Abstract
High-fat/high-fructose diet plus intermittent hypoxia exposure (HFDIH) causes metabolic disorders such as insulin resistance, obesity, nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes. The purpose of this study is to examine the effects and understand the mechanism of action of Lactobacillus rhamnosus GG culture supernatant (LGGs) on HFDIH-induced metabolic dysfunction. Mice were fed high-fat:high-fructose diet for 15 weeks. After 3 weeks of feeding, the mice were exposed to chronic intermittent hypoxia for the next 12 weeks (HFDIH), and LGGs was supplemented over the entire experiment. HFDIH exposure significantly led to metabolic disorders. LGGs treatment showed significant improvements in indices of metabolic disorders including fat mass, energy expenditure, glucose intolerance, insulin resistance, increased hepatic steatosis and liver injury. HFDIH mice markedly increased adipose inflammation and adipocyte size, and reduced circulating adiponectin, which was restored by LGGs treatment. LGGs treatment increased hepatic FGF21 mRNA expression and circulating FGF21 protein levels, which were associated with increased hepatic PPARα expression and fecal butyrate concentration. In addition, HFDIH-induced hepatic fat accumulation and apoptosis were significantly reduced by LGGs supplementation. In summary, LGGs treatment increased energy expenditure and insulin sensitivity and prevented metabolic abnormalities in HFDIH mice, and this is associated with the FGF21-adiponectin signaling pathway. LGGs may be a potential prevention/treatment strategy in subjects with the metabolic syndrome.
1. Introduction
Consumption of a diet containing high fat and high fructose is common in the United States and causes metabolic dysfunction, including nonalcoholic fatty liver disease (NAFLD), obesity, insulin resistance and type 2 diabetes. Obstructive sleep apnea (OSA) is a major health concern. OSA is associated with obesity and with metabolic dysfunction independently [1]. Although the pathophysiology of OSA-associated metabolic dysfunction is not completely clear, OSA-generated intermittent hypoxia (IH) seems to play a pivotal role in the exacerbation of diet-induced metabolic dysfunctions [1–3]). Accumulating evidence indicates that gut dysbiosis plays a key role in the development of metabolic disorders [4]. Probiotics have been used as a novel approach in the prevention and treatment of metabolic dysfunction, and this has gained significant attention in recent years [5]. However, live probiotic bacteria need to colonize the gut to exert their functions, and the colonization is not always efficient due to a variety of pathological circumstances, especially when the subjects are taking gut bacteria-disrupting drugs. As an alternative approach, probiotic fermentation supernatant, which does not need to colonize the gut, has been used in the prevention of several disease conditions [6,7]. Lactobacillus rhamnosus GG (LGG) is one the best characterized probiotic strains, and it has been widely used for the management of a variety of diseases [7]. Our previous studies showed that LGG supernatant (LGGs) improved intestinal barrier function, increased hepatic AMPK phosphorylation and regulated Treg/Th17 immune response in alcoholic liver disease (ALD) [8–12]. However, it is unknown whether this strategy is effective in the prevention/treatment of other metabolic disorders such as NAFLD and obesity.
The liver and adipose tissues are key organs for the control of lipid metabolism and insulin sensitivity. Studies have shown that liver-derived fibroblast growth factor 21 (FGF21) regulates liver and extrahepatic tissue glucose and lipid metabolism [13]. Adiponectin is a critical adipokine that regulates hepatic insulin sensitivity in an endocrine manner [14]. Recombinant FGF21 and derivatives have been shown to improve metabolic disorders in humans and mice [15,16]. Importantly, it has been demonstrated that adiponectin is required for the beneficial effects of FGF21, suggesting a liver–adipose interaction [17]. However, due to its short half-life and its aggregation property, therapy with recombinant FGF21 is problematic. However, stimulating endogenous FGF21 expression in the prevention/treatment of metabolic disorders is receiving increased investigative attention.
Here, we investigated the effectiveness of LGGs in the prevention of metabolic disorders in high-fat:high-fructose-fed mice exposed to intermittent hypoxia (HFDIH), and investigated the mechanisms of LGGs regulation of insulin resistance, energy expenditure and liver fat accumulation and injury. We found that LGGs up-regulated FGF21 and adiponectin expression in this mouse model. Our study supports the notion that LGG supernatant is effective in the prevention of metabolic disorders in HFDIH mice through FGF21–adiponectin signaling pathway.
2. Materials and methods
2.1. LGG culture and preparation of LGGs
LGG was purchased from American Type Culture Collection (ATCC 53103, Rockville, MD, USA) and cultured in MRS broth according to ATCC guidelines. The LGG culture and LGG supernatant (LGGs) collection were standardized. Briefly, LGGs was harvested after being filtered through 0.22-mm filters when the bacterial density reached 109 colony-forming units/ml (CFU/ml) [10]. The supernatant was stored at 4°C and used within a week after preparation.
2.2. Animals
Six-week-old C57BL/6J male mice from Jackson Laboratory (Bar Harbor, ME, USA) were housed in a conventional animal room. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisville prior to the start of the study. Mice were fed liquid high-fat:high-fructose diet (HFD) (made with culture media) (D12450JL, Research Diets, New Brunswick, NJ, USA), and one group of mice was supplemented with LGGs (added to food) at a dose equivalent to 109 CFU/day in addition to the HFD. In the control group, LGGs was replaced by phosphate-buffered saline. After 3 weeks feeding, both groups of mice were exposed to IH during their sleep cycle as described in previous reports [18] for 12 weeks. Briefly, the IH paradigm consisted of alternating cycles of 20.9% O2/8% O2 FiO2 (30 episodes per hour) with 20 s at the nadir FiO2 during the 12-h light phase. Body weight was measured weekly. Serum and tissue samples were collected for assays.
2.3. Measurement of fat mass by dual-energy x-ray absorptiometry (DEXA)
Mouse fat mass was measured by DEXA (GE Lunar Co, Wisconsin) scanning, and data were analyzed using Lunar PIXImus mouse software. Prior to scanning, mice were anesthetized by inhalation of isoflurane. During measurements, the animals were laid in the prone position, and the duration of each scan was 3 to 5 min. Body fat mass and percentage of fat mass were recorded.
2.4. Comprehensive metabolic monitoring
Oxygen consumption rates (VO2), carbon dioxide production rates (VCO2), respiratory exchange ratios, and food and water consumption were measured using a physiologic/metabolic cage system (TSE Phenomaster System, Bad Homberg, Germany). Mice were housed individually, acclimatized to respiratory chambers for 24 h, and allowed free access to food and water. VO2 and VCO2 were recorded simultaneously over a 24-h period with a 12-h light and dark cycles. The energy expenditure (kcal/h/kg) was calculated with the following formula: (3.815+1.232×VCO2/VO2)×VO2×0.001, and normalized to lean body mass as described previously [19].
2.5. Glucose tolerance and insulin sensitivity assays
Glucose tolerance test (GTT) (intraperitoneal injection of glucose at 2 g/kg) and insulin tolerance test (ITT) (intraperitoneal injection of insulin at 0.75 U/kg) were performed on mice fasted overnight or 6 h, respectively. Tail blood was collected at 0, 15, 30, 60 and 120 min, and glucose levels were determined using a glucometer.
2.6. Fecal short-chain fatty acid (SCFA) measurement
Fecal levels of SCFAs were analyzed as previous described [20].
2.7. Western blotting
Protein was extracted from frozen liver, epididymal white adipose (eWAT), intestinal and muscle tissues. Western blotting was performed as described previously [21]. Phospho-HSL, HSL, ATGL, phospho-AktS473, AKT, phospho-GSK-3β and GSK-3β antibodies were from Cell Signaling (Danvers, MA, USA). β-Actin antibody was from Abcam (Cambridge, MA, USA).
2.8. Real-time quantitative PCR
Total mRNA was extracted from mouse liver, adipose and muscle tissues by Trizol according to the manufacturer’s protocol. Total RNA was used for reverse transcription with the cDNA cycle kit (Invitrogen). Primers for the experiment are listed in Table S1. 18S and GAPDH were used as internal controls. Real-time PCR was performed by using SYBR green reaction mixture in the ABI 7300 fast real-time PCR system (Applied Biosystems). The relative gene expression was determined by the ΔΔCT method.
2.9. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Formalin-fixed paraffin liver sections were sectioned at 5 μm, and the sections were stained with the ApopTag Peroxidase in situ Apoptosis Detection Kit (Chemicon, CA, USA) as described previously [11]. In brief, the slides were deparaffinized and rehydrated, then treated with proteinase K. Slides were then treated with 3% hydrogen peroxide to quench endogenous peroxidases and incubated with terminal deoxynucleotidyl transferase and anti-digoxigenin-peroxidase, respectively. Diaminobenzidine was then applied. Hematoxylin was used as counterstaining. Under the microscope, apoptotic cells exhibited a brown nuclear stain, and the TUNEL positive cells were counted manually.
2.10. Liver injury and lipid accumulation
Hepatic fat accumulation and liver injury were evaluated by hepatic tissue hematoxylin and eosin (HE) staining, Oil red O staining and liver tissue triglyceride (TG) levels. Formalin-fixed paraffin tissue sections were processed for staining, and frozen tissue sections were processed for Oil red O staining. Liver TG levels were determined using a triglyceride kit (Thermo Scientific, Waltham, MA, USA). Serum ALT or AST activities were measured using ALT and AST assay kits (Thermo Scientific) according to the manufacturer’s instructions. Hepatic neutrophil accumulation was determined using a naphthol AS-D chloroacetate (CAE) kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s directions.
2.11. Blood biochemical assays
Serum variables were measured using commercial kits closely following the manufacturer’s instructions. Total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL), TG, low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were analyzed by the Piccolo Xpress chemistry analyzer (Abaxis, Inc., Union City, CA, USA). Adiponectin concentrations were measured using an ELISA kit from EMD Millipore Corporation (Billerica, MA, USA). FGF21 concentrations were measured using an ELISA kit from R&D Systems (Minneapolis, MN, USA).
2.12. Statistical analysis
Statistical comparisons were made using two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test or one-way ANOVA with Tukey’s post hoc test or Student t test where it was appropriate. Differences were considered to be significant at P<.05.
3. Results
3.1. LGGs improve energy expenditure
LGGs supplementation did not significantly change food consumption compared to control groups (Fig. 1A). LGGs decreased body weight gain over the 15-week experiment in mice treated with HFDIH (Fig. 1B). Body composition analysis showed that fat mass was significantly decreased (Fig. 1C) and lean mass was significantly increased (Fig. 1D) at the end of 15-week feeding with LGGs supplementation. Comprehensive metabolic chamber studies indicated that the rates of carbon dioxide production (VCO2) and oxygen consumption (VO2) were increased in the dark phase by LGGs supplementation (Fig. 1E and F). There were no changes in VCO2 and VO2 in the light phase. The calculated energy expenditure was increased 18% by LGGs supplementation (Fig. 1G). Moreover, the respiratory exchange ratio (VO2/ VCO2) was increased in the dark phase by LGGs (Fig. 1H). This may reflect an increase in the use of carbohydrate as an energy source induced by LGGs. Collectively, LGGs supplementation reduced body weight gain and body fat mass and increased energy expenditure and lean mass in HFDIH treated mice.
Fig. 1. LGGs improves energy expenditure.
C57BL/6J mice were treated with HFDIH or HFDIH+LGGs for 15 weeks as described in Materials and Methods. (A) Body weight changes. (B) Body fat mass and (C) lean mass were analyzed by DEXA. (D) Carbon dioxide production rate and (E) oxygen consumption rate were measured using a metabolic chamber system. Calculated energy expenditure (F) and respiratory exchange ratio (G). Data are presented as mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
3.2. LGGs increases glucose tolerance and insulin sensitivity
To determine whether LGGs supplementation improves glucose homeostasis, we performed glucose tolerance (GTT) and insulin tolerance (ITT) tests. Fasting insulin and glucose concentrations were not significantly different between LGGs treated and untreated groups (Fig. 2A and B). However, mice treated with LGGs supplementation had substantially reduced blood glucose levels after glucose loading (Fig. 2C). ITT revealed that insulin sensitivity was substantially increased after LGGs treatment (Fig. 2D). We further measured protein levels of glucose homeostasis-related molecules. Hepatic PI3K and p-AKTs473 were significantly increased by LGGs (Fig. 2E). GSK-3β was also increased by LGGs, indicating a likely increased glycogen formation. Indeed, hepatic glycogen was increased as indicated by PAS staining of hepatic tissue (Fig. 2F). Similarly, muscle PI3K, p-AKT and GSK-3β were increased by LGGs (Fig. 2G). Taken together, our results demonstrated that LGGs supplementation effectively and significantly improved insulin sensitivity in HFDIH treated mice.
Fig. 2. LGGs decreases glucose intolerance and insulin resistant.
C57BL/6J mice were treated by HFDIH and HFDIH+LGGs for 15 weeks as described in Materials and Methods. (A) HFDIH and HFDIH+LGGs mice were fasted overnight, and serum glucose and insulin concentrations were measured. (B) Blood glucose levels during GTT. Right panel: AUC quantification. (C) Blood glucose levels during ITT. Right panel: AUC quantification. (D) Hepatic protein levels of p-AKTs473, total AKT, PI3K and Gsk-3β. β-Actin was used as loading controls. Right panel: densitometry quantifications. (E) PAS staining of hepatic glycogen. (F) Muscle protein levels of p-AKTs473, total AKT, PI3K and Gsk-3β. β-Actin was used as loading controls. Right panel: densitometry quantifications. Data are presented as the mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
3.3. LGGs increases FGF21 and adiponectin expression in HFDIH mice
Previous research demonstrated that adiponectin, mainly produced in adipose tissue, plays an important role in lipid and glucose metabolism [22]. LGGs treatment in HFDIH mice increased serum adiponectin concentration about 4.2-fold compared to HFDIH mice without LGGs treatment (Fig. 3A). FGF21, a hepatokine and a critical mediator in the regulation of glucose and lipid metabolism by targeting adipose tissue to elevate adiponectin expression, was increased 3.2-fold at the mRNA level and at the serum protein level by LGGs (Fig. 3B, C). PPARα, a ligand-activated nuclear receptor and one of the major transcription factors regulating FGF21 expression in the liver, was also significantly increased by LGGs (Fig. 3D). To determine the role of LGGs supplementation in the regulation of gut microbiota-mediated SCFAs production, we performed an LC–MS measurement to analyze the fecal SCFA concentrations. LGGs marginally increased all SCFAs including formic acid, acetic acid, probionic acid, isobutyric acid, 2-methylbutanoic acid and valeric acid (Fig. 3E) and significantly elevated butyric acid (Fig. 3F). Previous studies have demonstrated that butyric acid increases FGF21 expression through activation of PPARα and that action was dependent on HDAC3 inhibition [23,24].
Fig. 3. LGGs increases adiponectin and FGF21 production.
C57BL/6J mice were treated by HFDIH and HFDIH+LGGs for 15 weeks as described in Materials and Methods. (A) Serum adiponectin concentrations. (B) Hepatic fgf21 mRNA expression; right panel. (C) Serum FGF21 protein concentrations. (D) Hepatic ppara mRNA levels. (E) Fecal SCFA concentrations were analyzed by LC–MS/MS. Data are presented as the mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
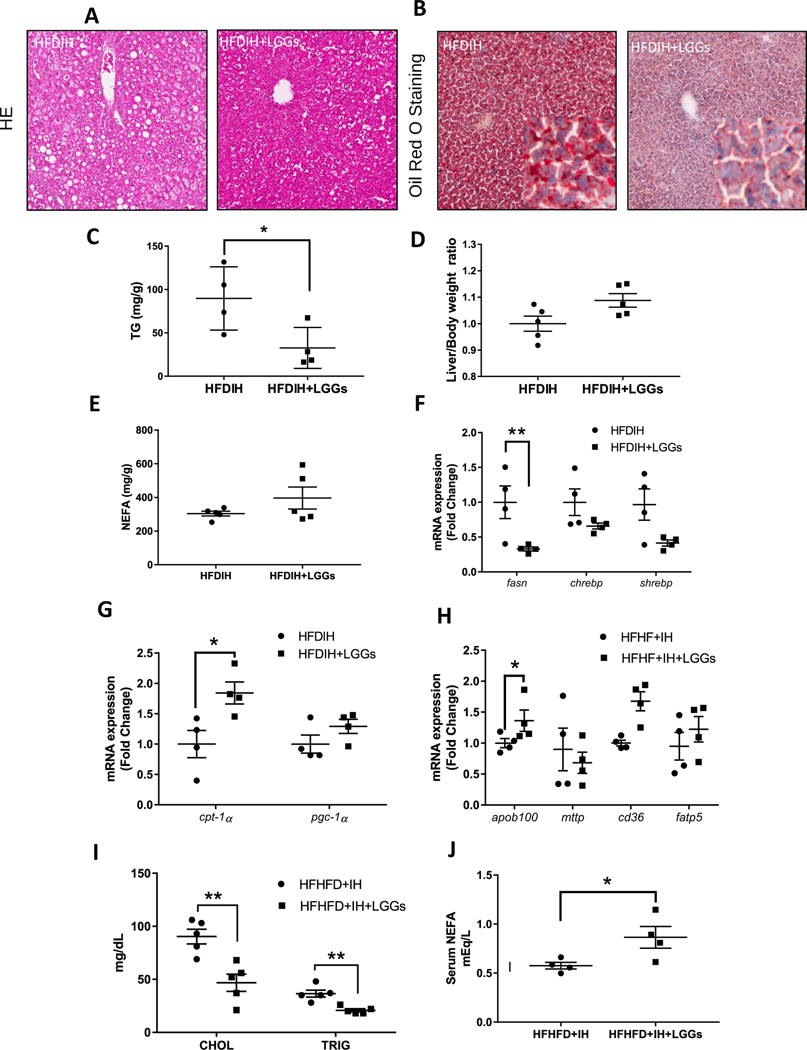
3.4. Effect of LGGs in hepatic lipid metabolism
LGGs supplementation decreased hepatic steatosis, as shown by HE staining and Oil red O staining of hepatic tissue, respectively (Fig. 4A and B). Confirming these results, liver triglyceride concentrations were significantly decreased by LGGs (Fig. 4C). Liver/body weight ratio and liver cholesterol levels were not affected by LGGs (Fig. 4D). Liver nonesterified fatty acid (NEFA) concentrations showed a marginal increase but did not reach statistical significance (Fig. 4E). In addition, we measured hepatic mRNA levels of genes involved in lipid metabolism. Fsan, a major enzyme in lipogenesis, was significantly reduced, while Chrebp and Shrebp, two important transcription factors in lipogenesis, were not affected by LGGs (Fig. 4F). Cpt-1a and Pgc-1a, two key genes in fatty acid β-oxidation, were increased by LGGs (Fig. 4G). Lipid uptake and output-related genes were also measured. Apob100 was increased significantly by LGGs, while Mttp was not altered (Fig. 4H). Genes involved in fatty acid uptake, Cd36 and Fatp5, were not altered by LGGs (Fig. 4H). Serological analysis showed that LGGs supplementation decreased serum cholesterol and triglycerides (Fig. 4I) but increased NEFA levels (Fig. 4J). Taken together, LGGs supplementation protected mice from HFDIH-induced hepatic steatosis and hyperlipidemia.
Fig. 4. LGGs supplementation decreases liver triglyceride.
C57BL/6J mice were treated by HFDIH and HFDIH+LGGs for 15 weeks as described in Materials and Methods. Histological examination of liver tissue sections stained with (A) HE (200×) and (B) Oil red O (200×). (C) Hepatic TG levels. (D) Liver/body weight ratios. (E) Liver NEFA concentrations. (F) Hepatic expression of genes related to DNL. (G) Hepatic expression of genes related to fatty acid β-oxidation. (H) Hepatic expression of genes related to lipid transport. (I) Serum lipid panels. (J) Serum NEFA concentrations. Data are presented as the mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
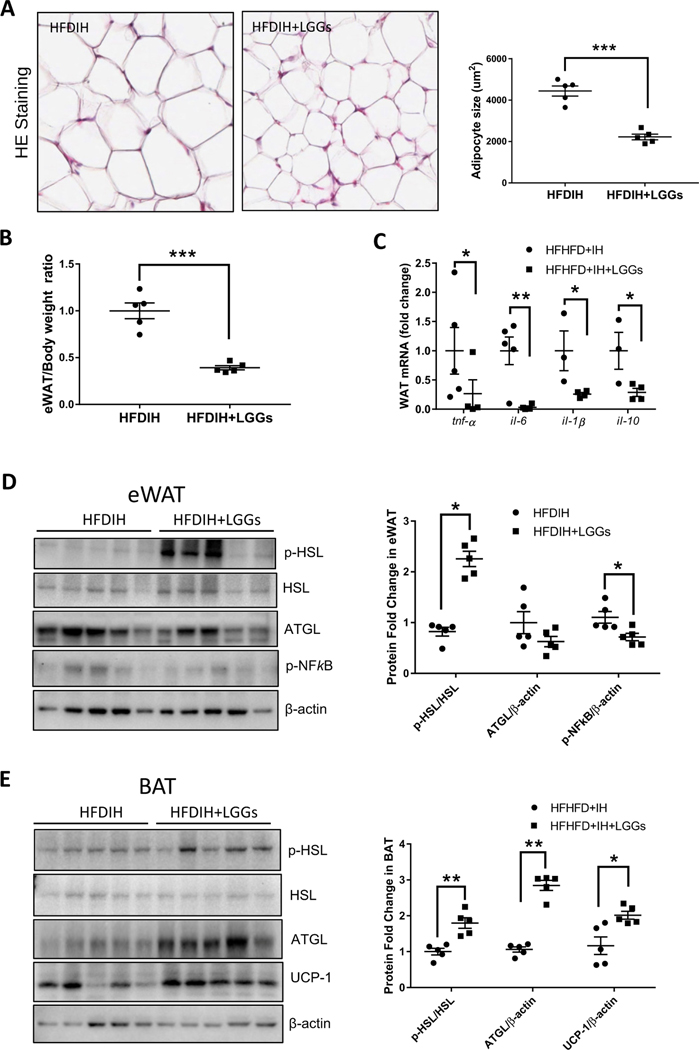
3.5. Effect of LGGs on adipose lipolysis
Decreased hepatic steatosis and increased serum NEFA led us to evaluate adipose lipolysis. LGGs significantly decreased adipocyte size and adipose/body weight ratio (Fig. 5A and B). Two major enzymes in adipose lipolysis pathways were measured. LGGs supplementation significantly increased p-HSL but not ATGL protein level in eWAT (Fig. 5C). Analyzing brown adipose tissue (BAT), we observed an increased expression of both of the lipolytic enzymes, p-HSL and ATGL (Fig. 5D). These results indicated an increased lipolysis by LGGs in eWAT and BAT. LGGs supplementation decreased eWAT p-NFkB protein level (Fig. 5E) and mRNA expression of proinflammatory cytokines Tnfa, Il-6 and Il-1b (Fig. 5F), indicating decreased inflammation. IL-10, an anti-inflammatory cytokine, was also significantly decreased by LGGs (Fig. 5F). Interestingly, protein level of UCP-1, a protein involved in fat burning, was increased by LGGs, indicating an increased effect by LGGs (Fig. 5G).
Fig. 5. LGGs increases adipose tissue lipolysis.
C57BL/6J mice were treated by HFDIH and HFDIH+LGGs for 15 weeks as described in Materials and Methods. (A) Representative HE staining of white adipose tissues (eWAT) (300×). Right panel: semiquantification of adipocyte size. (B) eWAT/body weight ratio. (C) Protein levels of p-HSL, HSL and ATGL of eWAT. β-Actin was used as loading controls. Right panel: densitometry quantification. (D) Protein levels of p-HSL, HSL and ATGL in BAT. Right panel: densitometry quantification. (E) Protein levels of p-NFkB in eWAT. (F) mRNA expression of tnf-a, il-6, il-1b and il-10 of eWAT. (G) UCP-1 protein levels in BAT. Data are presented as the mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
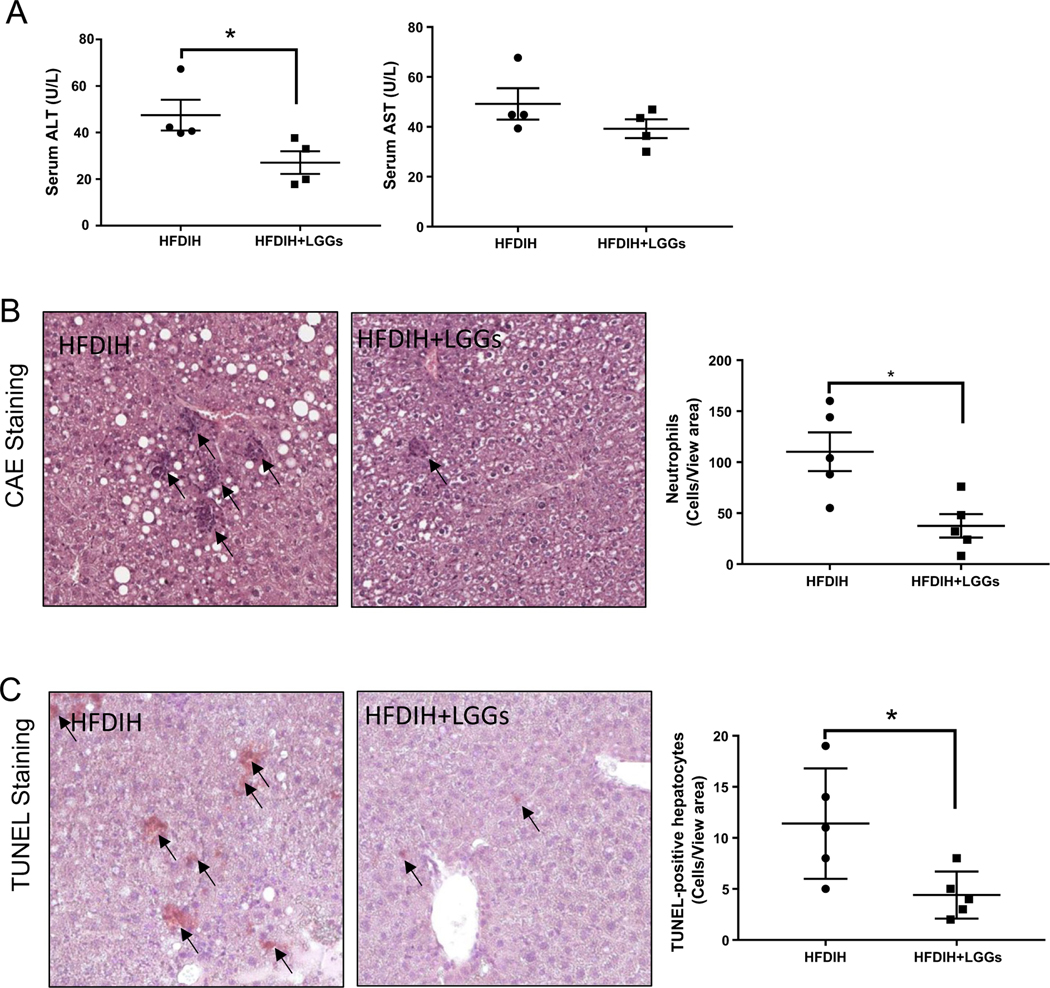
3.6. LGGs ameliorated HFDIH-induced liver injury
LGGs supplementation significantly decreased serum ALT and AST levels (Fig. 6A). Hepatic CAE staining showed a robust reduction of neutrophil infiltration by LGGs (Fig. 6B). LGGs also decreased hepatic apoptosis, as shown by TUNEL assay (Fig. 6C). These results indicated that LGGs protected mice from HFDIH-induced liver injury, neutrophil infiltration and apoptotic cell death.
Fig. 6. LGGs treatment decreases liver inflammation and injury.
C57BL/6J mice were treated by HFDIH and HFDIH+LGGs for 15 weeks as described in Materials and Methods. (A) Serum ALT and AST levels. (B) Representative photomicrographs of CAE staining of hepatic tissues. Right panel: quantification of CAE positive cells per 1000 hepatocytes. (E) Representative images of TUNEL staining of liver sections. Right panel: The number of apoptotic cells was determined by counting TUNEL-positive cells in at least 10 randomly selected high-power fields. Original magnification, ×10. Data are presented as the mean±S.E.M. (n=5) *P<.05, **P<.01, ***P<.001.
4. Discussion
The present study investigated the effects and mechanism of LGGs on HFDIH-induced metabolic syndrome. We demonstrated that the treatment with LGGs enhanced whole body energy expenditure and prevented HFDIH-induced insulin resistance and liver steatosis and injury in mice. We further demonstrated that the beneficial effects of LGGs are mediated through FGF21–adiponectin signaling.
Increasing evidence demonstrates the protective effect of probiotics on multiple metabolic pathways. Probiotics are being tested in many clinical trials to combat many metabolic diseases [25]. Probiotics exert their beneficial activities on the host through multiple mechanisms, including prevention of pathogenic bacterial growth and sustaining intestinal barrier integrity, which bestow protection on extraenteric tissues. However, these treatments are not always effective because, to confer their beneficial activity, live bacteria need to colonize the intestine to maintain their activity under various luminal conditions. Disease conditions vary from patient to patient due to the augmentation of pathogenic bacteria. It is therefore unclear whether probiotic treatment will result in sustained beneficial changes in the composition of the microbiota [26]. In addition, medications used by patients may be harmful to probiotics. This can cause a variable effect of probiotic treatment with live bacteria. Moreover, the clinically recommended dose of probiotics usually consists of billions of live bacteria. Probiotics are generally considered safe, but several reports have raised safety concerns about ingesting such large amounts of bacteria, especially when the intestinal function and the patient’s immune response are compromised [27,28].
Previous studies have suggested that the secreted factors from probiotic bacterial growth are likely to be major contributors to the beneficial effects of probiotics [29]. We demonstrated that LGGs is effective in the protection of alcohol-induced fatty liver disease in an animal model [10–12], implying that such a strategy may be effective in other metabolic diseases. However, this hypothesis has not been examined. Our current study showed that, in HFDIH mice, LGGs treatment significantly reduced body weight gain, body fat mass, adiposity, insulin resistance, hepatic fat accumulation and liver injury, and they also increased energy expenditure. This is the first evidence showing the beneficial effects of LGGs in HFDIH-induced metabolic syndrome.
Adiponectin, an adipokine that circulates at relative high concentrations, is one of the central players in glucose and lipid metabolism. Low levels of adiponectin have been shown to be correlated with the metabolic profile in patients with metabolic diseases [30,31]. The effects of probiotics on the circulating adiponectin in humans are not conclusive. While some studies showed that certain probiotics increased serum levels of adiponectin [32], other studies showed inconsistent regulation of adiponectin by probiotics [33]. These differences may be due to differences in populations and the probiotic strains and doses used. In animals, LGG application to HFD-fed mice stimulated adiponectin secretion with an associated improved insulin sensitivity and hepatic lipid accumulation [34]. In line with this observation, our study showed increased adiponectin by LGG culture supernatant without live bacteria.
It is clear that, in obese subjects, their adipose tissues are enlarged and inflamed, and there is a reduced ability to produce sufficient adiponectin. LGGs treatment decreased adipocyte size by increasing lipolysis and reduced adipose inflammation by reducing proinflammatory cytokines. These beneficial effects favor adiponectin production. Previous studies demonstrated that FGF21 is a strong regulator of adiponectin, and adiponectin is required for FGF21 function in metabolic regulation [17]. Our study also showed that the beneficial effects of probiotics treatment on the restoration of adiponectin production were blunted in FGF21-deficient mice (to be published separately). FGF21 is a hepatokine that has multiple metabolic functions, including improvement of dyslipidemia, NAFLD, obesity and hyperglycemia. FGF21-based therapeutic molecules, such as long-lasting FGF21 recombinant analogs, have been under development [35,36]. However, their short half-life and aggregation problem in recombinant protein have limited their application. Identifying endogenous mechanisms to increase FGF21 expression may represent an important approach to combat metabolic diseases. Our study suggests that LGGs is effective in increasing FGF21 expression in HFDIH mice likely via butyrate-mediated PPARα activation. LGGs exerts its function mainly through induction of hepatic FGF21 expression that increases adipose tissue adiponectin expression; however, other direct and indirect effects of LGGs on the metabolism cannot be ruled out. For instance, autophagy plays an important role in metabolic disease [37–39], and FGF21 was shown to stimulate autophagy in a variety of metabolic tissues [40–42], and autophagy deficiency protects against insulin resistance by inducing FGF21 [43]. It is unclear whether LGGs-stimulated FGF21 expression regulates tissue autophagic response in HFDIH mice to modulate metabolic function. Future studies are needed to elucidate the precise mechanisms of this regulation.
In addition to the improvement of body metabolism, including obesity, insulin resistance, energy expenditure and adipose function, we showed that LGGs supplementation ameliorated hepatic lipid accumulation. LGGs treatment increased lipid β-oxidation and decreased de novo lipogenesis, resulting in a reduced fatty liver. It has to be noted that LGGs decreased circulating triglyceride but increased circulating NEFA. Adipose tissue is the only significant site of NEFA liberation into circulation, and this process is mediated by lipolysis [44]. Recent studies suggest that increased lipolysis may increase FFA utilization and energy expenditure, resulting in resistance to obesity and insulin resistance [44]. In line with this study, our results showed that LGGs supplementation increased adipose lipolysis and energy expenditure, reduced obesity and increased brown fat activity, indicating an enhanced fat utilization. Although LGGs increased circulating NEFA, major hepatic transporters [45] responsible for fatty acid uptake were not altered. Taken together, our results suggest that elevated circulating NEFA is a result of increased adipose lipolysis and is utilized by extrahepatic tissues in accordance with enhanced energy expenditure, and reduced fatty liver is mainly a result of increased fatty acid β-oxidation and decreased lipogenesis.
Characterization of LGGs components will advance our understanding the regulatory mechanisms by which LGGs protects against HFDIH-induced metabolic dysfunction. Previous studies have identified ingredients secreted by probiotic culture [29,46]. These active ingredients have been demonstrated to be effective in the treatment of inflammatory bowel disease and liver disease through multiple mechanisms. However, the doses of these single molecules used in the applications were high in order to be effective. The effectiveness of LGGs is likely a combinatorial effect of multiple factors that work together additively or synergistically.
In conclusion, the present study demonstrates that LGGs is effective in reducing HFDIH-induced metabolic dysfunction in mice through FGF21–adiponectin signaling. Secreted factors from LGG probiotic bacteria increase intestinal butyrate concentrations and hepatic PPARα activation that lead to increased FGF21 expression. Circulating adiponectin is increased by LGGs, likely through hepatic FGF21 up-regulation. As a result, HFDIH-induced adiposity, hepatic fat accumulation and injury, insulin resistance and whole-body energy expenditure are improved by LGGs. This preclinical study suggests that LGGs treatment may be developed as a potential strategy in the prevention/treatment of metabolic disorders.
Supplementary Material
Acknowledgments
We thank Marion McClain for proofreading of the manuscript. This study was supported by NIH grants U01AA021901, U01AA021893-01, U01AA022489-01A1, R01AA023681, P20GM113226, P50AA024337 (C.J.M.); R21AA020848 and R01AA023190 (W.F.). Support was also provided by the VA (1I01BX002996, C.J.M.).
Abbreviations:
- AKT
protein kinase B
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- Apob100
apolipoprotein B
- AST
aspartate aminotransferase
- ATGL
adipose triglyceride lipase
- Bacs
Microtus ochrogaster solute carrier family 27 member 5
- BAT
brown adipose tissue
- Bsep
bile salt export pump
- CAE
naphthol AS-D chloroacetate
- CDCA
chenodeoxycholic acid
- CHOL
cholesterol Chrebp MLX interacting protein-like
- Cpt-1a
cricetulus griseus carnitine palmitoyltransferase 1A
- Cyp7a1
cytochrome P450 family 7, subfamily a, polypeptide 1
- Cyp8b1
cytochrome P450, family 8, subfamily b, polypeptide 1
- DEXA
dual-energy X-ray absorptiometry
- eWAT
Epididymal white adipose
- Fatp5
solute carrier family 27 (fatty acid transporter), member 5
- Fgf15
fibroblast growth factor 15
- FGF21
fibroblast growth factor 21
- Fasn
fatty acid synthase
- FXR
farnesoid X receptor
- GSK-3β
glycogen synthase kinase-3 beta
- GTT
glucose tolerance test
- HDAC3
histone deacetylase 3
- HDL
high-density lipoprotein
- HFD
high-fat:high-fructose diet
- HFDIH
high-fat/high-fructose diet plus intermittent hypoxia exposure
- HSL
hormone sensitive lipase
- IH
intermittent hypoxia
- IL-10
interleukin 10
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- ITT
insulin tolerance test
- LDL
low-density lipoprotein
- LGGs
Lactobacillus rhamnosus GG culture supernatant
- Mttp
microsomal triglyceride transfer protein
- NAFLD
nonalcoholic fatty liver disease
- NEFA
nonesterified fatty acid
- nHDLc
non-high-density lipoprotein cholesterol
- p-AKT
phospho-protein kinase B
- Pgc-1a
peroxisome proliferative activated receptor, gamma, coactivator 1 alpha
- p-GSK-3β
phospho-glycogen synthase kinase-3 beta
- P-HSL
phospho-hormone sensitive lipase
- PI3K
phosphoinositide 3-kinase
- p-NFkB
phospho-nuclear factor kappa B
- PPARα
peroxisome proliferator-activated receptor alpha
- SCFAs
short-chain fatty acids
- Shp
nuclear receptor subfamily 0, group B, member 2 (Nr0b2)
- Sptlc1
serine palmitoyltransferase long chain base subunit 1
- Sptlc2
serine palmitoyltransferase long chain base subunit 2
- Srebp
sterol regulatory element binding protein
- TG
triglyceride; TαMCA, tauro-α muricholic acid
- TβMCA
tauro-β muricholic acid
- UCP-1
uncoupling protein 1
- VLDL
very low-density lipoprotein
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jnutbio.2019.108256.
Conflicts of interest
The authors declare no competing interests.
References
- [1].Parikh MP, Gupta NM, McCullough AJ. Obstructive sleep apnea and the liver. Clin Liver Dis 2019;23:363–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 2010; 24:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ryan S, Arnaud C, Fitzpatrick SF, Gaucher J, Tamisier R, Pepin JL. Adipose tissue as a key player in obstructive sleep apnoea. Eur Respir Rev 2019;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 2019;15(5):261–73. [DOI] [PubMed] [Google Scholar]
- [5].Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr 2019;10:S49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cavallari JF, Fullerton MD, Duggan BM, Foley KP, Denou E, Smith BK, et al. Muramyl Dipeptide-Based Postbiotics Mitigate Obesity-Induced Insulin Resistance via IRF4. Cell Metab 2017;25:1063–1074 e1063. [DOI] [PubMed] [Google Scholar]
- [7].Capurso L Thirty years of lactobacillus rhamnosus GG: a review. J Clin Gastroenterol 2019;53(Suppl. 1):S1–S41. [DOI] [PubMed] [Google Scholar]
- [8].Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen RC, Xu LM, Du SJ, Huang SS, Wu H, Dong JJ, et al. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett 2016;241:103–10. [DOI] [PubMed] [Google Scholar]
- [10].Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 2012;303:G32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang M, Wang C, Wang C, Zhao H, Zhao C, Chen Y, et al. Enhanced AMPK phosphorylation contributes to the beneficial effects of lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem 2015;26:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao H, Zhao C, Dong Y, Zhang M, Wang Y, Li F, et al. Inhibition of miR122a by lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett 2015;234:194–200. [DOI] [PubMed] [Google Scholar]
- [13].Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab 2019;29:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fang H, Adiponectin regulation Judd RL. function. Compr Physiol 2018;8:1031–63. [DOI] [PubMed] [Google Scholar]
- [15].Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Investig 2005;115:1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS, et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring) 2019;27:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab 2013;17:779–89. [DOI] [PubMed] [Google Scholar]
- [18].Zhou S, Yin X, Jin J, Tan Y, Conklin DJ, Xin Y, et al. Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein. Free Radic Biol Med 2017;112:224–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 2014;146:539–549 e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He L, Prodhan MAI, Yuan F, Yin X, Lorkiewicz PK, Wei X, et al. Simultaneous quantification of straight-chain and branched-chain short chain fatty acids by gas chromatography mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2018;1092:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shao T, Zhao CQ, Li FY, Gu ZL, Liu LM, Zhang LH, et al. Intestinal HIF-1 alpha deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J Hepatol 2018;69:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(Suppl. 7):S13–8. [DOI] [PubMed] [Google Scholar]
- [23].Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 2012;61:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, et al. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One 2013;8:e68626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barengolts E Gut microbiota, prebiotics, probiotics, and synbiotics in manage-ment of obesity and prediabetes: review of randomized controlled trials. Endocr Pract 2016;22:1224–34. [DOI] [PubMed] [Google Scholar]
- [26].Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 2016;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–9. [DOI] [PubMed] [Google Scholar]
- [28].Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115: 178–81. [DOI] [PubMed] [Google Scholar]
- [29].Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007;132:562–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pyrzak B, Ruminska M, Popko K, Demkow U. Adiponectin as a biomarker of the metabolic syndrome in children and adolescents. Eur J Med Res 2010;15:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Savvidou S, Hytiroglou P, Orfanou-Koumerkeridou H, Panderis A, Frantzoulis P, Goulis J. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol 2009;43:765–72. [DOI] [PubMed] [Google Scholar]
- [32].Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010;64:636–43. [DOI] [PubMed] [Google Scholar]
- [33].Rouhani MH, Hadi A, Ghaedi E, Salehi M, Mahdavi A, Mohammadi H. Do probiotics, prebiotics and synbiotics affect adiponectin and leptin in adults? A systematic review and meta-analysis of clinical trials. Clin Nutr 2018;38(5):2031–7. [DOI] [PubMed] [Google Scholar]
- [34].Kim SW, Park KY, Kim B, Kim E, Hyun CK. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Bioph Res Co 2013;431: 258–63. [DOI] [PubMed] [Google Scholar]
- [35].Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019;392:2705–17. [DOI] [PubMed] [Google Scholar]
- [36].Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 2016;23: 427–40. [DOI] [PubMed] [Google Scholar]
- [37].Tan Y, Gong Y, Dong M, Pei Z, Ren J. Role of autophagy in inherited metabolic and endocrine myopathies. Biochim Biophys Acta Mol Basis Dis 1865;2019:48–55. [DOI] [PubMed] [Google Scholar]
- [38].Lahiri V, Hawkins WD, Klionsky DJ. Watch what you (self-) eat: autophagic mechanisms that modulate metabolism. Cell Metab 2019;29:803–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang S, Wang C, Turdi S, Richmond KL, Zhang Y, Ren J. ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1alpha deacetylation. Int J Obes (Lond) 2018;42:1073–87. [DOI] [PubMed] [Google Scholar]
- [40].Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle 2019;10:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, et al. FGF21 ameliorates nonalcoholic fatty liver disease by inducing autophagy. Mol Cell Biochem 2016;420:107–19. [DOI] [PubMed] [Google Scholar]
- [42].Cheng STW, Li SYT, Leung PS. Fibroblast growth factor 21 stimulates pancreatic islet autophagy via inhibition of AMPK-mTOR signaling. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 2013;19:83–92. [DOI] [PubMed] [Google Scholar]
- [44].Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology 2006;130:1245–58. [DOI] [PubMed] [Google Scholar]
- [46].Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr 2006;136: 1483–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.