Abstract
Objective
This research aimed to explore the effects of probiotic administration on glycemic control and renal function in patients with diabetic nephropathy (DN).
Methods
The 101 participants were randomly divided into two treatment groups and 76 patients were included in the final analysis. In 76 patients with diabetic nephropathy of type 2 diabetes, a randomized double‐blind and placebo‐controlled clinical trial was conducted to evaluate the administration of 3.2 × 109 CFU probiotic supplements per day (Bifidobacterium bifidum, 1.2 × 109 CFU, Lactobacillus acidophilus 4.2 × 109 CFU, Streptococcus thermophilus 4.3 × 109 CFU) for 12 weeks on glycemic control of patients, including fasting blood glucose, 2 h postprandial blood glucose, glycosylated hemoglobin (HbA1c), microalbuminuria/creatinine (mAlb/Cr) and estimated glomerular filtration rate (eGFR) levels. The placebo group daily received empty capsules filled with starch.
Results
After 12 weeks, the administration of probiotics demonstrated a significant reduction in fasting blood glucose (10.68 ± 3.24 mmol/L before vs. 7.81 ± 2.77 mmol/L after, p < 0.05), HbA1c (8.19 ± 1.60% before vs. 7.32 ± 1.20% after, p < 0.05) and mAlb/Cr (101.60 ± 22.17 mg/g before vs. 67.53 ± 20.11 mg/g after, p < 0.05), while only mAlb/Cr level was significantly lower in the probiotic group than in the placebo group after intervention (67.53 ± 20.11 mg/g vs. 87.71 ± 23.01, p < 0.05). Meanwhile, there was no significant reduction of 2 h postprandial blood glucose level (18.95 ± 5.23 mmol/L vs. 17.35 ± 6.28 mmol/L, p = 0.24) and eGFR (84.34 ± 6.97 ml/min vs. 82.8 ± 8.72 ml/min, p = 0.45) in patients before and after probiotic intake. In addition, the placebo group failed to show any significant change of these parameters.
Conclusion
This clinical study revealed probiotic administration could ameliorate glycemic control of patients with diabetic nephropathy, potentiating its therapeutic potential in clinical application.
Keywords: diabetic nephropathy, glycemic control, probiotics, type 2 diabetes mellitus
Probiotics decreases levels of fasting plasma glucose, 2 h postprandial blood glucose, glycated hemoglobin, and mAlb/Cr.
![]()
1. INTRODUCTION
Diabetic nephropathy (DN), one of serious diabetic microvascular complications of diabetes mellitus, is regarded as a dominative cause of end‐stage renal disease. 1 , 2 It was reported that patients with DN could result in end‐stage renal failure and disability worldwide with high mortality, affecting approximately 40% of diabetic patients. 3 , 4 Therefore, DN raised great concern in researchers in this field. Patients with DN were usually accompany by metabolic syndrome, such as elevated fasting blood glucose levels, postprandial blood glucose level 5 and microalbuminuria level. 6
In general, blood glucose elevated beyond the kidney's capacity and failed to reabsorb glucose from the renal ultrafiltration, resulting in redundant glucose diluted in the fluid in early stages of type 2 diabetic mellitus. 7 Consequently, redundant glucose level increased the urine volume and osmotic pressure. 8 Therefore, the treatments of DN based on reducing glucose level were limited, such as the control of glucose intake, 9 the supplementation of high‐quality protein diet, 10 intake of angiotensin‐converting enzyme inhibitors 11 and angiotensin II receptor blockers. 12 However, these controls failed to demonstrate efficacy and effectiveness on DN. Furthermore, recent studies focus on elucidating the long‐term effects of drugs, such as microcirculator, anti‐fibrosis chemicals and herbal extracts, on DN.
Upon sufficient intake as living microorganisms in dietary supplements, probiotics would efficiently improve the metabolism of the host and also increase its nutritional intake. 13 , 14 , 15 Numerous reports have illustrated the association between probiotics and gut. 16 , 17 The researchers demonstrated that the favorable effects of probiotics were closely associated with its abundance of certain beneficial bacteria, which produced anti‐inflammatory metabolites. Subsequently, these anti‐inflammatory metabolites would modulate the pro‐inflammatory immune cell line through the crosstalk between gut and other organs. 18 , 19 Specifically, it was suggested that probiotics exerted inhibitory effects of metabolic syndrome of diabetes. 20 In details, the results revealed that intake of probiotics had inhibitory effects on blood glucose level, which was essential for modulating the changes of the intestinal microflora in patients with type 2 diabetes mellitus. 20 In addition, it showed that intestinal dysbiosis remained a susceptible factor for the progress of chronic kidney disease in DN. Despite the clinical applications of the probiotic intake in the management of blood glucose, few studies were conducted to investigate the effects of probiotic intake on glycemic control and kidney function amelioration in DN patients.
Therefore, this study aimed to explore the effects of probiotic administration on glycemic control and renal function in patients with DN in type 2 diabetes. And our results revealed that probiotics showed potent effects in decreasing fasting blood glucose and postprandial glucose levels and other diabetic parameters of patients with diabetic nephropathy, potentiating its potential in clinical application.
1.1. Subjects
This randomized, parallel‐group, double‐blind, placebo‐controlled experiment was performed on patients with DN by China‐Japan Union Hospital of Jilin University at an endocrinology and metabolic clinics in Changchun, China, from January 2017 to July 2017. The inclusion criteria were as follows: (1) patient age was ≥18 years and ≤75 years; (2) patients were diagnosed with diabetic nephropathy according to world health organization's standard of type 2 diabetes mellitus 21 and did not intake any antidiabetic drugs within 3 months before the study; (3) the patients’ glycosylated hemoglobin (HbA1c) levels was between 7% and 10%; and (4) the microalbuminuria/creatinine (mAlb/Cr) level was ≥30 mg/g per 24 h. The exclusion criteria were as follows: (1) patients with type 1 diabetes mellitus; (2) patients with hypoglycemic coma, diabetic ketoacidosis, hyperosmotic nonketotic coma or diabetes mellitus acute complications; (3) fasting blood glucose was >13.3 mmol/L; (4) total bilirubin was >2.5 times of normal value; (5) serum creatinine was >133 μmol/L in male patients, and >124 μmol/L in female patients; (6) patients with history of hypertension, drug abuse, alcohol dependence or drug allergy; (7) patients' intake of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers within 3 months; and (8) patients' intake probiotic and/or synbiotic supplements within 3 months before this study. Following the selection criteria, the study was conducted on 101 volunteers. However, 25 volunteers failed to complete the trial, and 76 volunteers eventually received the treatment. This clinical trial was approved by the China‐Japan union hospital Ethics Committee (Approval number/2016ks095), and the approved date was December 20, 2016. Ethical clearance was taken from ethical clearance committee of the China‐Japan union hospital (Ethical clearance number/0431‐84995047) (ChiCTR2000038392). The informed consent was signed by all the participants participated in this study.
2. MATERIALS AND METHODS
2.1. Random allocation
Randomization of all participants was performed by means of a computer‐generated random‐numbers table, as to reduce the potential confounding effects. To balance the baseline characteristics between the two groups, subjects were assigned in a 1:1 ratio. The 76 participants were randomly divided into two treatment groups: probiotic group (n = 42) and placebo group (n = 34). The probiotic group received 3.2 × 109 CFU per day of probiotics containing (Bifidobacterium bifidum 1.2 × 109 CFU, Lactobacillus acidophilus 4.2 × 109 CFU, Streptococcus thermophilus 4.3 × 109 CFU) for 12 weeks and then stopped taking probiotics. Simultaneously, the placebo group received empty capsules with similar shape and weight for 12 weeks, and then stopped taking capsules. The probiotic and placebo (starch) were produced by LactoCare® and Tian San Qi Company, respectively. Moreover, all the participants were required to take their respective supplements on a daily base. Dietary assessment of all participants was performed by the nutritionist to assess the participants’ nutrient intake by Nutritionist IV software (First Databank). In order to follow up the treatment, our team contacted the participants once a week for general medical advice and diabetes education, including daily diet, physical exercise, and self‐monitoring of blood glucose.
2.2. Biochemical measurements
The height and weight of all patients were measured before and after 12 weeks treatment. Fasting blood glucose, 2 h postprandial blood glucose level, glycosylated hemoglobin (HbA1c) and serum creatinine were measured by automatic biochemical analyzer. Fasting insulin levels were measured by enzyme‐linked immunoassay kit (DRG Company). Microalbuminuria/creatinine (mAlb/Cr) was determined by automatic special protein dry immune scattering chromatographic analyzer (Ariel Afinion AS100). Estimated glomerular filtration rate (eGFR) was calculated by (CKD) ‐ EPI) formula. The formula was HOMA‐IR = fasting blood insulin (mIU/L) × fasting blood glucose (mmol/L)/22.5 was used to calculate the homeostasis model assessment index (HOMA‐IR).
2.3. Statistical analysis
Based on the difference between two sample rates, using the estimation formula to calculate the required sample size. 22 The inspection level (α) was 0.05 and power was 0.8 (β = 1; power = 0.2) were assumed. Based on the two‐sided tests, the sample size was estimated to be 74, with 37 subjects per group. Owing to possible patient withdrawal, we increased the sample size by 15%, and a total of 100 subjects were required, with 50 participants in each group. Statistical analysis of the data was performed using SPSS 19.0 (SPSS Inc). Kolmogorov–Smirnov test was used to check the normality of variables distribution. The data were presented as mean ± standard deviation (SD) or n (%). Student's t‐test or Mann–Whitney test was used to assess the statistical significance of the results between probiotic group and placebo group, or before and after intervention. p < 0.05 was regarded as statistically significant.
3. RESULTS
3.1. Characteristics of probiotic and placebo groups before treatment
101 volunteers were recruited into this research. At the end of the treatment, 25 participants failed to complete the trial, and only 76 participants completed the treatment (probiotic group (n = 42) and placebo group (n = 34)). Therefore, the glycemic control and renal function evaluations on these 76 participants were conducted in the following study (Figure 1). The mean value of age and the sex distribution in two groups are listed in Table 1. The results in two groups demonstrated no significant difference regarding levels of fasting blood glucose, 2 h postprandial blood glucose, HbA1c, serum creatinine, mAlb/Cr, BMI, insulin resistance index and eGFR (Table 1).
FIGURE 1.
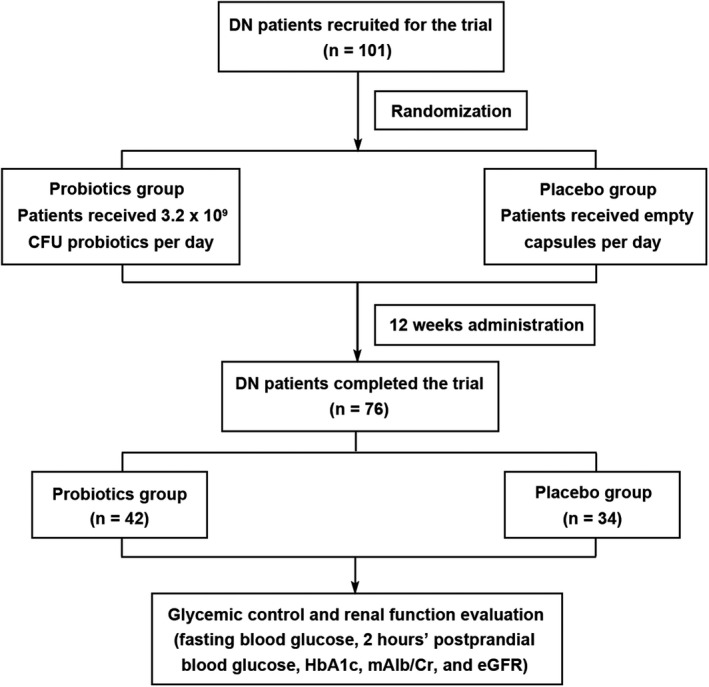
The flowchart of study design. DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; mAlb/Cr, microalbuminuria/creatinine
TABLE 1.
The comparison of baseline characteristics between probiotic group and placebo group
| probiotic group(n = 42) | placebo group(n = 34) | p value | |
|---|---|---|---|
| Age (year) | 55.96 ± 8.45 | 56.12 ± 8.23 | 0.35 |
| Sex (M/F, %) | M15(35.7%) | M12(35.3%) | 0.21 |
| F27(64.3%) | F22(64.7%) | ||
| BMI (kg/m2) | 27.51 ± 3.22 | 26.44 ± 2.78 | 0.47 |
| Fasting blood glucose (mmol/L) | 10.68 ± 3.24 | 9.83 ± 3.90 | 0.66 |
| 2 h postprandial blood glucose level (mmol/L) | 18.95 ± 5.23 | 19.00 ± 6.41 | 0.18 |
| Glycosylated hemoglobin (%) | 8.19 ± 1.60 | 8.25 ± 2.03 | 0.22 |
| HOMA‐IR | 2.73 ± 0.46 | 2.66 ± 0.52 | 0.42 |
| mAlb/Cr (mg/g) | 101.60 ± 22.17 | 99.66 ± 25.24 | 0.56 |
| eGFR(ml·min‐1(1.73m2)‐1) | 82.8 ± 8.72 | 83.12 ± 7.28 | 0.43 |
Data are mean ± SD, p values were obtained by using Student's t‐test or Mann–Whitney test and p < 0.05 was considered as significant difference between two groups.
Abbreviations: BMI, body mass index; reGFR, estimated glomerular filtration rate; rF, female; rHOMA‐IR, homeostasis model assessment index; rM, male; rmAlb/Cr, microalbuminuria/creatinine.
3.2. Probiotic administrations reduced glycemic control in DN patients
The probiotic group received 3.2 × 109 CFU per day of probiotics containing, while the placebo group received empty capsules filled with starch, for a total of 12 weeks. After 12 weeks of intervention, probiotic group demonstrated a significant reduction in fasting glucose (7.81 ± 2.77 mmol/L after treatment vs. 10.68 ± 3.24 mmol/L before treatment, p < 0.05, Figure 2) and HbA1c (%) (7.32 ± 1.20% after treatment vs. 8.19 ± 1.60% before treatment, Figure 4). However, the probiotic group revealed a slight reduction without significant change of 2 h postprandial blood glucose level (17.35 ± 6.28 mmol/L after intervention vs. 18.95 ± 5.23 mmol/L before intervention, p = 0.24, Figure 3). And the placebo group revealed a slight reduction without significant change of fasting glucose (8.78 ± 3.01 mmol/L after intervention vs. 9.83 ± 3.90 mmol/L before intervention, p = 0.48, Figure 2), 2 h postprandial blood glucose level (17.13 ± 6.05 mmol/L after intervention vs. 19.00 ± 6.41 mmol/L before intervention, p = 0.51, Figure 3) and HbA1c (%) (7.92 ± 1.21% after intervention vs. 8.25 ± 2.03% before intervention, p = 0.67, Figure 4). Meanwhile, the levels of fasting blood glucose, HbA1c and 2 h postprandial blood glucose showed no significant differences between the probiotic group and the placebo group after intervention (p > 0.05, Table 3).
FIGURE 2.
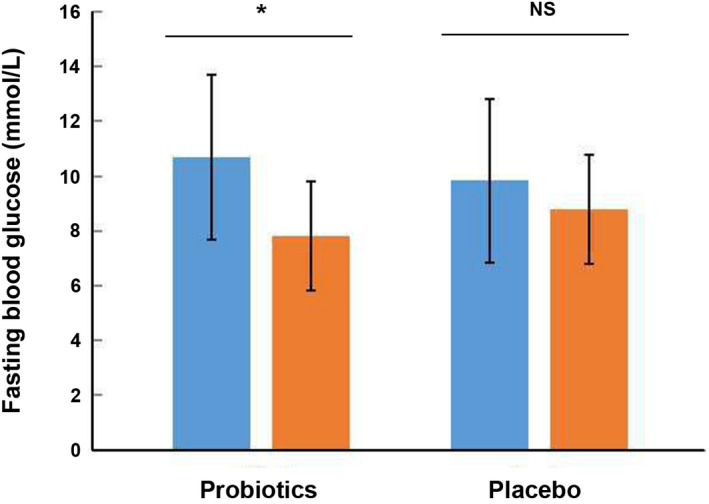
Effect of 12‐week intake with probiotics or placebo on fasting blood glucose (mmol/L) level of patients with DN. DN, diabetic nephropathy; NS, no significance. *: p < 0.05
FIGURE 4.
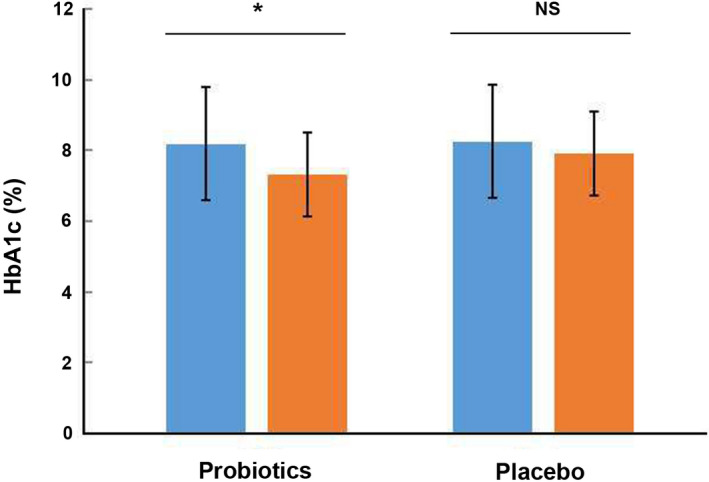
Effects of 12‐week intake with probiotics or placebo on HbA1c (%) of patients with DN. DN, diabetic nephropathy; HbA1c, glycosylated hemoglobin; NS, no significance. *: p < 0.05
FIGURE 3.
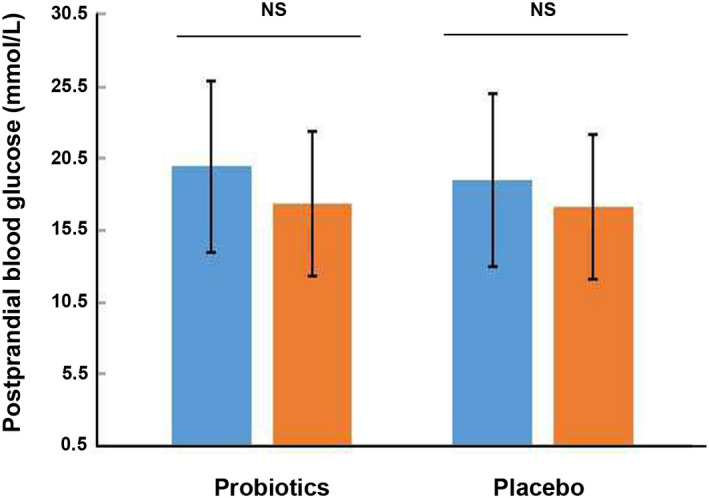
Effect of 12‐week intake with probiotics or placebo on 2 h postprandial blood glucose level (mmol/L) of patients with DN. DN, diabetic nephropathy; NS, no significance
TABLE 3.
The levels of fasting blood glucose, HbA1c, and 2 h postprandial blood glucose between probiotic group and placebo group
| Probiotic group (n = 42) | Placebo group (n = 34) | p value | |
|---|---|---|---|
| Fasting blood glucose (mmol/L) | 7.81 ± 2.77 | 8.78 ± 3.01 | 0.15 |
| 2 h postprandial blood glucose level (mmol/L) | 17.35 ± 6.28 | 17.13 ± 6.05 | 0.93 |
| Glycosylated hemoglobin (%) | 7.32 ± 1.20 | 7.92 ± 1.21 | 0.24 |
| mAlb/Cr (mg/g) | 67.53 ± 20.11 | 87.71 ± 23.01 | <0.05 |
| eGFR (ml·min−1(1.73 m2)−1) | 82.8 ± 8.72 | 84.28 ± 7.13 | 0.08 |
3.3. Probiotic administration reduced mAlb/Cr in DN patients
After 12 weeks of intervention, probiotic group demonstrated a significant reduction of mAlb/Cr level (67.53 ± 20.11 mg/g vs. 101.60 ± 22.17 mg/g, p < 0.05, Figure 5). However, the placebo group revealed a slight reduction without significant change of mAlb/Cr level (87.71 ± 23.01 mg/g vs. 99.66 ± 25.24 mg/g, p = 0.61, Figure 5). Meanwhile, mAlb/Cr level was found significantly different between the probiotic group and the placebo group after intervention (p < 0.05, Table 3).
FIGURE 5.
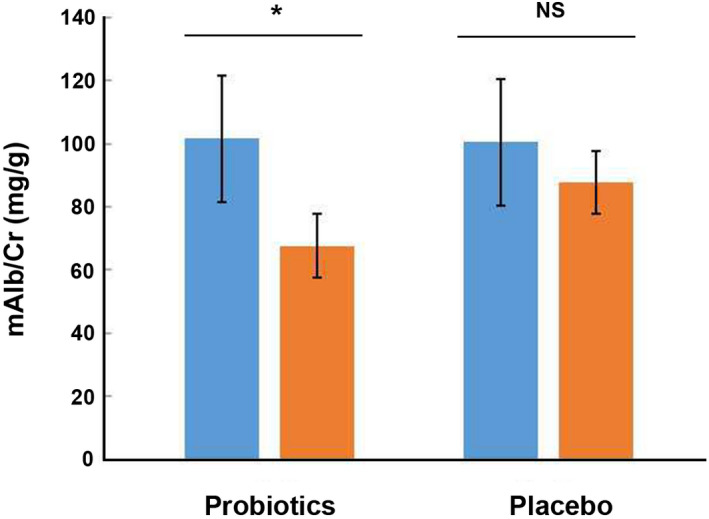
Effects of 12‐week intake with probiotics or placebo on mAlb/Cr (mg/g) level of patients with DN. DN, diabetic nephropathy; mAlb/Cr, microalbuminuria/creatinine; NS, no significance. *: p < 0.05
3.4. The effects of probiotic administration on eGFR
After 12 weeks of intervention, probiotic group demonstrated a reduction of eGFR level (82.8 ± 8.72 ml/min after treatment vs. 84.34 ± 6.97 ml/min before treatment, p = 0.45, Table 2). Meanwhile, the placebo group revealed no significant reduction of eGFR (84.28 ± 7.13 ml/min after treatment vs. 83.12 ± 7.28 ml/min before treatment, p = 0.77, Table 2). In addition, there was no significant difference of eGFR level between the probiotic group and the placebo group after intervention (p > 0.05, Table 3).
TABLE 2.
Changes of eGFR in probiotic and placebo group after 12 weeks
| Probiotic group (n = 42) | Placebo group (n = 34) | |
|---|---|---|
| onset treatment (ml/min) | 82.8 ± 8.72 | 83.12 ± 7.28 |
| 12‐week treatment (ml/min) | 84.34 ± 6.97 | 84.28 ± 7.13 |
| p value | 0.45 | 0.77 |
Data are mean ± SD, p values were obtained by using Student's t‐test or Mann–Whitney test and p < 0.05 was considered as significant difference between two groups.
Abbreviation: eGFR, estimated glomerular filtration rate.
4. DISCUSSION
DN is one of the most important complications of diabetes that can result in end‐stage renal failure and disability. Recent studies have revealed that the type 2 diabetes occurrence is associated with reduction of Lactobacillus and B. bifidum, and the alteration of intestinal flora. 23 Lu et al 24 used Lactobacillus reuteri GMNL‐263 to treat the diabetic rats with renal fibrosis that induced by streptozotocin and found Lactobacillus can reduce the HbA1c and blood glucose levels of the diabetic rats, and protect them from hyperglycemia‐enhanced renal fibrosis. Meanwhile, intake of soy milk with Lactobacillus plantarum A7 was shown to have a beneficial effect on the renal function of patients with DN. 25 Generally, the probiotics contain Lactobacillus and B. bifidum, and are commercially available. However, the systematical analysis of probiotic effect on DN patients in a clinical practice, especially regarding the blood glucose level and renal function, is rarely reported. In the present study, we aimed to explore the effects of probiotic administration on glycemic control of DN patients. The probiotics used here is produced by a company and is commercially available. Our results elucidated that probiotic administration ameliorated glycemic control of DN, including exerting beneficial effects on glycemic control. Our research revealed that after 12 weeks, probiotic intake significantly reduced levels of fasting blood glucose, HbA1c, serum creatinine and mAlb/Cr This proved probiotic effect on glycemic control of patients with DN may provide useful information in the clinical practice for DN patients.
It is well known that intestinal microbial populations vary between healthy individuals and patients with type 2 diabetes. 26 Moreover, DN is characterized by chronic hyperglycemia with the metabolic imbalance of carbohydrate, fat and protein digestion resulting from defects in insulin sensitivity. 27 , 28 Therefore, glycemic control is an essential factor for treatment of DN patients with metabolic syndrome. In this study, we evaluated the changes of glycemic control, indicating that probiotics significantly decreased the levels of blood glucose. In addition, the number of beta cells in the pancreas reduced in DN individuals due to pathological changes, leading to high level of blood glucose, leading to global hyperglycemia. 29 , 30 In our study, probiotics reduced the level of blood glucose, relieving the unbearable pain of DN patients. Moreover, probiotics reduced 2 h postprandial blood glucose level without significant difference, maybe due to the fact that it is difficult to control the postprandial spike in blood sugar in DN patients.
Furthermore, we estimated the levels of mAlb/Cr before and after the intake of probiotics, which revealed that probiotics reduced the level of mAlb/Cr mAlb level represents the glomerular filtration function, which is the primary indicator of glomerular filtration ability. 31 Under normal circumstances, the majority of mAlb would not get through filtration membrane due to selective barrier of the charge in glomerular filtration membrane with the effect of electrostatic repulsion. 32 However, in DN patients the levels of acetyl sulfate heparin and sialic acid were reduced, resulting in the reduction of the charge selectivity of glomerular filtration membrane, obstructing the extracellular basement affinity and protein polysaccharide, inducing the enlargement of diameter of glomerular filtration membrane filter hole and the change of negative charge structural constitution in glomerular filtration membrane, and this further led to the discharge of mAlb. Simultaneously, in patients who are attacked by kidney disease, serum creatinine variability has a strong effect on GFR level. 33 In previous studies, the researchers demonstrated that level of mAlb/Cr was significantly increased in DN patients by comparison with the healthy individuals. 34 This finding further proved our investigation that probiotics efficiently reduced the level of mAlb/Cr, indicating that probiotics would enhance the ability of glomerular filtration membrane.
In addition, exogenous stimuli, such as high level of blood sugar and urine toxin, may break the balance of angiotensin enzyme and angiotensin enzyme 2 in kidney, leading to a series of cascade reaction, increasing the renal injury and eventually promoting the progress of DN. 35 Furthermore, short‐chain fatty acids produced by probiotics bound with G protein‐coupled receptors in kidney, thus regulating the hormones, and further affecting the balance between carbohydrate, fat, and protein metabolism. Furthermore, SCFA increased the production of GLP‐2 and upregulated the transcriptional expression of tight junction proteins, thus decreasing gastrointestinal permeability; finally, it may improve insulin resistance. Therefore, probiotics exerted favorable effects on DN patients.
Several preclinical and clinical studies have demonstrated the antidiabetic effect of Lactobacillus and Bifidobacteria. 36 , 37 , 38 Soleimani et al 38 performed a randomized, double‐blind, placebo‐controlled trial to evaluate the effect of L. acidophilus, Lactobacillus casei, and B. bifidum on diabetes mellitus patients, indicating that probiotics would efficiently reduce the level of fasting blood glucose and HbA1c. Meanwhile, it showed that multi‐strains of probiotics significantly reduced the level of fasting blood glucose and HbA1c in diabetes mellitus patients of a randomized experiment. 39 The possible mechanism of hypoglycemic effect is that probiotics could affect intestinal flora to insulinotropic polypeptides and glucagon‐like peptide‐l, while these peptides induce glucose uptake by muscle. 40 In addition, Ejtahed et al 41 used probiotic yogurt in type 2 diabetic patients and claimed that the reduction of fast blood and HbA1c is probably related to antioxidant activity of probiotic yogurt by multiple interacting pathways. However, others pointed that the probiotic of Lactobacillus reuteri DSM 17938 failed to reduce the level of HbA1c in patients with diabetes mellitus. 42 Therefore, given that different kinds of probiotics may have different effects on blood glucose and HbA1c, we conducted this trial by selecting B. bifidum, L. acidophilus and S. thermophilus to prove the favorable effects on DN patients. Consistent with the previous results, probiotics significantly reduced the level of fasting blood glucose and HbA1c, simultaneously reducing the level of mAlb/Cr, although our results indicated that probiotics showed no significant reduction of 2 h postprandial blood glucose and eGFR. Together, our research provided the evidence that probiotics ameliorated glycemic control of DN patients via recovering the glycemic control, improving insulin resistance and reducing inflammatory response.
It was reported that probiotic honey consumption for 12 weeks among DN patients had beneficial effects on insulin metabolism, total‐/HDL‐cholesterol, serum hs‐CRP, and plasma MDA levels, but did not affect other metabolic profiles. 43 Another study showed that probiotic supplementation had beneficial effects on glycemic control and markers of cardiometabolic risk. 44 A meta‐analysis showed that probiotic supplementation decreased serum insulin and insulin resistance, but it had no beneficial effect regarding kidney function, body weight and lipid profiles, with a moderate positive effect regarding some oxidative stress biomarkers. 45 Another meta‐analysis showed the benefits of probiotic supplementation on the reduction of inflammation, oxidative stress and on the amelioration of renal function biomarkers in subjects with diabetic nephropathy. 46 These results were consistent with our study.
There are some limitations that present in this clinical trial. Firstly, the sample sizes analyzed in this study was not very large. This might result in no significant reduction of 2 h postprandial blood glucose and eGFR after probiotic administration. Secondly, the DN patients were not classified under different degrees of renal failure. Therefore, the probiotic effect on glycemic control of DN patients was not extensively evaluated in current study. Further investigations among DN patients with different degrees of renal failure and large number are suggested to reinforce these findings. In addition, the long‐term duration of study may be conducted to validate these data. Finally, the gut microbiota status of the stool sample was not evaluated in this study.
5. CONCLUSIONS
In summary, our results revealed that intake of probiotic formula B. bifidum, L. acidophilus, and S. thermophilus exerted favorable effects on regulating intestinal flora, glycemic control, reducing mAlb/Cr, thus ameliorating glycemic control of DN in patients with type 2 diabetes.
CONFLICT OF INTEREST
None.
CONSENT FOR PUBLICATION
Informed consent was obtained from all individual participants included in the study.
Jiang H, Zhang Y, Xu D, Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J Clin Lab Anal. 2021;35:e23650. 10.1002/jcla.23650
REFERENCES
- 1. Weng HB, Han WK, Xiong YW, et al. Taxus chinensis ameliorates diabetic nephropathy through down‐regulating TGF‐β1/Smad pathway. Chin J Nat Med. 2018;16(2):90. [DOI] [PubMed] [Google Scholar]
- 2. Završnik M, Letonja J, Makuc J, Šeruga M, Cilenšek I, Petrovič D. Interleukin‐4 gene (IL4) polymorphism rs2243250 is not associated with diabetic nephropathy (DN) in Caucasians with type 2 diabetes mellitus (T2DM). Bosn J Basic Med Sci. 2018;18(4):347‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soo KS, Ho KJ, Joo KI. Current challenges in diabetic nephropathy: early diagnosis and ways to improve outcomes. Endocrinol Metabolism. 2016;31(2):245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao M, Tian Z, Zhang L, Liu R, Guan Q, Jiang J. Effects ofCCR559029G/A polymorphism on the risk to diabetic nephropathy. Oncotarget. 2017;8(63):106926‐106934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108‐111. [DOI] [PubMed] [Google Scholar]
- 6. Pehlivan E, Ozen G, Taskapan H, Gunes G, Sahin I, Colak C. Identifying the determinants of microalbuminuria in obese patients in primary care units: the effects of blood pressure, random plasma glucose and other risk factors. J Endocrinol Invest. 2016;39(1):73‐82. [DOI] [PubMed] [Google Scholar]
- 7. Zhang R. Changes of Blood Glucose in Diabetic Nephropathy Patients and the Risk Control of Hypoglycemia. China Health Standard Management; 2016;18:44‐46. [Google Scholar]
- 8. Heise T, Jordan J, Wanner C, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38(10):2265‐2276. [DOI] [PubMed] [Google Scholar]
- 9. Alaveras AE, Thomas SM, Sagriotis A, Viberti GC Promoters of progression of diabetic nephropathy: the relative roles of blood glucose and blood pressure control. Nephrol Dialysis Transplant: Off Publ Eur Dialysis Transplant Assoc ‐ Eur Renal Assoc. 1997;12(Suppl. 2):71. [PubMed] [Google Scholar]
- 10. Nezu U, Kamiyama H, Kondo Y, Sakuma M, Morimoto T, Ueda S. Effect of low‐protein diet on kidney function in diabetic nephropathy: meta‐analysis of randomised controlled trials. BMJ Open. 2013;3(5):e002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melchior WR, Bindlish V, Jaber LA. Angiotensin‐converting enzyme inhibitors in diabetic nephropathy. Ann Pharmacother. 1993;27(3):344. [DOI] [PubMed] [Google Scholar]
- 12. Kalaitzidis R, Bakris GL. Effects of angiotensin II receptor blockers on diabetic nephropathy. J Hyperten Suppl Off J Int Soc Hyperten. 2009;27(5):15‐21. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. 2016;113(9):E1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobyliak N, Conte C, Cammarota G, et al. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metabolism. 2016;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sánchez B, Delgado S, Blanco‐Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2016;61(1). 10.1002/mnfr.201600240 [DOI] [PubMed] [Google Scholar]
- 16. Ji YY, Kim SS. Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients. 2016;8(3):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bron PA, Kleerebezem M, Brummer RJ, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117(1):93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aron‐Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non‐alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19(4):338‐348. [DOI] [PubMed] [Google Scholar]
- 19. Alard J, Lehrter V, Rhimi M, et al. Beneficial metabolic effects of selected probiotics on diet‐induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. 2016;18(5):1484‐1497. [DOI] [PubMed] [Google Scholar]
- 20. Li X, Xu Q, Jiang T, et al. A comparative study of the antidiabetic effects exerted by live and dead multi‐strain probiotics in the type 2 diabetes model of mice. Food Funct. 2016;7(12):4851‐4860. [DOI] [PubMed] [Google Scholar]
- 21. Chinese Diabetes Society . Prevention and treatment guideline of type 2 diabetes in China (2017). Chin J Diabetes Mellitus. 2018;10(1):4‐67. [Google Scholar]
- 22. Rodríguez Del Águila M, González‐Ramírez A. Sample size calculation. Allergol Immunopathol (Madr). 2014;42(5):485‐492. [DOI] [PubMed] [Google Scholar]
- 23. Lu CC, Ma KL, Ruan XZ, Liu BC. Intestinal dysbiosis activates renal renin‐angiotensin system contributing to incipient diabetic nephropathy. Int J Med Sci. 2018;15(8):816‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu YC, Yin LT, Chang WT, Huang JS. Effect of Lactobacillus reuteri GMNL‐263 treatment on renal fibrosis in diabetic rats. J Biosci Bioeng. 2010;110(6):709‐715. [DOI] [PubMed] [Google Scholar]
- 25. Miraghajani M, Zaghian N, Dehkohneh A, Mirlohi M, Ghiasvand R. Probiotic soy milk consumption and renal function among type 2 diabetic patients with nephropathy: a randomized controlled clinical trial. Probiotics Antimicrob Proteins. 2019;11(1):124‐132. [DOI] [PubMed] [Google Scholar]
- 26. Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS One. 2010;5(2):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Savitha AK, Gopalakrishnan S, Umadevi R, Rama R. The need for patient follow‐up strategies to confirm diabetes mellitus in large scale opportunistic screening. J Clin Diagn Res. 2016;10(2):LE01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xue R, Gui D, Zheng L, Zhai R, Wang F, Wang N. Mechanistic insight and management of diabetic nephropathy: recent progress and future perspective. J Diabetes Res. 2017;2017(1, supplement 1):1839809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of beta‐cell death in type 2 diabetes. Diabetes. 2005;54(Suppl. 2):S108. [DOI] [PubMed] [Google Scholar]
- 30. Inzucchi SE, Sherwin RS. The prevention of type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2005;34(1):199‐219. [DOI] [PubMed] [Google Scholar]
- 31. Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio‐renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rohani F. Glomerular filtration rate‐based cystatin C compared to microalbuminuria to detect early stage of diabetic nephropathy in children with type 1 diabetes mellitus. Int J Diabetes Developing Count. 2015;35(3):1‐7. [Google Scholar]
- 33. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929‐937. [DOI] [PubMed] [Google Scholar]
- 34. Chen DL, Zheng SH, Liu SM, Lu YL, Yang Y, Song XX. Value of combined detection of Cys‐C, HbA1c, GA and U‐mAlb/Cr in diagnosis of early renal damage in patients with type 2 diabetes mellitus. Int J Lab Med. 2014;(6):677–679. [Google Scholar]
- 35. Ma KL. Intestinal dysbiosis activates renal renin‐angiotensin system contributing to incipient diabetic nephropathy. Int J Med Sci. 2018;15(8):816‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 37. Moroti C, Souza Magri LF, de Rezende CM, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soleimani A, Zarrati Mojarrad M, Bahmani F, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2016;91(2):435. [DOI] [PubMed] [Google Scholar]
- 39. Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun‐Nisak MY. Effect of multi‐strain probiotics (multi‐strain microbial cell preparation) on glycemic control and other diabetes‐related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56(4):1535‐1550. [DOI] [PubMed] [Google Scholar]
- 40. Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double‐blind placebo‐controlled clinical trial. Iran J Public Health. 2015;44(2):228‐237. [PMC free article] [PubMed] [Google Scholar]
- 41. Ejtahed HS, Mohtadi‐Nia J, Homayouni‐Rad A, Niafar M, Asghari‐Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539‐543. [DOI] [PubMed] [Google Scholar]
- 42. Mobini R, Tremaroli V, Ståhlman M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2016;19(4):579. [DOI] [PubMed] [Google Scholar]
- 43. Mazruei Arani N, Emam‐Djomeh Z, Tavakolipour H, Sharafati‐Chaleshtori R, Soleimani A, Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double‐blind, controlled trial. Probiotics Antimicrob Proteins. 2019;11(4):1195‐1201. [DOI] [PubMed] [Google Scholar]
- 44. Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double‐blind, placebo‐controlled trial. Food Funct. 2018;9(9):4763‐4770. [DOI] [PubMed] [Google Scholar]
- 45. AbdelQadir YH, Hamdallah A, Sibaey EA, et al. Efficacy of probiotic supplementation in patients with diabetic nephropathy: a systematic review and meta‐analysis. Clin Nutr ESPEN. 2020;40:57–67. [DOI] [PubMed] [Google Scholar]
- 46. Vlachou E, Ntikoudi A, Govina O, Lavdaniti M, Kotsalas N, Tsartsalis A. Effects of probiotics on diabetic nephropathy: a systematic review. Curr Clin Pharmacol. 2020, 2. 10.2174/157488471566620030311275 [DOI] [PubMed] [Google Scholar]


