Abstract
Background
This study aimed to investigate the relationship of serum JNK pathway‐associated phosphatase (JKAP) expression with rheumatoid arthritis (RA) risk and clinical features, also to explore the longitudinal change of JKAP during etanercept treatment and its relationship with etanercept treatment response in RA patients.
Methods
A total of 87 RA patients and 44 healthy controls (HCs) were enrolled; then, their JKAP expression in serum was determined by enzyme‐linked immunosorbent assay (ELISA). Among 87 RA patients, 42 cases further received the 24‐week etanercept treatment; then, their JKAP level in serum (detected by ELISA) and clinical response (evaluated by disease activity score in 28 joints (DAS28) score) were evaluated at week 4 (W4), week 12 (W12), and week 24 (W24) after initiation of etanercept treatment.
Results
JKAP expression was decreased in RA patients compared to HCs, which disclosed a good predictive value for RA risk. JKAP expression was negatively associated with tender joint count, swollen joint count, erythrocyte sedimentation rate, C‐reactive protein, and DAS28 in RA patients, respectively. For RA patients who received 24‐week etanercept treatment, their clinical response rate was 0.0%, 33.3%, 50.0%, and 69% at W0, W4, W12, and W24, respectively. Importantly, JKAP was gradually increased during etanercept treatment, whose longitudinal elevation positively related to etanercept treatment response in RA patients.
Conclusion
Circulating JKAP links with decreased RA risk and mild disease activity, whose longitudinal elevation positively relates to etanercept treatment response.
Keywords: disease risk, disease severity, etanercept treatment, JNK pathway‐associated phosphatase, rheumatoid arthritis
This study aimed to investigate the relationship of serum JNK pathway‐associated phosphatase (JKAP) expression with rheumatoid arthritis (RA) risk and clinical features, also to explore the longitudinal change of JKAP during etanercept treatment and its relationship with etanercept treatment response in RA patients. A total of 87 RA patients and 44 healthy controls (HCs) were enrolled; then, their JKAP expression in serum was determined by enzyme‐linked immuno sorbent assay (ELISA). Among 87 RA patients, 42 cases further received the 24‐week etanercept treatment; then, their JKAP level in serum (detected by ELISA) and clinical response (evaluated by disease activity score in 28 joints (DAS28) score) were evaluated at week 4 (W4), week 12 (W12), and week 24 (W24) after initiation of etanercept treatment. JKAP expression was decreased in RA patients compared to HCs, which disclosed a good predictive value for RA risk. JKAP expression was negatively associated with tender joint count, swollen joint count, erythrocyte sedimentation rate, C‐reactive protein, and DAS28 in RA patients, respectively. For RA patients who received 24‐week etanercept treatment, their clinical response rate was 0.0%, 33.3%, 50.0%, and 69% at W0, W4, W12, and W24, respectively. Importantly, JKAP was gradually increased during etanercept treatment, whose longitudinal elevation positively related to etanercept treatment response in RA patients. Circulating JKAP links with decreased RA risk and mild disease activity, whose longitudinal elevation positively relates to etanercept treatment response.

1. INTRODUCTION
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory joint diseases, which affects about 1% of the population worldwide. 1 , 2 Primarily involves the joints, RA also exhibits other manifestations such as rheumatoid nodules, vasculitis, and pulmonary involvement, which make it substantial burdens for both the individual and the society. 3 , 4 Despite the fact that great progress has been achieved in treat‐to‐target strategy and novel treatment drugs such as tumor necrosis factor (TNF) inhibitors, interleukin (IL)‐6 inhibitor, and Janus kinase (JAK) inhibitors recently, the outcome of Chinese RA patients is still far more from satisfaction due to late diagnosis and high recurrence rate in clinical practice. 5 , 6 , 7 , 8 , 9 Therefore, the exploration of novel biomarkers for early diagnosis and disease monitoring in RA patients is of great importance.
JNK pathway‐associated phosphatase (JKAP), also called dual‐specificity phosphatase 22 (DUSP22), is a novel dual‐specificity phosphatase that can activate c‐Jun N‐terminal kinase (JNK) specifically, which is found in a variety of mammalian cells and involves in multiple biological activities. 10 , 11 , 12 , 13 , 14 , 15 Previous studies disclose that JKAP participates in the etiology of some autoimmune diseases via inhibition of T‐cell receptor signaling. 15 , 16 , 17 And the JKAP‐knockout mice are observed to be more susceptive to experimental autoimmune encephalomyelitis (EAE). 18 In addition, compared to healthy controls (HCs), JKAP expression is downregulated in peripheral blood T cells from patients with lupus nephritis (LN), which implies that determination of JKAP level in T cells may facilitate early diagnosis in LN patients. 19 Since JKAP is engaged in immune system activities, and the expression of JKAP is decreased in patients with autoimmune diseases such as systemic lupus erythematosus (SLE), it may serve as an important function in development and progress of RA as well. Nonetheless, no study has been done in evaluating the role of JKAP in diagnosis and disease management of RA until now. Therefore, the aim of the current study was to investigate the relationship of serum JKAP expression with RA risk and clinical features, also to explore the longitudinal change of JKAP during etanercept treatment and its relationship with treatment response in RA patients.
2. METHODS
2.1. Participants
A total of 87 RA patients admitted to Department of Rheumatology in the First Affiliated Hospital of Wenzhou Medical University, between June 2016 and Sep 2017, were consecutively enrolled in this study. The inclusion criteria were as follows: (1) diagnosis of RA according to the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) rheumatoid arthritis classification criteria 20 and (2) age above 18 years. Exclusion criteria were as follows: (1) severe deformity of joint; (2) history of hematological malignance disease or solid tumors; (3) history of severe infection, renal dysfunction, or hepatic dysfunction; and (4) pregnant or lactating women. During the same period, 44 healthy controls (HCs) with age and gender matched to RA patients were also consecutively recruited in the study when they underwent healthy examination in our hospital. This study was approved by the Ethics Committee of First Affiliated Hospital of Wenzhou Medical University. And all the participants signed the informed consents.
2.2. Data collection
After enrollment, comprehensive data of RA patients were collected including: age, gender, body mass index (BMI), disease duration, tender joint count (TJC), swollen joint count (SJC), erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), disease activity score in 28 joints (DAS28), rheumatoid factor (RF) status, anti‐citrullinated protein antibody (ACPA) status, and history of treatments (biologics, conventional disease‐modifying antirheumatic drugs [cDMARDs]). Age and gender of HCs were also recorded.
2.3. Sample collection and detection
After enrollment, peripheral blood (PB) samples were collected from all RA patients and HCs. Then, serum samples were subsequently isolated from the PB by centrifuging at 2000 g for 10 min in a refrigerated centrifuge, which was then stored at −80℃ for further detection. JKAP expression in serum was then determined by enzyme‐linked immunosorbent assay (ELISA) using commercial JKAP ELISA kit (Shanghai Enzyme‐linked Biotechnology Co.) according to the instructions.
2.4. Etanercept treatment cohort
Among 87 patients, there were 42 patients with poor efficacy to current treatment regimens (cDMARDs monotherapy or combination), and they selected to receive etanercept treatment combined with methotrexate or leflunomide for 24 weeks. In order to investigate the changes of JKAP levels with enactone treatment, these 42 RA patients were further enrolled into an etanercept treatment cohort and followed up at week 4 (W4), week 12 (W12), and week 24 (W24) after initiation of etanercept treatment. At the clinic visits of W4, W12, and W24, PB samples of patients were collected, and clinical response was assessed as well. The serum samples were separated from the collected PB samples as well as described above, and the JKAP levels in serum were also determined by commercial JKAP ELISA kit (Shanghai Enzyme‐linked Biotechnology Co.). Clinical response was evaluated by DAS28 score, which was defined as the DAS28 score decreased more than 1.2 points from baseline. 21 Besides, for the patients who lost follow‐up in the etanercept treatment cohort, the last follow‐up data were used as subsequent missing data in the analysis. All patients were included in the statistical analyses, and in terms of the RA patients who lost follow‐up, the clinical response to etanercept was analyzed with the last observation carried forward (LOCF) method for the missing data based on the intention‐to‐treat (ITT) principle.
2.5. Statistical analysis
Statistical analyses were performed using SPSS 22.0 software (IBM), and figures were constructed using GraphPad Prim 7.02 (GraphPad Software Inc.). Data were displayed as mean with standard deviation (SD), median with interquartile range (IQR), or number with percentage (No. [%]). Comparisons of JKAP level between two groups were determined by Wilcoxon rank‐sum test. The associations of continuous variables with JKAP level were analyzed by Spearman's rank correlation test. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the ability of JKAP level in distinguishing RA patients from HCs. Repeated measurement data were analyzed by Friedman's rank test. p value <.05 was considered as statistically significant.
3. RESULTS
3.1. RA patients’ characteristics
The mean age was 57.4 ± 11.3 years, and there were 18 (20.7%) and 69 (79.3%) females. Regarding serum markers, 62 (71.3%) patients had positive RF, and 63 (72.4%) patients had positive ACPA. In terms of disease status, the median TJC and SJC were 6.0 (5.0–9.0) joints and 5.0 (4.0–7.0) joints, respectively; the median ESR and CRP were 45.7 (27.0–62.0) mm/h and 23.0 (10.3–61.1) mg/L, respectively; and the mean DAS28 score was 5.2 ± 0.7. Other information of RA patients’ characteristics and treatment history was shown in Table 1.
TABLE 1.
RA patients’ characteristics.
| Items | RA patients (N = 87) |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 57.4 ± 11.3 |
| Gender, no. (%) | |
| Male | 18 (20.7) |
| Female | 69 (79.3) |
| BMI (kg/m2), mean ± SD | 22.1 ± 3.0 |
| Medical history | |
| Disease duration (years), median (IQR) | 4.1 (1.4–8.0) |
| History of biologics, no. (%) | 15 (17.2) |
| History of cDMARDs, no. (%) | 67 (77.0) |
| Serum markers | |
| RF status, no. (%) | |
| Negative | 18 (20.7) |
| Positive | 62 (71.3) |
| Not detected | 7 (8.0) |
| ACPA status, no. (%) | |
| Negative | 12 (13.8) |
| Positive | 63 (72.4) |
| Not detected | 12 (13.8) |
| Disease status | |
| TJC (joints), median (IQR) | 6.0 (5.0–9.0) |
| SJC (joints), median (IQR) | 5.0 (4.0–7.0) |
| ESR (mm/h), median (IQR) | 45.7 (27.0–62.0) |
| CRP (mg/L), median (IQR) | 23.0 (10.3–61.1) |
| DAS28ESR score, mean ± SD | 5.2 ± 0.7 |
Abbreviations: ACPA, anti‐citrullinated protein antibodies; BMI, body mass index; cDMARDs, conventional disease‐modifying antirheumatic drugs; CRP, C‐reactive protein; DAS28ESR score, 28 joints disease activity score based on erythrocyte sedimentation rate; ESR, erythrocyte sedimentation rate; HCs, healthy controls; IQR, interquartile range; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation; SJC, swollen joint count; TJC, tender joint count.
3.2. The predictive value of JKAP for RA risk
The JKAP expression in RA patients and HCs was assessed by ELISA, and the comparison of JKAP expression between RA patients and HCs was determined by Wilcoxon rank‐sum test, which revealed that JKAP expression in RA patients was decreased compared to HCs (p < .001, Figure 1A). ROC curve (Figure 1B) was further performed to assess the value of JKAP expression in distinguishing RA patients from HCs. The area under the curve (AUC) of JKAP expression for predicting RA risk was 0.819 (95% CI: 0.735–0.904), with a sensitivity of 94.3% and a specificity of 68.2% at best cutoff point (JKAP = 52.500 pg/ml), which was defined as the point with the maximum value of sensitivity plus specificity.
FIGURE 1.
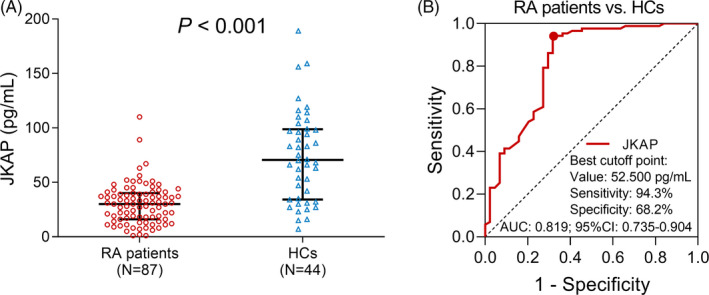
The JKAP expression, and its predictive value for RA risk by ROC curve. Comparison of JKAP expression between RA patients and HCs (A); the predictive value of JKAP for RA risk by ROC curve (B). JKAP, JNK pathway‐associated phosphatase; RA, rheumatoid arthritis; HCs, healthy controls; ROC, receiver operating characteristic; AUC, area under the curve.
3.3. The association of JKAP expression with characteristics of RA patients
The expression of JKAP was negatively associated with ESR (p = .005, Figure 2D), CRP (p = .001, Figure 2E) and DAS28 score (p < .001, Figure 2F) in RA patients. However, no association of JKAP expression with age (p = .958, Figure 2A), BMI (p = .326, Figure 2B), or disease duration (p = .882, Figure 2C) in RA patients was found. In addition, no difference of JKAP expression was found between male and female (p = .171, Figure 3A), RF‐negative status and RF‐positive status (p = .177, Figure 3B), ACPA‐negative status and ACPA‐positive status (p = .988, Figure 3C), no history of biologics and history of biologics (p = .170, Figure 3D), or no history of cDMARDs and history of cDMARDs (p = .876, Figure 3E) in RA patients.
FIGURE 2.
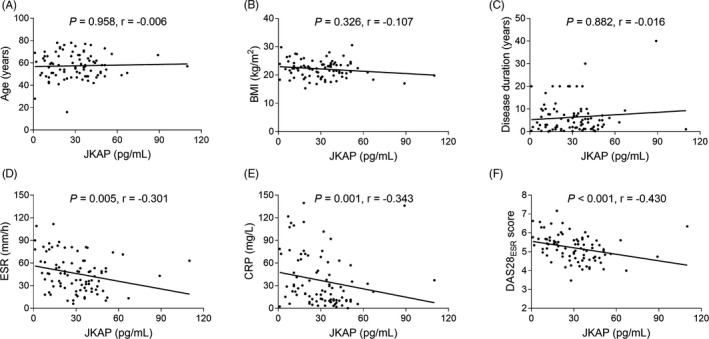
Correlation of JKAP expression with continuous variables of RA patients. Correlation of JKAP level with age (A), BMI (B), disease duration (C), ESR (D), CRP (E), and DAS28 score (F) in RA patients. JKAP, JNK pathway‐associated phosphatase; RA, rheumatoid arthritis; TJC, tender joint count; SJC, swollen joint count; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein; DAS28, disease activity score in 28 joints; BMI, body mass index.
FIGURE 3.
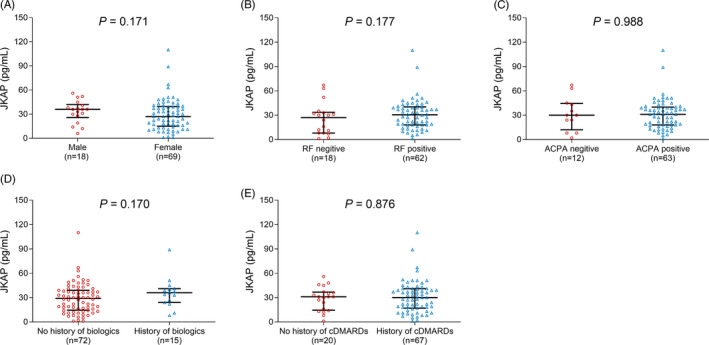
Relationship of JKAP expression with categorical variables of RA patients. Relationship of JKAP level with gender (A), RF status (B), ACPA status (C), history of biologics (D), and history of cDMARDs (E). JKAP, JNK pathway‐associated phosphatase; RA, rheumatoid arthritis; RF, rheumatoid factor; ACPA, anti‐citrullinated protein antibody; cDMARDs, conventional disease‐modifying antirheumatic drugs.
3.4. JKAP change during etanercept treatment and its relationship with treatment response
There were 0 (0.0%), 14 (33.3%), 21 (50.0%), and 29 (69%) patients achieved clinical response after etanercept treatment at W0, W4, W12, and W24, respectively (Figure 4). In total patients with etanercept treatment, JKAP expression was gradually increased with treatment time (W0, W4, W12, and W24) (p < .001, Figure 5). Regarding W24 responders, JKAP expression was also gradually increased with treatment time (W0, W4, W12, and W24) (p < .001, Figure 6A), whereas no difference in JKAP expression at different treatment time (W0, W4, W12, and W24) among W24 non‐responders (p = .093, Figure 6B).
FIGURE 4.
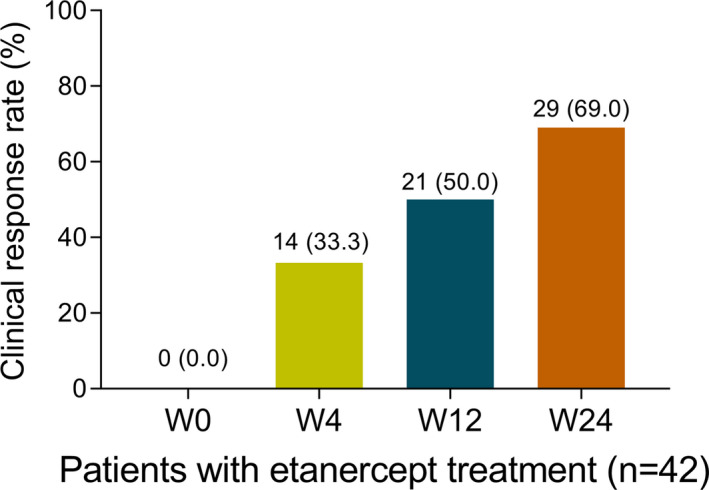
Clinical response after etanercept treatment in RA patients. RA, rheumatoid arthritis; W, week.
FIGURE 5.
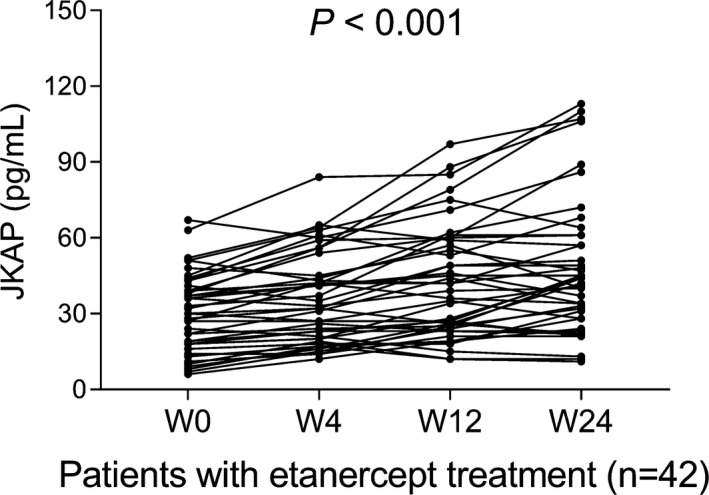
JKAP change during etanercept treatment in total RA patients. JKAP, JNK pathway‐associated phosphatase; RA, rheumatoid arthritis; W, week.
FIGURE 6.
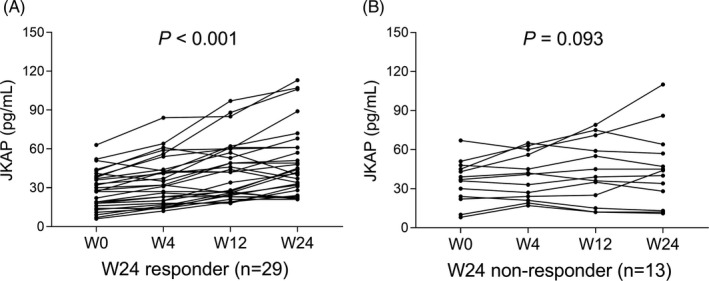
JKAP change during etanercept treatment in W24 responders and W24 non‐responders. JKAP change during etanercept treatment in W24 responders (A) and W24 non‐responders (B). JKAP, JNK pathway‐associated phosphatase; W, week.
4. DISCUSSION
JKAP belongs to the low molecular weight atypical dual‐specificity phosphatase (DUSPs) family, which is considered to participate in various biological processes via protein dephosphorylation. 14 , 22 , 23 , 24 A recent study shows that the expression of JKAP in T cells is downregulated in active LN patients compared to that of inactive LN patients. And the JKAP level in T cells could distinguish active LN patients from inactive LN patients with a sensitivity of 90.9% and a specificity of 88.2%. More interestingly, the AUC of JKAP expression for active LN risk is numerically higher than that of conventional biomarkers such as complement components C3, complement components C4, and anti‐double‐stranded DNA antibodies (ANA). 19 Another study discovers that the JKAP expression is decreased in intestinal mucosa of both active Crohn's disease (CD) and ulcerative colitis (UC) patients, and it shows a good predictive value for CD and UC susceptibility. 25 These results suggest that JKAP expression could be served as a biomarker for several immune diseases, and we then hypothesized that JKAP expression might related to RA risk as well. In this present study, the expression of JKAP was observed to be reduced in RA patients compared to HCs, which disclosed a good predictive value for RA risk. The possible reason for the reduction of JKAP expression in RA patients might be that JKAP acted as anti‐inflammatory gene through regulating T‐cell activity and the release of pro‐inflammatory cytokines. 18 , 19 , 26 , 27
An in vitro study finds that compared to wild‐type T cells, JKAP‐knockout T cells produce more IL‐2 and interferon‐γ (IFN‐γ); meanwhile, JKAP‐knockout CD4+ and CD8+ T cells increase T‐cell proliferation and activation by anti‐CD3 simulation compared to wild‐type CD4+ and CD8+ T cells. 18 In another study, JKAP is discovered to inhibit CD4+T‐cell activation, Th1/Th17 cell differentiation and negatively associated with CRP, ESR, disease activity, and pro‐inflammatory cytokines in inflammatory bowel disease (IBD). 25 These reports suggest that JKAP is engaged in T‐cell‐mediated activities, which subsequently intermediate inflammation and disease severity. In the present research, we observed that expression of JKAP was negatively associated with disease activity and inflammation in RA patients, which is partially in line with previous studies. The possible explanations for these results might be the following: Since JKAP could suppress T‐cell‐mediated immunity, the deficiency of JKAP expression would promote the release of pro‐inflammatory cytokines such as IL‐6, IL‐17, and TNF‐α through activating T cells, which inhibited inflammation and consequently result in mild disease activity in RA patients. 15 , 18 , 19 , 26 , 27
In addition, we also discovered that JKAP expression was increased during etanercept treatment and its longitudinal elevation was positively associated with treatment response in RA patients. One possible explanation was as follows: JKAP might mediate a variety of inflammation‐related mechanisms (such as T‐cell activation and the secretion of pro‐inflammation cytokines) to reflect inflammation level; thus, the longitudinal elevation of JKAP could reflect decreased inflammation and disease activity during etanercept treatment, thereby related to increased etanercept treatment response in RA patients. 15 , 18 , 19 , 26 , 27 Another possible reason was as follows: JKAP might regulate multiple inflammation‐related genes, and these genes might be directly or indirectly interacted with TNF‐α inhibitor, thereby promoted etanercept treatment response in RA patients.
There were some limitations in this study: (1) Most of RA patients in this study were in the active status; thus, the associations of JKAP level with inactive status RA patients were not assessed in our study. (2) The sample size was relatively small, and the number of patients and HCs was different, which would decrease the statistics power and make the result affected by extreme values greatly. (3) The majority of RA patients included in the study were from East China, which could cause selection bias in this study. Therefore, RA patients from other areas in China should be included in the future.
In conclusion, circulating JKAP expression could be served as a novel biomarker for disease risk and disease activity of RA. More interestingly, JKAP is increased during etanercept treatment and its longitudinal elevation positively associates with treatment response in RA patients.
CONFLICT OF INTEREST
The authors of this work have nothing to disclose.
ACKNOWLEDGEMENTS
This study was supported by the Science and Technology Planning Project of Wenzhou (Y20170060 and Y20170056), Science and Technology Action Plans on the Prevention and Treatment of Major Diseases—Trauma Repair Special Project, Development Research Center for Major Science and Technology National Health and Family Planning Commission of the PRC (ZX‐01‐C2016029), Medical Science Research Foundation of Zhejiang Province, China (2020374759), and Natural Science Foundation of Zhejiang Province, China (LY20H100002).
Li Sun and Jianxin Tu contributed equally to this work.
Contributor Information
Cailong Liu, Email: graceforcailong@hotmail.com.
Yan Zhou, Email: 215692141@qq.com.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338‐2348. [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328‐2337. [DOI] [PubMed] [Google Scholar]
- 3. Malmstrom V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. 2017;17(1):60‐75. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023‐2038. [DOI] [PubMed] [Google Scholar]
- 5. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205‐2219. [DOI] [PubMed] [Google Scholar]
- 6. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094‐1108. [DOI] [PubMed] [Google Scholar]
- 7. Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861‐1874. [DOI] [PubMed] [Google Scholar]
- 8. Wang G, Mu R, Xu H. Management of rheumatoid arthritis in People's Republic of China ‐ focus on tocilizumab and patient considerations. Int J Gen Med. 2015;8:187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Xu J, Zheng J, et al. Association between interleukin‐6 gene polymorphisms and rheumatoid arthritis in Chinese Han population: a case‐control study and a meta‐analysis. Sci Rep. 2014;4:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen AJ, Zhou G, Juan T, et al. The dual specificity JKAP specifically activates the c‐Jun N‐terminal kinase pathway. J Biol Chem. 2002;277(39):36592‐36601. [DOI] [PubMed] [Google Scholar]
- 11. Ju A, Cho YC, Kim BR, et al. Scaffold Role of DUSP22 in ASK1‐MKK7‐JNK Signaling Pathway. PLoS ONE. 2016;11(10):e0164259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez‐Mut JV, Aso E, Heyn H, et al. Promoter hypermethylation of the phosphatase DUSP22 mediates PKA‐dependent TAU phosphorylation and CREB activation in Alzheimer's disease. Hippocampus. 2014;24(4):363‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CP, Lin SP, Chern SR, Wu PS, Su JW, Wang W. A boy with cleft palate, hearing impairment, microcephaly, micrognathia and psychomotor retardation and a microdeletion in 6p25.3 involving the DUSP22 gene. Genet Couns. 2013;24(2):243‐246. [PubMed] [Google Scholar]
- 14. Sekine Y, Ikeda O, Hayakawa Y, et al. DUSP22/LMW‐DSP2 regulates estrogen receptor‐alpha‐mediated signaling through dephosphorylation of Ser‐118. Oncogene. 2007;26(41):6038‐6049. [DOI] [PubMed] [Google Scholar]
- 15. Melard P, Idrissi Y, Andrique L, et al. Molecular alterations and tumor suppressive function of the DUSP22 (Dual Specificity Phosphatase 22) gene in peripheral T‐cell lymphoma subtypes. Oncotarget. 2016;7(42):68734‐68748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xing X, Flotte TJ, Law ME, et al. Expression of the chemokine receptor gene, CCR8, is associated With DUSP22 rearrangements in anaplastic large cell lymphoma. Appl Immunohistochem Mol Morphol. 2015;23(8):580‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li JP, Fu YN, Chen YR, Tan TH. JNK pathway‐associated phosphatase dephosphorylates focal adhesion kinase and suppresses cell migration. J Biol Chem. 2010;285(8):5472‐5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li JP, Yang CY, Chuang HC, et al. The phosphatase JKAP/DUSP22 inhibits T‐cell receptor signalling and autoimmunity by inactivating Lck. Nat Commun. 2014;5:3618. [DOI] [PubMed] [Google Scholar]
- 19. Chuang HC, Chen YM, Hung WT, et al. Downregulation of the phosphatase JKAP/DUSP22 in T cells as a potential new biomarker of systemic lupus erythematosus nephritis. Oncotarget. 2016;7(36):57593‐57605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569‐2581. [DOI] [PubMed] [Google Scholar]
- 21. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LBA, van Riel PLCM. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 22. Hamada N, Mizuno M, Tomita H, Iwamoto I, Hara A, Nagata KI. Expression analyses of Dusp22 (Dual‐specificity phosphatase 22) in mouse tissues. Med Mol Morphol. 2018;51(2):111–117. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen MB, Hamilton‐Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK‐negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130(4):554‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King RL, Dao LN, McPhail ED, et al. Morphologic features of ALK‐negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou R, Chang Y, Liu J, et al. JNK pathway‐associated phosphatase/DUSP22 suppresses CD4(+) T‐cell activation and Th1/Th17‐cell differentiation and negatively correlates with clinical activity in inflammatory bowel disease. Front Immunol. 2017;8:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Csikesz CR, Knudson RA, Greipp PT, Feldman AL, Kadin M. Primary cutaneous CD30‐positive T‐cell lymphoproliferative disorders with biallelic rearrangements of DUSP22. J Invest Dermatol. 2013;133(6):1680‐1682. [DOI] [PubMed] [Google Scholar]
- 27. Arruga F, Gizdic B, Bologna C, et al. Mutations in NOTCH1 PEST domain orchestrate CCL19‐driven homing of chronic lymphocytic leukemia cells by modulating the tumor suppressor gene DUSP22. Leukemia. 2017;31(9):1882‐1893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.


