Keywords: iron, ferroptosis, myocardial infarction, cellular metabolism, lipid peroxidation
Abstract
Identification of effective cardiac biomarkers and therapeutic targets for myocardial infarction (MI) will play an important role in early diagnosis and improving prognosis. Ferroptosis, a cell death process driven by cellular metabolism and iron-dependent lipid peroxidation, has been implicated in diseases such as ischaemic organ damage, cancer and neurological diseases. Its modulators were involved in transferrin receptor, iron chelator, clock protein ARNTL, etc. Its mechanisms included the inhibition of system X−C, diminished GPX4 activity, change of mitochondrial voltage-dependent anion channels and rising intracellular reactive oxygen species level. Further, the inhibitors of apoptosis, pyroptosis and autophagy did not prevent the occurrence of ferroptosis, but iron chelating agents and antioxidants could inhibit it. Noticeably, ferroptosis is an important pattern of cardiomyocyte death in the infarcted area, which may play a vital role in support of the myocardial pathological process of heart disease. However, the molecular mechanism of ferroptosis in the pathogenesis and the development of MI is not clear. Therefore, a greater depth of exploration of the mechanism of ferroptosis and its inhibitors will undoubtedly improve the pathological process of MI, which may be expected to identify ferroptosis as novel diagnostic and therapeutic targets of MI.
1. Introduction
Myocardial infarction (MI) is a pathological process in which the local myocardium is necrotic due to severe persistent ischaemia and hypoxia after coronary occlusion. MI is the primary cause of sudden cardiac death in the world, and the local ischaemia induces extensive tissue injury, heart failure and other complications [1]. Identification of effective cardiac biomarkers and therapeutic targets for MI will play an important role in early diagnosis and the improving prognosis.
Iron is not only the fourth richest element in Earth's crust, but also vital to cell survival since it is part of the Fe–S cluster proteins or as part of the Heme molecule of haemoglobin and myoglobin. In addition, the free divalent iron greatly accelerates lipid peroxidation of saturated fatty acids through Fenton chemistry reaction in humans, and iron also participates in the process of oxidative phosphorylation (OXPHOS) of mitochondria and its productions of reactive oxygen species (ROS) and adenosine triphosphate (ATP). ATP production via OXPHOS is instead needed for cell function; however, lipid peroxidation and ROS will kill the cells. When the oxidation caused by iron deposition exceeds the antioxidant capacity of cells, it is attributed to produce oxidative stress in cells, which directly and/or indirectly damages macromolecular proteins, nucleic acids and lipids, and triggers cell injury and death. The new pattern of cell death is termed ferroptosis [2].
It is necessary for ferroptosis to keep the concomitant effects such as drug treatments, inhibition of glutathione peroxidase 4 (GPX4) activity and other concomitant effects. In its modulators, transferrin receptor 1 (TFRC) is the coding gene of transferrin receptor; cellular uptake of free iron (Fe3+) occurs via receptor-mediated endocytosis of ligand-occupied transferrin receptor (formation of transferrin-iron complex) into specialized endosomes. Fe3+ is converted to Fe2+ in endosomes by the metalloreductase six-transmembrane epithelial antigen of prostate 3 (Steap3) and released from endosomes by divalent metal transporter 1 (DMT1) [3,4] but it may promote ferroptosis by upregulating TFRC. Again, free divalent iron (Fe2+) is turned into Fe3+ by Fenton chemistry reaction in the cytoplasm, which propagates the peroxidation reaction; therefore it is the Fenton reaction, not Fe3+ that actives lipid peroxidation. Because the most chemically reactive species of activated oxygen is hydroxyl radical (OH−), which is a highly mobile, water-soluble form of ROS that can initiate lipid peroxidation. Fenton and Fenton-like reactions are the main sources of hydroxyl radical formation [5]. Subsequently, the generated Fe3+ and the increasing ROS activate lipoxygenases that damage cellular membranes, especially for phosphatidylethanolamine-containing polyunsaturated fatty acids (PUFAs) [4–6] (figure 1). On the other hand, transferrin and transferrin receptor, which import iron from the extracellular environment, are required for ferroptosis. A recent study shows that iron can still accumulate in the liver without transferrin and cause ferroptosis, and slc39a14 functions as the hepatic transport of iron [7]. Lately, ferritinophagy is an autophagic phenomenon that specifically involves ferritin to release intracellular free iron. A recent report demonstrates that ferritinophagy is required for the induction of ferroptosis by the degradation of ferritin [8]. Additionally, the loss of cardiac Ferritin H facilitates cardiomyopathy via slc7a11-mediated ferroptosis [9].
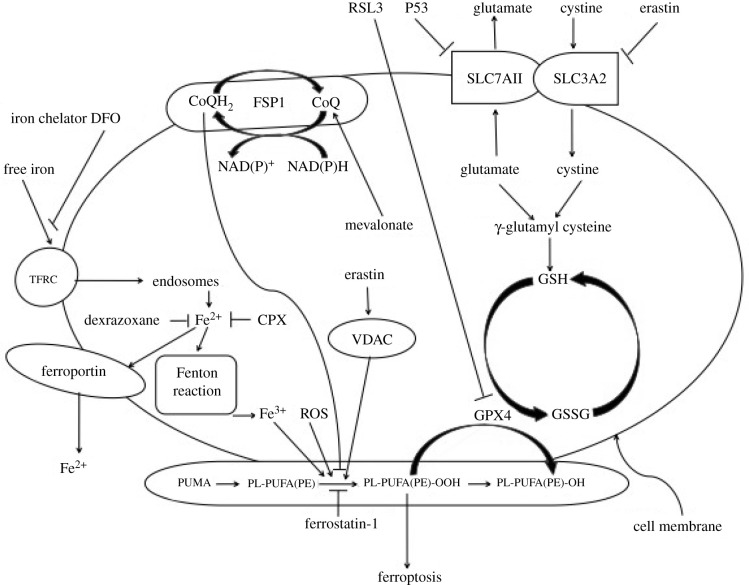
Figure 1.
The sketch of ferroptosis mechanism. SLC7A11, together with SLC3A2, encodes the heterodimeric amino acid transport System Xc−, a cystine/glutamine antiporter, and deprives cells of glutathione. Glutathione is a necessary substrate for GPX4, which uses glutathione to eliminate lipid peroxides formed in phospholipids containing PUFAs and prevents cell death. Ferroptosis is a newly identified pattern of cell death mediated by iron-induced lipid perxocidation in concomitance with the decreased activity/amount of GPX4. On the other hand, transferrin and transferrin receptor, which import iron from the extracellular environment, are required for ferroptosis. Free divalent iron (Fe2+) is turned into Fe3+ by Fenton chemistry reaction in cytoplasm, which propagates the peroxidation reaction. Notably, the myristoylation recruits FSP1 to the plasma membrane where it functions as an oxidoreductase that reduces CoQ 10, which acts as a lipophilic radical-trapping antioxidant that halts the propagation of lipid peroxides, thus FSP1 acts as a key component of a nonmitochondrial CoQ antioxidant system. Additionally, Fer-1 is a lipid antioxidant that inhibits ferroptosis. DFO binds Fe2+ mainly extracellularly, while dexrazoxane and CPX bind Fe2+ intracellularly. Ferroptosis inducers such as Ras Selective Lethal 3 (RSL3), directly inhibit GPX4, thus triggering the accumulation of lipid ROS and resulting in cell death.
Noticeably, the lipophilic iron chelator directly inactivates the iron-containing enzyme that promotes membrane lipid oxidation, permeates cell membrane and chelates the free ‘redox' iron pool, which reduces the products of catalytic soluble and lipid-free radicals in the iron pool to prevent cell ferroptosis. Alternatively, deferoxamine (DFO), the membrane-impermeable iron chelator, also prevents ferroptosis by chelating free iron (figure 1). Hence, the iron chelator prevents iron from transferring electrons to oxides and inhibits the generation of reactive oxygen species, thereby restricting ferroptosis [10]. Lately, ‘clockophagy', the selective degradation of the core circadian clock protein ARNTL (Aryl Hydrocarbon Receptor Nuclear Translocator Like Protein) by autophagy, facilitates ferroptosis induction [11].
Ferroptosis is generally caused by iron-dependent lipid peroxidation [12]. Regarded as programmed cell death, ferroptosis is involved in lipid metabolism, amino acid metabolism and iron metabolism. It is different from other patterns of cell death in biochemistry and morphology. In its biochemistry, ferroptosis is emerged with glutathione depletion, decreased cystine intake, iron deposition, rising lethal-free radicals and lipid peroxides [13]. On the other hand, ferroptosis sensitivity is also modulated by several other pathways and processes, including the mevalonate pathway. Since this pathway leads to the production of coenzyme Q10, its rate-limiting enzyme is HMG CoA reductase, which is crucial in myocardial infarction.
In its mechanisms, the inhibition of system X−C (membrane Na+-dependent cysteine-glutamate exchange transporter) can block the transport of intracellular glutamate to extracellular and extracellular cystine to intracellular, which induces ferroptosis. However, some cells make use of the transsulfuration pathway to biosynthesize cysteine from methionine and therefore bypass the requirement for cystine import via the cystine/glutamate antiporter system X−C, so this pathway is required for cellular metabolism. On the other hand, when erastin, the inducer of ferroptosis, depletes intracellular glutathione, which reduces the GPX4 activity and increases the iron-dependent ROS level in the cytoplasm and lipids, subsequently starting ferroptosis. Therefore, the degradation of glutamate via glutaminolysis is also a fuel of ferroptosis; glutaminolysis-targeted therapy may be effective in treating organ damage mediated by ferroptosis. The inhibition of glutaminolysis has been shown to attenuate ischaemia/reperfusion-induced heart damage [14].
Noticeably, erastin also binds to mitochondrial voltage-dependent anion channels (VDAC2 and VDAC3), changes the membrane permeability, slows down the oxidation of reduced form of nicotinamide-adenine dinucleotid (NADH), changes the iron selectivity of VDAC, whose channel only admits the cations to enter mitochondria, and causes mitochondrial dysfunction and oxidant release, and contributes to oxidation-dependent non-apoptotic cell death (i.e. ferroptosis [15]). In addition, the tumour suppressor gene (p53) increases the intracellular ROS level and triggers the stress, which eventually induces ferroptosis [16] (figure 1).
In the cellular morphology of ferroptosis, the cell membrane appeared rupture and vesicle; the mitochondria size was decreased; the double layers of mitochondria became thicker and denser; the mitochondrial ridges were decreased and even disappeared. The shape of the nucleus was natural without inside chromatin agglutination. The inhibitors of apoptosis, pyroptosis and autophagy did not prevent the occurrence of ferroptosis, but iron chelating agents and antioxidants could inhibit it [2].
Ferroptosis occurs not only in cancer cells [2], but also in neurons [17], even in cardiomyocytes, for example, with development of the reperfusion injury caused by revascularization after coronary occlusion, the cardiomyocytes arise ferroptosis and release inflammatory mediators to aggravate the heart injury. Notably, it has been found that doxorubicin can induce haemoglobin degradation and free iron release in the heart, and provoke ferroptosis in cardiomyocytes, which triggers heart failure [18]. Further, several clinical studies have shown that myocardial iron is an important independent risk factor for left ventricular remodelling after MI [19,20].
Apparently, searching new molecular targets for ferroptosis is gradually becoming the focus in the field of cardiovascular research. Importantly, quantitative proteomic analysis shows that the downregulation of GPX4 in MI contributes to the ferroptosis of cardiomyocytes [21]. However, Puerarin appears to alleviate ferroptosis and prevent heart failure via inducing ferritin production, reducing ROS and NOX4 (Non-phagocytic cell oxidase 4) [22]. In addition, Ferrostatin-1 (Fer-1), a ferroptosis inhibitor, reduces cardiomyocyte death, prevents the recruitment of neutrophils after heart transplantation, decreases the infarct size, diminishes left ventricular remodelling and improves left ventricular systolic function [23]. Thus, ferroptosis is an important pattern of cardiomyocyte death in the infarcted area, which may play a vital role in support of the myocardial pathological process of heart disease [4].
Ferroptosis, a cell death process driven by cellular metabolism and iron-dependent lipid peroxidation, has been implicated in diseases such as ischaemic organ damage; however, the molecular mechanism of ferroptosis in the pathogenesis and development of MI is not clear. Therefore, a greater depth of exploration of the mechanism of ferroptosis and its inhibitors will undoubtedly improve the pathological process of MI, which may be expected to identify ferroptosis as novel diagnostic and therapeutic targets of MI.
Abbreviations
ARNTL, aryl hydrocarbon receptor nuclear translocator like protein; ATP, adenosine triphosphate; CoQ, coenzyme Q; CPX, ciclopirox; DFO, deferoxamine; DMT1, divalent metal transporter 1; Fer-1, ferroptosis inhibitor Ferrostatin-1; FSP1, ferroptosis-suppressor-protein 1; GPX4, glutathione peroxidase 4; MI, myocardial infarction; NADH, reduced form of nicotinamide-adenine dinucleotid; NOX4, Non-phagocytic cell oxidase 4; OXPHOS, oxidative phosphorylation; p53, tumour suppressor gene; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; RSL3, Ras synthetic lethal 3; Steap3, metalloreductase six-transmembrane epithelial antigen of prostate 3; System X−C, membrane Na+-dependent cysteine-glutamate exchange transporter; TFRC, transferrin receptor 1; VDAC, voltage-dependent anion channels.
Data accessibility
This article has no additional data.
Authors' contributions
S.K conceived of the article, completed the literature search and wrote the draft. X.-d.W revised the manuscript and provided the fund support.
Competing interests
No authors have any potential conflicts of interest to be disclosed.
Funding
This work was supported in part by a Project of National Natural Science Foundation of China (grant no. 81870247, to S.K.), Beijing United Heart Foundation (grant no. BJUHFCSOARF201901-11, to X.-d.W), Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (grant no. PWZxq 2017-05) and Top-level Clinical Discipline Project of Shanghai Pudong District (grant no. PWYgf 2018-02).
References
- 1.Yamada Y, et al. 2018. S1P-S1PR2 axis mediates homing of muse cells into damaged heart for long-lasting tissue repair and functional recovery after acute myocardial infarction. Circ. Res. 122, 1069-1083. ( 10.1161/CIRCRESAHA.117.311648) [DOI] [PubMed] [Google Scholar]
- 2.Dixon SJ, et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060-1072. ( 10.1016/j.cell.2012.03.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen Z-N, Jiang X. 2019. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402-406. ( 10.1038/s41586-019-1426-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. 2019. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 99, 1765-1817. ( 10.1152/physrev.00022.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassannia B, Vandenabeele P, Vanden Berghe T. 2019. Targeting ferroptosis to iron out cancer. Cancer Cell 35, 830-849. ( 10.1016/j.ccell.2019.04.002) [DOI] [PubMed] [Google Scholar]
- 6.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. 2016. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966-E4975. ( 10.1073/pnas.1603244113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, et al. 2020. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136, 726-739. ( 10.1182/blood.2019002907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, Kang R, Tang D. 2016. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425-1428. ( 10.1080/15548627.2016.1187366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, et al. 2020. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ. Res. 127, 486-501. ( 10.1161/CIRCRESAHA.120.316509) [DOI] [PubMed] [Google Scholar]
- 10.Dixon SJ, Stockwell BR. 2014. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9-17. ( 10.1038/nchembio.1416) [DOI] [PubMed] [Google Scholar]
- 11.Yang M, et al. 2019. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 5, eaaw2238. ( 10.1126/sciadv.aaw2238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad M, Pratt DA. 2019. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137-1147. ( 10.1038/s41589-019-0408-1) [DOI] [PubMed] [Google Scholar]
- 13.Bersuker K, et al. 2019. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688-692. ( 10.1038/s41586-019-1705-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. 2015. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298-308. ( 10.1016/j.molcel.2015.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. 2016. Ferroptosis: process and function. Cell Death Differ. 23, 369-379. ( 10.1038/cdd.2015.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. 2015. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57-62. ( 10.1038/nature14344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. 2019. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 133, 221-233. ( 10.1016/j.freeradbiomed.2018.09.033) [DOI] [PubMed] [Google Scholar]
- 18.Fang X, et al. 2019. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. USA 116, 2672-2680. ( 10.1073/pnas.1821022116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Suhara T, Baba Y, Kawasaki NK, Higa JK, Matsui T. 2018. Pathological roles of iron in cardiovascular disease. Curr. Drug Targets 19, 1068-1076. ( 10.2174/1389450119666180605112235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba Y, et al. 2018. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 314, H659-H668. ( 10.1152/ajpheart.00452.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park TJ, et al. 2019. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 10, 835. ( 10.1038/s41419-019-2061-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S. 2018. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 497, 233-240. ( 10.1016/j.bbrc.2018.02.061) [DOI] [PubMed] [Google Scholar]
- 23.Li W, et al. 2019. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Invest. 129, 2293-2304. ( 10.1172/JCI126428) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.