Abstract
In response to novel environments, invasive populations often evolve rapidly. Standing genetic variation is an important predictor of evolutionary response but epigenetic variation may also play a role. Here, we use an iconic invader, the cane toad (Rhinella marina), to investigate how manipulating epigenetic status affects phenotypic traits. We collected wild toads from across Australia, bred them, and experimentally manipulated DNA methylation of the subsequent two generations (G1, G2) through exposure to the DNA methylation inhibitor zebularine and/or conspecific tadpole alarm cues. Direct exposure to alarm cues (an indicator of predation risk) increased the potency of G2 tadpole chemical cues, but this was accompanied by reductions in survival. Exposure to alarm cues during G1 also increased the potency of G2 tadpole cues, indicating intergenerational plasticity in this inducible defence. In addition, the negative effects of alarm cues on tadpole viability (i.e. the costs of producing the inducible defence) were minimized in the second generation. Exposure to zebularine during G1 induced similar intergenerational effects, suggesting a role for alteration in DNA methylation. Accordingly, we identified intergenerational shifts in DNA methylation at some loci in response to alarm cue exposure. Substantial demethylation occurred within the sodium channel epithelial 1 subunit gamma gene (SCNN1G) in alarm cue exposed individuals and their offspring. This gene is a key to the regulation of sodium in epithelial cells and may help to maintain the protective epidermal barrier. These data suggest that early life experiences of tadpoles induce intergenerational effects through epigenetic mechanisms, which enhance larval fitness.
This article is part of the theme issue ‘How does epigenetics influence the course of evolution?’
Keywords: Rhinella marina, epigenetic, alarm cue
1. Introduction
Epigenetic modifications (e.g. histone modifications, small RNA activity, DNA methylation) have important influences on development and can be induced by changes in the environment, including the environment provided by the parent [1]. Such changes can alter an individual's phenotype in an adaptive fashion but, because epigenetic marks are mostly cleared during early development in some taxa, the heritability of environmentally induced epigenetic modifications is controversial and their importance to evolution is unclear [2]. In addition to taxa-specific effects on heritability, epigenetic inheritance may depend on the environment and the mechanism of inheritance [3].
Invasive species provide ideal systems in which to study rapid responses to environmental change and the potential role of epigenetics in mediating these responses [4,5]. Small founding populations often have low levels of genetic diversity, limiting the amount of standing genetic variation available to selection. However, invasive populations with lowered genetic diversity often have increased epigenetic diversity compared with their native-range populations [4]. Further, introduced populations are likely to encounter environmental conditions different from those that exist in their native range, exposing these individuals to new selection regimes.
One intensively studied invasive species that has been shown to exhibit rapid evolutionary change is the cane toad (Rhinella marina; formerly Bufo marinus). Translocated from its native range in South America to sugar-cane plantations in many other countries, in an unsuccessful attempt at insect control, toads were released in north-eastern Australia in 1935 [6]. Over the course of their subsequent spread though that continent, toads have accumulated substantial phenotypic changes in a diverse suite of morphological, physiological and behavioural traits [7]. At least some of those changes are heritable [8,9]. However, phenotypic variation also occurs in response to environmental cues. For example, as for tadpoles of many anuran species [10], cane toad tadpoles often use inducible defences in response to predation risk. In cane toads, these defences can be induced by exposure to alarm cues produced by injured conspecifics [11]. Exposure to alarm cues causes tadpoles to modify their phenotype and behaviour in ways that can reduce predation risk, but producing a defence is also associated with fitness costs [12]; tadpoles exposed to alarm cues exhibit reduced rates of growth, development and survival [13–15], as well as altered glucocorticoid responses post-metamorphosis [16]. In addition, exposure to alarm cues alters tadpole DNA methylation [16], suggesting that this may provide a mechanistic basis through which the environment can alter phenotype. Chemical defences are a principal way in which cane toad tadpoles defend against Australian predators; the chemical cues and toxins produced by this phylogenetically distinct invader are repulsive or lethal to a wide variety of tadpole predators and thereby reduce predation risk [17,18]. Predator-induced plasticity in the potency of chemical cues could, therefore, be a highly effective strategy for dissuading predation [19], and may be an important component of the inducible defences produced by cane toad tadpoles. However, this possibility has not been assessed. In addition, the degree to which exposure to predation cues during the larval stage may influence offspring defences or performance remains unknown.
Intergenerational plasticity in inducible defences has been demonstrated in a variety of species and can improve offspring performance by preparing offspring for environmental conditions experienced by their parents [20–22]. In the current study, we investigate whether changing DNA methylation affects tadpole phenotype and whether these effects are intergenerational. We predicted that responses to tadpole alarm cues are mediated by changes to DNA methylation and that these effects would be transmitted to the next generation [21]. To test this hypothesis, we used two approaches known to affect DNA methylation in cane toad tadpoles: exposure to alarm cues from injured conspecifics and exposure to the DNA methylation inhibitor zebularine [16,23]. We predicted that within-generation exposure to alarm cues would increase the potency of the chemical cues produced by cane toad tadpoles, resulting in defensive costs such as reduced rates of tadpole growth, development and/or survival. We, therefore, predicted that the offspring of toads that had been exposed to predation cues during the tadpole stage may inherently exhibit stronger defences or reduced defensive costs (relative to the offspring of toads raised in the absence of these cues) [20,24].
2. Methods
(a). Sampling, experimental setup and animal husbandry
We collected wild cane toads from three locations each in the Australian range-core (R) and range-edge (E) and used them as founders (G0) for a common-garden experiment (see electronic supplementary material for locality details and full methods; figure 1, electronic supplementary material, table S1). We injected toads with leuprorelin acetate to induce spawning and collected Generation 1 (G1) egg clutches the following morning. Eggs were aerated prior to the start of experiments. In total, we produced 11 clutches from range-core pairs and 12 clutches from range-edge pairs. Because the sex of larval cane toads cannot be distinguished morphologically, all experiments described below contain mixed-sex tadpoles.
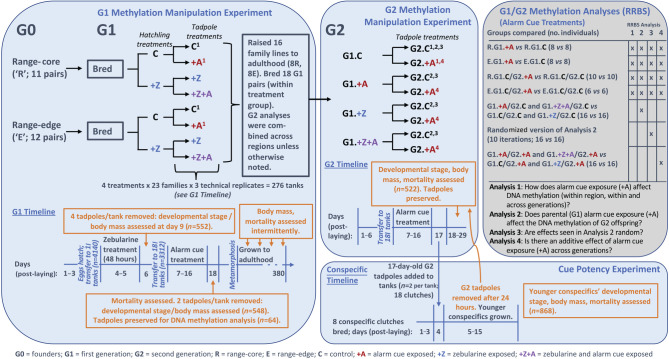
Figure 1.
Experimental design of common-garden experiment exploring intergenerational effects of methylation manipulation of cane toads. Left panel depicts the founders (G0) and first generation (G1), including the methylation manipulation experiment. Centre (upper) panel depicts the second generation (G2) manipulations, including parents' (G1) treatment. Bottom right panel depicts the cue potency experiment, in which conspecific tadpoles were exposed to tadpoles from the G2 methylation manipulation experiment (blue arrow). Top right panel describes which individuals were included in each DNA methylation analysis, including the geographic region where applicable, and treatment (G2 comparisons include their parents’ treatment codes). Timelines for each experiment are given, indicating the timepoints of assays (orange). Superscripts following treatment codes refer to the corresponding reduced representation bisulfite sequencing (RRBS) RRBS Analysis number in the methylation analyses table. Treatment codes are defined at the bottom of the figure.
(b). G1 DNA methylation manipulation experiment (zebularine and alarm cue treatment)
Seventy-two hours after egg deposition, we randomly selected a total of 180 hatchlings per clutch, and divided them evenly between zebularine (+Z) (100 µM; following [16]) and control (C) treatments, where they remained for 48 h (30 hatchlings per replicate; three replicates each treatment). Although little is known about developmental changes to DNA methylation in this species, zebularine treatment at days 4–5 post-laying has been shown to demethylate DNA in larval cane toads; on a global level, this treatment slightly decreases CpG methylation but has large locus-specific effects that may reduce or increase CpG methylation [16]. Post zebularine treatment, we removed four tadpoles from each tub, and at day 9 we euthanized, weighed and assessed them for development (Gosner) stage [25]. Of the remaining hatchlings, 72 from each zebularine treatment were allocated into 18 l tanks (density: 12 tadpoles per tank), which we randomly assigned to either alarm cue (A+) or control (C) treatments (3 replicates per treatment). This resulted in four treatment groups in a factorial design (C, +Z, +A, +Z+A; figure 1). We exposed tadpoles in each +A tank to approximately 4 ml bore water containing two crushed conspecifics strained through a fine mesh, at the same time each day (14.00–15.00 h) on 10 consecutive days (days 7–16 post-laying). We exposed control tanks to approximately 4 ml non-chlorinated water delivered in the same manner but without crushed conspecifics. On day 17, we assessed mortality in each tank and then removed two individuals per tank. On day 18, we euthanized, weighed, and assessed these two tadpoles per tank for developmental stage and preserved them in RNALater. We left the remaining tadpoles to metamorphose before transferring them to outdoor bins (see electronic supplementary material for details). We assessed mortality at the metamorph stage, and subsequently body mass and mortality at regular intervals until the toads were 380 days old.
(c). Second-generation DNA methylation manipulation experiment
After G1 toads reached adulthood, we bred a second generation (G2) from G1 toads paired by treatment group (n = 18 pairs), avoiding full-sibling pairings. Owing to low survival to adulthood of G1 toads in some treatment groups, region of origin (R and E) was ignored for G2 analyses. We split clutches and exposed G2 tadpoles either to alarm cues or to a sham control from days 7 to 16 post-laying, as described in the G1 DNA methylation manipulation experiment. G2 tadpoles were not directly exposed to zebularine (i.e. any zebularine effects on G2 tadpoles result from parental exposure in G1). On day 17, we assessed mortality in each tank and then removed two individuals per tank for the cue potency experiment (below). On day 18, the two G2 tadpoles that we removed from each tank were euthanized, weighed, staged and preserved in RNALater. The remaining G2 tadpoles in experimental tanks were euthanized, weighed and staged on days 18–29 for logistical reasons (see electronic supplementary material for further details).
(d). Cue potency experiment
To determine whether exposure to +A or +Z affected the potency of the chemical cues produced by cane toad tadpoles, we exposed the tadpoles from each treatment to conspecific hatchlings. Conspecific hatchlings exhibit clear, dose-dependent responses to the cues produced by cane toad tadpoles [26–28]. These effects are associated with the induction of defence against cannibalism by older conspecifics, which is a major source of mortality for cane toad eggs and hatchlings. To defend against this threat, hatchlings accelerate their development upon detecting cues produced by conspecific tadpoles [27]. The magnitude of the subsequent effects depends upon the level of risk perceived, such that stronger cues lead to larger reductions in viability during the tadpole stage [28]. Although stronger cues may generally indicate greater risk (i.e. that the cannibals are more abundant or closer), a shift in the potency of the cues produced by a tadpole could also lead to changes in the magnitude of this response (e.g. if a single tadpole produces disproportionately strong cues, the level of risk perceived by the hatchling may increase; J. L. DeVore 2020, personal communication). These effects are easily quantifiable in an experimental setting; indeed, the effects of exposure to conspecific tadpole cues are so pronounced that they were initially interpreted as allelopathic interference by older cannibal tadpoles [26]. Hatchlings, therefore, provide a useful indicator of the potency of the cues produced by conspecific tadpoles.
We assessed how our G1 and G2 treatments affected the impact of chemical cues from G2 tadpoles on conspecific hatchlings (figure 1, bottom right panel). To do so, we exposed hatchlings to 17-day-old G2 tadpoles from our DNA methylation manipulation experiments (details of experimental design are included in the electronic supplementary material). We separated hatchlings from older tadpoles by a fine mesh to prevent cannibalism. After 24 h, we removed the G2 tadpoles (see above, G2 methylation manipulation experiment) and left the hatchlings in the tubs containing G2 tadpole cues until they reached the free-swimming tadpole stage (stage 25; 48 h later). We then transferred them to new tubs, fed them ad libitum for the next 10 days (15 days old) and then euthanized them and assessed their weight and developmental stage, and calculated mortality for each tub.
(e). RRBS sequencing
We extracted DNA from whole G1 tadpoles (n = 11) for reduced representation bisulfite sequencing (RRBS) analyses and analysed these together with RRBS data from an additional 36 G1 tadpoles included in [16]. In total, we included the following G1 samples in our analyses (codes show region, generation and treatment group): R.G1.C (n = 8), R.G1.+A (n = 8), R.G1.+Z (n = 8), E.G1.C (n = 8), E.G1.+A (n = 8), E.G1.+Z (n = 7) (i.e. one individual per family per treatment). From G2, we extracted DNA from 64 individuals for RRBS analysis (n = 8 from each of the following groups; codes show parental G1 treatment/G2 treatment, see figure 1): G1.C/G2.C, G1.C/G2.+A, G1.+Z/G2.C, G1+Z/G2.+A, G1+A/G2.C, G1+A/G2.+A, G1.+Z+A/G2.C, G1.+Z+A/G2.+A). DNA was extracted using a Gentra Puregene Kit (QIAGEN, USA) following the manufacturer's instructions. The Ramaciotti Centre for Genomics (UNSW) conducted RRBS library preparation and sequencing using 100 ng of DNA and the Ovation RRBS Methyl-seq System (NuGEN Technologies, San Carlos, CA). Sequencing (1 × 100 bp reads) was conducted on the NovaSeq 6000 platform (Illumina, San Diego, CA).
3. Statistical analyses
(a). Ecological data
We analysed data on tadpole mass and stage with linear mixed-effects models using JMP Pro 14.2.0 (SAS Institute Inc., Cary, NC, 1989–2019). We log-transformed data prior to analysis to meet the assumption of normality, as required. For experiments that assessed treatment effects on mortality at a single time period, we classified mortality of tadpoles as a binomial response (dead versus alive) and analysed these data with logistic regression based on the quasi-binomial distribution to account for over-dispersion of data [29], using the package mass [30] in R [31]. We analysed treatment effects on mortality of post-metamorphic (terrestrial-stage) toads aged 6–12 months using mixed-effects Cox regression (coxme package [32]). Details for fixed effects and random effects for each specific analysis are given in the electronic supplementary material. We estimated the main effects by sequentially removing non-significant higher-order interaction terms, starting with the highest-order terms followed by highest p values. Experimental treatment effects were exposure to zebularine (yes/no), exposure to alarm cue (yes/no) and geographic origin (range-core/range-edge).
(b). DNA methylation analyses
RRBS data quality control and filtering parameters are included in the electronic supplementary material. We used the EDMR package [33] in R to identify differentially methylated regions (DMRs) that contained a minimum of 10% mean DNA methylation difference and at least five CpGs (cytosines followed by guanines). We aligned DMRs to the cane toad genome [34] using the closest-features algorithm in BEDOPS [35]. We used DMRs for four sets of analyses investigating alarm cue exposure (figure 1, top right panel). First, for Analysis 1, we compared DMRs identified in G1 tadpoles exposed to alarm cues (+A) versus controls (C) with DMRs identified in G2 tadpoles exposed to alarm cues (G1.C/G2.+A) versus controls (G1.C/G2.C). Note that the RRBS data for half of the G1 individuals used here were previously investigated, where it was determined that strong geographic regional differences exist in DNA methylation data [16]. For this reason, we conducted these comparisons within-region. This analysis allowed us to characterize effects of direct alarm cue exposure on two generations from two populations (i.e. four independent groups of tadpoles). For Analysis 2, we compared DNA methylation of G2 tadpoles whose parents were exposed (G1.+A/G2.C) versus G2 tadpoles from parents who had no exposure to alarm cues (G1.C/G2.C). Note that none of these individuals had direct exposure to alarm cues. We compared these results with those from RRBS Analysis 1 to determine whether the experience of G1 tadpoles affected the DNA methylation of their offspring. For RRBS Analysis 3, we created 10 randomized datasets containing all G2 individuals included in RRBS Analysis 2 so that each randomized treatment group contained 50% of the G2 tadpoles from G1 control family lines (G1.C/G2.C) and 50% of the G2 tadpoles from G1 families exposed to alarm cues (G1.+A/G2.C). This enabled us to discern whether RRBS Analysis 2 represented an actual effect or was just the result of random differences in DNA methylation between individuals. Finally, for RRBS Analysis 4, we compared DNA methylation of G2 tadpoles that were directly exposed to alarm cues and whose parents were exposed to alarm cues (G1.+A/G2.+A) versus control tadpoles that were directly exposed to alarm cues whose parents were controls (G1.C/G2.+A). We compared these results with those from RRBS Analysis 1, which also enabled us to determine whether the experience of G1 tadpoles affected G2 tadpoles and whether there was an additive effect of exposures in both generations on DNA methylation of G2 tadpoles. The individuals used in RRBS Analysis 2 were full-siblings of those used in RRBS Analysis 4. Finally, using the same methodology, we investigated methylation shifts in zebularine-exposed G1 tadpoles (G1.+Z versus G1.C, regions combined to be comparable with G2 individuals) and in their G2 unexposed offspring (G1.+Z/G2.C versus G1.C/G2.C).
4. Results
(a). Generation 1 DNA methylation manipulation experiment
At day 9, tadpoles that were exposed to zebularine exhibited greater mean body mass and developmental stage than controls (table 1a,b; electronic supplementary material, table S1a,b and figure S1a,b), but this effect was no longer evident at day 18 (electronic supplementary material, table S2a,b). There was no significant effect of geographic origin on body mass or developmental stage at day 9 (electronic supplementary material, table S1a,b). However, at day 18 there was an effect of geographic origin on tadpole body mass and development, with range-core tadpoles growing and developing faster than range-edge tadpoles (table 1c,d; electronic supplementary material, table S2a,b and figure S2a,b). At day 18, there was no significant effect of exposure to alarm cue on tadpole body mass or development (electronic supplementary material, table S2a,b). Tadpole density within the tanks also affected development (table 1d).
Table 1.
Summary of treatment effects for G1 (a–h) and G2 (i–l) (p-values <0.05 shown in bold). Full model results for all phenotypic analyses are listed in the electronic supplementary material. Zeb, zebularine treatment.
| response variable | fixed effects | estimate | d.f. | t/F/z | p |
|---|---|---|---|---|---|
| Generation 1 | |||||
| (a) day 9 tadpole growth | Zeb | −0.014 | 1, 476.5 | 6.529 | 0.011 |
| (b) day 9 tadpole development | Zeb | −0.047 | 1, 479.2 | 4.158 | 0.042 |
| (c) day 18 tadpole growth | Origin | 5.259 | 1, 18.97 | 4.714 | 0.043 |
| (d) day 18 tadpole development | Density | −0.463 | 1, 264.4 | 13.324 | <0.001 |
| Origin | 0.608 | 1, 18.94 | 8.679 | 0.008 | |
| (e) day 18 tadpole mortality | Alarm Cue | 0.487 | 250 | 2.599 | 0.010 |
| (f) metamorph mortality | Alarm Cue | 0.198 | 250 | 1.954 | 0.052 |
| (g) adult growth 6–12 months | Time | 0.006 | 1, 1221 | 2450.2 | <0.0001 |
| Density | −1.531 | 1, 1215 | 284.6 | <0.0001 | |
| Time × Origin × Zeb | 0.0002 | 1, 1212 | 4.369 | 0.037 | |
| Time × Origin × Alarm Cue | 0.01 | 1, 1212 | 3.766 | 0.053 | |
| (h) adult mortality 6–12 months | Density | −235.664 | 11.981 | −19.670 | <0.0001 |
| Generation 2 | |||||
| (i) tadpole growth | Density | 8.0968 | 1,64.85 | 19.6965 | <0.0001 |
| (j) tadpole mortality | G1 Zeb × G2 Alarm Cue | −1.8875 | 68 | −2.3750 | 0.0204 |
| G1 Alarm Cue × G2 Alarm Cue | −2.4516 | 68 | −3.1543 | 0.0024 | |
| (k) potency of G2 tadpole cues—younger conspecific tadpole growth | G1 Alarm Cue | 0.016 | 1,19.4 | 4.961 | 0.038 |
| G2 Alarm Cue | 0.045 | 1, 143.3 | 89.323 | <0.0001 | |
| (l) potency of G2 tadpole cues—younger conspecific tadpole development | G2 Alarm Cue | 0.379 | 1, 156.4 | 49.498 | <0.0001 |
Neither geographic origin nor exposure to zebularine affected tadpole mortality at day 18 (electronic supplementary material, table S3). However, exposure to alarm cues resulted in a higher mortality (table 1e; electronic supplementary material, figure S3). There was no significant effect of geographic origin or exposure to zebularine on the probability of mortality for metamorphs (electronic supplementary material, table S3a), but exposure to alarm cues resulted in a marginally non-significant increase in the probability of metamorph mortality (table 1f). Unsurprisingly, there was an effect of both time and tank density on toad growth from 6 to 12 months (table 1g). There was also significant Time × Origin × Zebularine interaction for toad growth at 6–12 months (table 1g; electronic supplementary material, figure S4). The Time × Origin × Alarm Cue interaction for toad growth was marginally non-significant (p = 0.053, table 1i; electronic supplementary material, table S3b). There was no significant effect of origin, zebularine or alarm cue on mortality of toads at 6–12 months, but density affected toad mortality (table 1h; electronic supplementary material, table S3c).
(b). Generation 2 DNA methylation manipulation experiment
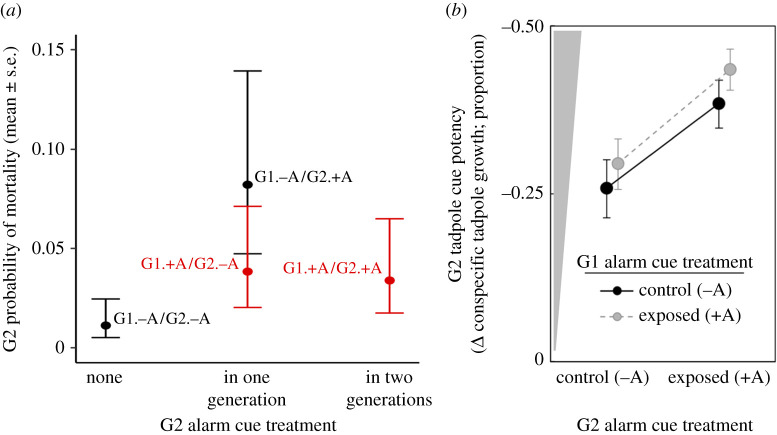
There was no significant effect of G1 treatment on growth or development of G2 tadpoles at days 18–29, nor was there an effect of direct exposure of G2 tadpoles to alarm cues on growth or development (electronic supplementary material, table S4a,b). There was an effect of tank density on tadpole growth (table 1i). With respect to G2 tadpole mortality, there were interactions between G2 tadpole alarm cue treatment and their G1 parents' treatments (table 1j). G2 tadpoles from control parents that were directly exposed to alarm cues had a higher probability of mortality than those with no direct exposure to alarm cues (figure 2a, black points; electronic supplementary material, table S4, p = 0.009). However, G2 tadpoles from alarm cue exposed parents that were themselves directly exposed to alarm cues were no more likely to die than were G2 tadpoles from control parents that were not themselves directly exposed to alarm cues (i.e. tadpoles that experienced the same environment as their parents performed similarly, regardless of the nature of that environment; figure 2a, right red (grey in printed version) data point and left black data point; electronic supplementary material, table S4, p = 0.52). The same pattern was found with respect to parental exposure to zebularine (electronic supplementary material, figure S5 and table S4).
Figure 2.
Phenotypic effects of methylation manipulations on second generation (G2) cane toad tadpoles. (a) Interaction of G1 alarm cue exposure × G2 alarm cue exposure on the probability of mortality of G2 tadpoles. G2 tadpoles whose parents were not exposed to alarm cues and were not directly exposed to alarm cues themselves had the lowest rates of mortality (G1.–A/G2.–A, left data point) and significantly lower mortality rates than those whose parents had no exposure to alarm cues but were directly exposed to alarm cues themselves (G1.–A/G2.+A, upper-middle data point; electronic supplementary material, table S4, p = 0.009). G2 tadpoles whose parents were exposed to alarm cues (G1.+A/G2.−A, lower-middle data point; G1.+A/G2.+A, right data point) did not have significantly different mortality rates compared with tadpoles in any other treatment group. (b) Effect of G1 and G2 alarm cue exposure on tadpole cue potency (measured by the change in conspecific growth). Grey wedge represents the strength of cue. (Online version in colour.)
(c). Cue potency experiment
We observed significant alarm cue-induced plasticity in the potency of tadpole chemical cues; G2 tadpoles directly exposed to alarm cues affected the growth and developmental stage of younger conspecifics more strongly than did G2 tadpoles that were raised in control conditions (figure 2b; electronic supplementary material, table S5a,b). There was also a significant carry-over effect of G1 alarm cue treatment on the potency of G2 tadpole cues in terms of the growth of younger conspecifics: the offspring of G1 toads that were exposed to alarm cues as tadpoles produced slightly stronger cues than did the offspring of G1 toads that were reared in control conditions (table 1k,l; figure 2b). This generational carry-over effect of exposure to alarm cues did not modify the induced reaction norm during G2 (i.e. the previous exposure had an additive effect [36] (G1 Alarm Cue × G2 Alarm Cue interaction, p = 0.419; electronic supplementary material, table S5a)). There was no significant carry-over effect of G1 alarm cue treatment on the potency of G2 tadpole cues in terms of development responses of younger conspecifics (electronic supplementary material, table S5b).
(d). DNA methylation analyses
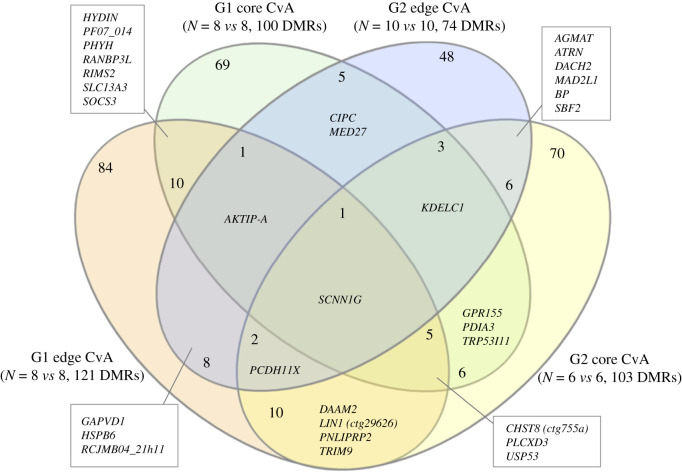
For G1 individuals in RRBS Analysis 1 (i.e. how direct alarm cue exposure affects DNA methylation, figure 1), we found 121 DMRs in range-edge samples and 100 DMRs in range-core samples (figure 3). Seventeen DMRs were identified in both regions, five of which were located in annotated genes (figure 3). In G2 individuals, we found 74 DMRs in range-edge samples and 103 DMRs in range-core samples (figure 3). Twelve DMRs were identified in both regions, eight of which were located in annotated genes (figure 3). Of the total of 328 distinct DMRs found in these four comparisons, 41 DMRs were found in both generations. Only one DMR was differentially methylated in all comparisons and this was located with the SCNN1G gene; six other DMRs were found in three out of four of these comparisons, and an additional 24 genes were found to be differentially methylated in two comparisons (electronic supplementary material, table S6). Most of the DMRs listed in electronic supplementary material, table S6 were located within gene bodies; three DMRs were located upstream and one DMR overlapped with the transcription start site.
Figure 3.
Venn diagram indicating the intersection of differentially methylated regions (DMRs) of the genome identified in first (G1) and second (G2) cane toad tadpoles in response to alarm cue exposure in range-core and range-edge samples (RRBS Analysis 1). Each comparison is alarm cue exposed (+A) versus control (C). Annotated genes associated with identified DMRs are shown.
For RRBS Analysis 2 (i.e. how parental (G1) alarm cue exposure affects DNA methylation in G2 offspring, figure 1), we found 332 DMRs between tadpoles from G1 control families and those from G1 families exposed to alarm cues (electronic supplementary material, figure S6 and table S7). Ninety-two of these DMRs were also found in RRBS Analysis 1, including the DMR in SCNN1G. For RRBS Analysis 3 (i.e. whether the result found in Analysis 2 is random, figure 1), we found fewer DMRs in random dataset comparisons (mean = 185, ranging from 140 to 253 DMRs) than in RRBS Analysis 2. We also found substantially fewer DMRs that overlapped with those identified in RRBS Analysis 1 (mean = 62, ranging from 50 to 75 overlapping DMRs). For RRBS Analysis 4 (i.e. whether there are additive effects of alarm cue exposure across generations, figure 1), we identified 678 DMRs, 110 of which overlapped with RRBS Analysis 1.
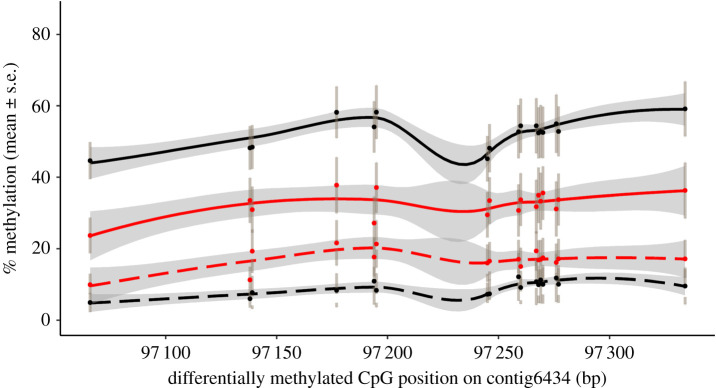
We examined DNA methylation of individual CpGs in the DMR found in SCNN1G (figure 4). This DMR contained 17 differentially methylated cytosines, all of which exhibited the highest levels of DNA methylation in G2s that were unexposed to alarm cues and were from G1 control families (figure 4, G1.C/G2.C). G2 tadpoles that experienced direct exposure to alarm cues but were from G1 control families (figure 4, G1.C/G2.+A) had less DNA methylation at this locus than did G1.C/G2.C individuals. G1 history had a larger impact on DNA methylation of this locus in G2 tadpoles than did the direct exposure to alarm cues on G2s (figure 4, top two lines represent G2 offspring from G1 control families, bottom two from G1 alarm cue exposed families).
Figure 4.
Mean percentage CpG methylation at the differentially methylated region contained in the SCNN1G gene of second generation (G2) cane toad tadpoles. Solid lines represent G2 individuals from first generation (G1) control families; dashed lines represent G1 alarm cue exposed families. Black lines represent unexposed G2 individuals; red lines represent alarm cue exposed G2s (grey in printed version). Mean methylation of differentially methylated CpGs is shown (dots; bars are standard errors). (Online version in colour.)
We identified 44 DMRs in tadpoles exposed to zebularine in G1 (G1.+Z versus G1.C) that were also identified in the analysis of their unexposed offspring (G1.+Z/G2.C versus G1.C/G2.C) (electronic supplementary material, table S8). Twenty of these DMRs were also found in tadpoles exposed to alarm cues.
5. Discussion
In the current study, we investigated how methylation manipulation affects the phenotypic traits of cane toads from early developmental stages through to adulthood. We also investigated which genomic regions were affected by our manipulations and asked whether associated changes in phenotype and DNA methylation were evident in the subsequent generation. Tadpole cue potency, growth, development and mortality were all affected by direct exposure to methylation manipulations. Interestingly, intergenerational effects were also identified: second-generation tadpoles of parents that had been exposed to alarm cues exhibited overall increases in cue potency and decreased mortality in environments where predation cues were present. Similarly, second-generation tadpoles of parents exposed to zebularine exhibited decreased mortality in environments where predation cues were present. DNA methylation changes were identified in all RRBS analyses, including in those that compared tadpoles of exposed parents that had not themselves been directly exposed to treatments. Many of these changes to DNA methylation were also seen in G2 tadpoles that had no direct exposure to treatments, demonstrating the stability of some of these shifts. Most DMRs identified were exclusive to a single comparison but one DMR within the SCNN1G gene was consistently differentially methylated; this gene plays an important role in the integrity of the epidermal barrier [37,38].
Exposure to both alarm cues and zebularine affected DNA methylation in cane toad tadpoles, albeit in different ways [16]. In the present study, we found no effect of alarm cue exposure on 18-day-old G1 tadpole growth or development, and only weak geographic region-specific support for impacts on growth in G1 juveniles aged between 6 and 12 months. However, we found differences in growth and development in 9-day-old G1 tadpoles exposed to zebularine. These zebularine-exposed tadpoles had accelerated development and they were larger than controls (results from both regions). Interestingly, juvenile (6–12 months old) toads from the range-core that were exposed to zebularine were smaller than controls but this effect was not seen in range-edge toads, suggesting the latter are less plastic in their response (as inferred by [39]).
We found evidence that direct exposure to alarm cues affects DNA methylation and, although dozens of DMRs were common between geographic regions and across generations, most DMRs were not found in multiple comparisons. We attribute this to three factors: (i) regional differences in population genetics within Australian toads [40] and the association of genetic similarity with DNA methylation patterns [16]; (ii) DNA methylation is labile and affected by environment, so analyses that included comparisons across generations (i.e. at different times) will likely include subtle experimental differences that could affect methylation; and (iii) given the apparent intergenerational similarities between DNA methylation patterns we see here and the differences between range-core and range-edge environments (hotter and drier at the range-edge; [40]), lineages from different geographic regions were likely to have had different DNA methylation profiles prior to our manipulations that may have persisted throughout the experiment. Further, there are substantial differences in behavioural, morphological and physiological traits between range-core and range-edge toads [7] and some of these traits may affect predator interactions. For example, range-edge toads are more exploratory than those from the range-core [41], which may increase their likelihood of encountering predators. Parotoid glands are smaller in native-range toads than invasive Australian toads, presumably because the toxin is more effective against naive predators in introduced ranges [42]. Range-edge toads in Australia have relatively larger parotoid glands ([43], but see [42]), which may be a response to increased predation threat to these animals or a more unpredictable predator landscape in newly colonized areas. Fewer and potentially more predictable interactions with predators at the range-core could result in a lower cost of transmitting epigenetic marks to offspring in response to alarm cue exposure, potentially favouring intergeneration effects. While we did find regional differences in where DMRs were located in the genome, our sample sizes in the second generation required that we combine samples across regions, preventing analysis of the magnitude of these effects with respect to geographic region.
Despite the differences we found in methylation patterns across regions and generations, we did identify a single DMR found in range-core and range-edge comparisons in G1 and G2 individuals directly exposed to alarm cues (figure 1, RRBS Analysis 1) as well as in comparisons of G2 controls whose parents were exposed versus not exposed to alarm cues (figure 1, RRBS Analysis 2). This suggests that this DMR (within the SCNN1G gene) is located in a functionally important region of the genome with respect to tadpoles’ response to alarm cues. The SCNN1G gene codes for the gamma subunit of the epithelial Na+ channel (ENaC), which regulates sodium absorption and secretion (reviewed in [44]), and is integral to the barrier function of skin [35,36]. The skin is the primary defence against physical, chemical or microbial exposure an individual might encounter. In amphibians, Na+ is absorbed into the skin (also urinary bladder and kidney, [45,46]) from the local environment or transcellular fluids [39]. ENaC also directs galvanotaxis in keratinocytes during the wound healing process [47]. Of the remaining six genes containing DMRs in three of four comparisons of direct exposure to alarm cues (RRBS Analysis 1), half were associated with gene ontology terms ‘plasma membrane’ or ‘cell junction’ (AKTIP-A, PCDH11X, USP53), as were an additional two genes found in two of four comparisons of direct exposure to alarm cues (RIMS2 and SLC13A3; electronic supplementary material, table S6). Range-edge toads have significantly lower cutaneous resistance to water loss than do toads from the range-core [48], suggesting a possible role of ENaC in the adaptation of cane toads to their environmental conditions. It is plausible that the differential methylation we found at this gene was caused by the physiological response that receiver tadpoles mounted when their skin contacted cues from crushed conspecifics. SCNN1G, PCDH11X and SLC13A3 were all differentially methylated in the G2 offspring whose parents were exposed to alarm cues (G1.+A/G2.C versus G1.C/G2.C; electronic supplementary material, table S7). Given the lability of DNA methylation, it is remarkable that we were able to identify intergenerational DNA methylation changes in these genes. While we provide evidence here of shifts in DNA methylation in response to our treatments, future gene expression studies of these genes are needed to clarify the functional significance of these changes.
Our treatments affected tadpole growth, development and mortality, so it is unsurprising that we identified DMRs in a range of genes involved in growth and development. The SOCS3 gene inhibits leptin signalling [49], which regulates food consumption in tadpoles [50]. DAAM2 regulates tissue and organ development [51] and is widely expressed across anuran embryogenesis [52]. TRIM9 is highly expressed in the mouse brain during embryogenesis [53], and is key to neuronal development [54]. ATRN contains multiple epidermal growth factor domains and is involved in myelination, cell adhesion and homeostasis [55]. A number of other genes we identified as differentially methylated are also important to development (e.g. DACH2, GPR155, HYDIN, MED27), any of which could be responsible for the phenotypic effects we have identified.
We found evidence of inducible plasticity in the potency of tadpole chemical cues. G2 tadpoles that were directly exposed to alarm cues had stronger effects on conspecific hatchlings than did control tadpoles, causing greater reductions in their growth and development. Here, we also found intergenerational effects: G2 tadpoles whose parents (i.e. G1) were exposed to alarm cues had stronger effects on the growth of conspecific hatchlings than did G2 tadpoles from control families. However, this effect was weaker than the effect induced by directly exposing tadpoles to alarm cues, suggesting that this signal is attenuated when parentally transmitted. Regardless, our finding of intergenerational inducible defences demonstrates that experiences individuals have in the aquatic larval environment, long before reaching the terrestrial reproductive stage, can affect the larval defences of their offspring.
Although tadpoles whose parents had been exposed to alarm cues produced more potent chemical cues, perhaps the greatest benefit of a shared parent–offspring environment was evident in terms of the costs of producing the inducible defence. Although direct exposure to alarm cues increased mortality in tadpoles (in G1 tadpoles and in G2 tadpoles whose parents were unexposed), we also found intergenerational effects of both alarm cue and zebularine exposure with respect to G2 tadpole mortality. However, rather than having a negative effect, both treatments increased offspring survival when offspring were directly exposed to alarm cues themselves (i.e. offspring of G1 controls exhibited high survival rates when they were also raised in control conditions, but were more likely to die than other treatment groups if exposed to alarm cues). This result implies that the costs of producing the inducible defence were reduced for tadpoles whose parents had also been reared in the presence of predation cues. In environments where predators are frequently encountered, this intergenerational effect may reduce the costs of producing inducible defences. As the costs of producing inducible defences can significantly reduce fitness, processes through which these costs can be offset by parental experiences could strongly influence the adaptive value of inducible defences. Interestingly, when we investigated DNA methylation responses in G2 tadpoles that were exposed to alarm cues and whose parents were or were not exposed to alarm cues (figure 1, RRBS Analysis 4), we found the highest number of DMRs of all comparisons (n = 678; compared with 332 in G2 tadpoles directly exposed, RRBS Analysis 2).
Our results suggest that experiences of G1 cane toads during early development affect the phenotype and DNA methylation of their offspring. Because primordial germ cells (PGCs) require totipotency, epigenetic marks are reprogrammed in many species during development [56,57]. Despite this, epigenetic inheritance has been demonstrated across a wide range of taxa (summarized in [2,58]). Nonetheless, it is not possible to determine from our experiments whether the methylation changes described here are transgenerational (i.e. heritable beyond a single generation). In amphibians, epigenetic reprogramming occurs in PGCs formed during Gosner stages 25–40 of their parents [57], which incorporates the window during which we exposed G1 tadpoles to alarm cues. Therefore, while epigenetic inheritance remains a possibility, it also is possible that G2 offspring were directly exposed as PGCs while their parents were developing.
Transgenerational plasticity can play an important role in enhancing offspring success and this may be especially true for invasive species. Because a multitude of environmental effects can shift rapidly during the invasion process (e.g. predator assemblages, conspecific densities, abiotic conditions), any process through which parents can use their experiences to facilitate the production of an adaptive phenotype in their offspring is likely to be favoured. Here, the intergenerational effects we detected appear to improve offspring fitness. These effects may, therefore, be under selection and have evolutionarily important implications. However, the degree to which these effects persist across generations is unknown; if conditions shift rapidly, the relevance of the parental environment may be limited beyond the first generation. In contrast, if the environment is relatively stable, we could see ‘run-away’ effects of these traits: within predator-rich environments, multigenerational exposure to predation cues could produce stronger and stronger defences until physiological limits are reached—especially because the cost of mounting this response appears to be low. Alterations in DNA methylation appear to underlie these effects; both exposure to alarm cues and artificially induced shifts in DNA methylation (using zebularine) resulted in similar phenotypic effects in both exposed individuals and their offspring. This similarity implies that the effect of alarm cue exposure is mediated by DNA methylation changes, and supports the hypothesis that epigenetic responses may provide a multigenerational bridging strategy until traits can be canalized or until the environmental driver dissipates.
Acknowledgements
We thank Paul Waters and Manish Kumar for conversations regarding analyses, Katarina Stuart for assistance with bioinformatics, Holly Trim for DNA extractions and Bill Sherwin for useful conversations regarding the interpretation of results.
Data accessibility
CpG reports from RRBS data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.pk0p2ngmv [59].
Authors' contributions
Conception and design of experiment: R.R.S., M.R.C., H.J.F.E., J.L.D., R.S., L.A.R. Data acquisition: R.R.S., M.R.C., H.J.F.E., M.C., J.Z. Analyses: R.R.S., M.R.C., J.L.D., R.J.E., G.P.B., L.A.R. Drafting manuscript: R.R.S., M.R.C., J.L.D., R.S., L.A.R. All authors critically revised and approved the final manuscript.
Competing interest
The authors have no competing interests.
Funding
We thank the Australian Research Council for research support through DP160102991 to R.S. and L.A.R. and DE150101393 to L.A.R.
References
- 1.Angers B, Castonguay E, Massicotte R. 2010. Environmentally induced phenotypes and DNA methylation: how to deal with unpredictable conditions until the next generation and after. Mol. Ecol. 19, 1283-1295. ( 10.1111/j.1365-294X.2010.04580.x) [DOI] [PubMed] [Google Scholar]
- 2.Legoff L, D'Cruz SC, Tevosian S, Primig M, Smagulova F. 2019. Transgenerational inheritance of environmentally induced epigenetic alterations during mammalian development. Cells 8, 1559. ( 10.3390/cells8121559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stajic D, Jansen LET. 2021. Empirical evidence for epigenetic inheritance driving evolutionary adaptation. Phil. Trans. R. Soc. B 376, 20200121. ( 10.1098/rstb.2020.0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawes NA, Fidler AE, Tremblay LA, Pochon X, Dunphy BJ, Smith KF. 2018. Understanding the role of DNA methylation in successful biological invasions: a review. Biol. Invasions 20, 2285-2300. ( 10.1007/s10530-018-1703-6) [DOI] [Google Scholar]
- 5.Mounger J, Ainouche ML, Bossdorf O, Cavé-Radet A, Li B, Parepa M, Salmon A, Yang J, Richards CL.. 2021. Epigenetics and the success of invasive plants. Phil. Trans. R. Soc. B 376, 20200117. ( 10.1098/rstb.2020.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shine R. 2018. The impact of an invasive amphibian: the cane toad Rhinella marina. In Status of conservation and decline of amphibians: Australia, New Zealand, and Pacific Islands (eds H Heatwole, JJL Rowley), pp. 107-124. Clayton, Australia: CSIRO Publishing. [Google Scholar]
- 7.Rollins LA, Richardson MF, Shine R. 2015. A genetic perspective on rapid evolution in cane toads (Rhinella marina). Mol. Ecol. 24, 2264-2276. ( 10.1111/mec.13184) [DOI] [PubMed] [Google Scholar]
- 8.Hudson CM, Brown GP, Shine R. 2016. It is lonely at the front: contrasting evolutionary trajectories in male and female invaders. R. Soc. Open Sci. 3, 160687. ( 10.1098/rsos.160687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GP, Phillips BL, Shine R. 2014. The straight and narrow path: the evolution of straight-line dispersal at a cane toad invasion front. Proc. R. Soc. B 281, 20141385. ( 10.1098/rspb.2014.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Buskirk J. 2000. The costs of an inducible defense in anuran larvae. Ecology 81, 2813-2821. ( 10.1890/0012-9658(2000)081[2813:TCOAID]2.0.CO;2) [DOI] [Google Scholar]
- 11.Alford R. 1999. Ecology: resource use, competition, and predation. In Tadpoles: the biology of anuran larvae (eds McDiarmid RW, Altig R), pp. 240-278. Chicago, IL: University of Chicago Press. [Google Scholar]
- 12.Harvell CD. 1990. The ecology and evolution of inducible defenses. Q. Rev. Biol. 65, 323-340. ( 10.1086/416841) [DOI] [PubMed] [Google Scholar]
- 13.Crossland MR, Salim AA, Capon RJ, Shine R. 2019. The effects of conspecific alarm cues on larval cane toads (Rhinella marina). J. Chem. Ecol. 45, 838-848. ( 10.1007/s10886-019-01111-2) [DOI] [PubMed] [Google Scholar]
- 14.Hagman M, Shine R. 2009. Factors influencing responses to alarm pheromone by larvae of invasive cane toads, Bufo marinus. J. Chem. Ecol. 35, 265. ( 10.1007/s10886-009-9592-x) [DOI] [PubMed] [Google Scholar]
- 15.Hagman M, Shine R. 2009. Larval alarm pheromones as a potential control for invasive cane toads (Bufo marinus) in tropical Australia. Chemoecology 19, 211. ( 10.1007/s00049-009-0027-5) [DOI] [Google Scholar]
- 16.Sarma RR, et al. 2020. Do epigenetic changes drive corticosterone responses to alarm cues in larvae of an invasive amphibian? Integr. Comp. Biol. 60, 1481-1494. ( 10.1093/icb/icaa082) [DOI] [PubMed] [Google Scholar]
- 17.Caller G, Brown C. 2013. Evolutionary responses to invasion: cane toad sympatric fish show enhanced avoidance learning. PLoS ONE 8, e54909. ( 10.1371/journal.pone.0054909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crossland MR, Alford RA. 1998. Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura: Bufonidae) to native Australian aquatic predators. Aust. J. Ecol. 23, 129-137. ( 10.1111/j.1442-9993.1998.tb00711.x) [DOI] [Google Scholar]
- 19.Hettyey A, Tóth Z, Van Buskirk J. 2014. Inducible chemical defences in animals. Oikos 123, 1025-1028. ( 10.1111/oik.01338) [DOI] [Google Scholar]
- 20.Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60-63. ( 10.1038/43425) [DOI] [Google Scholar]
- 21.Tariel J, Plénet S, Luquet É. 2020. Transgenerational plasticity in the context of predator-prey interactions. Front. Ecol. Evol. 8, 548660. ( 10.3389/fevo.2020.548660) [DOI] [Google Scholar]
- 22.Yin J, Zhou M, Lin Z, Li QQ, Zhang YY. 2019. Transgenerational effects benefit offspring across diverse environments: a meta-analysis in plants and animals. Ecol. Lett. 22, 1976-1986. ( 10.1111/ele.13373) [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Cheng X, Connolly B, Dickman M, Hurd P, Hornby D. 2002. Zebularine: a novel DNA methylation inhibitor that forms a covalent complex with DNA methyltransferases. J. Mol. Biol. 321, 591-599. ( 10.1016/S0022-2836(02)00676-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway LF, Etterson JR. 2007. Transgenerational plasticity is adaptive in the wild. Science 318, 1134-1136. ( 10.1126/science.1148766) [DOI] [PubMed] [Google Scholar]
- 25.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183-190. [Google Scholar]
- 26.Crossland MR, Hearnden MN, Pizzatto L, Alford RA, Shine R. 2011. Why be a cannibal? The benefits to cane toad, Rhinella marina [=Bufo marinus], tadpoles of consuming conspecific eggs. Anim. Behav. 82, 775-782. ( 10.1016/j.anbehav.2011.07.009) [DOI] [Google Scholar]
- 27.DeVore J, Crossland M, Shine R. 2020. Tradeoffs affect the adaptive value of plasticity: stronger cannibal-induced defenses incur greater costs in toad larvae. Ecol. Monogr. 91, e01426. ( 10.1002/ecm.1426) [DOI] [Google Scholar]
- 28.McCann S, Crossland M, Greenlees M, Shine R. 2020. Field trials of chemical suppression of embryonic cane toads (Rhinella marina) by older conspecifics. Ecol. Evol. 10, 10 177-10 185. ( 10.1002/ece3.6678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warton DI, Hui FK. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3-10. ( 10.1890/10-0340.1) [DOI] [PubMed] [Google Scholar]
- 30.Ripley B, Venables B, DM Bates, Hornik K, Gebhardt A, Firth D, Ripley MB.. 2013. Package ‘mass'. Cran r 538, 113-120. [Google Scholar]
- 31.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://wwwR-project.org/. [Google Scholar]
- 32.Therneau TM, Therneau MTM. 2015. Package ‘coxme’. Mixed effects Cox models R package version. 2. See https://cran.r-project.org/web/packages/coxme/index.html.
- 33.Li S, et al. (eds). 2013. An optimized algorithm for detecting and annotating regional differential methylation. BMC Bioinform. 14, S10. ( 10.1186/1471-2105-14-S5-S10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards RJ, et al. 2018. Draft genome assembly of the invasive cane toad, Rhinella marina. Gigascience 7, giy095. ( 10.1093/gigascience/giy095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neph S, et al. 2012. BEDOPS: high-performance genomic feature operations. Bioinformatics 28, 1919-1920. ( 10.1093/bioinformatics/bts277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luquet E, Tariel J. 2016. Offspring reaction norms shaped by parental environment: interaction between within- and trans-generational plasticity of inducible defenses. BMC Evol. Biol. 16, 209. ( 10.1186/s12862-016-0795-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charles R-P, Guitard M, Leyvraz C, Breiden B, Haftek M, Haftek-Terreau Z, Stehle J-C, Sandhoff K, Hummler E. 2008. Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J. Biol. Chem. 283, 2622-2630. ( 10.1074/jbc.M708829200) [DOI] [PubMed] [Google Scholar]
- 38.Frateschi S, Charles R-P, Hummler E. 2010. The epithelial sodium channel ENaC and its regulators in the epidermal permeability barrier function. Open Dermatol. J. 4, 27-35. ( 10.2174/1874372201004010027) [DOI] [Google Scholar]
- 39.Ducatez S, Crossland M, Shine R. 2016. Differences in developmental strategies between long-settled and invasion-front populations of the cane toad in Australia. J. Evol. Biol. 29, 335-343. ( 10.1111/jeb.12785) [DOI] [PubMed] [Google Scholar]
- 40.Selechnik D, Richardson MF, Shine R, DeVore JL, Ducatez S, Rollins LA. 2019. Increased adaptive variation despite reduced overall genetic diversity in a rapidly adapting invader. Front. Genet. 10, 1221. ( 10.3389/fgene.2019.01221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruber J, Brown G, Whiting MJ, Shine R. 2017. Geographic divergence in dispersal-related behaviour in cane toads from range-front versus range-core populations in Australia. Behav. Ecol. Sociobiol. 71, 38. ( 10.1007/s00265-017-2266-8) [DOI] [Google Scholar]
- 42.Hudson CM, Brown GP, Blennerhassett RA, Shine R. 2021. Variation in size and shape of toxin glands among cane toads from native-range and invasive populations. Scient. Rep. 11, 936. ( 10.1038/s41598-020-80191-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips BL, Shine R. 2005. The morphology, and hence impact, of an invasive species (the cane toad, Bufo marinus): changes with time since colonisation. Anim. Conserv. 8, 407-413. ( 10.1017/S1367943005002374) [DOI] [Google Scholar]
- 44.Garty H, Palmer LG. 1997. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359-396. ( 10.1152/physrev.1997.77.2.359) [DOI] [PubMed] [Google Scholar]
- 45.Jensik P, Holbird D, Cox T. 2002. Cloned bullfrog skin sodium (fENaC) and xENaC subunits hybridize to form functional sodium channels. J. Comp. Physiol. B 172, 569-576. ( 10.1007/s00360-002-0285-9) [DOI] [PubMed] [Google Scholar]
- 46.Konno N, Hyodo S, Yamada T, Matsuda K, Uchiyama M. 2007. Immunolocalization and mRNA expression of the epithelial Na+ channel α-subunit in the kidney and urinary bladder of the marine toad, Bufo marinus, under hyperosmotic conditions. Cell Tissue Res. 328, 583-594. ( 10.1007/s00441-007-0383-9) [DOI] [PubMed] [Google Scholar]
- 47.Yang H-y, Charles R-P, Hummler E, Baines DL, Isseroff RR. 2013. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J. Cell Sci. 126, 1942-1951. ( 10.1242/jcs.113225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kosmala GK, Brown GP, Shine R, Christian K. 2020. Skin resistance to water gain and loss has changed in cane toads (Rhinella marina) during their Australian invasion. Ecol. Evol. 10, 13 071-13 079. ( 10.1002/ece3.6895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjørbæk C, et al. 2000. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275, 40 649-40 657. ( 10.1074/jbc.M007577200) [DOI] [PubMed] [Google Scholar]
- 50.Crespi EJ, Denver RJ. 2006. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc. Natl Acad. Sci. USA 103, 10 092-10 097. ( 10.1073/pnas.0507519103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsh IC, Thomsen M, Gludish DW, Alfonso-Parra C, Bai Y, Martin JF, Kurpios NA. 2013. Integration of left-right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Dev. Cell. 26, 629-644. ( 10.1016/j.devcel.2013.07.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakaya M-A, Habas R, Biris K, Dunty WC Jr, Kato Y, He X, Yamaguchi TP. 2004. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr. Patterns 5, 97-105. ( 10.1016/j.modgep.2004.06.001) [DOI] [PubMed] [Google Scholar]
- 53.Berti C, Messali S, Ballabio A, Reymond A, Meroni G. 2002. TRIM9 is specifically expressed in the embryonic and adult nervous system. Mech. Dev. 113, 159-162. ( 10.1016/S0925-4773(02)00013-8) [DOI] [PubMed] [Google Scholar]
- 54.Winkle CC, Olsen RH, Kim H, Moy SS, Song J, Gupton SL. 2016. Trim9 deletion alters the morphogenesis of developing and adult-born hippocampal neurons and impairs spatial learning and memory. J. Neurosci. 36, 4940-4958. ( 10.1523/JNEUROSCI.3876-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuramoto T, et al. 2001. Attractin/Mahogany/Zitter plays a critical role in myelination of the central nervous system. Proc. Natl Acad. Sci. USA 98, 59-64. ( 10.1073/pnas.98.2.559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos F, Dean W. 2004. Epigenetic reprogramming during early development in mammals. Reproduction 127, 643-651. ( 10.1530/rep.1.00221) [DOI] [PubMed] [Google Scholar]
- 57.Han JY. Epigenetic programming of germline, nonmammalian vertebrates. In Encylopedia of reproduction, vol. 6 (ed. Skinner MK), pp. 152-158. New York, NY: Academic Press. [Google Scholar]
- 58.Richards CL, Pigliucci M. 2020. Epigenetic inheritance. A decade into the extended evolutionary synthesis. Paradigmi 38, 463-494. ( 10.30460/99624) [DOI] [Google Scholar]
- 59.RR Sarma, et al. 2021. Data from: Intergenerational effects of manipulating DNA methylation in the early life of an iconic invader. Dryad Digital Repository. ( 10.5061/dryad.pk0p2ngmv) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- RR Sarma, et al. 2021. Data from: Intergenerational effects of manipulating DNA methylation in the early life of an iconic invader. Dryad Digital Repository. ( 10.5061/dryad.pk0p2ngmv) [DOI] [PMC free article] [PubMed]
Data Availability Statement
CpG reports from RRBS data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.pk0p2ngmv [59].