Abstract
The byssus is a structure unique to bivalves. Byssal threads composed of many proteins extend like tendons from muscle cells, ending in adhesive pads that attach underwater. Crucial to settlement and metamorphosis, larvae of virtually all species are byssate. By contrast, in adults, the byssus is scattered throughout bivalves, where it has had profound effects on morphological evolution and been key to adaptive radiations of epifaunal species. I compare byssus structure and proteins in blue mussels (Mytilus), by far the best characterized, to zebra mussels (Dreissena polymorpha), in which several byssal proteins have been isolated and sequenced. By mapping the adult byssus onto a recent phylogenomic tree, I confirm its independent evolution in these and other lineages, likely parallelisms with common origins in development. While the byssus is superficially similar in Dreissena and Mytilus, in finer detail it is not, and byssal proteins are dramatically different. I used the chromosome-scale D. polymorpha genome we recently assembled to search for byssal genes and found 37 byssal loci on 10 of the 16 chromosomes. Most byssal genes are in small families, with several amino acid substitutions between paralogs. Byssal proteins of zebra mussels and related quagga mussels (D. rostriformis) are divergent, suggesting rapid evolution typical of proteins with repetitive low complexity domains. Opportunities abound for proteomic and genomic work to further our understanding of this textbook example of a marine natural material. A priority should be invasive bivalves, given the role of byssal attachment in the spread of, and ecological and economic damage caused by zebra mussels, quagga mussels and others.
This article is part of the Theo Murphy meeting issue ‘Molluscan genomics: broad insights and future directions for a neglected phylum’.
Keywords: byssal threads, Dreissena polymorpha, bivalve genome
1. Introduction
The byssus is an attachment structure made of protein filaments; a character unique to bivalve molluscs and key to their success. Stretchable, strong and shock absorbing, it is unlike the silks of spiders, silkworms or caddisflies, because its non-living fibres are attached directly to living cells, rather than being deposited in a detached structure. The mussel byssal thread, one of ‘… nature's most peculiar tendons…’ [1, p. 1830], at one end is bundled fibres attached to the membranes of retractor muscle cells, at the other end an adhesive pad that affixes it to underwater substrates.
The adult byssus is scattered throughout the bivalves, where it has been modified to function in a wide variety of habitats. Stanley [2] concluded that the byssus first served in bivalves as an anchor in infaunal species, then later spread slowly, driving adaptive radiations of species that adopted it for hard substrate attachment. The trait is widespread in their modern descendants, especially in the Pteriomorphia, such as blue mussels, ark clams and many scallops. It is absent from all adult true oysters (Ostreidae) [3], where the larval shell cements to substrate and the adult remains so [4]. It is also absent from clay-, rock- or wood-boring piddocks (Pholadidae) and shipworms (Teredinidae), but rock-boring date mussels (Mytilidae) use it to anchor within their boreholes [5]. Most but not all soft sediment-burrowing species lack an adult byssus, including most venerids [6], but a byssus anchoring into gravel and sand occurs in the pen shells (Pinnidae), and in scattered members of Subclass Imparidentia such as Corbula and Varicorbula (Corbulidae) [7], Sphenia binghami (Myidae), Kelliella (Kelliellidae), Kellia and Lasaea (Lasaeidae) [8,9] and several infaunal cockles (Cardiidae) [10].
Yonge emphasized the initial functions of the byssus in larvae at settlement [3]. The larval foot secretes a single byssal filament that the crawling larva drags behind, anchoring it for the duration of metamorphosis. This is the ‘pediveliger’ stage in which the foot first appears; the locomotory velum is still functional. Histological studies of the pediveliger foot reveal a series of glands, including secretory byssal glands, homologous across species that span the full range in development of the adult structure—from oyster Ostrea edulis (foot lost and shell cemented at metamorphosis) to scallop Pecten maximus (byssus lost in swimming adult) to blue mussel Mytilus edulis (byssus well developed) [11]. Regardless of adult life habit, larvae of all bivalve species appear to be byssate [3] with the following few exceptions. Earlier reports to the contrary, species in the basal subclass Protobranchia appear to lack byssus and byssal glands [12]. Larvae of some paleoheterodonts form a larval thread; in some like Mutela [13] a very long filament [14], but the structure is not homologous to the byssus [15].
After metamorphosis and loss of the velum, a byssate, benthic stage known as the plantigrade occurs in most bivalves, with exceptions including oysters and shipworms [16]. In some cases, the plantigrade byssus appears briefly, but is necessary to settle and initiate benthic life. This is true for pediveligers of the clay-boring Zirfaea (Pholadidae) and wood-boring Bankia (Teredinidae) [3]. Byssal plantigrades of the hard clam Mercenaria mercenaria lack the mobility needed to burrow, so they attach using the byssus, then soon after lose it and develop the burrowing morphology of the sedentary juvenile [17]. By contrast, plantigrades of steamer clams Mya arenaria use their byssus and foot as highly active burrowers to a size of greater than 1 cm [16]. Plantigrades of Mytilus edulis byssally attach first to filamentous algae or eelgrass, then detach and move to hard substrate in mussel beds where they reattach with byssal threads as juveniles [18,19]. Planktonic dispersal to this secondary settlement site is aided by a byssal sail produced by stem glands in the foot [19]. Cases of postlarval byssal drifting have been found in at least 22 other families outside the mytilids [16]. Hence, during this complex and sometimes lengthy transition between larva and final settlement, the byssus plays several critical roles.
Byssus structure has become highly modified throughout adult bivalves [20], particularly in the Pteriomorphia. The adult mussel byssus consists of multiple threads, each 0.2 mm in diameter or less, bundled into a stem: at the proximal end rooted in the foot, at the distal end terminating in attachment plaques. Mytilus has 50–100 threads organized this way. Ark shells (Arcidae) and pen and fan shells (Pinnidae) lack a stem [21,22]. In fan shells (the source of sea silk), the threads converge near the foot, and in ark shells the threads are fused into a sheet or plug-like structure. Instead of threads, the byssus in scallops consists of ribbons that lack attachment plaques. These are secreted in sedentary cavity-nestlers, temporary byssal-attachers and a few highly mobile species [23]. Outside the Pteriomorphia, other notable modifications include the single byssal thread, highly calcified, of jingle shells (Anomiidae) [24].
The byssal attachment has led to dramatic modifications to adult morphology. The most familiar of these is the heteromyarian form. This is the term used for the evolution of unequally sized adductor muscles—posterior enlarged relative to anterior—from the ancestral state of equally sized. Stanley [2] examined the evolution and functional consequences of heteromyarianism and correlated traits in the mytilids. Heteromyarian musculature evolves in concert with a triangular, wedge-shaped shell and the shifting of the byssal retractor muscles that control tensioning of the threads towards the posterior, to lie above the byssus. Species with less reduced anterior adductors have more ovoid shells, the byssal retractors are lateral to the byssus (as in the horsemussel Modiolus), and there exists a graded transformation in these designs in the family. The result in Mytilus is the generation of the greatest downward attachment force, adaptive in its wave swept environment, with less force in Modiolus, where structural support is provided in part by the sediment and marsh grasses among which it lives. Heteromyarian form has also evolved along a convergent series of character transformations in the byssate dreissenids (zebra mussels, quagga mussels, Mytilopsis), unrelated species in a different subclass (Imparidentia) [25].
Ideas about the evolution of the adult byssus have been strongly influenced by Yonge, who regarded it as a neoteny—the retention of a juvenile trait in the adult [3,26]. Stanley [2] and Morton [25] suggested that independent events of neoteny accounted for the repeated appearance of the trait in adults throughout bivalve phylogeny. More recently, biomechanical, ultrastructural and biochemical analyses of the byssus structure have demonstrated similarities, as well as pronounced differences in this structure outside of the Mytilidae. In this paper, I review some of these findings, with emphasis on the byssus of the zebra mussel, Dreissena polymorpha, the most well studied apart from Mytilus. I also examine new insights from our work on the byssal genes in the zebra mussel genome, comparing them to Mytilus and to the quagga mussel, D. rostriformis. Then I revisit some questions of broader significance to bivalve biology, and examine some avenues for future work.
2. Results and discussion
(a). The adult byssus mapped onto bivalve phylogeny
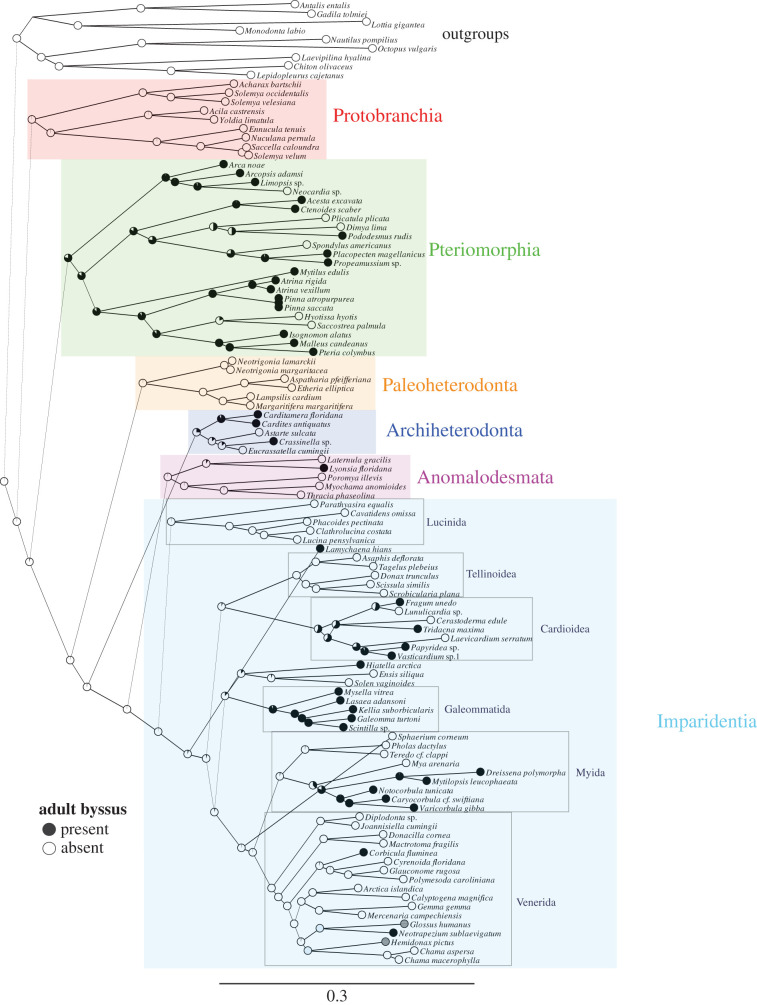
We begin by examining the character of the adult byssus, mapped onto a recent phylogenomic tree of the bivalves [27] (figure 1). At the outset, I will mention a few caveats. First, while the analysis of deeply sequenced transcriptomes provides robust support for major clades [27] relevant to the topic at hand, the study examined only 99 bivalve species. As a result, the resolution of evolutionary transitions will be limited. Second, the reader should be aware that some exceptions (e.g. byssate paleoheterodont species [28,29]) are missing from this phylogeny. And third, we examine a single trait, so cannot evaluate correlations between the presence of the byssus and other characters, as was thoroughly addressed in scallops [23] and ark clams [30].
Figure 1.
Phylogenetic history of the evolution of the adult byssus in bivalves. The tree is based on the phylogenomic analysis of Lemer et al. [27]. Branch lengths are proportional to genetic distances from maximum likelihood. Pie diagrams at internal and terminal nodes represent likelihoods of ancestral states based on the Mk1 model in Mesquite. Major clades are labelled in colour and minor clades are labelled within the Imparidentia. (Online version in colour.)
The tree demonstrates what Yonge and Stanley concluded to be a scattered pattern of byssus presence across bivalve phylogeny, and it provides support for independent evolutionary events of both the gain and loss of the adult byssus. Examples of the latter exist in Pteriomorphia, in which an adult byssus is predominant—Spondylus americanus (Spondylidae) and Plicatula plicata (Plicatulidae) appear to have lost the trait present in byssate ancestors. Stanley's analysis of the fossil record [2] considered these to be reversions to ancestral states. An adult byssus is far less widespread across Imparidentia, which includes several lineages in which a byssus has evolved from non-byssate ancestors—the Cardiidae (cockles and giant clams), the Galeommatida (clams such as Kellia) and the Myida, a diverse lineage that includes shipworms and piddocks (non-byssate), the Myidae (non-byssate clams like Mya), the Dreissenidae and Corbulidae (both byssate). Along this branch, the dreissenids are a story of remarkable evolutionary transformation, from infaunal marine ancestors through diversification of four modern genera (including the subterranean Congeria) in fresh and marine habitats [31,32], through the biological invasions of Dreissena and Mytilopsis, all aided by independent evolution of the adult byssus and heteromyarian form [25].
(b). Comparisons of byssus structure across bivalve families
Biochemical, ultrastructural, biophysical and mechanical studies, pioneered by Waite and colleagues, have made the Mytilus byssus the most well characterized of any marine natural material. The structure consists of 25–30 proteins (table 1 shows the 10 most prominent) in highly organized, structurally differentiated stem, thread and plaque regions [35]. The threads consist mainly of three different collagen-based precursors (preCOLs), each with protein building blocks of a collagenous core and two flanking domains, composed of either a soft (elastin-like), a stiff (silk fibroin-like) or an intermediate (amorphous polyglycine) chain [36]. The distribution of the three preCOLs creates a proximal to distal gradient of stiffness and extensibility (ability to stretch without breaking), and a mechanical transition from living to non-living material. In the attachment plaques, several divergent mussel foot proteins (Mfps), encoded by polymorphic gene families and then processed by alternative splicing, mediate adhesion underwater [35]. Only one of these—Mfp-1, a major protein of the byssus cuticle—is not confined to the plaque. These are proteins with high pI values, rich in repetitive low complexity domains (RLCDs). Post-translationally modified tyrosine residues (3,4-dihydroxyphenyl-l-alanine, or DOPA), uncommon elsewhere in animals, are particularly abundant in the plaque proteins, ranging from 2 to 28 mol%. DOPA has multiple functions, ranging from cross-linking for thread structural rigidity, to adhesion of plaques to metal oxide surfaces. For a readable review of the material science of mussel byssus proteins, see Waite et al. [37]. Recent transcriptome sequencing has added 15 transcripts expressed in the foot and secreted as byssal proteins to the list for further study [38].
Table 1.
Comparison of byssal proteins.
| byssal protein | species | mass (kDa) | pIb | DOPA (mol%) | sequence or consensus repeatc | repeat lengthd | featurese |
|---|---|---|---|---|---|---|---|
| A. Blue mussels Mytilus | |||||||
| Mfp-1 | Med | 108 | 10.5 | 15 | [AKPSYO*OTYK]n | 750 | protein in cuticle coating threads and plaques |
| Mfp-2 | Mgallo | 45 | 9.5 | 5 | [TDKAYKPNPCVVSKPCKNRGKCIWNGKAYRCKCAYGYGGRHC]n | 417 | 25% of plaque protein mass; EGF-like repeats |
| Mfp-3 | Med | 6 | 20 | ADYYGPNYGPPRRYGGGNYNRYNRYGRRYGGYKGWNNGWNRGRRGKYW | 0 | highly polymorphic plaque protein | |
| Mfp-4 | Mcal | 93 | 2 | [HVHTHRVLHK]n, [DDHVNDIAQTA]n | 533 | large plaque protein | |
| Mfp-5 | Med | 9 | 9 | 28 | SSEEYKGGYYPGNAYHYSGGSYHGSGYHGGYKGKYYGKAKKYYYKYKNSGKYKYLKKARKYHRKGYKYYGGSS | 14 | DOPA-rich plaque protein; low polymorphism |
| Mfp-6 | Mcal | 11 | 9.5 | 3 | GGGNYRGYCSNKGCRSGYIFYDNRGFCKYGSSSYKYDCGNYACLPRNPYGRVKYYCTKKYSCPDDFYYYNNKGYYYYNDKDYGCFNCGSYNGCCLRSGY | 0 | donates electrons to restore DOPA and surface binding proteins at the plaque interface |
| Tmp-1 | Mgallo | 57 | 9.5 | 3 | [YGYGNIYGYNGYGNGKTNIIVNKSGYGYGNGYGYGNGYIYYG]n, [YGNGKTTKIVVNKGNGYGYD]n | 270 | thread matrix protein separating collagen fibres |
| PreCOL-P | Med | 261 | 12.1 | <1 | [GX1X2]n | 435 | elastic protein in proximal thread; collagen flanked by elastin domains |
| PreCOL-D | Med | 240 | 10.5 | <1 | [GX1X2]n, [A]n | 528 | stiff protein in distal thread; collagen flanked by silk fibroin-like domains |
| PreCOL-NG | Med | 230 | 7.5 | <1 | [GX1X2]n | 429 | medium stiffness protein with non-graded distribution in threads; collagen flanked by glycine rich domains |
| byssal protein | mass (kDa) | pI | DOPA (mol%) | repeat consensus sequence | repeat length | featuresf | |
|---|---|---|---|---|---|---|---|
| B. Zebra mussels Dreissena polymorpha | |||||||
| Dpfp1 | 49.3 | 5.2 | ∼7 | [PEYPTPSKYPVYPDQSPAYPNQY]n, [DKKPGPYDYDGPY]n, [KKPNPYGTDWQYDKKTGPYVPDKPDD]n,[DKKPGPYDYDGPY]n,[KTPEYPTKVPEYPTKDPTYPTF]n | 279 | block copolymer structure of basic N-terminal and acidic C-terminal repeats; 17 exons, 9 of which also encode Dpfp6 | |
| Dpfp2 | 15.3 | 9.0 | ∼7 | [KTY(P/E)AYPTK(Q/D)SYPVYPEKKYTE]n | 100 | basic with no block structure | |
| Dpfp5 | 20.1 | 6.4 | ? | [SYPAYPPKQ]n,[NAVGGKGNDVGEQG]n, [QPQ]n | 87 | block structure of N-terminal and acidic C-terminal regions | |
| Dpfp7α | 11.2 | 6.5 | ? | [VGSQGNSVDGYGNA]n | 50 | some similarity to Dpfp5; paralogs located on four chromosomes | |
| Dpfp7β | 11.0 | 8.7 | ? | [VGSQGNDVRGYGNA]n | 30 | ||
| Dpfp7δ | 8.7 | 8.7 | ? | — | 0 | ||
| Dpfp8 | 6.9 | 9.5 | ? | — | 0 | non-repetitive; possible plaque protein | |
| Dpfp9α | 6.8 | 9.3 | ? | [YGYGGN]n | 28 | glycine-rich with some similarity to other glycine-rich invertebrate proteins; 14 loci, most are clustered on chromosome 5 | |
| Dpfp9β | 6.8 | 8.8 | ? | [YGYGGN]n,[NYGDYD]n | 43 | ||
| Dpfp10 | 6.7 | 8.8 | ? | — | 0 | cysteine-rich, possible plaque proteins | |
| Dpfp11α | 6.4 | 7.6 | ? | — | 0 | cysteine-rich, possible plaque proteins | |
| Dpfp11β | 6.1 | 8.0 | ? | — | 0 | ||
| Dpfp12 | 4.1 | 9.6 | ? | [YPSYPDKK]n | 17 | possible cuticle protein | |
aSpecies are Mytilus californianus (Mcal), M. edulis (Med), and M. galloprovincialis (Mgallo).
bIsoelectric point (pI).
cFull sequence is provided for Mfp-3 and Mfp-6; all others are consensus repeat sequences. Posttranslational modifications to amino acids in repeat sequences are trans-4-hydroxyproline (O), trans-2,3-cis-3,4-dihydroxyproline (O*), DOPA (Y), 4-hydroxyarginine (R), and O-phosphoserine (S). X = any amino acid.
dLength of entire repeat region (number of residues).
eData from Lee et al. [32].
A much smaller but growing literature exists on byssal threads outside of the mytilids. Analyses of protein composition and organization of the fibres using X-ray diffraction and electron microscopy have shown, not surprisingly (given differing ecological functions and deep evolutionary divergence), that structures in other taxa differ dramatically from Mytilus. The pearl oyster Pinctada fucata (Margaritidae) attaches its byssus to interstices in coral reef habitats where it is exposed to calmer water flow, and the fan shells Atrina pectinata and Pinna nobilis (Pinnidae) use their byssus to anchor their massive shells (from 0.5 to 1 m in length, respectively) into sandy substrates. The pinnids produce a large number (up to 30 000) of small and thin threads that are used in entangling (instead of attaching), whereas the pearl oyster has fewer, much shorter and thicker (attaching) threads. These three species lack the collagenous byssal proteins of blue mussels, and the structural arrangement is of helical fibrils imbedded in an unstructured matrix, very different from the units of block copolymers in blue mussels [39]. Analysis of Pinctada fucata byssus [21] found a protein (and its mRNA) highly expressed in foot with multiple thrombospondin-1 repeats (as in extracellular calcium-binding glycoproteins in fungi and animals) and implicated it in byssus extensibility.
Even more evolutionarily divergent are the coral-burrowing species of the giant clam Tridacna (Cardiidae), which attach with a massive byssus. Their byssal threads are described to exhibit a mechanical response like tendon, but the threads are not constructed of collagen triple helices, rather they exhibit a rope-like structure consisting of four coiled-coil alpha-helical proteins that are further organized into hexagonal arrays [40]. The proteins are enriched in basic and acid residues that may help stabilize the structure.
(c). The byssus of dreissenid mussels
Interest in the byssus of dreissenids grew following the spread of the notorious zebra mussel throughout North America starting in the mid to late 1980s. Among the world's most widely distributed bivalves, zebra mussels and quagga mussels spread across Europe, Eurasia and North America in the last two centuries, and are now among the most damaging of the world's aquatic invasive species [41,42]. Byssal threads fasten adults and juveniles to boats and other equipment—the vectors of spread to new water bodies—where byssal attachment to lake and river bottom, aquatic plants and conspecifics leads to massive densities of mussels that transform aquatic systems [43,44]. Byssally attached zebra and quagga mussels foul water intakes, turbines and dams of water treatment, hydroelectric and other facilities, contributing to hundreds of millions USD in economic damages annually [45,46].
At the macro scale, the byssus of dreissenids resembles the structure in mytilids. Both consist of a stem rooted in retractor muscle cells, bundling together the threads. The threads each are continuous at their distal ends with adhesive pads, superficially similar to Mytilus plaques, and the entire byssus in both species is coated with a sealing cuticle of cross-linked protein [33,47]. From micro scale down to constituent proteins, however, the dreissenid byssus is substantially different. While the Dreissena proteins have not been well localized, the gradient in construction and protein composition found in Mytilus appears to be absent from the Dreissena threads [34,48,49], and there is no proximal to distal gradient in byssal thread mechanical properties in zebra mussels [50]. Electron microscopic and biochemical studies also reveal differences in the D. polymorpha plaques, including much lower levels of DOPA in plaque proteins. Whether this relates to biophysical differences in adhesion in freshwater compared to marine environments remains to be determined [34,47,48].
Despite considerable difficulties in studying these highly cross-linked molecules, Waite et al. [51,52] sequenced Dpfp1, and Sone and colleagues sequenced the remaining byssal proteins from zebra [34,48] and quagga [49] mussels. The results (table 1) confirm that the Dreissena and Mytilus byssal proteins are highly dissimilar. The Dreissena proteins lack collagen, silk fibroin or elastin-like domains. And while posttranslational modifications to amino acids have not been well established, DOPA levels appear lower, with D. polymorpha foot proteins Dpfp1 and Dpfp2 being the most DOPA-rich reported to date at 7 mol%; the entire byssus contains only about 0.6 mol% DOPA [33,53]. Zebra mussel byssal proteins, like the Mytilus proteins and others involved in invertebrate adhesion, are rich in RLCDs, including several tandem repeats. But the Dreissena repeats tend to be shorter and fewer, and they lack sequence similarity to the Mytilus repeats (table 1). A 26-residue region of Dpfp12 shows only modest similarity (E-value = 0.011) to foot protein 1 from Mytilus californianus (Mcfp-1) [48]. I aligned the 732-residue Mcfp-1 to Dpfp12 and Dpfp2 (the N- and C-terminal portions, respectively, of a single gene: see below). When trimmed, 63 of 220 aligned positions (28.6%) are gaps, and the amino acid distance at the un-gapped positions is 1.239. The byssal proteins in the two genera otherwise cannot be aligned with confidence.
Examination of the composition of byssal proteins in both genera (table 2) show that some are rich in the same amino acids. Several are glycine rich, due to tandem repeats in the Mytilus plaque proteins and several of the Dpfps. Dpfp7(α,β,γ), Dpfp8 and Dpfp9(α,β) proteins are rich in both glycine and tyrosine, as are the plaque proteins Mfp-3 and Mfp-5. Tyrosine is abundant throughout the Mytilus proteins, where it is often modified to DOPA. Dpfp1, 2 and 12 are proline rich, as are Mfp-1 and the preCOLs. Dpfp10 and 11 are cysteine and proline rich. Cysteine is abundant in Mfp-2 and Mfp-6, otherwise it is uncommon in byssal proteins. Gantayet et al. [48] discuss the possible functional significance of these and other patterns. Each of the Dpfps show strongly biased amino acid composition (electronic supplementary material, table S3), a hallmark of byssal proteins, often but not always within regions of tandem repeats (electronic supplementary material, figures S1–S5).
Table 2.
Amino acid composition of byssal proteins (mol%). Proteins from Mytilus are: Mfps (mussel foot proteins), Tmp (thread matrix protein), preCOL-P (precursor collagen proximal), preCOL-D (precursor collagen distal), preCOL-NG (precursor collagen non-graded). Proteins from D. polymorpha are Dpfps (D. polymorpha foot proteins). (Online version in colour.)
 |
Structure–function relationships in the zebra mussel byssal proteins are unknown. For example, while amino acid composition is similar in thread and plaque regions [33], the locations of the proteins themselves have not been determined [48]. Dpfp1 has been immunolocalized to a subset of the glands lining the secretory portions of the foot [52], and expressed sequence tags for the byssal genes are differentially expressed in the stem and thread glands [54]. But extensive cross-linking makes identifying the location of individual proteins within the byssus itself difficult—this was avoided in proteomic studies by extracting the proteins from newly extruded threads, prior to cross-linking in the mature structure [48]. Progress on this would greatly improve understanding of byssal protein function in zebra mussels, as it did in blue mussels.
It may also shed light on the mechanical performance of the dreissenid byssus. Remarkably, mechanical studies show that zebra mussel byssal threads are stiffer and stronger, and they recover from partial deformation more rapidly than threads of marine species (e.g. Mytilus, Modiolus, Geukensia) that are exposed to harsher water motion in their native environments [50]. This and the smaller mass of dreissenid mussels makes possible a different mechanical solution; nevertheless, at present, we know little about it.
(d). Byssal genes in the zebra mussel genome
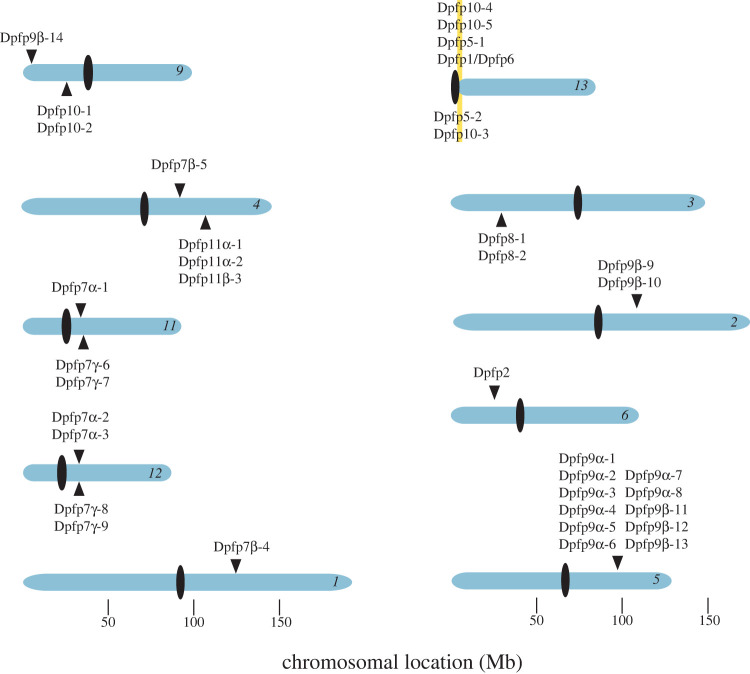
I found full-length coding DNA sequences (cds) for complete byssal proteins at a total of 37 loci. In addition, there are other BLAST hits at chromosome positions nearby that are incomplete, encoding products that are N- or C-truncated. Byssal loci were found on 10 of the 16 D. polymorpha chromosomes (electronic supplementary material, table S1 and figure 2). The genome confirmed the coding gene structure for four of the byssal proteins. Dpfp2, the sequence of which lacked a signal sequence in the proteome [48], is encoded by the C-terminal portion of a gene, the N-terminal portion of which encodes the signal sequence and Dpfp12 (the previous peptide sequence of Dpfp12 lacked a stop codon). The proteomic sequence of Dpfp8 also lacked a signal sequence [48]. This protein is predicted to be encoded by two loci on the antisense strand of chromosome 3 (figure 2). Dpfp8 (195 base pairs (bp) in length) is at the C-terminus of two large coding regions, 7194 bp and 1881 bp, respectively, and neither locus encodes a signal peptide. Finally, there is the most complex gene of all—the 14.2 kilobase (kb) locus that encodes Dpfp1 and most likely Dpfp6 as well (electronic supplementary material, figure S1). The locus is in a 600 kb region on (81.15 megabase) chromosome 13 that contains five other byssal loci (figure 2). Dpfp6 was isolated from extruded byssal threads by Gantayet et al. [48] in their proteomic study. It showed strong similarity to a portion of Dpfp1 but Dpfp6 lacked a start codon and signal sequence. In the genome, Dpfp1 consists of one long exon (encoding 207 residues), followed by exons 2–15 encoding 13 residues each, exon 16 encoding 19 residues and C-terminal exon 17 encoding three residues. Dpfp6 appears to be composed of exons 2–4, 7–9, 10, 12 and 13 of Dpfp1 (electronic supplementary material, figure S1). Its confirmation as a splice variant awaits long-read RNA sequencing.
Figure 2.
Chromosomal locations of byssal genes in D. polymorpha. Chromosome numbers are labelled in italics. The vertical black ovals represent positions of the centromeres based on cytogenetics. Genes labelled above the chromosomes are on + strands, those below are on − strands. The yellow shaded region on chromosome 13 marks a cluster of byssal genes from three gene families.
Of the 12 byssal proteins only two are encoded by a single locus in the genome; all others are from small gene families. In all cases, except for one of the Dpfp7 copies, all the genes in each family showed identical intron/exon structure. Dpfp9 showed the largest number of duplicates, with 14 copies in total on chromosomes 2, 5 and 9. Gene duplication clearly contributes to the diversity of the byssal genes and proteins. Amino acid divergence is extensive between paralogs (table 3), and indels are common (electronic supplementary material, figures S1–S5). Of the several theories to account for the evolutionary maintenance of duplicated genes [55], those that involve the acquisition of novel functions are particularly worth considering for the byssal genes. Orb-weaving spiders spin their web like all spiders using several mechanically different silks, each extruded from a different silk gland. Mechanical differences in part are determined by differences in amino repeat regions between duplicated genes in the silk spidroin gene family [56]. Moreover, the spidroins, like the dreissenid byssal genes, are RLCD-containing proteins, a common theme in the construction of silk-like proteins in mussels, spiders, silkworms, caddisflies, sandcastle worms and other animals [57].
Table 3.
Sequence analysis of D. polymorpha byssal gene families. Amino acid distances are based on the JTT model. Nucleotide distances are based on the Kimura 2-parameter model. dN and dS are estimated using the Nei-Gojobori method, Jukes-Cantor adjusted for multiple hits. dN, the number of nonsynonymous DNA substitutions per nonsynonymous site; dS, the number of synonymous DNA substitutions per synonymous site.
| byssal protein(s) | chromosomes | number of loci | amino acid distance | nucleotide distance | dN | dS | dN/dS |
|---|---|---|---|---|---|---|---|
| Dpfp1/Dpfp6 | 13 | 1 | |||||
| Dpfp2 | 6 | 1 | |||||
| Dpfp5 | 13 | 2 | 0.061 | 0.024 | 0.027 | 0.055 | 0.491 |
| Dpfp7 | 1,4,11,12 | 9 | 0.180 | 0.092 | 0.088 | 0.270 | 0.326 |
| Dpfp8 | 3 | 2 | 0.159 | 0.090 | 0.086 | 0.069 | 1.246 |
| Dpfp9 | 2,5,9 | 14 | 0.111 | 0.053 | 0.051 | 0.129 | 0.395 |
| Dpfp10 | 9,13 | 5 | 0.061 | 0.024 | 0.025 | 0.081 | 0.309 |
| Dpfp11 | 4 | 3 | 0.229 | 0.118 | 0.111 | 0.132 | 0.841 |
The dN/dS ratios calculated between duplicates show that substitutions are under moderate purifying selection in the Dpfp5, 7, 9 and Dpfp10 families (table 3). This is important, because it provides evidence that these variants experience selection for the maintenance of function, indicating that each are expressed. Multiple variants are also a feature of the Mytilus proteins, especially Mfp-3, a small protein located near the plaque/substrate interface, of which variants have been postulated to play a role in surface adhesion. Dpfp9 is small and highly variable, so perhaps it is also an adhesive plaque protein.
Based on fossil and molecular evidence [32], the split between D. rostriformis and the clade containing D. polymorpha dates to just 12.7 million years ago (Ma) in the Miocene, yet evolutionary divergence between their byssalomes is extensive. Of 15 of the named byssal proteins from D. rostriformis (foot protein 6, the splice variant in D. polymorpha, was not analysed), seven are missing from the D. polymorpha genome (this study) and proteome [49]. The remaining proteins are found in both species: Dfp1, 2, 5, 7, 8, 9 and 10 (Dfp, Dreissena foot protein). Alignments showed domains of similarity separated by multiple gaps (electronic supplementary material, file 3). On average, 34.3% of the positions in the alignments were gapped, while the distance at aligned residues averaged 1.175 per site (table 4). These results contrast with those from eight other extracellular and fibrous proteins from the two species, which showed many fewer gapped positions (2.8%), and much smaller genetic distances (0.485) on average. Perhaps the byssal genes are prone to rapid evolution, like other RLCD-containing proteins, such as the matrix proteins of bivalve shells [58,59].
Table 4.
Analysis of orthologous groups of byssal proteins in D. polymorpha and D. rostriformis.
| proteina | alignment lengthb | number of gapsc | proportion alignable | amino acid sequence divergenced |
|---|---|---|---|---|
| byssal proteins | ||||
| Dfp1 | 152 | 92 | 0.395 | 2.548 |
| Dfp2 | 185 | 48 | 0.741 | 0.548 |
| Dfp5 | 206 | 22 | 0.893 | 0.484 |
| Dfp7 | 133 | 55 | 0.586 | 0.559 |
| Dfp8 | 88 | 24 | 0.727 | 2.246 |
| Dfp9 | 79 | 11 | 0.861 | 0.441 |
| Dfp10 | 88 | 29 | 0.670 | 1.027 |
| Dfp11 | 124 | 77 | 0.379 | 1.546 |
| mean | 0.657 | 1.175 | ||
| s.e.m. | 0.068 | 0.298 | ||
| other extracellular and fibrous proteins | ||||
| CollagenVI alpha 5.5 | 232 | 3 | 0.987 | 0.234 |
| CollagenVI alpha 5.6 | 257 | 0 | 1.000 | 0.086 |
| Ficolin | 145 | 7 | 0.952 | 0.571 |
| Fibrinogen-like 1 | 219 | 27 | 0.877 | 0.952 |
| Myosin H chain | 558 | 0 | 1.000 | 0.181 |
| Myosin IX | 193 | 6 | 0.969 | 1.484 |
| FRAS1 (ECM) | 743 | 0 | 1.000 | 0.046 |
| COL4A2 (ECM) | 272 | 2 | 0.993 | 0.324 |
| mean | 0.972 | 0.485 | ||
| s.e.m. | 0.015 | 0.177 | ||
aDfp, Dreissena foot protein; ECM, extracellular matrix.
bAlignments for all byssal proteins except Dfp1 included the entire cds. For the ECM and fibrous proteins, fragments from tBLASTn hits were aligned because, in the D. rostriformis genome, complete coding regions for their genes have not been characterized.
cFor gaps not present across all members of byssal gene families in D. polymorpha, gaps were tallied by weighing their frequencies.
dBased on the JTT model [57].
3. Conclusion
Biologists have concluded that the byssus is nearly universally present and crucial in larval settlement and dispersal, and that foot glands that secrete it are homologous across the bivalves. This suggests that the many gains and losses of the adult structure may represent parallelism, or independent evolution (from different ancestors) of a trait that is determined by common developmental mechanisms or pathways, rather than convergence, which implies different developmental programmes for homoplasies [60]. A classic example is the parallel evolution of butterfly and moth wing colour patterns, underlain by common generating rules for pigment deposition in wing cells [61]. Other examples include parallel evolution of pelvic armour reduction in sticklebacks [62], and repeated appearance of cartilage across deep animal phylogeny (in cuttlefish, horseshoe crabs and vertebrates [63]). More work on bivalve development with this parallelism hypothesis in mind is warranted.
By contrast to this deep homology of the secretory anatomy is the dramatic divergence of the proteins used to construct the byssus itself. In addition to the results discussed in the present paper, results from proteomes and genomes of scallops and oysters have revealed many new putative byssal proteins that are also very different from mussels (reviewed in [64]). We will have to wait and see what all of this means; proteomes from the M. edulis foot have also revealed many previously unstudied proteins [65].
I end by pointing to the need for research on this trait in invasive species. I tallied that, of 34 invasive bivalve species listed by Sousa et al. [66], 21 (62%) are byssate, including all five freshwater species. Work in Dreissena is particularly called for. There is growing interest in explanations for the ecological replacement of zebra mussels by quagga mussels in North America, and evidence for this is growing in Europe [67]. Zebra mussels spread more rapidly between inland waters and they are more abundant hitchhikers on watercraft, even where they are outnumbered by quagga mussels in nearby populations [27,67]. Does this result from differences in in tenacity of attachment, and are mechanical and structural differences in byssal threads responsible? Conversely, quagga but not zebra mussels are abundant in soft sediments in deeper lakes [40]. Are quagga byssal threads better suited to this semi-infaunal habit? These are interesting questions, important for invasion biology.
Acknowledgements
The author thanks Daryl Gohl (University of Minnesota Genomics Center: UMGC) and Eli Sone (University of Toronto: U of T) for advice and comments, and Ben Auch (UMGC) and Angelico Obille (U of T) for sharing analyses that initiated this work. Comments of the editor and three anonymous reviewers substantially improved the manuscript and are gratefully acknowledged.
Data accessibility
Additional tables and figures and all sequence alignments used in the paper have been uploaded as part of the electronic supplementary material.
Competing interests
I have no competing interests.
Funding
The zebra mussel genome project was funded by the Environment and Natural Resources Trust Fund of Minnesota and by the UMGC. The author provided funding for data analysis and preparation of this manuscript.
References
- 1.Coyne KJ, Qin X-X, Waite JH. 1997. Extensible collagen in mussel byssus: a natural block copolymer. Science 277, 1830-1832. ( 10.1126/science.277.5333.1830) [DOI] [PubMed] [Google Scholar]
- 2.Stanley SM. 1972. Functional morphology and evolution of byssally attached bivalve mollusks. J. Paleontol. 46, 165-212. [Google Scholar]
- 3.Yonge CM. 1962. On the primitive significance of the byssus in the Bivalvia and its effects in evolution. J. Mar. Biol. Assoc. UK 42, 113-125. [Google Scholar]
- 4.Cranfield HJ. 1973. Observations on the function of the glands of the foot of the pediveliger of Ostrea edulis during settlement. Mar. Biol. 22, 211-223. ( 10.1007/bf00389175) [DOI] [Google Scholar]
- 5.Owada M. 2007. Functional morphology and phylogeny of the rock-boring bivalves Leiosolenus and Lithophaga (Bivalvia: Mytilidae): a third functional clade. Mar. Biol. 150, 853-860. ( 10.1007/s00227-006-0409-y) [DOI] [Google Scholar]
- 6.Mikkelsen PM, Bieler R, Kappner I, Rawlings TA. 2006. Phylogeny of Veneroidea (Mollusca: Bivalvia) based on morphology and molecules. Zool. J. Linn. Soc. 148, 439-521. ( 10.1111/j.1096-3642.2006.00262.x) [DOI] [Google Scholar]
- 7.Bieler R. 2001. Varicorbula (Bivalvia: Corbulidae) of the western Atlantic: taxonomy, anatomy, life habits, and distribution. Veliger 44, 271-293. [Google Scholar]
- 8.Oldfield E. 1955. Observations on the anatomy and mode of life of Lasaea rubra (Montagu) and Turtonia minuta (Fabricius). J. Molluscan Stud. 31, 226-249. ( 10.1093/oxfordjournals.mollus.a064746) [DOI] [Google Scholar]
- 9.Oldfield E. 1961. The functional morphology of Kellia suborbicularis (Montagu), Montacuta ferruginosa (Montagu) and M. substriata (Montagu), (Mollusca, Lamellibranchiata). J. Molluscan Stud. 34, 255-295. ( 10.1093/oxfordjournals.mollus.a064872) [DOI] [Google Scholar]
- 10.Hylleberg J. 1994. Indo-Pacific cockles (Bivalvia: Cardiidae). Part 1. Generic diagnoses and an overview of species with mention of taxonomic problems encountered in Thailand. Phuket Mar. Biol. Cent. Spec. Publ. 13, 113-136. [Google Scholar]
- 11.Gruffydd LD, Lane DJW, Beaumont AR. 2009. The glands of the larval foot in Pecten maximus L. and possible homologues in other bivalves. J. Mar. Biol. Assoc. UK 55, 463-476. ( 10.1017/S0025315400016064) [DOI] [Google Scholar]
- 12.Gustafson RG, Reid RGB. 1988. Larval and post-larval morphogenesis in the gutless protobranch bivalve Solemya reidi (Cryptodonta: Solemyidae). Mar. Biol. 97, 373-387. ( 10.1007/BF00397768) [DOI] [Google Scholar]
- 13.Fryer G, Yonge M. 1961. The developmental history of Mutela bourguignati (Ancey) Bourguignat (Mollusca: Bivalvia). Phil. Trans. R. Soc. Lond. B 244, 259-298. ( 10.1098/rstb.1961.0009) [DOI] [Google Scholar]
- 14.Wächtler K, Dreher-Mansur MC, Richter T. 2001. Larval types and early postlarval biology in naiads (Unionoida). In Ecology and evolution of the freshwater mussels Unionoida) (eds K Wächtler, G Bauer), pp. 93-125. Berlin, Heidelberg: Springer. [Google Scholar]
- 15.Carter JG, et al. 2012. Illustrated glossary of the Bivalvia. Treatise Online 48(part N/vol 1), 1-209. [Google Scholar]
- 16.Baker P, Mann R. 1997. The postlarval phase of bivalve mollusks: a review of functional ecology and new records of postlarval drifting of Chesapeake Bay bivalves. Bull. Mar. Sci. 61, 409-430. [Google Scholar]
- 17.Carriker MR. 2001. Embryogenesis and organogenesis of veligers and early juveniles. In Biology of the hard clam (eds JN Kraeuter, M Castagna), pp. 77-115. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 18.Bayne BL. 1964. Primary and secondary settlement in Mytilus edulis L. (Mollusca). J. Anim. Ecol. 33, 513-523. [Google Scholar]
- 19.Newell CR, Short F, Hoven H, Healey L, Panchang V, Cheng G. 2010. The dispersal dynamics of juvenile plantigrade mussels (Mytilus edulis L.) from eelgrass (Zostera marina) meadows in Maine, U.S.A. J. Exp. Mar. Biol. Ecol. 394, 45-52. ( 10.1016/j.jembe.2010.06.025) [DOI] [Google Scholar]
- 20.Waite JH. 1983. Adhesion in byssally attached bivalves. Biol. Rev. 58, 209-231. ( 10.1111/j.1469-185X.1983.tb00387.x) [DOI] [Google Scholar]
- 21.Liu C, Li S, Huang J, Liu Y, Jia G, Xie L, Zhang R. 2015. Extensible byssus of Pinctada fucata: Ca2+-stabilized nanocavities and a thrombospondin-1 protein. Sci. Rep. 5, 1-3. ( 10.1038/srep15018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J, Hennebert E, Flammang P, Hwang DS. 2020. A sugar-lectin rich interface between soft tissue and the stiff byssus of Atrina pectinata. Biomater. Sci. 8, 3751-3759. ( 10.1039/c9bm01932d) [DOI] [PubMed] [Google Scholar]
- 23.Alejandrino A, Puslednik L, Serb JM. 2011. Convergent and parallel evolution in life habit of the scallops (Bivalvia: Pectinidae). BMC Evol. Biol. 11, 164. ( 10.1186/1471-2148-11-164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzholtz JR, Birkedal H. 2009. Architecture of the biomineralized byssus of the saddle oyster (Anomia sp). J. Adhes. 85, 590-600. ( 10.1080/00218460902996820) [DOI] [Google Scholar]
- 25.Morton B. 1993. The anatomy of Dreissena polymorpha and the evolution and success of the heteromyarian form in the Dreissenoidea. In Zebra mussels: biology, impacts and control (eds Nalepa TF, Schloesser DW), pp. 185-215. Boca Raton, FL: CRC Press. [Google Scholar]
- 26.Yonge CM, Campbell JI. 1968. On the heteromyarian condition in the Bivalvia with special reference to Dreissena polymorpha and certain Mytilacea. Trans. R. Soc. Edinb. 68, 21-42. ( 10.1017/S0080456800014502) [DOI] [Google Scholar]
- 27.Lemer S, Bieler R, Giribet G. 2019. Resolving the relationships of clams and cockles: dense transcriptome sampling drastically improves the bivalve tree of life. Proc. R. Soc. B 286, 20182684. ( 10.1098/rspb.2018.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archambault JM, Cope WG, Kwak TJ. 2013. Burrowing, byssus, and biomarkers: behavioral and physiological indicators of sublethal thermal stress in freshwater mussels (Unionidae). Mar. Freshw. Behav. Physiol. 46, 229-250. ( 10.1080/10236244.2013.805891) [DOI] [Google Scholar]
- 29.Bradley ME. 2011. Byssus production in freshwater mussels (Bivalvia: Unionoidea). MS thesis, Missouri State University, Springfield, MO.
- 30.Audino JA, Serb JM, Marian JEAR. 2019. Ark clams and relatives (Bivalvia: Arcida) show convergent morphological evolution associated with lifestyle transitions in the marine benthos. Biol. J. Linn. Soc. 126, 866-884. ( 10.1093/biolinnean/blz017) [DOI] [Google Scholar]
- 31.Geda SR, Lujan NK, Perkins M, Abernethy E, Sabaj MH, Gangloff M. 2018. Multilocus phylogeny of the zebra mussel family Dreissenidae (Mollusca: Bivalvia) reveals a fourth Neotropical genus sister to all other genera. Mol. Phylogenet. Evol. 127, 1020-1033. ( 10.1016/j.ympev.2018.07.009) [DOI] [PubMed] [Google Scholar]
- 32.Bilandžija H, Morton B, Podnar M, Ćetković H. 2013. Evolutionary history of relict Congeria (Bivalvia: Dreissenidae): unearthing the subterranean biodiversity of the Dinaric Karst. Front. Zool. 10, 5. ( 10.1186/1742-9994-10-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzepecki LM, Waite JH. 1993. The byssus of the zebra mussel, Dreissena polymorpha. I: Morphology and in situ protein processing during maturation. Mol. Mar. Biol. Biotech. 2, 255-266. [PubMed] [Google Scholar]
- 34.Gantayet A, Ohana L, Sone ED. 2013. Byssal proteins of the freshwater zebra mussel, Dreissena polymorpha. Biofouling 29, 77-85. ( 10.1080/08927014.2012.746672) [DOI] [PubMed] [Google Scholar]
- 35.Lee BP, Messersmith PB, Israelachvili JN, Waite JH. 2011. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 41, 99-132. ( 10.1146/annurev-matsci-062910-100429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waite JH, Lichtenegger HC, Stucky GD, Hansma P. 2004. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry 43, 7653-7662. ( 10.1021/bi049380h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waite JH, Andersen NH, Jewhurst S, Sun C. 2005. Mussel adhesion: finding the tricks worth mimicking. J. Adhes. 81, 297-317. ( 10.1080/00218460590944602) [DOI] [Google Scholar]
- 38.DeMartini DG, Errico JM, Sjoestroem S, Fenster A, Waite JH. 2017. A cohort of new adhesive proteins identified from transcriptomic analysis of mussel foot glands. J. R. Soc. Interface 14, 20170151. ( 10.1098/rsif.2017.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasche D, Horbelt N, Marin F, Motreuil S, Macías-Sánchez E, Falini G, Hwang DS, Fratzl P, Harrington MJ. 2018. A new twist on sea silk: the peculiar protein ultrastructure of fan shell and pearl oyster byssus. Soft Matter 14, 5654-5664. ( 10.1039/c8sm00821c) [DOI] [PubMed] [Google Scholar]
- 40.Miserez A, Li Y, Cagnon J, Weaver JC, Waite JH. 2012. Four-stranded coiled-coil elastic protein in the byssus of the giant clam, Tridacna maxima. Biomacromolecules 13, 332-341. ( 10.1021/bm2013394) [DOI] [PubMed] [Google Scholar]
- 41.Karatayev AY, Burlakova LE, Padilla DK. 2015. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746, 97-112. ( 10.1007/s10750-014-1901-x) [DOI] [Google Scholar]
- 42.Lowe S, Browne M, Boudjelas S, De Poorter M. 2004. 100 of the world's worst invasive alien species: a selection from the Global Invasive Species database. Aukland, New Zealand: Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC), World Conservation Union (IUCN). [first published in Aliens12, December 2000].
- 43.Strayer DL. 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 55, 152-174. ( 10.1111/j.1365-2427.2009.02380.x) [DOI] [Google Scholar]
- 44.Higgins SN, Vander Zanden MJ. 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol. Monogr. 80, 179-196. ( 10.1890/09-1249.1) [DOI] [Google Scholar]
- 45.Bossenbroek JM, Finnoff DC, Shogren JF, Warziniack TW. 2009. Advances in ecological and economical analysis of invasive species: dreissenid mussels as a case study. In Bioeconomics of invasive species: integrating ecology, economics, policy, and management (eds Keller RP, Lodge DM, Lewis MA, Shogren JF), pp. 244-265. New York, NY: Oxford University Press. [Google Scholar]
- 46.O'Neill CR Jr. 2008. The silent invasion: finding solutions to minimize the impacts of invasive quagga mussels on water rates, water infrastructure and the environment. Washington, DC: US House of Representatives Committee on Natural Resources – Subcommittee on Water and Power.
- 47.Farsad N, Sone ED. 2012. Zebra mussel adhesion: structure of the byssal adhesive apparatus in the freshwater mussel, Dreissena polymorpha. J. Struct. Biol. 177, 613-620. ( 10.1016/j.jsb.2012.01.011) [DOI] [PubMed] [Google Scholar]
- 48.Gantayet A, Rees DJ, Sone ED. 2014. Novel proteins identified in the insoluble byssal matrix of the freshwater zebra mussel. Mar. Biotechnol. 16, 144-155. ( 10.1007/s10126-013-9537-9) [DOI] [PubMed] [Google Scholar]
- 49.Rees DJ, Hanifi A, Obille A, Alexander R, Sone ED. 2019. Fingerprinting of proteins that mediate quagga mussel adhesion using a de novo assembled foot transcriptome. Sci. Rep. 9, 1-4. ( 10.1038/s41598-019-41976-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brazee SL, Carrington E. 2006. Interspecific comparison of the mechanical properties of mussel byssus. Biol. Bull. 211, 263-274. ( 10.2307/4134548) [DOI] [PubMed] [Google Scholar]
- 51.Anderson KE, Waite JH. 1998. A major protein precursor of zebra mussel (Dreissena polymorpha) byssus: deduced sequence and significance. Biol. Bull. 194, 150-160. ( 10.2307/1543045) [DOI] [PubMed] [Google Scholar]
- 52.Anderson KE, Waite JH. 2000. Immunolocalization of Dpfp1, a byssal protein of the zebra mussel Dreissena polymorpha. J. Exp. Biol. 203, 3065-3076. [DOI] [PubMed] [Google Scholar]
- 53.Rzepecki LM, Waite JH. 1993. The byssus of the zebra mussel, Dreissena polymorpha. II: structure and polymorphism of byssal polyphenolic protein families. Mol. Mar. Biol. Biotech. 2, 267-279. [PubMed] [Google Scholar]
- 54.Xu W, Faisal M. 2010. Gene expression profiling during the byssogenesis of zebra mussel (Dreissena polymorpha). Mol. Genet. Genom. 283, 327-339. ( 10.1007/s00438-010-0517-8) [DOI] [PubMed] [Google Scholar]
- 55.Innan H, Kondrashov F. 2010. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 11, 97-108. ( 10.1038/nrg2689) [DOI] [PubMed] [Google Scholar]
- 56.Sponner A, Schlott B, Vollrath F, Unger E, Grosse F, Weisshart K. 2005. Characterization of the protein components of Nephila clavipes dragline silk. Biochemistry 44, 4727-4736. ( 10.1021/bi047671k) [DOI] [PubMed] [Google Scholar]
- 57.de Brevern AG, McDougall C, Woodcroft BJ, Degnan BM. 2016. The widespread prevalence and functional significance of silk-like structural proteins in metazoan biological materials. PLoS ONE 11, e0159128. ( 10.1371/journal.pone.0159128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson DJ, et al. 2010. Parallel evolution of nacre building gene sets in molluscs. Mol. Biol. Evol. 27, 591-608. ( 10.1093/molbev/msp278) [DOI] [PubMed] [Google Scholar]
- 59.McDougall C, Aguilera F, Degnan BM. 2013. Rapid evolution of pearl oyster shell matrix proteins with repetitive, low-complexity domains. J. R. Soc. Interface 10, 20130041. ( 10.1098/rsif.2013.0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Futuyma DJ. 1998. Evolutionary biology. Sunderland, MA: Sinauer. [Google Scholar]
- 61.Nijhout HF. 1991. The development and evolution of butterfly wing patterns. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 62.Cresko WA, Amores A, Wilson C, Murphy J, Currey M, Phillips P, Bell MA, Kimmel CB, Postlethwait JH. 2004. Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations. Proc. Natl Acad. Sci. USA 101, 6050-6055. ( 10.1073/pnas.0308479101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarazona OA, Slota LA, Lopez DH, Zhang G, Cohn MJ. 2016. The genetic program for cartilage development has deep homology within Bilateria. Nature 533, 86-89. ( 10.1038/nature17398) [DOI] [PubMed] [Google Scholar]
- 64.Yang Z, Zhang L, Hu J, Wang J, Bao Z, Wang S. 2020. The evo-devo of molluscs: insights from a genomic perspective. Evol. Dev. 22, 409-424. ( 10.1111/ede.12336) [DOI] [PubMed] [Google Scholar]
- 65.Sansoucy M, Tremblay R, Carrington E, Marcotte I, Sleno L. 2021. Investigating byssogenesis with proteomic analysis of byssus, foot, and mantle in Mytilus mussels by LC-MS/MS. Proteomics 21, 2000014. ( 10.1002/pmic.202000014) [DOI] [PubMed] [Google Scholar]
- 66.Sousa R, Gutiérrez JL, Aldridge DC. 2009. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions 11, 2367-2385. ( 10.1007/s10530-009-9422-7) [DOI] [Google Scholar]
- 67.Nalepa TF, Schloesser DW. 2014. Quagga and zebra mussels: biology, impacts, and control, 2nd edn. Boca Raton, FL: CRC Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional tables and figures and all sequence alignments used in the paper have been uploaded as part of the electronic supplementary material.