Abstract
Ever since the pioneering research efforts on their utility in biomedicine, polyphosphazene polymers have witnessed enormous growth and expansion in several biomedical applications due to their unique properties. The development of this exceptional biodegradable system with extraordinary design flexibility, property tunability and neutral bioactivity could stimulate an unprecedented paradigm in biomaterial design. Thus, polyphosphazenes are, undoubtedly, the next-generation biomaterials. This editorial provides a brief perspective on the promising prospects of polyphosphazene-based biomaterials for medical device technology, focusing mainly on the authors’ work on this particular polymeric system.
Keywords: biocompatibility, biodegradability, drug delivery, medical device, tissue regeneration
1 |. INTRODUCTION
Although numerous conventional organic polymers have been utilized in biomedical applications, a few numbers of them have had expected and desired success for essential medical use such as scaffolding materials for regenerative engineering, controlled drug delivery systems, and surgical sutures (Ogueri, Jafari, Ivirico, & Laurencin, 2019). This is because most organic polymers (with few exceptions) lack the design flexibility and property tunability as materials scientist and engineers often focus on harnessing and optimizing the physicochemical properties of polymers during design, scale-up, and commercialization (Ogueri, Ivirico, Nair, Allcock, & Laurencin, 2017; Ogueri, Jafari, et al., 2019; Song et al., 2018). Meanwhile, the biocompatibility and degradability are often ignored and not considered as target properties (Allcock, 2018; Ogueri, Jafari, et al., 2019). Thus, polyphosphazenes provide a versatile tool that can manage this trade-off, and they offer an optimal biologic interface between the surface of medical implants and native tissues (Z. Huang et al., 2018; Ogueri, Ogueri, Allcock, & Laurencin, 2020a; Potta, Chun, & Song, 2010). Polyphosphazene-based polymers are a class of inorganic–organic hybrid polymers with an unusual polymer skeletal backbone composed of alternating phosphorous and nitrogen atoms (Ogueri, Allcock, & Laurencin, 2019; Strasser & Teasdale, 2020). Each phosphorous atom is linked with two substituents (e.g. organic groups, drug molecules and organometallic species) available for property optimizations. The smooth integration of the substituent groups with the polyphosphazene skeleton has yielded a library of polymers with a broad spectrum of properties and applications such as biomaterials for regenerative engineering and drug delivery (Ogueri et al., 2020a). Polyphosphazenes that hydrolyse and degrade, when exposed to aqueous media, may be useful for medical devices, including controlled drug release and tissue scaffolding matrices (Allcock, 2016). These degradable polyphosphazene polymers are usually characterized by substituents which are hydrolytically sensitive and susceptible to hydrolysis (Ogueri, Allcock, & Laurencin, 2019; Ogueri et al., 2017). The ones with substituents such as amino acid esters and peptide esters constitute the largest group of degradable polyphosphazenes and have been extensively investigated and developed as tissue constructs for bone regenerative engineering (Allcock & Morozowich, 2012; Ogueri & Laurencin, 2018). Also, degradable polyphosphazenes have shown promise in their applications as carriers for the controlled release of bioactive and therapeutic molecules (Ogueri et al., 2020a; Salinas et al., 2020; Strasser & Teasdale, 2020). The process of tissue regeneration and the release of immobilized molecules can be controlled and regulated by modulating the degradation of polyphosphazenes (Ni et al., 2020; Ogueri, Allcock, et al., 2019; Ogueri et al., 2020a). The adjustment of the polyphosphazene materials’ degradation pattern can be managed by using the hydrophilic–lipophilic balance of the substituent groups (Allcock & Morozowich, 2012; Ogueri et al., 2020a). Thus, polyphosphazene design and properties can be customized to the needs of medical materials. The structural multiplicity, property tunability and functional diversity of this unique family of phosphorous-containing polymers have distinguished them as potentially relevant and attractive materials for biomedical applications (Ogueri et al., 2017).
2 |. PREPARATION
Polyphosphazenes for medical applications have mostly been produced using the original two-step reaction process, which encompasses the ring-opening polymerization (ROP), and the macromolecular nucleophilic substitution (Figure 1; Allcock, 2016; Ogueri, Allcock, et al., 2019; Ogueri, Escobar Ivirico, et al., 2019). The first step starts with the controlled thermal ROP of commercially available hexachlorocyclotriphosphazene monomers (HCCTP) at 250°C under vacuum (Ogueri, Escobar Ivirico, et al., 2019). This initial step results in a highly reactive organic precursor, called poly(dichloro)phosphazene (PDCP) with labile P–Cl bonds that are later utilized to substitute in organic nucleophiles for chlorine atoms (Allcock, 2016, 2018; Ogueri, Escobar Ivirico, et al., 2019). Though ROP is the conventional synthetic route to obtain a linear and high molecular weight PDCP, other alternative technique routes such as living cationic polymerization and direct synthesis have been reported to yield PDCP intermediates with low molecular weight (Ogueri et al., 2019, 2020a). The drawbacks of ROP (such as high-temperature requirements and uncontrollable molecular weight) can be outmanoeuvred using these alternative methods (Ogueri et al., 2020a).
FIGURE 1.

General reaction schematic of polyphosphazenes showing the two-step process. First step encompasses the thermal ring-opening polymerization of hexachlorocyclotriphosphazene, and the subsequent step involves the nucleophilic macromolecular substitution of polydichlorophosphazene with similar and dissimilar substituent groups
The second step, which is the macromolecular substitution reaction, entails the functionalization of PDCP with a variety of organic groups (Allcock, 2016; Ogueri et al., 2020a). In principle, the nucleophilic substitution provides a flexible platform for the easy incorporation of organic or inorganic nucleophiles to obtain stable polymer species whose properties are dependent upon these side groups (Allcock, 2016; Ogueri et al., 2017). In reality, certain constraints can hamper the substitution reaction as bulky nucleophiles may not be able to substitute all the chlorine atoms along the polyphosphazene chain due to steric hindrance (Ogueri, Escobar Ivirico, et al., 2019). This can lead to undesirable effects such as uncontrollable degradation of medical implants (Allcock & Morozowich, 2012; Ogueri & Laurencin, 2018). It is crucial to ensure the complete substitution of the chlorine atoms during macromolecular substitution reaction. Otherwise, the unsubstituted chlorine atoms could leach out and react with water or other bodily fluid to form hydrochloric acid (HCl). This acid is extremely unfavourable for the living cells and can trigger a chain effect on the migration of more chlorine atoms (Allcock, 2016; Linhardt et al., 2016; Ogueri & Laurencin, 2018).
Furthermore, macromolecular substitution can be used to obtain mixed-substituent polyphosphazenes with two or more organic substituent side groups (Figure 1; Ogueri et al., 2019; Ogueri, Allcock, et al., 2019). This can be achieved by simultaneous or sequential reactions of organic nucleophilic species, where, for the later, the large groups are reacted first, followed by the small groups in a solution phase (Ogueri, Allcock, et al., 2019; Ogueri, Escobar Ivirico, et al., 2019). The nature and ratios of the substituent side groups provide a window with which researchers could use to fine-tune material properties (such as degradation, mechanical strength, among others) and also could exploit to create certain physical interactions (such as hydrogen bonds) with other biologically relevant materials for the fabrication of polymer blends and composites (Ogueri et al., 2017, 2020a). This flexibility is vital to the design of new materials for various biomedical applications as biomaterials are tailored to specific medical needs by incorporating different chemical functionalities (Figure 2a–e).
FIGURE 2.

Images showing the versatile utility of polyphosphazene polymers: (a) poly(organo)phosphazene polymers demonstrating the multiplicity of macromolecular substitution, (b) matrix-based strategy for bone regenerative engineering, (c) stent can be coated with polyphosphazenes to reduce platelet adhesion and increase thrombus resistance, and (d) polyphosphazene coating can be employed on cardiac patch to enhance biocompatibility and function
3 |. BIOMEDICAL ASPECTS AND IMPLICATIONS
Among all the practical implications of polyphosphazene technology, their applications in biomedicine may be the most significant contributions to polymer science (Baillargeon & Mequanint, 2014; Ogueri et al., 2019; Ogueri, Jafari, et al., 2019). The biomedical research on polyphosphazenes has garnered relatively considerable interest, especially in bone regenerative engineering and drug delivery (Allcock, 2016; Allcock & Morozowich, 2012; Baillargeon & Mequanint, 2014; Ni et al., 2020; Ogueri et al., 2019). One of the most intriguing parts of macromolecular substitution reaction is its variability that allows the incorporation of several biologically relevant groups onto the skeletal backbone of polyphosphazenes (Ogueri et al., 2020a). Hence, polyphosphazenes can possess immobilization capabilities and tend to degrade to release the immobilized molecules, which can have therapeutic effects (Figure 2; Ni et al., 2020; Ogueri et al., 2020a; Strasser & Teasdale, 2020).
Polymers that undergo degradation to produce benign end-products are generally desired in many biomedical applications that range from vehicles for controlled drug release to scaffolds for regeneration of musculoskeletal tissues (Allcock & Morozowich, 2012; Linhardt et al., 2016). One typical example of this biodegradable system is amino acid ester-substituted polyphosphazene, whose substituent groups form the active centre that sensitizes the backbone to hydrolysis (Khan et al., 2020; Ogueri, Allcock, et al., 2019; Ullah et al., 2017). The hydrolysis of this type of polyphosphazene usually results in benign small molecules (such as ammonium phosphate, amino acid and ethanol) that can be easily metabolized or excreted from the body (Figure 3; DeCollibus, Marin, & Andrianov, 2010; Linhardt et al., 2016; Ogueri et al., 2017; Ogueri & Laurencin, 2018; Teasdale & Brüggemann, 2013; Wilfert et al., 2014). It is also worth mentioning that ammonium phosphate from the breakdown of polyphosphazene backbone constitutes a natural buffer due to its amphoteric nature (Allcock, 2016; Allcock & Morozowich, 2012; DeCollibus et al., 2010; Ullah et al., 2017; Wilfert et al., 2014). The self-neutralizing effect of the polyphosphazenes’ degradation products has become a subject of keen interest in the design of blends composed of polyphosphazenes and clinical polyesters (Ogueri, Ogueri, Allcock, & Laurencin, 2020b). This is crucial to tissue regeneration and drug delivery as the resulting hydrolysis products do not only stabilize the pH of the tissue microenvironment but neutralize the acidic degradation products from the bulk erosion of polyesters in polyphosphazene-polyester blends (Figure 3b; Z. Huang et al., 2018; Ogueri et al., 2020a; Ogueri et al., 2020b; Zhou et al., 2020). Besides, the rate at which amino acid ester-based polyphosphazenes degrade can be influenced by the type of amino acid and the ester function (Allcock, 2016; Khan et al., 2020; Ullah et al., 2017). More recently, dipeptide moieties as side groups have been exploited to provide a significant number of sites for physical interactions with PLGA (Ogueri, Escobar Ivirico, et al., 2019; Ogueri et al., 2020b). The peptide molecules in the polyphosphazene-PLGA blends presented unique intra- and intermolecular hydrogen-bonding interactions that allowed the formation of miscible blends with distinct phase distribution morphologies (Ogueri, Allcock, et al., 2019). The peptide-based blend system exhibits a unique erosion mechanism that presents 3D interconnected porosity (Ogueri et al., 2020b; Figure 3a,d). The blend’s intrinsic pore-forming tendency has been demonstrated to enhance tissue ingrowth, osteoconductivity, osteoinduction, vascularization and osseointegration in bone regenerative engineering (Baillargeon & Mequanint, 2014; Deng, Nair, Nukavarapu, Kumbar, et al., 2010; Z. Huang et al., 2018; Ogueri, Allcock, et al., 2019; Ogueri et al., 2020b). This is one of the earliest experimental shreds of evidence of a biomaterial presenting dynamic in situ pores in the course of degradation. This precludes the need for the prefabrication of porous structures, which can negatively impact the initial mechanical properties of the matrix.
FIGURE 3.
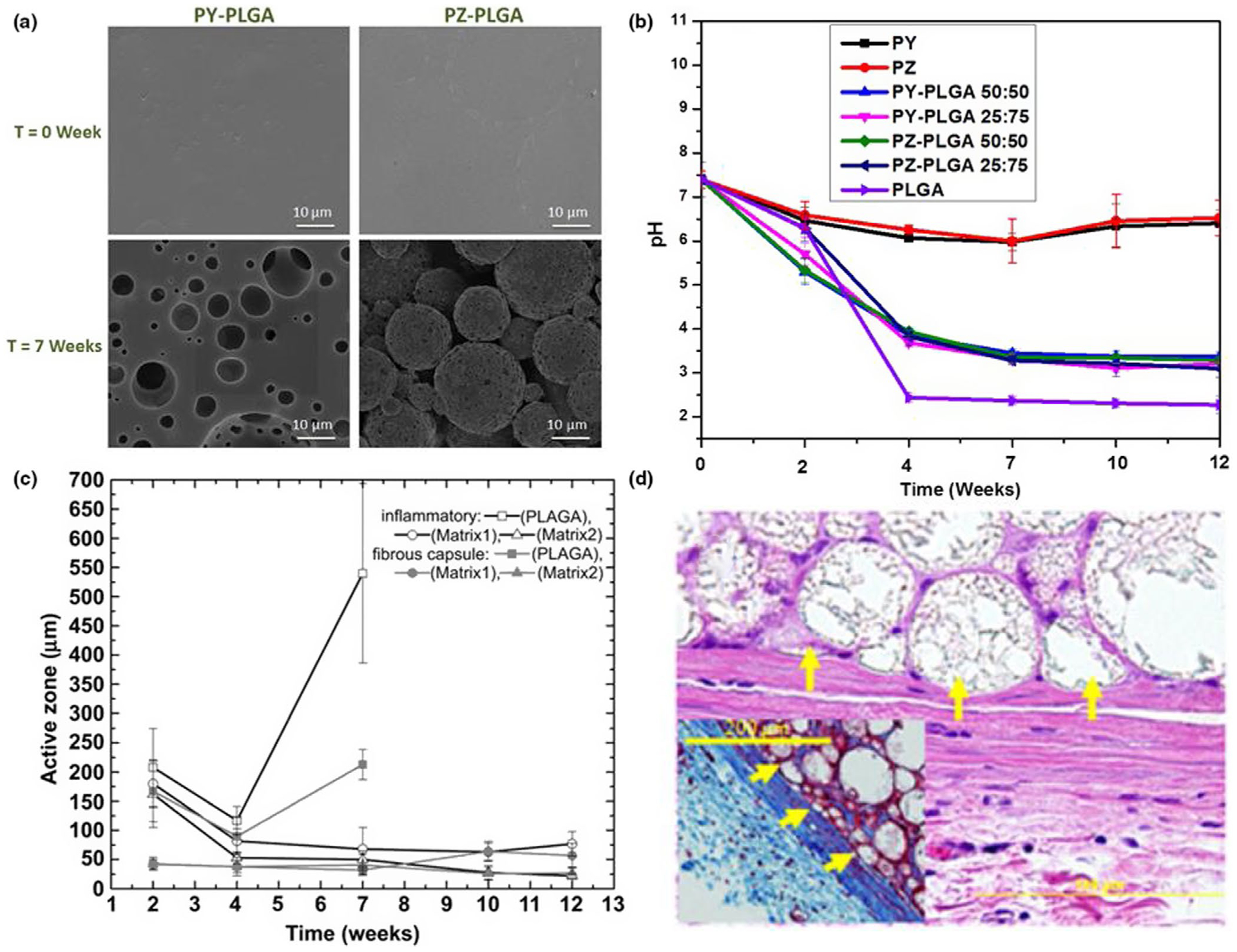
Studies confirming the optimal biocompatibility and degradability of polyphosphazene-based biomaterials. a, SEM images of time-dependent morphological changes of polyphosphazene-PLGA blends. Blend films presented interconnected porosity upon degradation. PY is peptide ester/amino acid ester co-substituted polyphosphazenes, while PZ is peptide ester/phenylphenol co-substituted polyphosphazenes. b, Hydrolytic degradation study of PY, PZ, PLGA and their respective blends in PBS medium at physiological conditions. PLGA showed a lower pH value than the polyphosphazene polymers as PY and PZ exhibited a near-neutral pH and demonstrated the ability to neutralize the degradation products of PLGA (Ogueri et al., 2020b). c, PZ-PLGA 25:75 (matrix1) and PZ-PLGA 50:50 (matrix2) exhibited minimal tissue responses as compared to PLGA. The blends showed less inflammatory responses, and the thickness of the fibrous capsules remained at low level (Deng, Nair, Nukavarapu, Jiang, et al., 2010). d, H & E-stained section illustrating the in vivo biocompatibility of the PZ-PLGA. The histological image is also demonstrating the formation of polymer spheres with pore system and collagen tissue infiltration within the pores (Deng, Nair, Nukavarapu, Kumbar, et al., 2010)
As one of the advantageous properties, the biocompatibility of polyphosphazene-based polymers has been proven by several studies (Figure 3c, d; Deng, Nair, Nukavarapu, Jiang, et al., 2010; Deng, Nair, Nukavarapu, Kumbar, et al., 2010). In one of such studies, Deng et al. (Deng, Nair, Nukavarapu, Jiang, et al., 2010) demonstrated that blend matrix composed of polyphosphazenes have higher osteoblast growth rates and lower in vivo tissue reactions (minimal inflammatory response and fibrous capsule formation) in a rat subcutaneous implantation than PLGA (Figure 3c). The MTS and live/dead stain assays in this study showed robust proliferation and well-spread morphology of primary osteoblast cells on the matrices. Histological analysis also showed that these polyphosphazene-containing matrices enabled the infiltration of cells and the ingrowth of collagen tissues during regeneration (Figure 3d; Deng, Nair, Nukavarapu, Jiang, et al., 2010). In a similar biocompatibility study by another group, Huang et al. (Y. Huang et al., 2020) showed that aniline tetramer/glycine ethyl ester co-substituted polyphosphazenes (PATGP) possesses excellent antioxidant and osteoinductive capacities for effective bone regeneration. The PATGP polymers presented ideal electrochemical cues that up-regulated cellular activities and facilitated neobone formation in rat calvarial defects.
Additionally, recent advances in nanotechnology have stimulated efforts to design innovative nanomaterials based on polyphosphazene platforms for engineering tissues and organs (Ogueri et al., 2019). Interestingly, polyphosphazenes offer the dynamism to personalize and adapt the nanofibre properties to precisely meet the needs of intended applications. Polyphosphazene nanofibres have shown excellent prospects as a carrier matrix for controlling and sustaining therapeutic drug release (Deng et al., 2011; Ogueri et al., 2020a). Currently, there is increasing interest in the utility of polyphosphazene in nanolayer device surface modification (Bates, Yousaf, Sun, Barakat, & Kueller, 2019; L.-C. Xu et al., 2018; L. C. Xu et al., 2020). Polyphosphazenes have been coated on medical devices such as bone implants, heart pumps, stent and other vascular prostheses, to improve the biologic interface between implants and native tissues (Figure 2d, e; Koppara et al., 2016; Mori et al., 2018; L.-C. Xu et al., 2018; L. C. Xu et al., 2020). Polyphosphazene coatings have excellent resistance to thrombosis and decreased platelet adhesion. Thus, biomaterials coated with polyphosphazene have enhanced biocompatibility and suitability for the applications of blood-contacting medical devices (Koppara et al., 2016; Mori et al., 2018; L.-C. Xu et al., 2018).
Since mechanical strength is one of the required criteria for the suitability of biomaterials for biomedical applications, a number of investigations have confirmed the mechanical competence of polyphosphazene-based biomaterials (Deng et al., 2011; Deng, Nair, Nukavarapu, Jiang, et al., 2010). For example, Deng et al. (Deng et al., 2011) demonstrated the feasibility of developing a mechanically competent nanofibre matrix made up of dipeptide polyphosphazene-polyester blends. The biomimetic polyphosphazene matrix exhibited excellent mechanical properties and allowed the in situ ECM secretion (by the cells), which sustained its structural features throughout degradation. In general, this study illustrates the suitability and promising prospects of polyphosphazenes as materials for biomedical applications.
Despite the remarkable progress in the development and utility of polyphosphazene-based biomaterials in biomedicine, there are still concerns regarding their scale-up and commercialization (Ogueri et al., 2020a). The conventional synthetic technique of a linear polyphosphazene is cumbersome and requires severe reaction conditions such as high temperature, anhydrous environment and an airtight closed system (Allcock, 2018; Ogueri, Escobar Ivirico, et al., 2019). These stringent conditions pose a formidable hurdle to the manufacturing process development and scale-up (Ogueri et al., 2020a). Additionally, the properties of a linear polyphosphazene can sometimes be problematic to control due to its high molecular weight and broad molecular weight distribution (Ogueri et al., 2019, 2020a). The molecular weight issues are attributed to the ROP, which has irregular polymerization stages of initiation and propagation (Ogueri & Laurencin, 2018; Ogueri et al., 2020a). Scaling up polyphosphazenes from bench through pilot to commercialization can be achieved using a more facile synthetic method called ‘one-pot polymerization’ (Kühl, Deniz, Gau, & Pich, 2020; Mehmood et al., 2020; Zhou et al., 2020). Unlike the conventional ROP, this method can be carried out at room temperature, and it involves the precipitation polycondensation of HCCTP with any difunctional compounds such as dichlorofluorescein, 4, 5-dibromofluorescein, 4, 4 sulfonyldiphenol, dopamine, and the likes (Kühl et al., 2020; Ogueri et al., 2020a; Zhou et al., 2020). The products of this polymerization method are referred to as cyclomatrixpolyphosphazenes, and they are morphologically structured as nanoparticles with enhanced surface area, making them suitable microspheres for cell and vaccine delivery vehicles (Ogueri et al., 2020a). Lastly, the ratios of the reactants and the solvent type during polymerization can be used to adjust the size of the microspheres, which consequently affects the release profile of the cyclomatrixpolyphosphazene delivery system (Kühl et al., 2020; Ogueri et al., 2020b).
4 |. CONCLUSION
As far as biodegradable polymers continue to be an indispensable component of biomedical device technology, polyphosphazenes have a significant role in ensuring an ideal platform for innovation in biomaterial design. Degradable polyphosphazenes are a promising class of biomaterials for many applications in biomedicine due to their unique characteristics. Their synthetic flexibility, structural multiplicity and neutral end-products have proven to be immensely important for regenerative engineering. Therefore, polyphosphazenes represent an emerging and disruptive force that could change the blueprint of the growing biomaterial industry.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from National Institutes of Health (NIH) through the Award No. NIH DP1-AR068147. Support from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences is also acknowledged.
Funding information
National Institutes of Health, Grant/Award Number: DP1-AR068147
REFERENCES
- Allcock HR (2016). The expanding field of polyphosphazene high polymers. Dalton Transactions, 45(5), 1856–1862. [DOI] [PubMed] [Google Scholar]
- Allcock HR (2018). Synthesis, structures, and emerging uses for poly (organophosphazenes). In Andrianov AK & Allcock HR (Ed.), Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis (pp. 3–26). Washington, DC: ACS Publications. [Google Scholar]
- Allcock HR, & Morozowich NL (2012). Bioerodible polyphosphazenes and their medical potential. Polymer Chemistry, 3(3), 578–590. [Google Scholar]
- Baillargeon AL, & Mequanint K (2014). Biodegradable Polyphosphazene Biomaterials for Tissue Engineering and Delivery of Therapeutics. BioMed Research International, 2014, 1–16. 10.1155/2014/761373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MC, Yousaf A, Sun L, Barakat M, & Kueller A (2019). Translational Research and Early Favorable Clinical Results of a Novel Polyphosphazene (Polyzene-F) Nanocoating. Regenerative Engineering and Translational Medicine, 5(4), 341–353. [Google Scholar]
- DeCollibus DP, Marin A, & Andrianov AK (2010). Effect of environmental factors on hydrolytic degradation of water-soluble polyphosphazene polyelectrolyte in aqueous solutions. Biomacromolecules, 11(8), 2033–2038. [DOI] [PubMed] [Google Scholar]
- Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, & Laurencin CT (2011). Biomimetic structures: biological implications of dipeptide-substituted polyphosphazene–polyester blend nanofiber matrices for load-bearing bone regeneration. Advanced Functional Materials, 21(14), 2641–2651. [Google Scholar]
- Deng M, Nair LS, Nukavarapu SP, Jiang T, Kanner WA, Li X, … Allcock HR (2010). Dipeptide-based polyphosphazene and polyester blends for bone tissue engineering. Biomaterials, 31(18), 4898–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, … Laurencin CT (2010). In situ porous structures: a unique polymer erosion mechanism in biodegradable dipeptide-based polyphosphazene and polyester blends producing matrices for regenerative engineering. Advanced Functional Materials, 20(17), 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Du Z, Wei P, Chen F, Guan B, Zhao Z, … Leng H (2020). Biodegradable microspheres made of conductive polyorganophosphazene showing antioxidant capacity for improved bone regeneration. Chemical Engineering Journal, 397, 125352. 10.1016/j.cej.2020.125352 [DOI] [Google Scholar]
- Huang Z, Gao C, Huang Y, Zhang X, Deng X, Cai Q, & Yang X (2018). Injectable polyphosphazene/gelatin hybrid hydrogel for biomedical applications. Materials & Design, 160, 1137–1147. [Google Scholar]
- Khan RU, Yu H, Wang L, Teng L, Nazir A, Fahad S, Shen D (2020). Synthesis of amino-cosubstituted polyorganophosphazenes and fabrication of their nanoparticles for anticancer drug delivery. Journal of Applied Polymer Science, 49424. 10.1002/app.49424 [DOI] [Google Scholar]
- Koppara T, Sakakura K, Pacheco E, Cheng Q, Zhao X, Acampado E, … Ren J (2016). Preclinical evaluation of a novel polyphosphazene surface modified stent. International Journal of Cardiology, 222, 217–225. [DOI] [PubMed] [Google Scholar]
- Kühl S, Deniz A, Gau E, & Pich A (2020). Cyclophosphazene microgels with adjustable number of crosslinks and deformability by precipitation polycondensation of mono-and bifunctional amines with hexachlorocyclotriphosphazene. Polymer, 192, 122314. 10.1016/j.polymer.2020.122314 [DOI] [Google Scholar]
- Linhardt A, König M, Schöfberger W, Brüggemann O, Andrianov AK, & Teasdale I (2016). Biodegradable polyphosphazene based peptide-polymer hybrids. Polymers, 8(4), 161. 10.3390/polym8040161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood S, Wang L, Yu H, Haq F, Fahad S, Alim Uddin M, & Haroon M (2020). Recent progress on the preparation of cyclomatrix-polyphosphazene based micro/nanospheres and their application for drug release. ChemistrySelect, 5(20), 5939–5958. 10.1002/slct.201904844 [DOI] [Google Scholar]
- Mori H, Jinnouchi H, Diljon C, Torii S, Sakamoto A, Kolodgie FD, … Finn AV (2018). A new category stent with novel polyphosphazene surface modification. Future Cardiology, 14(03), 225–235. 10.2217/fca-2017-0103 [DOI] [PubMed] [Google Scholar]
- Ni Z, Yu H, Wang L, Shen D, Elshaarani T, Fahad S, … Teng L (2020). Recent research progress on polyphosphazene-based drug delivery systems. Journal of Materials Chemistry B, 8(8), 1555–1575. 10.1039/C9TB02517K [DOI] [PubMed] [Google Scholar]
- Ogueri KS, Allcock HR, & Laurencin CT (2019). Polyphosphazene Polymer. Encyclopedia of Polymer Science and Technology, 2, 1–23. 10.1002/0471440264.pst284.pub2 [DOI] [Google Scholar]
- Ogueri KS, Allcock HR, & Laurencin CT (2019). Generational biodegradable and regenerative polyphosphazene polymers and their blends with poly (lactic-co-glycolic acid). Progress in Polymer Science, 98, 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueri KS, Escobar Ivirico JL, Li Z, Blumenfield RH, Allcock HR, & Laurencin CT (2019). Synthesis, Physicochemical Analysis, and Side Group Optimization of Degradable Dipeptide-Based Polyphosphazenes as Potential Regenerative Biomaterials. ACS Applied Polymer Materials, 1(6), 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueri KS, Ivirico JLE, Nair LS, Allcock HR, & Laurencin CT (2017). Biodegradable polyphosphazene-based blends for regenerative engineering. Regenerative Engineering and Translational Medicine, 3(1), 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueri KS, Jafari T, Ivirico JLE, & Laurencin CT (2019). Polymeric biomaterials for scaffold-based bone regenerative engineering. Regenerative Engineering and Translational Medicine, 5(2), 128–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueri KS, & Laurencin CT (2018). Polyphosphazene-based biomaterials for regenerative engineering. Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis (pp. 53–75). Columbus, OH: ACS Publications. [Google Scholar]
- Ogueri KS, Ogueri KS, Allcock HR, & Laurencin CT (2020a). Polyphosphazene polymers: The next generation of biomaterials for regenerative engineering and therapeutic drug delivery. Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena, 38(3), 30801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueri KS, Ogueri KS, Allcock HR, & Laurencin CT (2020b). A regenerative polymer blend composed of glycylglycine ethyl ester-substituted polyphosphazene and poly (lactic-co-glycolic acid). ACS Applied Polymer Materials, 2(3), 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potta T, Chun C, & Song S-C (2010). Dual cross-linking systems of functionally photo-cross-linkable and thermoresponsive polyphosphazene hydrogels for biomedical applications. Biomacromolecules, 11(7), 1741–1753. 10.1021/bm100197y [DOI] [PubMed] [Google Scholar]
- Salinas Y, Kneidinger M, Fornaguera C, Borrós S, Brüggemann O, & Teasdale I (2020). Dual stimuli-responsive polyphosphazene-based molecular gates for controlled drug delivery in lung cancer cells. RSC Advances, 10(46), 27305–27314. 10.1039/D0RA03210G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Murphy M, Li C, Ting K, Soo C, & Zheng Z (2018). Current development of biodegradable polymeric materials for biomedical applications. Drug Design, Development and Therapy, 12, 3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser P, & Teasdale I (2020). Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications. Molecules, 25(7), 1716. 10.3390/molecules25071716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale I, & Brüggemann O (2013). Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers, 5(1), 161–187. 10.3390/polym5010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah RS, Wang L, Yu H, Abbasi NM, Akram M, Saleem M, … Khan RU (2017). Synthesis of polyphosphazenes with different side groups and various tactics for drug delivery. RSC Advances, 7(38), 23363–23391. 10.1039/C6RA27103K [DOI] [Google Scholar]
- Wilfert S, Iturmendi A, Schoefberger W, Kryeziu K, Heffeter P, Berger W, … Teasdale I (2014). Water-soluble, biocompatible polyphosphazenes with controllable and pH-promoted degradation behavior. Journal of Polymer Science Part A: Polymer Chemistry, 52(2), 287–294. 10.1002/pola.27002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LC, Chen C, Zhu J, Tang M, Chen A, Allcock HR, & Siedlecki CA (2020). New cross-linkable poly [bis (octafluoropentoxy) phosphazene] biomaterials: Synthesis, surface characterization, bacterial adhesion, and plasma coagulation responses. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 67, 87–98. 10.1016/j.actbio.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L-C, Li Z, Tian Z, Chen C, Allcock HR, & Siedlecki CA (2018). A new textured polyphosphazene biomaterial with improved blood coagulation and microbial infection responses. Acta Biomaterialia, 67, 87–98. 10.1016/j.actbio.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Zhou N, Zhi Z, Liu D, Wang D, Shao Y, Yan K, … Yu D (2020). Acid Responsive and Biologically Degradable Polyphosphazene Nanodrugs for Efficient Drug Delivery. ACS Biomaterials Science & Engineering, 6(7), 4285–4293. 10.1021/acsbiomaterials.0c00378 [DOI] [PubMed] [Google Scholar]


