Abstract
Hematopoietic cancers are among the most common malignancies worldwide, which are divided into different types depending on the origin of tumor cells. In recent years, the pivotal role of different signaling pathways in the onset and progression of these cancer types has been well established. One of these pathways, whose role in blood malignancies has been well‐defined, is PI3K/mTOR/AKT axis. The signaling pathway involves in a wide variety of important biological events in cells. It is clear that dysregulation of mediators involved in PI3 kinase signaling takes a pivotal role in cancer development. Considering the undeniable role of miRNAs, as one of the well‐known families of non‐coding RNAs, in gene regulation, we aimed to review the role of miRNAs in regulation of PI3 kinase signaling effectors in hematopoietic cancers.
Keywords: AKT, gene regulation, hematopoietic cancers, miRNA, mTOR, PI3 kinase
PI3K pathway involves in a wide verity of important biological events in cells such as proliferation, differentiation, apoptosis, and even drug resistance. It is confirmed that there is a relationship between dysregulation of mediators involved in this signaling pathway and different cancer types such as hematopoietic malignancies. Considering the pivotal role of miRNAs as the key regulators in gene regulation, overexpression and downregulation of various miRNAs have been reported to take a role in development of different hematopoietic cancers through targeting mediators of PI3K signaling pathway.
1. INTRODUCTION
Today, the number of patients suffering from cancer is growing as a result of increasing environmental risk factors in both developing and developed countries. 1 , 2 , 3 Hematopoietic cancers are heterogenous malignancies which show various prognosis, etiology, and incidence rate. According to numerous population studies, these types of cancer are classified into different subtypes including leukemias, lymphomas, and myelomas. 4 , 5 , 6 , 7 Currently, enormous studies are investigating the possible mechanisms involved in the onset and progression of hematopoietic malignancies. Different signaling pathways have been suggested to have important role in this context. Accordingly, dysregulation of PI3K/mTOR/AKT signaling pathway which is paramount important in various cellular events, such as cell growth, differentiation, and proliferation, has been observed in different cancer types, especially in hematopoietic cancers. Hence, changes in the expression of mediators of PI3K/mTOR/AKT axis not only participate in cancer onset, but also it would affect the responses of tumor cells to the common therapies. 8 , 9
Gene regulation is a complex process that delicately regulates cellular activities and homeostasis. Numerous reports have highlighted the role of non‐coding RNAs, such as miRNAs, in regulation of various gene expressions. miRNAs are small RNAs that do not encode any protein, but they regulate gene expression mostly at the translational levels. 10 In recent years, with increasing information regarding the miRNA's structure and its gene expression regulatory function, many studies have been conducted to clarify the relationship between dysregulation of miRNA's expression and development of different cancer types such as blood cancers. Since the PI3K signaling pathway has a central role in blood cancer development, we tried to compile studies that examined the relationship between dysregulation of different miRNA targeting PI3K/mTOR/AKT signaling mediators and development of hematopoietic malignancies.
2. PI3 KINAS SIGNALING AND CANCER
Disturbance of the balance between cell proliferation and apoptosis may lead to cancer. Numerous proteins and signaling pathways regulate this delicate process. These signaling pathways are the main regulators of cell proliferation, differentiation, and cell growth, so that any imbalanced expression of their mediators may lead to cancer development 11 (Figure 1). One of the most important signaling pathways in this context is the PI3K/AKT/mTOR signaling. Until now, relationship between this biological pathway and various cancers has been well established and new treatments have been designed based on targeting the mediators involved in the signaling pathway. 12
FIGURE 1.
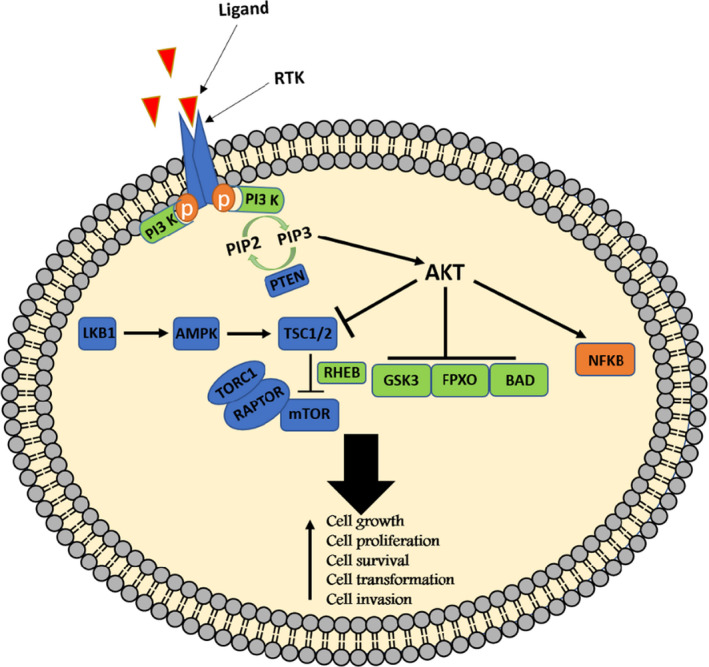
PI3 kinase signaling pathway. As shown in the figure, activation of PI3K/mTOR/AKT signaling activates a cascade that eventually changes several critical activities including cell growth, cell proliferation, cell survival, cell transformation, and motility in cell
Phosphatidylinositol 3‐kinases (PI3Ks) are a highly conserved family of lipid kinases. In general, the members of this family are divided into three categories: 1—class I kinases, which are divided into two subclasses including IA and IB, 2—class II with three isoforms including PI3K‐C2α, PI3K‐C2β, and PI3K‐C2γ, and 3—Class III, which includes a member called PI3K‐C3. The kinases in the class IA group are heterodimers consisting of a catalytic component (p110) and a regulating subunit (p85). 13 Each of these components has several isoforms including 3 isoforms for p110 (p110α, p110β, and p110δ) and 5 isoforms for p85 subunit (p85α, p55α, p50α, p85β, and p55γ). Class Iβ kinases consist of two catalytic and regulatory subunits, including p110γ and p101, respectively. PI3K activation occurs following binding to the tyrosine kinase receptor (RTK) directly or through the IRS protein. 14 However, this enzyme can be activated by G protein‐coupled receptor (GPCR) and RAS protein activation. 15 After receiving extracellular signal, the tyrosine kinase (RTK) receptor and PI3K regulatory subunit bind together directly or in an IRS protein‐mediated manner. 16 Afterward, the catalytic subunit of the enzyme is activated, leading to phosphorylation of the phosphatidyl inositol 4,5 (PIP2) located in cell membrane that turns it into the phosphatidyl inositol 3,4,5 (PIP3). Phosphorylating of PIP2 and converting it into PIP3 make the latter molecule able to bind to proteins that possess the FYVE and Pleckstrin homology domain (PHIP) domains such as AKT protein. After binding the PHIP domain of AKT protein to PIP3, its thr308 site will be exposed to phosphoinositide‐dependent kinase 1 (PDK1). Full activation of AKT occurs following phosphorylation at the ser473 site. 13 After activation, AKT phosphorylates and inhibits numerous proteins within cytosol and nucleus such as PRAS40, GSK‐3β, and BAD. On the other hand, AKT activates mTOR by inhibitory phosphorylation of TSC2 protein. TSC2 protein is a serine/threonine kinase that forms two distinct complex structures including mTORC1 and mTORC2. By activating mTORC1, this protein causes phosphorylation of 4EBP1 and S6K1 proteins, which ultimately increases protein synthesis in cell through activating S6 protein. In addition, PIP3 activates Rac by triggering a number of Rac‐GEF factors such as P‐Rex and Vav, resulting in activation of different biological events including cell migration, growth, adhesion, and even changes in cell morphology. 17
In general, the PI3K/AKT/mTOR signaling is a very important and vital pathway in cells which plays a critical role in biological processes such as cell growth, proliferation, survival, and cell mobility by converting extracellular signals into an intracellular response. It is obvious that disturbing the balance of the expression of any of mediators involved in this biological pathway may lead to malignancy. 18 , 19 For example, overexpression of the mTOR2 protein has been reported in a number of cancers. In this regard, unbalanced expression of Rictor protein, as a component of the mTOR2 complex is frequently observed in samples obtained from patients with CLL. 20 There is also an intertwined correlation between PI3K signaling and other cancer‐related signaling networks. For example, AKT activation elevates the SOX2 stability and its nuclear localization that introduces AKT as an upstream regulator of SOX2 transcription factor. However, the relationship between SOX2 and AKT is reciprocal which is exemplified in glioblastoma, when SOX2 accumulation in nucleus increases PI3KCA gene expression. 21
Although the role of this signaling pathway in cancer has been well studied, various regulatory factors appear to play a role in dysregulation of this biological pathway. In recent years, with highlighting the crucial role of non‐coding RNAs such as miRNAs in regulation of signaling effectors, numerous researches have been conducted on the effect of dysregulation of various miRNAs in these pathways.
3. miRNA AND GENE REGULATION
MiRNAs are small RNAs which are not translated into proteins, but they contribute to different cellular processes by regulating the expression of various proteins. Today, the role of miRNAs in a wide range of biological events, including cell growth, proliferation, cell cycle, differentiation, and cellular metabolism has been well‐documented. 22 , 23 , 24 It is found that there are more than 2,000 non‐coding RNAs in the human genome, about 3–4% of which are miRNAs. miRNAs are mainly divided into the two groups based on their locations in genome, intragenic and intergenic. 25 Intergenic miRNAs are placed in non‐coding regions of genome and synthesized by the RNA polymerase III enzyme, while intragenic miRNAs are found at the exon and introns regions of different encoding genes which are transcribed by RNA polymerase II. A small group of miRNAs can be found in duplicated sequences which are transcribed by RNA polymerase III. 26 , 27 The process of developing a miRNA starts from the nucleus and ends in the cytoplasm. The synthesis of miRNAs is initiated by RNA polymerase II or III in the cell nucleus, resulting in production of a long primary miRNA (Pri‐miRNA). Pri‐miRNA undergoes post‐transcriptional changes including cleavage, 3′ UTR polyadenylation, and adding 5′‐7‐methylguanosine cap. Following cleavage by Drosha and DGCR8, Pri‐miRNA converts into pre‐miRNA with approximately 70 nt long which is transported from nucleus to the cytoplasm via a GTP‐dependent transporter named exportin 5. In the cytoplasm, pre‐miRNA undergoes further cleavages by the Dicer enzyme, which is turned to mature miRNA by losing its hairpin's end. Subsequently, miRNA along with AGO proteins form a RISC complex that mainly targets 3′ UTR of the target mRNA, causing mRNA degradation. 10 In addition to 3′ UTR, miRNA can target 5′ UTR and even coding region of mRNA. Beside this, miRNA may even target the promoter region of the coding gene, resulting in regulation of gene expression at the transcriptional level. Although miRNAs mostly downregulate their target genes through targeting 3′ UTR, 5′ UTR, and coding regions, they may overexpress their targets when they bind to the gene promoters. 28 At the translational level, various studies have confirmed the miRNA‐mediated gene overexpression in several situations such as food starvation. In the mentioned circumstance, AGO2 and other miRNA‐binding proteins, such as FXR1, bind to the AU‐rich sequence in the 3՝ UTR of the target mRNA and increase its translation. 29 In the amino acid deficiency condition, miRNAs can bind to the 5՜ UTR region of mRNAs encoding ribosomal proteins, leading to increase the translation of these proteins. 30 Despite increasing information about miRNA's mechanism of action, there are still dark areas in this context that need to be elucidated in future studies.
4. miRNA AND PI3 KINASE SIGNALING IN HEMATOPOIETIC CANCERS
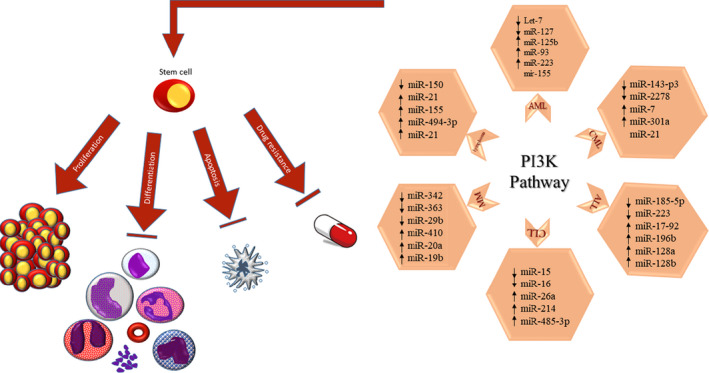
As mentioned earlier, precise regulation of PI3 kinase signaling is important so that avoid unbridle proliferation of hematopoietic cells. In the following subsections, we will describe how different miRNAs regulate the mediators involved in PI3 kinase signaling in various hematopoietic malignancies (Table 1).
TABLE 1.
List of miRNAs that directly or indirectly target mediators involve in PI3 kinase signaling
| Disease | miRNA | Function | Target gene/genes | Sample | Regulation | References | |
|---|---|---|---|---|---|---|---|
| AML | miR‐127 | Tumor suppressor | BCL6 | CBF leukemias with inv(16) and AML with t(8;21) | Downregulated | 77 | |
| Mir‐155 | Apoptosis/tumor suppressor | Hematopoietic transcription factor PU.1, SHIP1, and C/EBPβ | Patients' with acute leukemia | – | 78 | ||
| miR‐125b | Increase proliferation rate | Bak1 |
HEK‐293T cells NB4 HL‐60 |
Upregulated | 79 | ||
| miR‐93 | Increase cell proliferation | Integrin‐β8 | AML patients | Upregulated | 80 | ||
| miR‐223 | Cooperative with tumor suppressor gene | IGF1‐R | AML patients | Upregulated in patients with favorable prognosis | 81 | ||
| CML | miR‐143‐p3 | Oncogene/promote apoptosis |
_/DNMT3A BCR‐ABL |
K562 cell line | Downregulated | 82 | |
| miR−7 | Tumor suppressor | ABL1 | K562 cell line | Upregulated | 31 | ||
| MiRNA‐301a | Oncogene | TIMP2 | K562 cell line | Upregulated | 36 | ||
| MiR‐2278 | Tumor suppressor | AKT2, STAM2, and STAT5A |
K562/IMA‐3 μM K562 |
Downregulated in resistance cell line | 37 | ||
| miR‐21 | Resistant to apoptosis |
PTEN CDC25A PDCD4 RHOB hMSH2 |
CML patients | – | 38 | ||
| ALL | miR‐17‐92 | Oncogene | Retinoblastoma protein like 1/homeodomain‐interacting protein kinase 3/BCL2 | BCR‐ABL positive patients | Upregulated | 83 | |
| miR‐196b | Oncogene | Unknown | ALL patients | Upregulated | 84 | ||
|
miR‐128a miR‐128b |
Antiapoptotic | PHF6 | Jurkat cell line/Pediatric ALL | Upregulated | 43 | ||
| miR‐185‐5p | Sensitize to chemotherapy | mTORC2 | CEM‐C1 | Downregulated | 43 | ||
|
miR‐128b miR‐223 |
Prognostic factor |
PTEN/E2F1 CEBPα E2A |
Mn60 ‘Jurkat HL60/child patients with ALL |
Upregulated Downregulated |
48 | ||
| CLL |
miR‐15 miR‐16 |
Induce apoptosis | BCL2 |
MEG‐01 cell line CLL patients |
Deleted/downregulated | 85 | |
|
miR‐26a miR‐214 |
Oncogene | PTEN | 293T/CLL patient | Upregulated | 57 | ||
| miR‐485‐3p | Tumor suppressor | FOXD3 | CLL patient | Upregulated | 86 | ||
| Lymphoma |
miR‐21 miR‐155 |
Oncogene |
C1RL TCAP |
Raji cell line | Upregulated | Nasal natural killer cell lymphoma | 58 |
| miR‐494‐3p | Oncogene | PTEN | NK92 cells/patient sample | Upregulated | NK/T cell lymphoma | 60 | |
| miR‐150 | Sensitize to radiotherapy |
AKT2 AKT3 |
NK‐92 cell line Hank‐1 cell line NODSCID mice |
Downregulated | Diffuse large B‐cell lymphoma (DLBCL) | 61 | |
| miR‐21 | Oncogene |
FOXO1 PTEN |
DLBCL patients | Upregulated | 4.3/MM1 | 59 | |
| Multiple myeloma |
miR‐342 miR‐363 |
Tumor suppressor |
RUNX‐2 RANKL DKK1 |
CAG cell line MM patients |
Downregulated | 67 | |
| miR‐29b | Tumor suppressor | Sp1 |
SKMM1 NCL‐H‐929 |
Downregulated | 68 | ||
| miR‐410 | Oncogene | KLF10 |
RPMI‐8266 U266 NCI‐H929/patients’ samples |
Upregulated | 87 | ||
| miR‐20a | Oncogene | PTEN | MM1S, U266, and RPMI‐8226 cell line/patients’ samples | Upregulated | 70 | ||
| miR‐19b | Oncogene | TSC1 | Patients’ samples | Upregulated | 71 | ||
4.1. miRNA and leukemias
Chronic myeloid leukemia (CML) is a type of blood malignancy that affects hematopoietic stem cells, leading them to produce a large numbers of immature white blood cells. The main feature of CML is reciprocal translocation between ABL1 and BCR genes that causes formation of BCR/ABL fusion gene and shorten chromosome 22 which is called Philadelphia chromosome. The fusion gene shows an elevated tyrosine kinase activity that increases proliferation of immature myeloid cells. 31 According to the reports, miRNAs regulate the expression of BCR/ABL gene via binding to its 3՝ UTR and promoter. 32 , 33 mir‐7 is a tumor suppressor that targets BCR/ABL gene (Figure 2). As the downstream signaling pathway of BCR/ABL, PI3K/Akt is indirectly downregulated by mir‐7. 34 , 35 Jiang et al. investigated the possible synergistic effect of miR‐7 with imatinib in the K562 cell line. 31 In that study, miR‐7 directly targeted ABL1, resulting in the decreased expression of BCR/ABL and PI3K/Akt proteins. Accordingly, miR‐7 inhibited proliferation of K562 cells, induced apoptosis in the cells, and increased their sensitivity to imatinib through the BCR/ABL/PI3K/Akt signaling pathway. 31 In another study, Huang et al. reported that the expression of miR‐310a was significantly higher in patients with CML compared with normal individuals. Moreover, overall survival rate in the CML patients with overexpression of miR‐310a was lower than those with normal expression of this oncomir. A study on the K562 cell line showed that upregulation of miR‐310a increased proliferation and inhibited apoptosis and activities of Cas‐3 and Cas‐9 in these cells. Indeed, Bax/Bcl2 ratio, TIMP2 expression, ERK1/2 phosphorylation, and eventually, AKT phosphorylation decreased in the miR‐310a overexpressed K562 cells, suggesting the regulatory role of this miRNA in TIMP2/ERK1/2 and AKT signaling pathways. 36 miRNAs also involve in drug resistance in CML patients via targeting AKT signaling. Comparison of two K562 and imatinib‐resistant K562 (K562/IMA‐3 μM) cell lines showed the lower expression rate of miR‐2278 in the drug‐resistant cells. Further investigations revealed that miR‐2278 had a tumor suppressor role which induced apoptosis by reducing STAT5, AKT2, and STAM2 proteins in both transcriptional and translational levels. 37 As mentioned before, BCR/ABL tyrosine kinase is the main fusion protein in CML. Despite development of effective therapeutic strategies based on targeting tyrosine kinase activity, full elimination of CML stem cells is challenging and these cells exhibit resistance to tyrosine kinase inhibitors. Although the exact mechanism is not well‐understood, recent studies have highlighted the role of miRNAs in the context. It is reported that targeting miR‐21 by an antagomir significantly increased the imatinib‐induced apoptosis in CD34+ leukemic cells. The cells treated with miR‐21‐antagomir or PI3 kinases inhibitors exhibited a decreased AKT phosphorylation and c‐myc expression compared with control. It implies that miR‐21 may induce resistance to imatinib via PI3 kinase signaling. 38
FIGURE 2.
The role of various miRNAs in development of hematopoietic cancers by targeting proteins involved in PI3K signaling
Acute myeloid leukemia is one of the most common types of invasive hematopoietic cancers in adults that exhibits different clinical manifestations. Its pathogenesis can vary based on the different pathways involved in the disease. 39 Despite the discovery of different effective drugs for treatment of AML, which have been mostly designed based on targeting different signaling pathways, the overall prognosis is still unsatisfactory. 40 Increased proliferation, suppression of apoptosis, and inhibition of differentiation are the most important features of AML leukemia. Previous studies have shown that miR‐29a and miR‐29b act as the tumor suppressors. A study conducted by Gong et al. on the miR‐29 family showed the lower expression rate of miR‐29 in peripheral blood mononuclear cells and bone marrow CD34+ cells in patients with AML compared with healthy individuals. Forced overexpression of miR‐29 in AML‐derived cell lines including THP1 and NB4 cells significantly reduced proliferation and induced apoptosis in these cells. Further evaluations suggested that the AKT2 and CCND2 are the main targets of miR‐29. Patients with decreased expression of miR‐29 showed the upregulation of CCND2, AKT2, and c‐myc. 40 miR‐628 indirectly decreased the phosphorylation rate of PI3K/Akt signaling in patients with AML. The overexpression of IGF‐R1, as the direct target of miR‐628, has been observed in different cancers. Chen et al. reported the high levels of miR‐628 expression in the samples obtained from patients with AML and AML‐derived cell lines (HL‐60, Kasumi‐1 and THP‐1). Ectopic expression of miR‐628 in AML cell lines inhibited cell proliferation and cell cycle and induced apoptosis. Similar to the other cancer types, the adverse correlation between IGF‐R1 and miR‐628 expression rates was confirmed in the patients suffering from AML. Since activation of IGF‐R1 results in PI3K/AKT activation, miR‐628 by suppressing IGF‐R1 consequently inhibits phosphorylation and activation of PI3K/AKT pathway. 41 In the WHO classification of patients with AML, those with the FLT3‐ITD mutation have a poor prognosis. Following FLT3‐ITD activation, different important signaling pathways such as MAPK/ER, PI3K/AKT, NFĸB, and STAT5 will be activated. FLT3‐ITD can also suppress PU1 that is one the key transcription factors involved in differentiation of myeloid linage. According to reports, miR‐155 expression was higher in FLT3‐ITD‐positive patients, suggesting that FLT3‐ITD signaling induced miR‐155 expression. In fact, downstream proteins of the FLT3‐ITD signaling including P65 and STAT5 regulate miR‐155 expression. As a result of silencing miR‐155 expression or increasing the expression of PU1 transcription factor, cell proliferation of AML‐derived cells decreased. 42
Acute lymphoblastic leukemia (ALL) is a type of blood cancer that affects both adults and children. The onset age in children is between 2 and 5 years. 43 ALL is one of the most common blood malignancies among children, accounting for about 80–85% of cases. Given that some poor clinical outcomes in ALL are related to mTOR‐mediated signaling, different drugs targeting mTOR are available for ALL treatment which are divided into three groups including allosteric inhibitors, ATP‐competitive dual PI3K/mTOR inhibitors, and mTOR kinase inhibitors. 44 Despite being a favorable therapeutic strategy, mTOR inhibitors have not met expectations in treatment of cancers. There are several reasons for failure of these drugs. It is reported that targeting mTOR activates the mediators involved in the PI3K or other signaling pathways. 45 , 46 For example, rapalogs increase AKT phosphorylation while suppressing mTORC1 function. Moreover, mTOR inhibition is reported to activate MAPK/ERK signaling in cancer cells. 46 The existence of an intertwined signaling network seems to pose serious challenges to the use of mTOR inhibitors.
The first‐line chemotherapy drugs for patients with ALL are glucocorticoids such as prednisone and dexamethasone. 47 Chen et al. reported an upregulation of miR‐185‐5p in the ALL cell lines as well as samples obtained from glucocorticoid‐sensitive ALL patients. The expression of mTORC2 as the direct target of miR‐185‐5p is downregulated in ALL patients. Chen and colleagues reveled a direct relationship between miR‐185‐5p expression and sensitivity of ALL cells to glucocorticoid drugs. To confirm this claim, they assessed the effect of miR‐185‐5p on drug‐resistant CEM‐C1 cell lines. Accordingly, overexpression of this miRNA increased apoptosis rate and cell cycle suppression through targeting mTORC2. 43
Despite favorable results, 15–20% of children suffering from ALL still do not respond to therapy. By analyzing the miRNA expression profile of these patients, Nemes et al. confirmed the possible role of miRNAs in the context. Based on their report, the expression levels of miR‐128b were high in the ALL‐derived cell lines, while these cells showed a low level of miR‐223 expression. miR‐128 affected the PI3K/AKT/mTOR signaling pathway by reducing PTEN expression. On the other hand, overactivation of IGFR1 signaling decreased the expression of miR‐223 via overactivating the mTOR signaling. Nemes et al. reported that there was a relationship between increased levels of miR‐128b and response to prednisolone in patients with ALL. 48
Chronic lymphocytic leukemia (CLL) is one of the most common blood cancers in Western countries, which occurs as a result of accumulation of neoplastic B lymphocytes in the bone marrow and peripheral blood and other lymphatic organs. 49 These leukemic lymphocytes protect themselves against apoptosis by stimulating the production of autocrine cytokines and, of course, activation of receptors involved in the related signaling pathways. 50 The role of BCR receptor in the pathophysiology of CLL disease and subsequent activation of downstream pathways (MEK/ERK, PI3K/Akt, NFKB) that are involved in cell survival is well described in literature. 51 The PTEN protein can act as a tumor suppressor by inhibiting the PI3K/Akt pathway, which regulates cell metabolism, growth, and survival. Recent studies in lung, 52 breast, 53 glioblastoma, intestine, 54 endometrial, 55 and hematologic cancers have shown a decrease in the levels of this protein. Zou and colleagues found the relationship between PTEN levels and the prognosis of CLL patients. They reported that decreased PTEN levels were associated with a poor prognosis in these patients. 56 In the next study, they showed that miR‐26a and miR‐214 reduced PTEN protein expression, suggesting PTEN as the main target of miR‐26a and miR‐214. Therefore, these miRNAs indirectly increased the PI3K/AKT levels by decreasing PTEN expression in patients with CLL. 57
4.2. miRNA and lymphomas
Lymphomas are a group of clonal malignancies of the lymphatic system. The tumor cells in these cancer types origin from lymphocytes and NK cells that underwent mutation in different maturation stages. In recent years, the role of PI3K/mTOR/AKT signaling pathway in the onset and progression of lymphomas has been well studied. In this regard, the role of miRNAs in regulating the mediators involved in this signaling pathway is prominent. In patients suffering from Burkitt's lymphoma, which is known as an invasive non‐Hodgkin's lymphoma, the expression of miRNA‐21 and miR‐155 miRNAs in their blood samples was significantly higher than in normal individuals. Further evaluations by knocking down of these miRNAs in Raji cell line showed that these miRNAs increased the activity of the PI3K/AKT pathway by targeting C1RL and TCAP, so that downregulating of these miRNAs inhibited the proliferation of tumor cells through increasing apoptosis and cellular arrest at the stage S. 58 The overexpression of mir‐21 has been also reported in diffuse large B‐cell lymphoma (DLBCL). The analyzing of samples obtained from patients with diffuse large B‐cell lymphoma (DLBCL) showed that the expression of miR‐21 in these patients was higher than normal samples. It was found that the expression of this miRNA was inversely correlated with the expression of FOXO1 and PTEN proteins. Decreased expression of these proteins would ultimately increase cell growth through overactivation of PI3K/AKT/mTOR pathway. 59
Another study on patients with nasal natural killer cell lymphoma (NNL) revealed that overexpression of miR‐494‐3p in these patients increased the AKT activity. It was reported that miR‐494‐3p by targeting PTEN indirectly increased AKT activity in the NK92 cells. 60 miRNAs are also involved in the sensitivity of NK/T lymphoma cells to radiotherapy via AKT signaling. For example, decreased miR‐150 expression in 36 patients with NK/T cell lymphoma was associated with increased resistance to radiotherapy. The results of the luciferase reporter assay showed that miR‐150 directly targeted the 3′ UTR region of AKT2 and AKT3 mRNAs. It was reported that NK‐92 and Hank‐1 cells overexpressing miR‐150 showed the higher apoptosis rate after exposure to IR compared with the control group. Wu et al. created a tumor model by injection of Hank‐1‐miR‐control and Hank‐1‐miR‐150 in the NODSCID mice to evaluate the effect of radiotherapy treatment on the tumor size. Accordingly, the tumors in the Hank‐1‐miR‐150 injected mice were more sensitive to radiotherapy than the control group. This study showed that miR‐150 could play a pivotal role in the treatment of NK/T cell lymphoma by inhibiting the PI3K/AKT/mTOR signaling pathway. 61
4.3. miRNA and myelomas
Multiple myeloma (MM) is a malignant disease that is known with infiltration and accumulation of plasma cells in the bone marrow, resulting in dysfunction of hematopoietic cells and fragility of bone tissue. 62 Bone tissue malfunction is the main cause of death in 90% of patients with MM. 63 Multiple myeloma is the second most common blood malignancy that still shows poor prognosis despite many advances in diagnosis and treatment of the disease. 64 , 65 Plasma cell infiltration results in different complications including lytic lesions in the bone, hypercalcemia, renal dysfunction, and spinal cord compression syndrome. 66 RUNX‐2 is one of the bone‐specific transcription factors that is reported to be increased in myeloma cells. Gowda et al. reported that there was an inverse relationship between the expression of miR‐342/miR‐363 and RUNX‐2 in the patients with MM. Forced overexpression of miR‐342/miR‐363 in the CAG cell line significantly decreased the expression of RUNX‐2 and its target genes including DKK1 and RANKL. Moreover, these miRNAs downregulated the downstream signaling of RUNX‐2, the AKT/β‐catenin/surviving, leading to inhibition of MM progression. 67
Sp1 protein is one of the important transcription factors involved in various biological events such as apoptosis, cell cycle, and differentiation. Amodio et al. described the regulatory role of miR‐29b in regulation of SP1 expression. They showed that the expression of SP1 is increased in the patients with MM. It is reported that induction of miR‐29b expression in the multiple myeloma‐derived cell lines (SKMM1 and NCL‐H‐929) reduced the expression of Sp1 at both mRNA and protein levels. Overexpression of miR‐29b significantly decreased the tumor size in MM‐xenograft mice models.
Proteasome inhibitors are the major drugs used to treat MM. Amodio et al. reported that combination of bortezomib and miR‐29b induced apoptosis in myeloma cells. In that study, concomitant use of bortezomib and miR‐29b induced apoptosis in cancer cells which emphasized the role of PI3K/Akt pathway in regulating miR‐29b/Sp1 loop and inducing apoptosis in MM cells. In fact, increased AKT levels led to a decrease in the miR‐29b rate which consequently upregulated the Sp1 expression. This effect was reversed by inhibition of PI3K activity using LY294002 drug. These findings highlighted the negative regulatory role of PI3K/Akt pathway on miR‐29b expression. 68
Various studies in cancer biology have shown that the PTEN/PI3K/AKT oncogenic pathway is one of the important pathways in tumorigenesis, growth, proliferation, and invasion of malignant cells. 69 Jiang et al. determined the effect of miR‐20a on PTEN/PI3K/AKT pathway in MM‐derived cell lines and plasma cells obtained from MM patients. According to their report, there was an inverse correlation between miR‐20a and PTEN expression. Suppressing the expression of miR‐20a significantly increased the PTEN levels, which confirmed that the PTEN is the main target of this miRNA. Further evaluations exhibited the lower proliferation, colony formation, and migration in the miR‐20a‐suppressed MM‐derived cells. These cells also showed lower PI3K expression and AKT phosphorylation. 70 In a study on MM cancer stem cells, Wang and colleagues surveyed the role of miR‐19b in the progression of MM. Q‐PCR results showed the high expression levels of miR‐19b and low expression levels of TSC1 in these cells. By targeting TSC1, miR‐19b significantly elevated the proliferation and survival of MM‐derived stem cells, in vitro. 71
5. PHARMACOLOGICAL MODULATION OF miRNAs
As aforementioned, dysregulation of miRNAs is frequently observed in various cancer types. Considering the important influence of these molecules on cell biological process, restoring their expression pattern is paramount important. To aim this, transfection of the desired sequences that mimic or inhibit a specific miRNA offers an extraordinary strategy to treat cancer cells. However, delivering naked miRNA mimic or inhibitor constructs is problematic due to degradation by various RNases and even self‐hydrolysis. 72 To overcome this barrier, numerous approaches are developing each day to protect and increase the half‐life of the developed constructs. Beside this, restoring some miRNA expression has been achieved using small molecules.
Chemical modifications of miRNAs dramatically increase their stability within the body. Removing or modifying the 2′‐OH in the RNA structure makes them resistance to the enzymatic degradation. For example, locked nucleic acid (LNA) modification of hsa‐miR‐15a‐5p mimic increased its stability, so that treating MM cell lines with this tumor suppressor construct significantly suppressed their proliferation. 73 Virus vectors are also used as an efficient delivery system for expressing miRNAs in the target cells. In this delivery system which is mostly applied for constantly expression of miRNA mimics, the miRNAs are expressed under a cell‐specific promoter in the target cell. Yamamoto et al. used a retroviral expression vector encoding miR‐133 to target Evi1 gene in the leukemic cells. Accordingly, the transfected cells showed an elevated level of miR‐133 expression, leading to downregulation of Evi1 and promote apoptosis in the leukemic cells. 74 Despite favorable results, viral delivery system suffers from some disadvantages such as provoking immune responses, notable toxicity, and limited loading capacity, encouraging researchers to find alternative solutions.
Non‐viral delivery system has been extensively used to transfect miRNA mimics or inhibitors to cancer cells. These vectors include a wide range of synthetic and natural materials such as lipides, polymers, nanoparticles, and even exosomes. 72 For example, Cosco et al. successfully used a chitosan/PLGA nanoparticle to deliver miR‐34a into the multiple myeloma cell lines and human multiple myeloma xenograft mice model. 75 In addition to the miRNA delivery systems, the expression of these non‐coding RNAs can be modulated through targeting with different small molecules. Zhao et al. reported that targeting BET bromodomain and EZH2 proteins by two small molecules restored the expression of miR‐26a in the aggressive and primary lymphoma cell lines. According to their results, lymphoma cells treated with JQ1 and DZNep small molecules showed the lower expression of MYC and consequently lower survival and growth compared with control. 76
6. FUTURE PERSPECTIVE
Increasing advances in molecular genetics have highlighted the role of non‐coding RNAs in regulating the expression of various genes. In this light, extensive studies have been performed on miRNAs as the most well‐known non‐coding RNAs, suggesting them as the key regulators of signaling pathways involved in cell growth, proliferation, and differentiation. To date, dysregulation of the PI3K/AKT/mTOR signaling pathway has been observed in many different cancer types. In hematopoietic cancers, this signaling pathway is reported to be responsible for cancer onset, progression, and even drug resistance. Considering the regulatory role of miRNAs in the expression of mediators involved in PI3K/AKT/mTOR signaling, future studies provide a plan to overcome drug resistance in hematopoietic malignancies using different miRNAs.
AUTHOR CONTRIBUTIONS
E.R. and L.N. designed the structure of article, collected the related articles, and wrote sections 1 and 2. B.F. wrote the section 3 and prepared Figures 1 and 2. M.M. wrote the sections 4.1 and 4.2. M.H.K. prepared Table 1 as well as sections 4.3 and 5. S.P. devised the main conceptual idea and revised the manuscript.
Elham Roshandel and Leila Noorazar contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available.
REFERENCES
- 1. Jafari A, Rezaei‐Tavirani M, Farhadihosseinabadi B, Taranejoo S, Zali HJCI. HSP90 and co‐chaperones: impact on tumor progression and prospects for molecular‐targeted cancer therapy. Cancer Invest. 2020 ;38:1‐19. [DOI] [PubMed] [Google Scholar]
- 2. Zali H, Jafari AJJ. Anticancer properties of amniotic membrane epithelial cells. 2016;12:22‐29. [Google Scholar]
- 3. Jafari A, Rezaei‐Tavirani M, Salimi M, Tavakkol R, Jafari ZJSWiPH. Oncological emergencies from pathophysiology and diagnosis to treatment: a narrative review. Soc Work Public Health. 2020;35:1‐21. [DOI] [PubMed] [Google Scholar]
- 4. Yao JC, Link DCJSC. Concise review: the malignant hematopoietic stem cell niche. Stem Cells. 2017;35(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 5. Ardakani MT, Mehrpooya M, Mehdizadeh M, Beiraghi N, Hajifathali A, Kazemi MHJBmt. Sertraline treatment decreased the serum levels of interleukin‐6 and high‐sensitivity C‐reactive protein in hematopoietic stem cell transplantation patients with depression; a randomized double‐blind, placebo‐controlled clinical trial. Bone Marrow Transplant. 2020;55(4):830‐832. [DOI] [PubMed] [Google Scholar]
- 6. Hajifathali A, Parkhideh S, Kazemi MH, Chegeni R, Roshandel E, Gholizadeh MJJoCP. Immune checkpoints in hematologic malignancies: What made the immune cells and clinicians exhausted! 2020. [DOI] [PubMed]
- 7. Ghasemi K, Parkhideh S, Kazemi MH, et al. The role of serum uric acid in the prediction of graft‐versus‐host disease in allogeneic hematopoietic stem cell transplantation. J Clin Lab Anal. 2020;34:e23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang N, Dai Q, Su X, Fu J, Feng X, Peng JJMBR. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47:1‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassan B, Akcakanat A, Holder AM, Meric‐Bernstam FJSOC. Targeting the PI3‐kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am. 2013;22(4):641‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filipowicz W, Bhattacharyya SN, Sonenberg NJNrg. Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102‐114. [DOI] [PubMed] [Google Scholar]
- 11. Matsui WHJM. Cancer stem cell signaling pathways. Medicine. 2016;95(Suppl 1):S8‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu F, Na L, Li Y, Chen LJC, Bioscience . Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch EJAom. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372‐383. [DOI] [PubMed] [Google Scholar]
- 14. Owusu‐Brackett N, Shariati M, Meric‐Bernstam F. Role of PI3K/AKT/mTOR in cancer signaling. Predictive Biomarkers in Oncology. Springer. 2019;263‐270.
- 15. Nakano N, Matsuda S, Ichimura M, et al. PI3K/AKT signaling mediated by G protein‐coupled receptors is involved in neurodegenerative Parkinson's disease. Int J Mol Med. 2017;39(2):253‐260. [DOI] [PubMed] [Google Scholar]
- 16. Vanhaesebroeck B, Guillermet‐Guibert J, Graupera M, Bilanges BJNrMcb. The emerging mechanisms of isoform‐specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329‐341. [DOI] [PubMed] [Google Scholar]
- 17. Vara JÁF, Casado E, de Castro J, Cejas P, Belda‐Iniesta C, González‐Barón MJCtr. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193‐204. [DOI] [PubMed] [Google Scholar]
- 18. Li B, Xu WW, Lam AKY, et al. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti‐metastasis therapy. Oncotarget. 2017;8(24):38755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campa CC, Martini M, De Santis MC, Hirsch EJFic, Biology D . How PI3K‐derived lipids control cell division. Front Cell Dev Biol. 2015;3:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jordaan G, Liao W, Gera J, Sharma S. Rictor overexpression and mTORC2 signaling in chronic lymphocytic leukemia. Am Soc Hematol. 2012;3884‐3884. [Google Scholar]
- 21. Schaefer T, Lengerke CJO. SOX2 protein biochemistry in stemness, reprogramming, and cancer: the PI3K/AKT/SOX2 axis and beyond. Oncogene. 2020;39(2):278‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee RC, Feinbaum RL, Ambros VJc. The C. elegans heterochronic gene lin‐4 encodes small RNAs with antisense complementarity to lin‐14. Cell. 1993;75(5):843‐854. [DOI] [PubMed] [Google Scholar]
- 23. Hong BS, Ryu HS, Kim N, et al. Tumor suppressor miRNA‐204‐5p regulates growth, metastasis, and immune microenvironment remodeling in breast cancer. Cancer Res. 2019;79(7):1520‐1534. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Li Y, Xie XJE, Medicine T. MicroRNA‐425 inhibits proliferation of chronic lymphocytic leukaemia cells through regulation of the Bruton's tyrosine kinase/phospholipase Cγ2 signalling pathway. Exp Ther Med. 2020;20(2):1169‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stojic L, Lun AT, Mascalchi P, et al. A high‐content RNAi screen reveals multiple roles for long noncoding RNAs in cell division. Nat Commun. 2020;11(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ipsaro JJ, Joshua‐Tor LJNs, Biology M . From guide to target: molecular insights into eukaryotic RNA‐interference machinery. 2015;22(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Zhou W, Liu Y, Liu T, Li C, Wang LJOl. Oncogenic role of microRNA‐532‑5p in human colorectal cancer via targeting of the 5′UTR of RUNX3. Oncol Lett. 2018;15(5):7215‐7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brien J, Hayder H, Zayed Y, Peng CJFie. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vasudevan S, Steitz JAJC. AU‐rich‐element‐mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128(6):1105‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ørom UA, Nielsen FC, Lund AHJMc. MicroRNA‐10a binds the 5′ UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30(4):460‐471. [DOI] [PubMed] [Google Scholar]
- 31. Jiang M‐j, Dai J‐j, Gu D‐n, Huang Q, Tian LJB, Communications BR . MicroRNA‐7 inhibits cell proliferation of chronic myeloid leukemia and sensitizes it to imatinib in vitro. Biochem Biophys Res Commun. 2017;494(1‐2):372‐378. [DOI] [PubMed] [Google Scholar]
- 32. Yates LA, Norbury CJ, Gilbert RJJC. The long and short of microRNA. Cell. 2013;153(3):516‐519. [DOI] [PubMed] [Google Scholar]
- 33. Yeh C‐H, Moles R, Nicot CJMc. Clinical significance of microRNAs in chronic and acute human leukemia. Mol Cancer. 2016;15(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu D‐N, Jiang M‐J, Mei Z, et al. microRNA‐7 impairs autophagy‐derived pools of glucose to suppress pancreatic cancer progression. Cancer Lett. 2017;400:69‐78. [DOI] [PubMed] [Google Scholar]
- 35. Fang Y, Xue JL, Shen Q, Chen J, Tian LJH. MicroRNA‐7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3‐kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55(6):1852‐1862. [DOI] [PubMed] [Google Scholar]
- 36. Huang X, Qi L, Lu W, et al. miRNA‐301a induces apoptosis of chronic myelogenous leukemia cells by directly targeting TIMP2/ERK1/2 and AKT pathways. Oncol Rep. 2017;37(2):945‐952. [DOI] [PubMed] [Google Scholar]
- 37. Kaymaz BT, Günel NS, Ceyhan M, et al. Revealing genome‐wide mRNA and microRNA expression patterns in leukemic cells highlighted “hsa‐miR‐2278” as a tumor suppressor for regain of chemotherapeutic imatinib response due to targeting STAT5A. Tumour Biol. 2015;36(10):7915‐7927. [DOI] [PubMed] [Google Scholar]
- 38. Wang W‐Z, Pu Q‐H, Lin X‐H, et al. Silencing of miR‐21 sensitizes CML CD34+ stem/progenitor cells to imatinib‐induced apoptosis by blocking PI3K/AKT pathway. Leuk Res. 2015;39(10):1117‐1124. [DOI] [PubMed] [Google Scholar]
- 39. Cheung E, Perissinotti AJ, Bixby DL, et al. The leukemia strikes back: a review of pathogenesis and treatment of secondary AML. Ann Hematol. 2019;98(3):541‐559. [DOI] [PubMed] [Google Scholar]
- 40. Döhner H, Weisdorf DJ, Bloomfield CDJNEJoM. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136‐1152. [DOI] [PubMed] [Google Scholar]
- 41. Chen L, Jiang X, Chen H, et al. microRNA‐628 inhibits the proliferation of acute myeloid leukemia cells by directly targeting IGF‐1R. Onco Targets Ther. 2019;12:907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Gerloff D, Grundler R, Wurm A, et al. NF‐κB/STAT5/miR‐155 network targets PU. 1 in FLT3‐ITD‐driven acute myeloid leukemia. Leukemia. 2015;29(3):535‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen P, Shen T, Wang H, et al. MicroRNA‐185‐5p restores glucocorticoid sensitivity by suppressing the mammalian target of rapamycin complex (mTORC) signaling pathway to enhance glucocorticoid receptor autoregulation. Leuk Lymphoma. 2017;58(11):2657‐2667. [DOI] [PubMed] [Google Scholar]
- 44. Simioni C, Martelli AM, Zauli G, Melloni E, Neri LMJC. Targeting mTOR in acute lymphoblastic leukemia. Cells. 2019;8(2):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi GJFip. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front Pharmacol. 2016;7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun S‐YJFoM. mTOR‐targeted cancer therapy: great target but disappointing clinical outcomes, why? 2020;1‐11. [DOI] [PubMed]
- 47. Ahmad N, Abosoudah IF, Sobaihi MM, et al. Adrenal function following acute discontinuation of glucocorticoids in children with acute lymphocytic leukemia: a prospective study. Pediatr Hematol Oncol. 2019;36(7):422‐431. [DOI] [PubMed] [Google Scholar]
- 48. Nemes K, Csóka M, Nagy N, et al. Expression of certain leukemia/lymphoma related microRNAs and its correlation with prognosis in childhood acute lymphoblastic leukemia. Pathol Oncol Res. 2015;21(3):597‐604. [DOI] [PubMed] [Google Scholar]
- 49. Kr CNR, Ferrarini MJNEJM. Chronic lymphocytic leukemia. 2005;352:804‐815. [DOI] [PubMed] [Google Scholar]
- 50. Caligaris‐Cappio F, Bertilaccio MT, Scielzo C. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Paper presented at: Seminars in cancer biology; 2014. [DOI] [PubMed] [Google Scholar]
- 51. Burger JA, Chiorazzi NJTii. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dong L, Li G, Li Y, Zhu ZJMsmimjoe, Research C . Upregulation of long noncoding RNA GAS5 inhibits lung cancer cell proliferation and metastasis via miR‐205/PTEN axis. Med Sci Monit. 2019;25:2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu L, Meng T, Zheng X, et al. Transgelin 2 promotes paclitaxel resistance, migration, and invasion of breast Cancer by directly interacting with PTEN and activating PI3K/Akt/GSK‐3β pathway. Mol Cancer Ther. 2019;18(12):2457‐2468. [DOI] [PubMed] [Google Scholar]
- 54. Gu J‐J, Fan K‐C, Zhang J‐H, Chen H‐J, Wang S‐SJIjomm. Suppression of microRNA‐130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int J Mol Med. 2018;41(1):284‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q‐a‐z, Yang Y, Liang XJJoOR. LncRNA CTBP1‐AS2 sponges miR‐216a to upregulate PTEN and suppress endometrial cancer cell invasion and migration. 2020;13:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Zou Z‐J, Zhang R, Fan L, et al. Low expression level of phosphatase and tensin homolog deleted on chromosome ten predicts poor prognosis in chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(6):1159‐1164. [DOI] [PubMed] [Google Scholar]
- 57. Zou Z‐J, Fan L, Wang L, et al. miR‐26a and miR‐214 down‐regulate expression of the PTEN gene in chronic lymphocytic leukemia, but not PTEN mutation or promoter methylation. Oncotarget. 2015;6(2):1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han B, Wang S, Zhao HJIJoC, Pathology E . MicroRNA‐21 and microRNA‐155 promote the progression of Burkitt's lymphoma by the PI3K/AKT signaling pathway. Int J Clin Exp Pathol. 2020;13(1):89. [PMC free article] [PubMed] [Google Scholar]
- 59. Go H, Jang J‐Y, Kim P‐J, et al. MicroRNA‐21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B‐cell lymphoma. Oncotarget. 2015;6(17):15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen H‐H, Huang W‐T, Yang L‐W,Lin C‐WJTAjop. The PTEN‐AKT‐mTOR/RICTOR pathway in nasal natural killer cell lymphoma is activated by miR‐494‐3p via PTEN but inhibited by miR‐142‐3p via RICTOR. Am J Pathol. 2015;185(5):1487‐1499. [DOI] [PubMed] [Google Scholar]
- 61. Wu SJ, Chen J, Wu B, Wang YJ, Guo KYJJoE, Research CC . MicroRNA‐150 enhances radiosensitivity by inhibiting the AKT pathway in NK/T cell lymphoma. J Exp Clin Cancer Res. 2018;37(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Avet‐Loiseau H. Introduction to a review series on advances in multiple myeloma. Washington, DC: American Society of Hematology; 2019. [DOI] [PubMed] [Google Scholar]
- 63. Gavriatopoulou M, Βoultadaki A, Koutoulidis V, et al. The role of low dose whole body CT in the detection of progression of patients with smoldering multiple myeloma. Blood Cancer J. 2020;10(9):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Naymagon L, Abdul‐Hay MJJOH, Oncology . Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9(1):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson KC, Carrasco RDJARoPMoD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249‐274. [DOI] [PubMed] [Google Scholar]
- 66. Roodman GDJNEJoM. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655‐1664. [DOI] [PubMed] [Google Scholar]
- 67. Gowda PS, Wildman BJ, Trotter TN, et al. Runx2 suppression by miR‐342 and miR‐363 inhibits multiple myeloma progression. Mol Cancer Res. 2018;16(7):1138‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Amodio N, Di Martino M, Foresta U, et al. miR‐29b sensitizes multiple myeloma cells to bortezomib‐induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis. 2012;3(11):e436‐e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Z, Fang S, Di Y, Ying W, Tan Y, Gu WJPo. Modulation of NF‐κB/miR‐21/PTEN pathway sensitizes non‐small cell lung cancer to cisplatin. PLoS ONE. 2015;10(3):e0121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jiang Y, Chang H, Chen GJOl. Effects of microRNA‐20a on the proliferation, migration and apoptosis of multiple myeloma via the PTEN/PI3K/AKT signaling pathway. Oncol Lett. 2018;15(6):10001‐10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang N, Liang X, Yu W, Zhou S, Fang MJCP, Biochemistry . Differential expression of MicroRNA‐19b promotes proliferation of cancer stem cells by regulating the TSC1/mTOR signaling pathway in multiple myeloma. Cell Physiol Biochem. 2018;50(5):1804‐1814. [DOI] [PubMed] [Google Scholar]
- 72. Lee TJ, Yuan X, Kerr K, et al. Strategies to modulate microRNA functions for the treatment of cancer or organ injury. Pharmacol Rev. 2020;72(3):639‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Z, Liu L, Du C, et al. Therapeutic effects of oligo‐single‐stranded DNA mimicking of hsa‐miR‐15a‐5p on multiple myeloma. Gene Cancer Ther. 2020;27:1‐9. [DOI] [PubMed] [Google Scholar]
- 74. Yamamoto H, Lu J, Oba S, et al. miR‐133 regulates Evi1 expression in AML cells as a potential therapeutic target. Sci Rep. 2016;6:19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cosco D, Cilurzo F, Maiuolo J, et al. Delivery of miR‐34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci Rep. 2015;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao X, Lwin T, Zhang X, et al. Disruption of the MYC‐miRNA‐EZH2 loop to suppress aggressive B‐cell lymphoma survival and clonogenicity. Leukemia. 2013;27(12):2341‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jongen‐Lavrencic M, Sun SM, Dijkstra MK, Valk PJ, Löwenberg BJB, The Journal of the American Society of Hematology . MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111(10):5078‐5085. [DOI] [PubMed] [Google Scholar]
- 78. Zhang X, Wang Y, Guo Q, et al. Prognostic role of microRNA‐155 in patients with leukemia: a meta‐analysis. Clin Chim Acta. 2018;483:6‐13. [DOI] [PubMed] [Google Scholar]
- 79. Zeng Q, Xu L, Liu X, Liao W, Yan MJZxyxzzZXZ. miR‐125b promotes proliferation of human acute myeloid leukemia cells by targeting Bak1. Zhonghua Xue Ye Xue Za Zhi. 2013;34(12):1010‐1014. [DOI] [PubMed] [Google Scholar]
- 80. Zhi F, Cao X, Xie X, et al. Identification of circulating microRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS ONE. 2013;8(2):e56718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gentner B, Pochert N, Rouhi A, et al. MicroRNA‐223 dose levels fine tune proliferation and differentiation in human cord blood progenitors and acute myeloid leukemia. Exp Hematol. 2015;43(10):858.e857‐868.e857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liang B, Song Y, Zheng W, Ma WJMsmimjoe, Research C . MiRNA143 induces K562 cell apoptosis through downregulating BCR‐ABL. Med Sci Monit. 2016;22:2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Swellam M, El‐Khazragy NJTB. Clinical impact of circulating microRNAs as blood‐based marker in childhood acute lymphoblastic leukemia. Tumor Biol. 2016;37(8):10571‐10576. [DOI] [PubMed] [Google Scholar]
- 84. Mets E, Van Peer G, Van der Meulen J, et al. MicroRNA‐128‐3p is a novel oncomiR targeting PHF6 in T‐cell acute lymphoblastic leukemia. Haematologica. 2014;99(8):1326‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cimmino A, Calin GA, Fabbri M, et al. miR‐15 and miR‐16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102(39):13944‐13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Namazi F, Ketabchi F, Moghimi M, Hadi NJIJoML. Expression of miR‐485‐3p and its target FOXD3 in chronic lymphocytic leukemia. Int J Med Lab. 2020;7(1):23‐29. [Google Scholar]
- 87. Yang N, Chen J, Zhang H, et al. LncRNA OIP5‐AS1 loss‐induced microRNA‐410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8(8):e2975‐e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available.


