Abstract
Background
Although quality improvement interventions for acute heart failure have been studied in high-income countries, none have been studied in low- or middle-income country settings where quality of care can be lower. We evaluated the effect of a quality improvement toolkit on process of care measures and clinical outcomes in patients hospitalized for acute heart failure in 8 hospitals in Kerala, India utilizing an interrupted time series design from February 2018 to August 2018.
Methods
The quality improvement toolkit included checklists, audit-and-feedback reports, and patient education materials. The primary outcome was rate of discharge guideline-directed medical therapy for patients with heart failure with reduced ejection fraction. We used mixed effect logistic regression and interrupted time series models for analysis.
Results
Among 1400 participants, mean (SD) age was 66.6 (12.2) years, and 38% were female. Mean (SD) left ventricular ejection fraction was 35.2% (9.7%). The primary outcome was observed in 41.3% of participants in the intervention period and 28.1% of participants in the control period (difference 13.2%; 95% CI 6.8, 19.0; adjusted OR = 1.70; 95% CI 1.17, 2.48). Interrupted time series model demonstrated highest rate of guideline-directed medical therapy at discharge in the initial weeks following intervention delivery with a concomitant decline over time. Improvements were observed in discharge process of care measures, including diet counseling, weight monitoring instructions, and scheduling of outpatient clinic follow-up but not hospital length of stay nor inpatient mortality.
Conclusions
Higher rates of guideline-directed medical therapy at discharge were observed in Kerala. Broader implementation of this quality improvement intervention may improve heart failure care in low- and middle-income countries.
Summary Tweet:
QI toolkit in Kerala, India shows improvements in GDMT at discharge for patients with HFrEF. Centre for Chronic Disease Control, Cardiological Society of India, @DukeGHI, @Fogarty_NIH, @NMCardioVasc, @FSMGlobalHealth
Keywords: Heart failure, Quality improvement, India
Introduction
The burden of heart failure (HF) is increasing worldwide and has disproportionately shifted toward low- and middle-income countries due to population growth, aging, and a greater prevalence of major heart failure risk factors, including hypertension, diabetes, and ischemic heart disease.1–3 The outcomes of HF patients in low- and middle-income countries remain poor, with inpatient mortality rates around 8% (95% CI: 6% to 10%), which are almost triple to some high-income country groups.4–6 Almost 50% of participants died at 3 years in the vanguard HF registry in India, reaching mortality rates observed much later in high-income country settings.7,8 Improvements in HF mortality in high-income countries over time have been attributed to increased adherence to guideline-directed medical and device therapy which is a key heart failure performance measure.9,10 Despite high-quality evidence that guideline-directed medical therapy reduces morbidity and mortality of patients with heart failure with reduced ejection fraction (HFrEF), only 25% of patients were discharged on guideline-directed medical therapy in the Trivandrum Heart Failure Registry in Kerala, India revealing a potential target for intervention.5
Improving the quality and safety of health systems, which are increasingly recognized as key strategies for improving clinical outcomes, is a global health priority.11,12 Quality improvement initiatives have been developed in high-income countries to improve health system quality and subsequent clinical outcomes in patients with HF with limited effect, but none have been studied in low- or middle-income countries where there is a greater potential effect in the setting of lower baseline quality of care.4,13 Most quality improvement research in India has been focused on acute coronary syndrome care, and HF remains understudied despite the potential to improve clinical outcomes and population health.14,15 To fill this gap, we developed, implemented, and evaluated a locally-contextualized HF quality improvement toolkit-based intervention compared to usual care for patients with acute HF in 8 hospitals in Kerala, India using an interrupted time series study design.16
Methods
Study design
The Heart Failure Quality Improvement in Kerala (HF QUIK) study was a quasi-experimental study evaluating the effect of a locally-contextualized quality improvement toolkit on process of care measures and clinical outcomes in patients hospitalized for acute HF in 8 hospitals in Kerala, India from February 2018 to August 2018. The study utilized an interrupted time series study design. The pre-intervention period was from February 5th 2018 to May 6th 2018, and the post-intervention period was from May 6th 2018 to August 5th 2018. All data were entered online by study coordinators at each site using a Health Insurance Portability and Accountability Act (HIPAA)-compliant electronic data capture tool (REDCap, Vanderbilt University, Nashville, TN, USA).17
The study protocol was reviewed by and received ethics board approval from Duke University (Durham, NC, USA), Centre for Chronic Disease Control (New Delhi, Delhi, India), Cardiological Society of India-Kerala Chapter (Kochi, Kerala, India) and Indian Health Ministry Screening Committee (New Delhi, Delhi, India) in November 2017. No changes were made to the study protocol during the course of the study. Because data were used at the local hospitals for the purpose of quality improvement, sites were granted a waiver of informed consent under the Common Rule.
Hospitals and study participants
We purposively recruited 8 hospitals in Kerala, India from a sampling frame of hospitals (n = 63) that had previously participated in the Acute Coronary Syndrome Quality Improvement in Kerala (ACS QUIK) trial.15 All hospitals that were approached participated in the study. Hospitals varied in region and type, including government, non-profit/charity, and private hospitals to capture a range of implementation settings. Hospitals enrolled consecutive patients with a primary admission diagnosis of acute HF. Patients were eligible for inclusion if they were adults aged 18 years or older and met at least 2 out of 3 criteria for the diagnosis of HF (i.e. clinical symptoms and signs of HF, natriuretic peptide elevation, or echocardiographic evidence of left ventricular systolic or diastolic dysfunction) as defined by the European Society of Cardiology.18 These criteria were similar to those used in the Trivandrum Heart Failure Registry, the first HF registry in Kerala.5,7,19
Intervention
We used formative, mixed-methods research to contextualize previously tested components of a quality improvement toolkit to target process of care measures and clinical outcomes in patients hospitalized with acute HF through previously identified gaps in HF care in Kerala5,7,19, a systematic review13 key informant interviews16, and prior acute cardiovascular quality improvement trial experience.15 The HF QUIK toolkit included an in-hospital and discharge checklist to prompt physicians and nurses to order guideline-recommended in-hospital diagnostics (i.e. electrocardiogram, natriuretic peptide, transthoracic echocardiogram), guideline-directed medical therapy, patient education for HF-specific health behaviors, and follow-up recommendations (e.g. referral for implantable cardioverter defibrillator or cardiac resynchronization therapy in eligible patients, referral for outpatient cardiac rehabilitation, scheduled outpatient clinic follow-up). Sites received personalized audit-and-feedback reports, which included site-specific performance measures guided by established HF quality metrics (Online Supplement).6 Based on prior research in Kerala demonstrating limited goal-setting among hospital administrators, each hospital site investigator set a HF quality metric to improve based on their personalized audit-and-feedback report.20 Lastly, the HF QUIK toolkit included patient education materials on lifestyle, diet, and smoking cessation written in the local language of Malayalam.
The study team performed on-site training of the HF QUIK toolkit at each hospital site with the hospital investigator, site study coordinator, and other members of the local quality improvement team. Each 2-hour session included training of cardiac care unit and general cardiology ward nurses on guideline-directed medical therapy for patients with HF and HF QUIK toolkit use. The pre-intervention control period consisted of usual care according to local hospital practice.
Outcomes
The primary outcome was the prescription of guideline-directed medical therapy for patients with HFrEF at discharge including: angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), beta-blocker, and aldosterone antagonist measured separately and as a combined outcome. Secondary outcomes included rates of: in-hospital electrocardiogram, in-hospital echocardiogram, discharge tobacco and alcohol cessation counseling in eligible participants, diet counseling, weight monitoring instructions at discharge, referral for outpatient cardiac rehabilitation, referral for implantable cardioverter defibrillator or cardiac resynchronization therapy, outpatient follow-up appointment scheduled at discharge, hospital length of stay, and inpatient mortality.
Statistical analysis
We initiated this study with a calculated total sample size of 156 participants, which would provide 90% power with a two-sided significance level of 0.05 to detect a 24% relative improvement in the primary outcome by the intervention from the anticipated 17% baseline rate of guideline-directed medical therapy (i.e. ACE-I or ARB, beta blocker, and aldosterone antagonist) among patients with HFrEF without contraindications to these medications. This baseline proportion was estimated from the Trivandrum Heart Failure Registry.5 Date of admission was used to allocate participants to pre-intervention, control period or the intervention period in the analysis. Baseline characteristics are summarized for control and intervention periods. Continuous variables are reported as means with standard deviations or medians with interquartile range if data were skewed, and categorical variables as counts with percentages. We performed multivariable logistic regression analysis to evaluate the odds of adherence to in-hospital process of care measures pre- and post-intervention and quantile regression for the hospital length of stay outcome. The unadjusted model included group variable only (control and intervention period), and the second model was adjusted for age and gender. In the primary analysis, the mixed effect logistic regression model was used adjusting for age, gender, and random effect for hospital. The final model was further adjusted for covariates that are predictors of mortality in the HF risk score developed by the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC).21 We used interrupted time series models to compare the slopes of monthly control and intervention rates for GDMT, ACE-I or ARB, beta blocker and aldosterone antagonist.22 We performed a complete case analysis due to low rate of missing data (0.1%). For statistical analyses, Stata, version 14 (Stata Corp), SAS, version 9.4 (SAS Institute Inc), and R, version 3.5.1 (R Foundation), were used.
Results
We recruited 1,469 participants from 8 hospitals in Kerala, India. The hospitals varied in type, including government, non-profit, and private hospitals (Online Table 1). Participants were excluded if there were duplicate data entries for the same participant (n = 13), had missing data (n = 2), or were admitted to the hospital after the final date of study enrollment (n = 54). The complete case analysis was performed on 1,400 participants with 758 participants in the control period and 642 participants in the intervention period (Online Figure 1).
Table 1 describes the baseline characteristics of study participants by control and intervention period. Mean (SD) age of participants was 66.6 (12.2) years, 62% were men, and 36% had a history of tobacco use. More than half of participants had diabetes mellitus (55%), hypertension (58%) and coronary heart disease (56%). The primary etiology of HF was ischemic heart disease (n = 1,204, 86%). Prior to hospital admission, 24% of participants were prescribed ACE-I or ARB, 34% were prescribed beta-blocker, and 16% were prescribed an aldosterone antagonist. Mean (SD) left ventricular ejection fraction was 35.9% (10.1%) in the control period and 35.1% (9.9%) in the intervention period (p = 0.12).
Table 1.
Baseline characteristics of HF QUIK participants by control and intervention period.
| Control | Intervention | P-value | |||
|---|---|---|---|---|---|
| Participant characteristics | N | n (%) | N | n (%) | |
| Age, mean (SD), y | 758 | 66.7 (12.4) | 642 | 66.5 (12.0) | 0.78 |
| Male | 758 | 441 (58.2) | 642 | 423 (65.9) | 0.003 |
| Transferred from another facility | 758 | 282 (37.2) | 642 | 225 (35.0) | 0.57 |
| Ischemic etiology of HF | 757 | 634 (83.8) | 642 | 570 (88.8) | <0.001 |
| Medical history prior to HF admission | |||||
| Tobacco use, | 756 | 267 (35.3) | 642 | 233 (36.3) | 0.21 |
| Alcohol use | 756 | 187 (24.7) | 642 | 154 (24.0) | 0.24 |
| Coronary heart disease | 758 | 409 (54.0) | 642 | 377 (58.7) | 0.07 |
| Percutaneous coronary intervention | 758 | 67 (8.8) | 642 | 62 (9.7) | 0.60 |
| Diabetes mellitus | 758 | 428 (56.5) | 642 | 339 (52.8) | 0.17 |
| Hypertension | 758 | 447 (59.0) | 642 | 371 (57.8) | 0.66 |
| Hyperlipidemia | 758 | 161 (21.2) | 642 | 134 (20.9) | 0.87 |
| Valvular heart disease | 758 | 52 (6.9) | 642 | 36 (5.6) | 0.34 |
| Rheumatic heart disease, | 758 | 24 (3.2) | 642 | 21 (3.3) | 0.91 |
| Chronic kidney disease | 758 | 129 (17.0) | 642 | 97 (15.1) | 0.33 |
| Stroke | 758 | 54 (7.1) | 642 | 42 (6.5) | 0.67 |
| Implantable cardioverter defibrillator | 758 | 5 (0.7) | 642 | 4 (0.6) | 0.93 |
| Cardiac resynchronization therapy | 758 | 5 (0.7) | 642 | 4 (0.6) | 0.93 |
| Medications prior to HF admission | |||||
| Loop diuretic | 758 | 297 (39.2) | 642 | 258 (40.2) | 0.70 |
| Thiazide diuretic | 758 | 8 (1.1) | 642 | 8 (1.2) | 0.74 |
| ACE-I or ARB | 758 | 179 (23.6) | 642 | 156 (24.3) | 0.77 |
| Beta-blocker | 758 | 233 (30.7) | 642 | 247 (38.5) | 0.002 |
| Aldosterone antagonist | 758 | 135 (17.8) | 642 | 87 (13.6) | 0.03 |
| ARNi | 758 | 10 (1.3) | 642 | 3 (0.5) | 0.10 |
| Digoxin | 758 | 78 (10.3) | 642 | 52 (8.1) | 0.16 |
| Ivabradine | 758 | 34 (4.5) | 642 | 20 (3.1) | 0.19 |
| Aspirin | 758 | 334 (44.1) | 642 | 318 (49.5) | 0.04 |
| Statin | 758 | 347 (45.8) | 642 | 330 (51.4) | 0.04 |
| Physical exam, laboratory and imaging | |||||
| Weight, mean (SD), kg | 400 | 63.6 (11.4) | 399 | 64.2 (10.6) | 0.45 |
| Systolic blood pressure, mean (SD), mmHg | 755 | 138.7 (30.1) | 639 | 141.4 (30.5) | 0.10 |
| Diastolic blood pressure, mean (SD), mmHg | 755 | 82.4 (15.5) | 639 | 83.5 (15.5) | 0.18 |
| Heart rate, mean (SD), bpm | 757 | 93.5 (23.3) | 640 | 94.1 (23.8) | 0.65 |
| Sodium, mean (SD), mEq/L | 746 | 134.6 (6.3) | 638 | 134.8 (5.1) | 0.68 |
| Creatinine, median (IQR), mg/dL | 749 | 1.2 (1.0, 1.6) | 638 | 1.2 (1.0, 1.7) | 0.87 |
| BNP, median (IQR), pg/dL | 72 | 2,665 (1,295, 7,398) | 84 | 2,331 (1,441, 8,780) | 0.66 |
| NT pro-BNP, median (IQR), pg/mL | 53 | 4,322 (2,215, 12,071) | 39 | 6,487 (2,957, 12,260) | 0.23 |
| Ejection fraction, mean (SD), % | 752 | 35.9 (10.1) | 638 | 35.1 (9.9) | 0.12 |
ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, ARNi: angiotensin receptor neprilysin inhibitor, BNP: B-type natriuretic peptide, IQR: interquartile range, NT pro-BNP: N-terminal pro hormone B-type natriuretic peptide
Table 2 describes in-hospital evaluation and management by control and intervention period. An electrocardiogram was performed during the hospitalization on almost all participants. Less than one quarter (21%) of participants underwent coronary angiography during the hospitalization, and 6% received percutaneous coronary intervention. Approximately 18% of participants were treated with an inotrope during the hospitalization. Although non-invasive positive pressure ventilation was utilized in the care of 28% of participants, use of invasive support devices, such as intra-aortic balloon pump and dialysis or ultrafiltration, was rare.
Table 2.
In-hospital tests, procedures, and treatment of HF QUIK participants by control and intervention period.
| Diagnostic tests and treatment | Control (n = 758)1 | Intervention (n = 642)1 | P-value |
|---|---|---|---|
| In-hospital tests and procedures | |||
| ECG, No. (%) | 751 (99.1) | 641 (99.8) | 0.06 |
| Cardioversion, No. (%) | 23 (3.0) | 14 (2.2) | 0.32 |
| Stress testing, No. (%) | 1 (0.1) | 0 (0.0) | 0.36 |
| Coronary angiography, No. (%) | 163 (21.5) | 133 (20.7) | 0.72 |
| Percutaneous coronary intervention, No. (%) | 45 (5.9) | 44 (6.9) | 0.48 |
| Coronary artery bypass graft, No. (%) | 6 (0.8) | 9 (1.4) | 0.27 |
| Implantable cardioverter defibrillator, No. (%) | 4 (0.5) | 3 (0.5) | 0.87 |
| Cardiac resynchronization therapy, No. (%) | 1 (0.1) | 3 (0.5) | 0.24 |
| Intra-aortic balloon pump, No. (%) | 0 (0.0) | 1 (0.2) | 0.28 |
| Dialysis or ultrafiltration, No. (%) | 8 (1.1) | 9 (1.4) | 0.56 |
| Non-invasive positive pressure ventilation, No. (%) | 218 (28.8) | 173 (26.9) | 0.45 |
| Mechanical ventilation, No. (%) | 68 (9.0) | 57 (8.9) | 0.95 |
| In-hospital treatment | |||
| Loop diuretic, No. (%) | 713 (94.1) | 631 (98.3) | <0.001 |
| Thiazide diuretic, No. (%) | 16 (2.1) | 16 (2.5) | 0.63 |
| ACE-I or ARB, No. (%) | 310 (40.9) | 283 (44.1) | 0.23 |
| Beta-blocker, No. (%) | 542 (71.5) | 478 (74.5) | 0.22 |
| Aldosterone antagonist, No. (%) | 436 (57.5) | 431 (67.1) | <0.001 |
| ARNi, No. (%) | 17 (2.2) | 8 (1.2) | 0.16 |
| Digoxin No. (%) | 153 (20.2) | 107 (16.7) | 0.09 |
| Ivabradine, No. (%) | 78 (10.3) | 70 (10.9) | 0.71 |
| Aspirin, No. (%) | 632 (83.4) | 556 (86.6) | 0.09 |
| Statin, No. (%) | 638 (84.2) | 559 (87.1) | 0.12 |
| Hydralazine-nitrate, No. (%) | 45 (5.9) | 23 (3.6) | 0.04 |
| Nitroglycerin, No. (%) | 219 (28.9) | 204 (31.8) | 0.24 |
| Inotrope, No. (%) | 129 (17.0) | 126 (19.6) | 0.21 |
ECG: electrocardiogram, ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, ARNi: angiotensin receptor neprilysin inhibitor
Data were completely reported for all variables in Table 2.
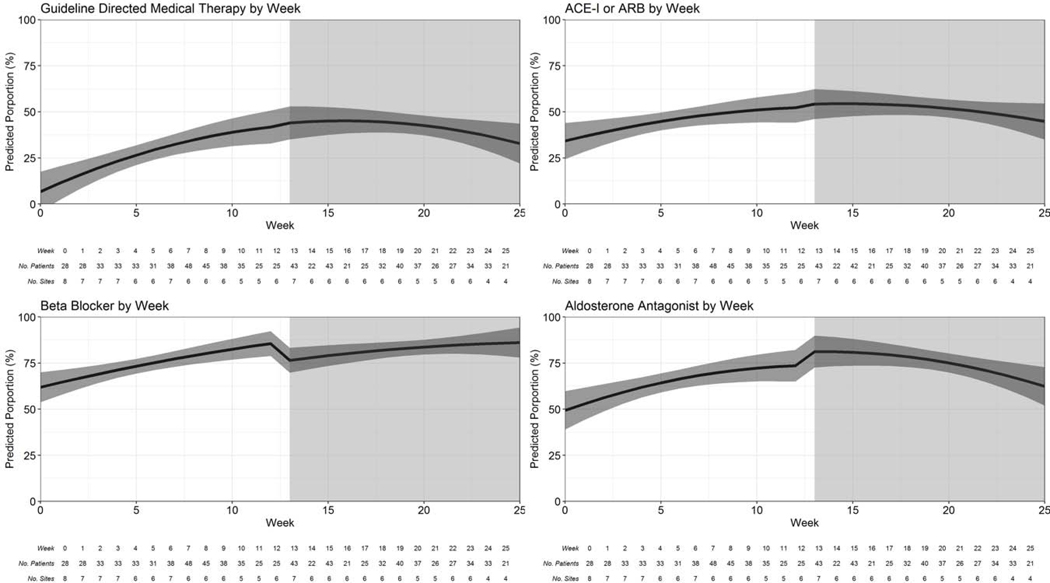
Table 3 describes crude differences in outcomes between participants in the control and intervention periods, and Table 4 describes the unadjusted and adjusted analyses. The primary outcome of prescription of guideline-directed medical therapy at discharge for participants with HFrEF was achieved in 167 (41.3%) participants in the intervention period compared to 124 (28.2%) participants in the control period (difference 13.2%; 95% CI 6.8, 19.5). The intervention led to 70% higher odds of guideline-directed medical therapy at discharge (adjusted OR 1.70, 95% CI 1.17, 2.46). The highest rate of guideline-directed medical therapy at discharge was observed in the initial weeks following intervention delivery with an insignificant concomitant decline over time (Figure 1, Online Table 2, Online Table 3). There was a 4.9% (95% CI 2.2, 7.5) higher rate in the prescription of diuretics at discharge in participants in the intervention period compared with the control period (adjusted OR 3.15; 95% CI 1.33, 7.46). Discharge process of care measures were higher in participants in the intervention period compared to control period including diet counseling (adjusted OR 2.37; 95% CI 1.38, 4.08), weight monitoring instructions (adjusted OR 2.52; 95% CI 1.49, 4.28) and scheduling of outpatient clinic follow-up appointment (adjusted OR 3.81; 95% CI 2.27, 6.39). There was a 3% (95 CI −5.9, −0.1) lower rate of referral for implantable cardioverter defibrillator among participants in the intervention period compared with the control period. There was no difference in hospital length of stay (adjusted beta coefficient 0.0; 95% CI −0.92, 0.92) nor inpatient mortality (adjusted OR 1.04, 95% CI 0.70, 1.54) between participants enrolled during both control and intervention periods.
Table 3.
Process of care measures and clinical outcomes of HF QUIK participants by control and intervention period.
| Control (n=758) | Intervention (n=642) | Difference (95% CI)1 | |||
|---|---|---|---|---|---|
| N | n (%) | N | n (%) | ||
| Discharge process of care measures2 | |||||
| GDMT at discharge3 | 440 | 124 (28.2) | 404 | 167 (41.3) | 13.2 (6.8, 19.5) |
| ACE-I or ARB at discharge3 | 440 | 200 (45.5) | 403 | 207 (51.4) | 5.9 (−0.8, 12.7) |
| Beta-blocker at discharge3 | 440 | 333 (75.7) | 404 | 332 (82.2) | 6.5 (1.0, 12.0) |
| Aldosterone antagonist at discharge3 | 440 | 286 (65.0) | 404 | 305 (75.5) | 10.5 (4.4, 16.6) |
| Diuretic at discharge3 | 440 | 411 (93.4) | 404 | 397 (98.3) | 4.9 (2.2, 7.5) |
| Tobacco cessation counseling4 | 420 | 266 (63.3) | 355 | 229 (64.5) | 1.2 (−5.6, 8.0) |
| Alcohol cessation counseling4 | 407 | 246 (60.4) | 343 | 213 (62.1) | 1.7 (−5.3, 8.7) |
| Diet counseling | 689 | 589 (85.5) | 587 | 510 (86.9) | 1.4 (−2.4, 5.2) |
| Weight monitoring instructions | 689 | 576 (83.6) | 587 | 507 (86.4) | 2.8 (−1.2, 6.7) |
| Referral to outpatient cardiac rehabilitation | 690 | 30 (4.3) | 586 | 16 (2.7) | −1.6 (−3.6, 0.4) |
| Referral for ICD therapy5 | 364 | 20 (5.5) | 322 | 8 (2.5) | −3.0 (−5.9, −0.1) |
| Outpatient clinic follow-up scheduled | 690 | 618 (89.6) | 587 | 565 (96.3) | 6.7 (3.9, 9.4) |
| In-hospital process of care measures | |||||
| ECG | 758 | 751 (99.1) | 642 | 641 (99.8) | 0.8 (0.02, 1.5) |
| Transthoracic echocardiogram | 757 | 710 (93.8) | 642 | 591 (92.1) | −1.7 (−4.4, 1.0) |
| Clinical outcomes | |||||
| Hospital length of stay, median (IQR), days | 756 | 4 (3–6) | 641 | 4 (3–6) | 0.0 (−0.3, 0.3) |
| Inpatient mortality | 756 | 66 (8.7) | 642 | 55 (8.6) | −0.2 (−3.1, 2.8) |
Crude difference: Intervention minus control calculated either as a risk difference percentage for count data or difference in medians for continuous nonparametric data.
Among participants discharged; N = 1279 with 692 in control period and 587 in intervention period
Among participants discharged with LVEF <40%; N = 846 with 442 in control period and 404 in intervention period
Among participants who reported tobacco or alcohol use; N = 775 and N = 750 for tobacco and alcohol use respectively
Among participants with LVEF ≤35%; N = 688 with 366 in control period and 322 in intervention period
ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, GDMT: guideline-directed medical therapy, ICD: implantable cardioverter defibrillator, LVEF: center ventricular ejection fraction, ECG: electrocardiogram
Table 4.
Unadjusted and adjusted odds of process of care measures and clinical outcomes in HF QUIK.
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| N | Unadjusted | Age and sex adjusted | Primary analysis1 | MAGGIC adjusted analysis2 | |
| Discharge process of care measures3 | |||||
| GDMT at discharge4 | 844 | 1.80 (1.35, 2.39) | 1.82 (1.37, 2.43) | 1.70 (1.17, 2.46) | 1.81 (1.21, 2.70) |
| ACE-I or ARB at discharge4 | 843 | 1.27 (0.97, 1.66) | 1.30 (0.99, 1.71) | 1.00 (0.72, 1.39) | 0.94 (0.66, 1.34) |
| Beta-blocker at discharge4 | 844 | 1.48 (1.06, 2.07) | 1.48 (1.06, 2.07) | 2.15 (1.38, 3.35) | 2.26 (1.44, 3.56) |
| Aldosterone antagonist at discharge4 | 844 | 1.66 (1.23, 2.24) | 1.67 (1.24, 2.26) | 2.16 (1.47, 3.17) | 2.26 (1.51, 3.37) |
| Diuretic at discharge4 | 844 | 4.00 (1.73, 9.24) | 4.02 (1.74, 9.30) | 3.15 (1.33, 7.46) | 2.98 (1.25, 7.11) |
| Tobacco cessation counseling5 | 775 | 1.05 (0.78, 1.41) | 0.87 (0.61, 1.25) | 1.50 (0.91, 2.48) | 1.44 (0.86, 2.40) |
| Alcohol cessation counseling5 | 750 | 1.07 (0.80, 1.44) | 0.93 (0.66, 1.32) | 1.64 (0.99, 2.72) | 1.67 (0.99, 2.82) |
| Diet counseling | 1276 | 1.12 (0.82, 1.55) | 1.11 (0.81, 1.54) | 2.37 (1.38, 4.08) | 2.29 (1.33, 3.95) |
| Weight monitoring instructions | 1276 | 1.24 (0.91, 1.70) | 1.23 (0.90, 1.68) | 2.52 (1.49, 4.28) | 2.45 (1.44, 4.16) |
| Referral to outpatient cardiac rehabilitation | 1276 | 0.62 (0.33, 1.14) | 0.62 (0.33, 1.15) | 0.60 (0.32, 1.13) | 0.57 (0.30, 1.08) |
| Referral for ICD therapy6 | 686 | 0.44 (0.19, 1.01) | 0.44 (0.19, 1.01) | 0.39 (0.16, 0.94) | 0.39 (0.16, 0.94) |
| Outpatient clinic follow-up scheduled | 1277 | 2.99 (1.83, 4.89) | 3.02 (1.84, 4.93) | 3.81 (2.27, 6.39) | 3.88 (2.29, 6.57) |
| In-hospital process of care measures | |||||
| ECG | 1400 | 5.97 (0.73, 48.69) | 6.03 (0.74, 49.30) | 5.21 (0.62, 43.80) | 4.66 (0.54, 40.06) |
| Transthoracic echocardiogram | 1399 | 0.77 (0.51, 1.16) | 0.75 (0.50, 1.14) | 0.90 (0.55, 1.46) | 0.89 (0.53, 1.48) |
| Clinical outcomes | |||||
| Hospital length of stay7 | 1397 | 0.0 (−0.50, 0.50) | 0.0 (−0.50, 0.50) | 0.0 (−0.92, 0.92) | 0.0 (−0.48, 0.48) |
| Inpatient mortality | 1398 | 0.98 (0.67, 1.42) | 0.98 (0.67, 1.42) | 1.04 (0.70, 1.54) | 1.03 (0.69, 1.55) |
Adjusted for age, sex and random cluster effect for hospital.
Adjusted for age, sex, random cluster effect for hospital, and measured MAGGIC risk predictors including ejection fraction, serum creatinine, and diabetes mellitus.
Among participants discharged; N = 1279 with 692 in control period and 587 in intervention period.
Among participants discharged with LVEF <40%; N = 846 with 442 in control period and 404 in intervention period.
Among participants who reported tobacco or alcohol use; N = 775 and N = 750 for tobacco and alcohol use respectively.
Among participants with LVEF ≤35%; N = 688 with 366 in control period and 322 in intervention period.
Effect estimates are Beta coefficients from quantile regression models.
GDMT: guideline-directed medical therapy, ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, ICD: implantable cardioverter defibrillator, LVEF: left ventricular ejection fraction, ECG: electrocardiogram, MAGGIC: Meta-Analysis Global Group in Chronic Heart Failure
Figure 1.
Rate of guideline-directed medical therapy over time in HF QUIK. Figure Legend: Interrupted time series model graphs for the primary outcome of guideline-directed medical therapy (A); its components: ACE-I or ARB (B), beta-blocker (C) and aldosterone antagonist (D) are shown. The white background indicates control period and grey background indicates intervention period.
ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker.
Discussion
Among 1,400 participants admitted with acute HF in Kerala, a locally-contextualized quality improvement toolkit significantly increased the prescription of guideline-directed medical therapy with 70% higher odds at hospital discharge among participants in the intervention period compared to the control period. The intervention also increased the rates of discharge process of care measures including tobacco cessation counseling, alcohol cessation counseling, diet counseling, weight monitoring instructions, and scheduling of outpatient clinic follow-up appointments. There was no effect on hospital length of stay nor inpatient mortality. To our knowledge, this is the first quasi-experimental study evaluating the effect of an in-hospital quality improvement intervention for patients with acute HF in a low-or middle-income country.13
Guideline-directed medical therapy substantially reduces morbidity and mortality of patients with HFrEF and is considered among the highest priority interventions for inclusion in universal health coverage packages.1 Among patients with HFrEF, the relative risk reduction in mortality for ACE-I or ARB is 17% (number needed to treat [NNT] standardized to 36 months for mortality reduction = 26), beta blocker is 34% (NNT = 9), and aldosterone receptor antagonist is 30% (NNT = 6).23 There is increased clinical effectiveness and incremental reduction in risk of death when these pharmacotherapies are used in combination.24 Survival benefits from these medications can translate to large gains in clinical outcomes of HF patients by increasing prescription of guideline-directed medical therapy, which remain low in low- and middle-income countries. For example, a 2014 systematic review of HF care in low-and middle-income countries including 53 studies of 237,908 patients revealed suboptimal treatment rates with ACE-I (57%; 95% CI 49%, 64%), beta-blockers (34%; 95% CI 28%, 41%), and aldosterone receptor antagonists (32%; 95% CI 25%, 39%).4 The Trivandrum Heart Failure Registry demonstrated even lower rates of optimal treatment with combined guideline-directed medical therapy at discharge in only 25% of patients with HFrEF.5 Low prescription rates of ACE-I or ARB, beta-blocker, and aldosterone receptor antagonist prior to admission in our study highlight the opportunity to increase guideline-directed medical therapy during hospitalization in India, particularly as pre-discharge initiation improves future adherence.25
Randomized trials have not demonstrated a consistent effect of in-hospital quality improvement interventions on process of care measures or clinical outcomes for patients with acute HF.13 The largest trial to date (147 hospitals, 71,829 participants in the Get With The Guidelines-Heart Failure [GWTG-HF] quality improvement program) demonstrated no improvement in prescription of guideline-directed medical therapy at discharge.6 In comparison to the GWTG-HF trial in the United States, baseline quality of care was lower in the current study, which might partially explain the observed differences between these two studies.
The Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study assessed whether public release of hospital-specific quality indicators could improve quality of care of patients hospitalized with acute myocardial infarction or HF in 86 hospitals (n = 17,544 participants) in Canada.26 Although the investigators found no improvement in their primary outcome of a composite HF process-of-care metric, there was 5.9% (95% CI 1.0%, 10.7%) increase in ACE-I or ARB prescription for patients with left ventricular dysfunction in the intervention group. Our intervention demonstrated a similar increase in prescription of ACE-I or ARB, as well as higher rates of beta-blocker and aldosterone receptor antagonist at discharge. The larger effect of the current study’s intervention on process of care measures compared with the EFFECT study may be due to lower baseline medication rates, greater acceptability among providers and health systems, higher adoption and fidelity of the intervention, and more actionable information presented in the hospital-specific audit-and-feedback reports.
Prior mixed methods research in Kerala suggests low levels of targetable goal-setting behavior amongst hospital managers is associated with worse cardiovascular health outcomes in the region.20 By incorporating these data into the development of our intervention and enabling each hospital investigator to target one process of care measure to improve at their site based on the personalized audit-and-feedback report, the feedback-action loop may have been tightened. Whether gains in process of care measures at discharge translate to improvements in long-term clinical outcomes remains uncertain and warrants further investigation.15
This study has several strengths. First, to our knowledge, this is the first quasi-experimental study evaluating the effect of an in-hospital, quality improvement intervention for patients with acute HF in India, or any a low- or middle-income country.13 Second, we used formative, mixed-methods research to contextualize previously tested components of a quality improvement toolkit through previously identified gaps in HF care in Kerala 5,7,19, a systematic review 13 and key informant interviews.16 Third, our collaboration with local and national stakeholders, including prior cardiovascular quality improvement trial experience15, supported the implementation of this study. Fourth, we utilized a rigorous interrupted time series study design in an unselected population leading to greater external validity.27,28
This study has several limitations. First, it is unknown which components of the complex intervention led to improvements in process of care measures. Second, the short duration of the study limited evaluation of the sustained effect of the intervention. Third, this study is susceptible to selection bias due to lack of randomization, even though interrupted time series design is considered a rigorous quasi-experimental study design.27,28 Fourth, baseline temporal trends in guideline directed medical therapy over the study period suggest that the observed results may be partially driven by the Hawthorne effect wherein the process of observation changes the measure that is being observed.
Conclusion
This quasi-experimental study in Kerala demonstrated improvements in guideline-directed medical therapy at hospital discharge using a HF-specific quality improvement toolkit. Implementation of this intervention may improve HF care in other settings in India and other low- or middle-income countries.13 Although significant gains in process of care measures were demonstrated in this study, further investigation is needed to continue to improve clinical outcomes for patients with HF.
Supplementary Material
Highlights.
Although quality improvement interventions for acute heart failure have been studied in high-income countries without a consistent effect on process of care measures and clinical outcomes, none have been studied in low- or middle-income country settings where quality of care can be lower.
To our knowledge, this is the first quasi-experimental study evaluating the effect of an in-hospital quality improvement intervention for patients with acute heart failure in a low-or middle-income country. Findings from this study support implementation of locally-contextualized quality improvement toolkits to increase rates of guideline-directed medical therapy for patients with acute heart failure at hospital discharge.
Broader implementation of this quality improvement intervention may improve heart failure care in low- and middle-income countries.
Acknowledgements:
We acknowledge Alex Irudayaraj for his assistance with program management and study coordinators at each study site for their contributions.
Funding: AA received funding from the Fogarty International Center of the National Institutes of Health, Duke Global Health Institute and Duke Hubert-Yeargan Center for Global Health for this research. Research reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are independent from the funders.
All authors are requested to disclose any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work. If there are no conflicts of interest, the COI should read: “The authors report no relationships that could be construed as a conflict of interest”.
Abbreviations
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
Footnotes
This statement is to certify that all authors have seen and approved the manuscript being submitted, have contributed significantly to the work, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the International Journal of Cardiology.
Disclosures: MDH received funding from the World Heart Federation to serve as its senior program advisor for the Emerging Leaders program, which is supported by Boehringer Ingelheim and Novartis with previous support from BUPA and AstraZeneca. MDH also receives support from the American Heart Association, Verily, and AstraZeneca and American Medical Association for work unrelated to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We attest that the article is the Authors’ original work, has not received prior publication and is not under consideration for publication elsewhere. We adhere to the statement of ethical publishing as appears in the International of Cardiology (citable as: Shewan LG, Rosano GMC, Henein MY, Coats AJS. A statement on ethical standards in publishing scientific articles in the International Journal of Cardiology family of journals. Int. J. Cardiol. 170 (2014) 253-254 DOI:10.1016/j.ijcard.2013.11).
References
- 1.Huffman MD, Roth GA, Sliwa K, Yancy CW, Prabhakaran D. Heart failure. In: Disease Control Priorities (third edition): Volume 5, Cardiovascular, Respiratory and Related Disorders, edited by Prabhakaran D, Anand S, Gaziano T, Mbanya J, Wu Y, Nugent R. Washington, DC: World Bank. [Google Scholar]
- 2.Roth GA, Abate D, Abate K, Abay SM, Abbafati C, Abbasi N, Abbastabar H, AbdAllah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader R, Abebe H, Abebe M, Abebe Z, Abejie A, Abera SF, Abil O, Abraha H, Abrham A, Abu-Raddad L, Accrombessi M, Acharya D, Adamu AA, Adebayo OM, Adedoyin R, Adekanmbi V, Adetokunboh OO, Adhena B, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray C. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667–1678. [DOI] [PubMed] [Google Scholar]
- 4.Callender T, Woodward M, Roth G, Farzadfar F, Lemarie J-C, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto B, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLOS Med 2014;11:e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harikrishnan S, Sanjay G, Anees T, Viswanathan S, Vijayaraghavan G, Bahuleyan CG, Sreedharan M, Biju R, Nair T, Suresh K, Rao AC, Dalus D, Huffman MD, Jeemon P. Clinical presentation, management, in hospital and 90 day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail 2015;17:794–800. [DOI] [PubMed] [Google Scholar]
- 6.DeVore AD, Cox M, Heidenreich PA, Fonarow GC, Yancy CW, Eapen ZJ, Peterson ED, Hernandez AF. Cluster-randomized trial of personalized site performance feedback in Get With The Guidelines-Heart Failure. Circulation Cardiovasc Qual Outcomes 2018;8:421–427. [DOI] [PubMed] [Google Scholar]
- 7.Sanjay G, Jeemon P, Agarwal A, Viswanathan S, Shreedharan M, Vijayaraghavan G, Bahuleyan C, Biju R, Nair T, Prathapkumar N, Krishnakumar G, Rajalekshmi N, Suresh K, Park LP, Huffman MD, Harikrishnan S. In-hospital and three-year outcomes of heart failure patients in south India: the Trivandrum Heart Failure Registry. J Card Fail 2018;12:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho K, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. New Engl J Medicine 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich PA, Fonarow GC, Breathett K, Jurgens CY, Pisani BA, Pozehl BJ, Spertus JA, Taylor KG, Thibodeau JT, Yancy CW, Ziaeian B. 2020 ACC/AHA Clinical performance and quality measures for adults with heart failure. J Am Coll Cardiol 2020;76:2527–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind M, Ferguson JF, Fornage M, Jordan L, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop M, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 11.Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S, Salomon JA. Mortality due to low-quality health systems in the universal health coverage era: a systematic analysis of amenable deaths in 137 countries. Lancet 2018;392:2203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Academies of Sciences, Engineering and Medicine. Improving quality of care in low- and middle-income countries. 2015. Available at: https://www.nap.edu/read/21736/chapter/1. Accessed October 28th, 2019.
- 13.Agarwal A, Bahiru E, Yoo S, Berendsen MA, Harikrishnan S, Hernandez AF, Prabhakaran D, Huffman MD. Hospital-based quality improvement interventions for patients with heart failure: a systematic review. Heart 2019;105:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhakaran D, Singh K, Roth GA, Banerjee A, Pagidipati NJ, Huffman MD. Cardiovascular diseases in India compared with the United States. J Am Coll Cardiol 2018;72:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, Viswanathan S, Stigi J, Joseph J, Chozhakkat S, Lloyd-Jones DM, Prabhakaran D. Effect of a quality improvement intervention on clinical outcomes in patients in India with acute myocardial infarction: the ACS QUIK randomized clinical trial. JAMA 2018;319:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal A, Davies D, Goenka S, Prabhakaran D, Huffman MD, Mohanan PP. Facilitators and barriers of heart failure care in Kerala, India: a qualitative analysis of healthcare providers and administrators. Indian Hear J 2019;71:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey J, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, Meer P van der. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 19.Harikrishnan S, Sanjay G, Agarwal A, Kumar PN, Kumar KK, Bahuleyan C, Vijayaraghavan G, Viswanathan S, edharan M, Biju R, Rajalekshmi N, Nair T, Suresh K, Jeemon P. One-year mortality outcomes and hospital readmissions of patients admitted with acute heart failure: data from the Trivandrum Heart Failure Registry in Kerala, India. Am Heart J 2017;189:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SK, Davies D, Mohanan PP, Baldridge AS, Charles PM, Schumacher M, Bhalla S, Devarajan R, Hirschhorn LR, Prabhakaran D, Huffman MD. Hospital-level cardiovascular management practices in Kerala, India. Circulation Cardiovasc Qual Outcomes 2019;12:e005251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocock SJ, Ariti CA, McMurray J, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 22.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Incremental reduction in risk of death associated with use of guideline recommended therapies in patients with heart failure: a nested case control analysis of IMPROVE HF. J Am Heart Assoc 2012;1:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004;43:1534–1541. [DOI] [PubMed] [Google Scholar]
- 26.Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, Ko DT. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA 2009;302:2330–2337. [DOI] [PubMed] [Google Scholar]
- 27.Bernal LJ, Soumerai S, Gasparrini A. A methodological framework for model selection in interrupted time series studies. J Clin Epidemiol 2018;103:82–91. [DOI] [PubMed] [Google Scholar]
- 28.Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017;46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.