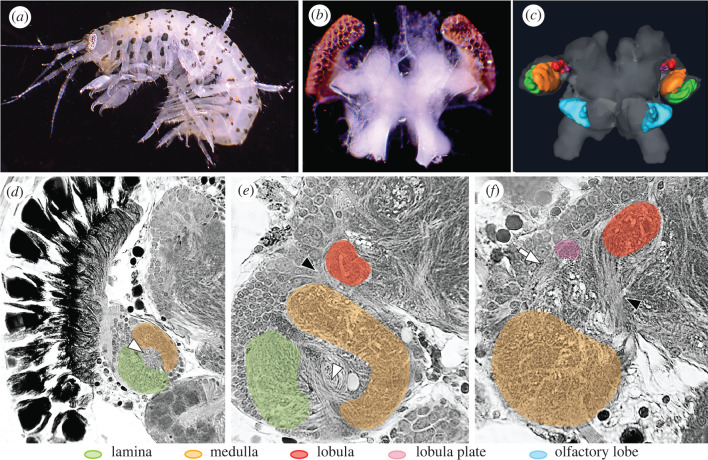
Using histology and three-dimensional brain reconstructions, we first identified the existence of the four optic neuropils with their respective characteristic optic chiasmata and uncrossed neural connections in G. mucronatus, a non-hyperiid amphipod found in intertidal marine habitats such as bays, estuaries and mangroves (figure 2). This amphipod is equipped with a typical, modest pair of compound eyes (figure 2a) and a small pair of optic lobes (figure 2c) consisting of all four expected optic neuropils (figure 2d–f), comparable to other malacostracans studied thus far. Fundamental visual processing pathways are, therefore, expected to be conserved within Amphipoda as well.
Figure 2.
The brain and optic lobe organization of the near shore, non-hyperiid amphipod, Gammarus mucronatus. (a–c) The animal, its brain and eyes, and three-dimensional reconstruction of the brain with highlighted optic and olfactory lobes. (d–f) Osmium-ethyl gallate-stained optic lobe sections in different planes showing the characteristic optic lobe first and second chiasmata (white and black arrowheads, respectively), uncrossed neural connections between medulla and lobula plate (white arrow in f), and all four optic neuropils. (Online version in colour.)
(b). Highly variable eyes and optic lobe organization among hyperiids
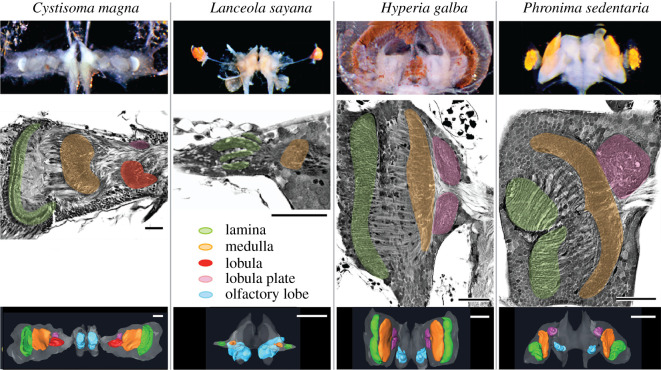
In stark contrast to Gammarus and other malacostracans, we found that hyperiid amphipods exhibit extreme variability in brain morphology, brain-to-body size and especially in the number of optic neuropils in their optic lobes. The early branching hyperiid C. magna [27–29] possesses a gigantic pair of dorsally directed eyes whose retinas consist of a thin, mesh-like sheet of suspended rhabdoms (figure 1a). Within an unusually large optic lobe (73% total brain volume, figure 3), photoreceptor axons of C. magna project to an optic lobe consisting of the same four retinotopic optic neuropils that are also found in other malacostracans (figure 3).
Figure 3.
Hyperiid optic lobe arrangements and brain morphologies. Cystisoma magna, enlarged optic lobe with all four optic neuropils as seen in other malacostracans. Lanceola sayana, reduced optic lobe with lamina and medulla only and enlarged olfactory lobes. Hyperia galba, enlarged optic lobe comprise lamina, medulla and dorsal and ventral lobula plates. Phronima sedentaria, enlarged optic lobe comprise dorsal and ventral laminas, a fused medulla and dorsal lobula plate receiving inputs solely from the dorsal half of the medulla. Scale bars, 100 µm (black) and 200 µm (white). (Online version in colour.)
The deep-living L. sayana, on the other end of the spectrum, possesses a tiny pair of eyes (figure 1b). Photoreceptor axons project to a minute optic lobe (6% total brain volume) that consists of only the lamina and medulla, lacking both the lobula and lobula plate (n = 15, figure 3) that are typical of other malacostracans. The reduction of optic neuropils in the L. sayana brain is offset by the size of the L. sayana olfactory lobe (24% total brain volume) compared to the other hyperiids examined here, whose olfactory lobes account for just 2% of total brain volume (figure 3).
Hyperia galba possesses a single pair of large dome-like eyes (figure 1c). Photoreceptor axons from each retina project to a single planar lamina. There, visual information is relayed retinotopically with second-order neurons, through the first chiasma, to a single medulla. Third-order medullary neurons then project uncrossed axons to a pair of lobula plates that are partially connected to each other (figures 3 and 4c). No second optic chiasma or lobula is present in H. galba (n = 18). Despite the absence of the lobula, H. galba optic lobes still account for 62% of total brain volume (figure 3), a number that is drastically larger than any other non-hyperiid malacostracans [30,31].
Figure 4.
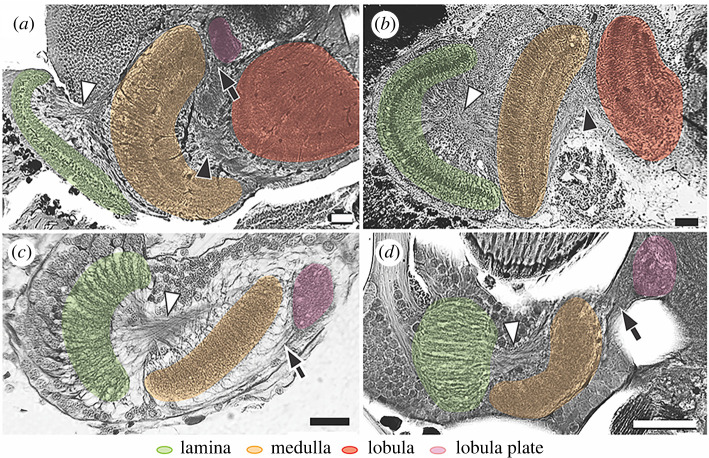
Osmium-ethyl gallate-stained optic lobe sections of various crustaceans at the antero-posterior plane showing the characteristic optic lobe first and second chiasmata (white and black arrowheads, respectively) and optic neuropils, including the structurally simplified medulla found in hyperiids. (a) Alima pacifica (mantis shrimp), (b) Procambarus clarkii (crayfish), (c) Hyperia galba (hyperiid) and (d) Phronima sedentaria (hyperiid). Black arrows indicate the uncrossed neuronal connections between medulla and lobula plate. Scale bars, 50 µm. (Online version in colour.)
Phronima sedentaria possesses two pairs of eyes (figure 1d)— a dorsally directed pair with greatly enlarged facets attached to a specialized array of long light guides [32], and a smaller pair of laterally directed eyes. Each of the four eyes has a highly condensed, darkly pigmented retina [33] (figure 1d), with photoreceptor axons connecting to one of the four lenticular-shaped laminas (figure 3). Axon output from the dorsal- and lateral-eye laminas project to a single medulla on each side (figure 3). A small neuropil lies beneath the medulla and receives uncrossed projections from the dorsal, but not the lateral region of the medulla (figures 3 and 4d). The lobula is absent. Thus, like H. galba, P. sedentaria also lacks both the second optic chiasma and the lobula (n = 24), yet the remaining optic lobes still account for 61% of total brain volume (figure 3).
(c). Additional optic neuropil simplifications
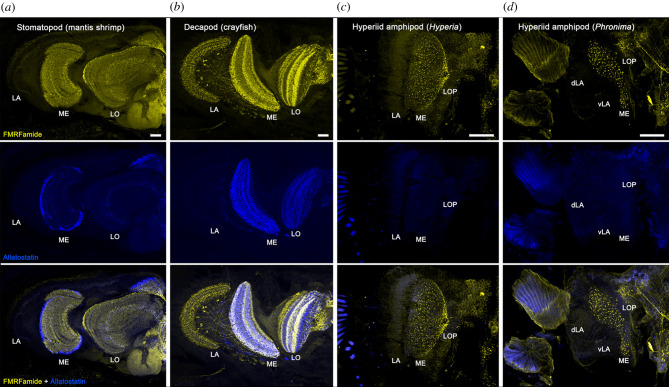
In addition to the lost optic neuropils described above, the medulla also appeared to be structurally simplified in hyperiids compared to that of other malacostracans. In all four representative hyperiids we observed a lack of neuronal stratification within optic neuropils (figures 3 and 4), an anatomical feature typifying distinct neuronal layers for serial and parallel visual processing [34]. To further demonstrate the lack of neuronal stratification in hyperiid optic neuropils, we employed immunohistochemistry with antisera against allatostatin and FMRFamide, two neuropeptides that are highly expressed in distinctive cell types of the optic lobes of malacostracans [35]. The representative stomatopod (N. oerstedii, a mantis shrimp) and decapod (P. clarkii, a crayfish) showed distinct allatostatin- and FMRFamide-like stratified immunoreactive layers in both the medulla and lobula following the expected malacostracan optic lobe ground pattern (figure 5a,b). However, in H. galba and P. sedentaria, FMRFamide-like immunoreactivity dispersed throughout the entire medulla and lobula plate without stratifications. In addition, no allatostatin immunoreactivity was found in the optic lobes of either hyperiid (figure 5c,d), although positive labelling was found in their central brains in both species. Immunolabelling data in C. magna and L. sayana were not possible because fresh specimens were not available. However, based on histology, their medullas also appear to be reduced in thickness and without clear neuronal stratifications (compare figure 3 with figure 4a,b). The absence of several morphological distinct allatostatinergic medulla neurons typically found in malacostracans [35] demonstrates a further reduction in neuronal complexity and diversity of cell types in the hyperiid optic lobes.
Figure 5.
FMRFamide-like (yellow, top row) and allatostatin-like (blue, middle row) immunoreactivity reveals distinct layers of neuronal organization in (a) a stomatopod (mantis shrimp, Neogonodactylus oerstedii) lamina (LA), medulla (ME) and lobula (LO) and in (b) a decapod (crayfish Procambarus clarkii). However, in (c) Hyperia galba and (d) Phronima sedentaria FMRFamide-like immunoreactivity is scattered throughout the entire medulla and lobula plate (LOP). No allatostatin-like immunoreactivity is detected in the hyperiid optic lobes. Scale bars, 100 µm. (Online version in colour.)
4. Discussion
Because nervous tissue is one of the most energetically expensive tissues to build and maintain, neural arrangements in a given animal group are typically highly conserved [36,37]. This is most evident when comparing the arthropod brains, where three nested optic neuropils connected with two axonal chiasmata typify the optic lobe organization of malacostracan crustaceans [10–12]. Our study nevertheless demonstrates an unusual nervous system diversification among sister taxa. Figure 6 illustrates the unusual variation found in the hyperiid optic lobes compared to all other malacostracan lineages. Our examined hyperiids, with the exception of C. magna, the earliest branching hyperiid available for this study, show losses of optic neuropils in multiple ways. Based on the finding that other malacostracans and the basal hyperiid Cystisoma are equipped with both lobula and lobula plate, and the second optic chiasma (figure 3), the most parsimonious explanation would be a loss of the lobula in H. galba, a loss of the lobula and the ventral lobula plate in P. sedentaria and a loss of both the lobula and the lobula plate in L. sayana (figures 3 and 4). In addition, given that neurons in the lobula are used for computing object features [17], object motion [18–22] and certain flow field information [23], the complete absence of the lobula in H. galba, P. sedentaria and L. sayana are significant secondary reductions of the optic lobes (figures 3 and 4). Likewise, the loss of ventral or entire lobula plate in P. sedentaria and L. sayana, respectively, are additional significant secondary reductions of the optic lobes (figures 3 and 4). Despite the loss of these optic neuropils, H. galba and P. sedentaria have substantially enlarged optic lobes (62% and 61% total brain volume, respectively, figure 3), which is similar to C. magna (73% total brain volume, figure 3) but contrasts with L. sayana (6% total brain volume, figure 3), and G. mucronatus (representative non-hyperiid amphipod, 15% total brain volume, figure 2). These findings indicate that in H. galba and P. sedentaria the remaining optic neuropils (lamina, medulla) are enlarged in size to compensate for the loss of the lobula (and partial lobula plate in P. sedentaria). In L. sayana, on the other hand, the dramatic reduction of optic lobes is compensated for by other sensory modalities, as discussed below.
Figure 6.
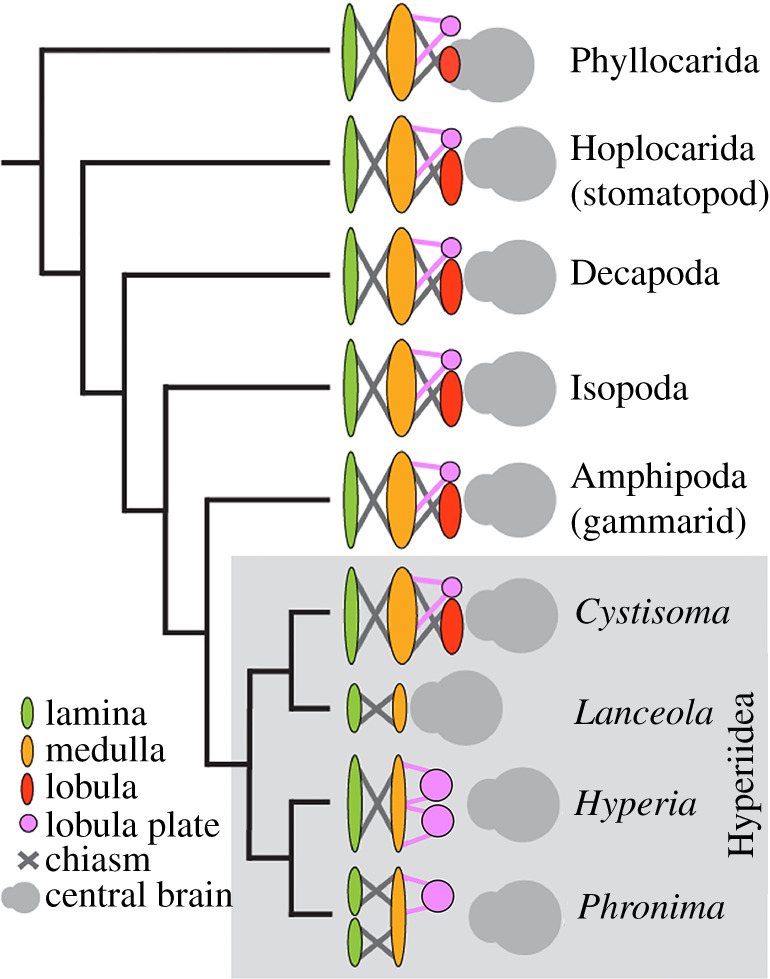
Optic lobe organization in malacostracan crustaceans with compound eyes. Hyperiid amphipods exhibit dramatic variation in the number of optic neuropils unlike all other known malacostracans, including all previously known amphipods [10–12]. Phylogeny modified from [27,38]. (Online version in colour.)
(a). Ecological implications
The unexpected variability in hyperiid optic lobes may be due to the unique set of selective pressures that act on organisms living in the midwater. The high predatory pressure, limited illumination and a highly structured mesopelagic light regime, absence of substrate and hence hiding places and limited food availability, has likely driven the adaptive radiation that resulted in the extreme diversity of lifestyles and eye forms among close hyperiid relatives (electronic supplementary material, table S1). Observed differences in depth of occurrence, swimming ability, free-living or host-associated lifestyles, body size and crypsis create different visual needs for each hyperiid group, and these differences may have driven the evolution of dramatically different eyes and subsequently the visual circuits in the brains that support those eyes. Given the large proportion of energy dedicated to the development and maintenance of their greatly enlarged eyes, it is not surprising to find that a similarly disproportionate amount of central brain tissue is also dedicated to vision. In addition, finding that there is a clear trade-off between the allocation of peripheral and central nervous system tissue to one sensory modality over another is not unexpected. What is unexpected is the degree to which the central brain tissues have been selectively modified within different hyperiids to focus on specific visual needs. These modifications are seen as the reduced complexity of the medulla in all hyperiids examined here (figures 3–5), the loss of the lobula in L. sayana, H. galba and P. sedentaria that may compromise their ability to distinguish object features and object motion, and the loss of the lobula plate in L. sayana and the ventral lobula plate in P. sedentaria that may limit their ability to process wide-field motion. The loss of the ability to distinguish an object's features and motion would seem detrimental to finding prey or a host, nevertheless, all three species lacking the lobula in this study are often observed free-swimming—an indication of prey searching. Do they simply attack anything they can catch? Given that the principal prey of H. galba and L. sayana are gelatinous zooplankton including slow-moving jellies and pelagic tunicates, this may not be a bad strategy. Phronima sedentaria, on the other hand, feeds additionally on other hyperiids and invertebrates. Behavioural work is needed to determine how they recognize and track their prey. One possibility would be the exploitation of the lobula plate, which is peculiarly enlarged in association with the enlarged eyes of H. galba and the dorsal eyes of P. sedentaria (figures 3 and 4). While the abundant marine snow, other plankton and bioluminescent point sources in the surrounding packet of water would provide the needed visual flow field feedback for navigation, moving objects against this flow-field background can potentially be detected using the same neural circuitry, as shown in the wide-field motion-sensitive neurons found in the modified lobula plates of several predatory larval insects in support of their prey hunting [39,40].
(b). Brain evolution in the midwater
Examples of sensory modality compensation have been observed in animals living in light-limited environments, such as in the deep sea or caves. For example, the loss of eyes in many cave-dwelling animals is compensated by enlarged tactile and/or olfactory organs. In the brain, likewise, the eyeless amphipod Niphargus puteanus and other blind peracarids from cave habitats show reduced optic lobes, with complete losses of lamina and medulla, but extensively elaborated olfactory and mechanosensory neuropils [41,42]. The hyperiid brains examined in this study show a similar compensatory pattern between optic and olfactory lobes. While the huge eyes and enlarged optic lobes in C. magna, H. galba and P. sedentaria are accompanied by much smaller olfactory lobes, the reduction of optic neuropils in L. sayana is offset by the enlargement of their olfactory lobes (figure 3). These results provide the first support for sensory modality compensation in deep-sea hyperiids.
In addition to the sensory system trade-offs between vision and olfaction, our findings indicate a variety of trade-offs even within the hyperiid visual system. While their eyes and optic lobes are greatly enlarged, they have eliminated selected visual processing centres (lobula and lobula plate) and reduced neuronal complexity in the remaining visual centres (figures 3–6). Two prominent hypotheses have been proposed to explain the variation in brain structures seen among mammalian lineages. The ‘concerted brain evolution’ hypothesis states that the brain evolves as a single unit and correlated changes between major brain regions exist due to developmental constraints, suggesting that natural selection cannot act independently on individual brain regions [43]. Our observation of the changes to the size and complexity of the hyperiid visual systems directly contradict the ‘concerted brain evolution’ hypothesis. Alternatively, our findings support the ‘mosaic brain evolution’ hypothesis, which postulates that different brain regions can evolve independently of each other [44]. In hyperiids, we see that strong selective pressure has individually increased eye and selected optic neuropil sizes but decreased neuronal complexity within neuropils and eliminated other optic neuropils. These changes should yield increased sensitivity, higher contrast and, in H. galba, better wide-field motion vision, but reduced object recognition. Our findings thus provide new insights into the patterns of brain evolution and sensory adaptation among animals that live in extreme habitats such as the largest living space on the planet, the deep pelagic realm or the midwater.
Supplementary Material
Acknowledgements
We thank Bruce Robison and Kakani Katija for the invitations to participate in MBARI field expeditions, the crew of MBARI's R/V Western Flyer and GEOMAR's Poseidon, the pilots of the ROV Doc Ricketts and the Jago Team and Stephanie Bush, Kyra Schlining, Kate Thomas, Kim Reisenbichler and Robert Sherlock for providing assistance with specimen collection. We also thank Freya Goetz, Scott Whittaker and Tagide DeCarvalho for microscopy and imaging support.
Data accessibility
All data are included in the manuscript.
Authors' contributions
C.L. and K.J.O. conceptualized and designed the study. C.L. carried out the study. C.L. and K.J.O. analysed the results and wrote the manuscript. H.-J.T.H. provided access to live specimens and edited the manuscript. T.W.C. provided research space, equipment and edited the manuscript. All authors approved the final manuscript.
Competing interests
The authors declare no competing interests.
Funding
C.L. was supported by a Smithsonian Peter Buck Postdoctoral Fellowship and SI-NMNH Fenner Chase and Mary Jane Rathbun Crustacean Endowments. H.-J.T.H. was funded by the German Research Foundation's Emmy Noether Junior Research Group grant (HO 5569/2-1) and GEOMAR's POF III.
References
- 1.Robison BH. 2004. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 300, 253-272. ( 10.1016/j.jembe.2004.01.012) [DOI] [Google Scholar]
- 2.Warrant EJ, Locket NA. 2004. Vision in the deep sea. Biol. Rev. 79, 671-712. ( 10.1017/S1464793103006420) [DOI] [PubMed] [Google Scholar]
- 3.Zeidler W. 2012. A review of the hyperiidean amphipod families Mimonectidae and Proscinidae (Crustacea: Amphipoda: Hyperiidea: Scinoidea). Zootaxa 3533, 1-74. ( 10.11646/zootaxa.3533.1.1) [DOI] [Google Scholar]
- 4.Land MF. 2000. Eyes with mirror optics. J. Opt. A: Pure Appl. Opt. 2, R44-R50. ( 10.1088/1464-4258/2/6/204) [DOI] [Google Scholar]
- 5.Land MF, Nilsson D-E. 2012. Animal eyes, 2nd ed. New York, NY: Oxford University Press. [Google Scholar]
- 6.Land MF. 1981. Optics and vision in invertebrates. In Handbook of sensory physiology (ed. Autrum H), pp. 471-592. Berlin, Germany: Springer. [Google Scholar]
- 7.Land MF. 1989. The eyes of hyperiid amphipods: relations of optical structure to depth. J. Comp. Physiol. A 164, 751-762. ( 10.1007/BF00616747) [DOI] [Google Scholar]
- 8.Meyer-Rochow VB. 1978. The eyes of mesopelagic crustaceans, II. Streetsia challenger (Amphipoda). Cell Tissue Res. 186, 337-349. ( 10.1007/bf00225542) [DOI] [PubMed] [Google Scholar]
- 9.Baldwin Fergus JL, Johnsen S, Osborn KJ. 2015. A unique apposition compound eye in the mesopelagic hyperiid amphipod Paraphronima gracilis. Curr. Biol. 25, 473-478. ( 10.1016/j.cub.2014.12.010) [DOI] [PubMed] [Google Scholar]
- 10.Elofsson R, Dahl E. 1970. The optic neuropiles and chiasmata of Crustacea. Z. Zellforsch. Mikrosk. Anat. 107, 343-360. ( 10.1007/BF00336672) [DOI] [PubMed] [Google Scholar]
- 11.Sinakevitch I, Douglass JK, Scholtz G, Loesel R, Strausfeld NJ. 2003. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J. Comp. Neurol. 467, 150-172. ( 10.1002/cne.10925) [DOI] [PubMed] [Google Scholar]
- 12.Strausfeld NJ. 2005. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct. Dev. 34, 235-256. ( 10.1016/j.asd.2005.04.001) [DOI] [Google Scholar]
- 13.Bengochea M, Beron de Astrada M, Tomsic D, Sztarker J. 2018. A crustacean lobula plate: morphology, connections, and retinotopic organization. J. Comp. Neurol. 526, 109-119. ( 10.1002/cne.24322) [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258-261. ( 10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 15.Strausfeld NJ, Ma X, Edgecombe GD. 2016. Fossils and the evolution of the arthropod brain. Curr. Biol. 26, R989-R1000. ( 10.1016/j.cub.2016.09.012) [DOI] [PubMed] [Google Scholar]
- 16.Aria C, Caron JA. 2019. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586-589. ( 10.1038/s41586-019-1525-4) [DOI] [PubMed] [Google Scholar]
- 17.Medan V, Oliva D, Tomsic D. 2007. Characterization of lobula giant neurons responsive to visual stimuli that elicit escape behaviors in the crab Chasmagnathus. J. Neurophysiol. 98, 2414-2428. ( 10.1152/jn.00803.2007) [DOI] [PubMed] [Google Scholar]
- 18.de Astrada MB, Tomsic D. 2002. Physiology and morphology of visual movement detector neurons in a crab (Decapoda: Brachyura). J. Comp. Physiol. A 188, 539-551. ( 10.1007/s00359-002-0328-4) [DOI] [PubMed] [Google Scholar]
- 19.de Astrada MB, Bengochea M, Sztarker J, Delorenzi A, Tomsic D. 2013. Behaviorally related neural plasticity in the arthropod optic lobes. Curr. Biol. 23, 1389-1398. ( 10.1016/j.cub.2013.05.061) [DOI] [PubMed] [Google Scholar]
- 20.Medan V, de Astrada MB, Scarano F, Tomsic D. 2015. A network of visual motion-sensitive neurons for computing object position in an arthropod. J. Neurosci. 35, 6654-6666. ( 10.1523/JNEUROSCI.4667-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarano F, Sztarker J, Medan V, de Astrada MB, Tomsic D. 2018. Binocular neuronal processing of object motion in an arthropod. J. Neurosci. 38, 6933-6948. ( 10.1523/JNEUROSCI.3641-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarano F, Tomsic D, Sztarker J. 2020. Direction selective neurons responsive to horizontal motion in a crab reflect an adaptation to prevailing movements in flat environments. J. Neurosci. 40, 5561-5571. ( 10.1523/JNEUROSCI.0372-20.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horseman BF, Macauley MWS, Barnes WJP. 2011. Neuronal processing of translational optic flow in the visual system of the shore crab Carcinus maenas. J. Exp. Biol. 214, 1586-1598. ( 10.1242/jeb.050955) [DOI] [PubMed] [Google Scholar]
- 24.Tomsic D. 2016. Visual motion processing subserving behavior in crabs. Curr. Opin. Neurobiol. 41, 113-121. ( 10.1016/j.conb.2016.09.003) [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Cronin TW. 2018. Two visual systems in one eyestalk: the unusual optic lobe metamorphosis in the stomatopod Alima pacifica. Dev. Neurobiol. 78, 3-14. ( 10.1002/dneu.22550) [DOI] [PubMed] [Google Scholar]
- 26.Lin C, Strausfeld NJ. 2013. A precocious adult visual center in the larva defines the unique optic lobe of the split-eyed whirligig beetle Dineutus sublineatus. Front. Zool. 10, 7. ( 10.1186/1742-9994-10-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurt C, Haddock SHD, Browne WE. 2013. Molecular phylogenetic evidence for the reorganization of the hyperiid amphipods, a diverse group of pelagic crustaceans. Mol. Phylogenet. Evol. 67, 28-37. ( 10.1016/j.ympev.2012.12.021) [DOI] [PubMed] [Google Scholar]
- 28.Copilaş-Ciocianu D, Borko Š, Fišer C. 2020. The late blooming amphipods: global change promoted post-Jurassic ecological radiation despite Palaeozoic origin. Mol. Phylogenet. Evol. 143, 106664. ( 10.1016/j.ympev.2019.106664) [DOI] [PubMed] [Google Scholar]
- 29.Biancani LM. 2019. Multi-locus phylogenetic analysis of Amphipoda indicates a single origin of the pelagic suborder Hyperiidea. College Park, MD: University of Maryland. [Google Scholar]
- 30.Sandeman DC, Scholtz G, Sandeman RE. 1993. Brain evolution in decapod Crustacea. J. Exp. Zool. 265, 112-133. ( 10.1002/jez.1402650204) [DOI] [Google Scholar]
- 31.Krieger J, Hornig MK, Sandeman RE, Sandeman DC, Harzsch S. 2020. Masters of communication: the brain of the banded cleaner shrimp Stenopus hispidus (Olivier, 1811) with an emphasis on sensory processing areas. J. Comp. Neurol. 528, 1561-1587. ( 10.1002/cne.24831) [DOI] [PubMed] [Google Scholar]
- 32.Ball EE. 1977. Fine structure of the compound eyes of the midwater amphipod Phronima in relation to behavior and habitat. Tissue Cell 9, 521-536. ( 10.1016/0040-8166(77)90010-6) [DOI] [PubMed] [Google Scholar]
- 33.Land MF. 1981. Optics of the eyes of Phronima and other deep sea amphipods. J. Comp. Physiol. 145, 209-226. ( 10.1007/BF00605034) [DOI] [Google Scholar]
- 34.Millard SS, Pecot MY. 2018. Strategies for assembling columns and layers in the Drosophila visual system. Neural. Dev. 13, 11. ( 10.1186/s13064-018-0106-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meth R, Wittfoth C, Harzsch S. 2017. Brain architecture of the Pacific white shrimp Penaeus vannamei Boone, 1931 (Malacostraca, Dendrobranchiata): correspondence of brain structure and sensory input? Cell Tissue Res. 369, 255-271. ( 10.1007/s00441-017-2607-y) [DOI] [PubMed] [Google Scholar]
- 36.Karten HJ, Shimizu T. 1989. The origins of neocortex: connections and lamination as distinct events in evolution. J. Cogn. Neurosci. 1, 291-301. ( 10.1162/jocn.1989.1.4.291) [DOI] [PubMed] [Google Scholar]
- 37.Shanahan M, Bingman VP, Shimizu T, Wild M, Güntürkün O. 2013. Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 7, 89. ( 10.3389/fncom.2013.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwentner M, Richter S, Rogers DC, Giribet G. 2018. Tetraconatan phylogeny with special focus on Malacostraca and Branchiopoda: highlighting the strength of taxon-specific matrices in phylogenomics. Proc. R. Soc. B 285, 20181524. ( 10.1098/rspb.2018.1524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizutani A, Toh Y. 1995. Optical and physiological properties of the larval visual system of the tiger beetle, Cicindela chinensis. J. Comp. Physiol. A 177, 591-599. ( 10.1007/BF00207188) [DOI] [Google Scholar]
- 40.Okamura JY, Toh Y. 2004. Morphological and physiological identification of medulla interneurons in the visual system of the tiger beetle larva. J. Comp. Physiol. A 190, 449-468. ( 10.1007/s00359-004-0509-4) [DOI] [PubMed] [Google Scholar]
- 41.Stegner ME, Stemme T, Iliffe TM, Richter S, Wirkner CS. 2015. The brain in three crustaceans from cavernous darkness. BMC Neurosci. 16, 19. ( 10.1186/s12868-015-0138-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramm T, Scholtz G. 2017. No sight, no smell? — Brain anatomy of two amphipod crustaceans with different lifestyles. Arthropod Struct. Dev. 46, 537-551. ( 10.1016/j.asd.2017.03.003) [DOI] [PubMed] [Google Scholar]
- 43.Finlay BL, Darlington RB. 1995. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578-1584. ( 10.1126/science.7777856) [DOI] [PubMed] [Google Scholar]
- 44.Barton RA, Harvey PH. 2000. Mosaic evolution of brain structure in mammals. Nature 405, 1055-1058. ( 10.1038/35016580) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.