Abstract
Morbidity associated with hepatic and urogenital schistosomiasis stems primarily from the host immune response directed against schistosome eggs. When eggs become entrapped in host tissues, the development of fibrotic plaques drives downstream pathology. These events occur due to the antigenic nature of egg excretory/secretory products (ESPs). Both Schistosoma mansoni and S. japonicum ESPs have been shown to interact with several cell populations in the host liver including hepatocytes, macrophages, and hepatic stellate cells, with both immunomodulatory and pathological consequences. Several protein components of the ESPs of S. mansoni and S. japonicum eggs have been characterised; however, studies into the collective contents of schistosome egg ESPs are lacking. Utilising shotgun mass spectrometry and an array of in silico analyses, we identified 266, 90 and 50 proteins within the S. mansoni, S. japonicum and S. haematobium egg secretomes respectively. We identified numerous proteins with already established immunomodulatory activities, vaccine candidates and vesicle markers. Relatively few common orthologues within the ESPs were identified by BLAST, indicating that the three egg secretomes differ in content significantly. Having a clearer understanding of these components may lead to the identification of new proteins with uncharacterised immunomodulatory potential or pathological relevance. This will enhance our understanding of host-parasite interactions, particularly those occurring during chronic schistosomiasis, and pave the way towards novel therapeutics and vaccines.
Keywords: Proteomics, Excretory/secretory protein, Schistosoma mansoni, Schistosoma japonicum, Schistosoma haematobium, Egg
1. Introduction
Schistosomiasis is a disease caused by infection with parasitic blood flukes of the genus Schistosoma. It affects over 200 million people worldwide, primarily in sub-Saharan Africa, Asia and South America, and causes as many as 200,000 deaths per year [1]. Three species of schistosomes are primarily responsible for human cases; Schistosoma mansoni, S. japonicum and S. haematobium [1]. Human infection occurs when water-borne larval cercariae penetrate the skin of the mammalian host; following penetration, cercariae transform into immature schistosomula which enter the circulation and migrate towards the mesenteric veins of the intestines (S. mansoni and S. japonicum) or the venous plexus of the bladder (S. haematobium) [2]. During this migratory phase the schistosomula mature into dioecious adult worms which, upon reaching the terminal point of their migration, pair up to produce eggs which are then shed in the faeces or urine [2].
The egg burden produced by mature worm pairs varies significantly between the three schistosome species. Female S. japonicum worms are the most fecund, laying ~1,000–2,500 eggs per day per pair in an established murine infection, with 30–50 % being successfully shed in the faeces [3]. By comparison, S. mansoni females lay approximately 350 eggs per day in murine models, with 30 % being successfully shed [3], while S. haematobium females produce around 100–200 eggs per day in human infections [4]. Eggs that fail to shed become swept up in the circulation and deposited within the host tissues, either the liver sinusoids (S. mansoni and S. japonicum) [5], or the bladder, cervical, and vaginal wall mucosa (S. haematobium) [6].
The chronic pathology associated with hepatoenteric and urogenital schistosomiasis arises due to a shift from a moderate host type 1 helper (Th1)-mediated inflammatory immune response against the migrating schistosomes to a strong type 2 helper (Th2)-mediated granulomatous response against schistosome eggs [5]. This change occurs alongside the commencement of egg laying and is brought about by components of the soluble egg antigens (SEA) released by eggs acting on host immune cells to alter the balance of cytokines and chemokines towards a Th2 phenotype [5]. This shift is orchestrated to assist the migrating egg in successfully shedding from the host; by reducing local inflammation and acquiring a layer of host cells in the form of a granuloma, the eggs will encounter fewer obstacles to transmission and can better pass through the lamina propria and intestinal and pelvic organ walls [7]. The modulation away from a Th1-mediated response also ensures host survival. Studies in murine models have shown that preventing the Th1-to-Th2 shift and enforcing a dominant Th1 response throughout the chronic stages of schistosomiasis leads to substantial host death due to enhanced egg-related immunopathology, including larger granuloma and increased inflammation in response to increasing egg burden [8]. Certain SEA components released by S. mansoni eggs have been shown to be hepatotoxic, causing cell death in hepatocytes [9], driving the formation of granuloma around the eggs. This last mechanism benefits the host by containing harmful antigens and protecting the liver tissue from necrosis. Eggs that become entrapped in host tissues will eventually be destroyed within the granuloma; however, the resolution of these granuloma following egg death leaves behind fibrotic plaques. These plaques accumulate throughout the course of chronic infection and eventually lead to impaired liver function and downstream schistosomiasis pathology.
Several components of schistosome egg ESPs have previously been characterised, and the most extensively characterised of these proteins include IPSE/α−1, omega-1, and major egg antigen P40. IPSE/α−1 is a hepatotoxic glycoprotein released by S. mansoni and S. haematobium eggs. In S. mansoni infections, IPSE/α−1 inhibits neutrophil migration to the site of the granuloma and assists in driving the host immune shift from a Th1 to a Th2 mediated response that follows egg laying [10,11]. Omega-1 is another hepatotoxic glycoprotein released by S. mansoni eggs that functions as a T2 ribonuclease (RNase). Omega-1 is internalised by dendritic cells (DCs), where it degrades DC ribosomal and messenger RNA (rRNA, mRNA) to reduce the expression of pro-Th1 mediators [12]. Ribonucleases released by S. japonicum eggs have similar functions [13,14]. P40 is a component of both S. mansoni and S. japonicum egg ESPs. Unlike IPSE/α−1 and omega-1, P40 has been linked with anti-fibrotic roles; Sm-P40 stimulation of murine lymphocytes induces interleukin (IL)-2 and interferon-gamma (IFN-γ) expression, both of which are considered Th1 cytokines, while Sj-P40 has been linked with decreasing hepatic stellate cell (HSC) activity and contribution to fibrosis by inducing apoptosis and senescence in these stellate cells [15–18]. Together, these proteins act to reduce local inflammation at the site of the granuloma and promote the regulated development of a fibrotic phenotype that assists egg survival and transmission.
Identifying the full range of (immunomodulatory) protein components that comprise schistosome egg ESPs is advantageous, as it could further advance knowledge of the host-parasite dynamic that is established during chronic schistosomiasis. The egg stage secretomes for S. mansoni, S. japonicum and S. haematobium have been reported previously [19–21]. However, this is the first comparative study that aims to identify common, or species-specific, antigens within the egg secretions of the three species in an effort to link these findings to differential host pathology. While most of these previously published secretomes used a gel-based MS approach, we have utilised shotgun proteomics and found differing ESP profiles in the 3 secretomes.
2. Materials and methods
2.1. Animal work
All animal ethics were approved by the Animal Ethics Committee of the QIMR Berghofer Medical Research Institute (Brisbane, Australia) and the Biomedical Research Institute (BRI; Rockville, Maryland, United States of America).
Female four-week-old Swiss Webster mice were percutaneously infected with 150–200 S. mansoni cercariae (Puerto Rico strain) via tail exposure or with 20–30 S. japonicum cercariae (Chinese Anhui strain) via abdominal exposure for 45 min. Mice were sacrificed after 6–7 weeks post infection. Five to six-week-old, male and female Golden Syrian LVG hamsters were percutaneously infected with 350 S. haematobium cercariae (Egyptian strain) via abdominal exposure for 30 min. Hamsters were sacrificed after 14–16 weeks post infection.
2.2. Egg isolation and culture
S. mansoni and S. japonicum eggs were isolated from perfused murine livers by repeated homogenisation and sieving of the liver tissue using a Waring blender and several sieves with pore sizes ranging from 420 μm to 45 μm. The homogenate was maintained in cold 1.2 % (w/v) NaCl throughout to prevent egg hatching. Mature eggs were enriched by gentle swirling in a petri dish, with the mature eggs collecting in the centre of the dish. These mature eggs were concentrated using a 40 μm cell strainer. S. haematobium eggs were obtained from perfused hamster livers and intestines using the same method.
Approximately 500,000 mature eggs of each schistosome species were cultured separately in 10 ml of RPMI-1640 media (ThermoFisher, Waltham, USA) supplemented with 300 units/mL penicillin, 300 μg/mL streptomycin and 500 μg/mL gentamycin antibiotics (ThermoFisher), in the absence of phenol red or any serum supplements. Eggs were maintained at 37 °C and 5% CO2 for 72 h, after which the culture medium was collected, eggs pelleted and stored at −80 °C. Egg viability was assessed using an egg hatching assay [20] and preparations were only retained with 85 % minimum hatch rate. The egg culture media were then concentrated down using 3 kDa MW cut-off centrifugal filter units (Merck, Kenilworth, USA) to a final concentration of approximately 15 μg/mL each, as determined by the bicinchoninic acid (BCA) assay (ThermoFisher). Approximately 15 μg of total protein from each sample were submitted for MS/MS analysis.
2.3. Mass spectrometry analysis
Three technical replicates of S. mansoni, S. japonicum and S. haematobium egg secretions were analysed by LC–MS/MS. Samples were digested with 100 ng/μl sequencing grade trypsin (Sigma-Aldrich, St. Louis, USA) at 37 °C overnight. The samples were then dried in a vacuum centrifuge and reconstituted with 10 μl of 0.1 % trifluoroacetic acid before analysis. Five μl of the resulting suspension were delivered to an analytical column (S. mansoni; Acquity UPLC Peptide BEH C18 nano column, 130A, 1.7 um, 75 umx200 mm, S. japonicum/S. haematobium; Eksigen C18-CL NanoLC Column, 3 μm; 75 μm x15 cm) equilibrated in 5% acetonitrile/0.1 % formic acid (FA). Elution was carried out with a linear gradient of 5–35 % buffer B in buffer A for 30 min (buffer A: 0.1 % FA; buffer B: acetonitrile, 0.1 % FA) at a flow rate of 300 nl/min. Peptides were analysed in an Orbitrap Fusion mass spectrometer (ThermoFisher) (S. mansoni) or a nanoESI QqTOF mass spectrometer (5600 TripleTOF, ABSCIEX- Warrington, UK) (S. japonicum/S. haematobium) operating in information-dependent acquisition mode, in which a 0.25-s TOF MS scan from was performed (S. mansoni; 380–1500 m/z, S. japonicum/S. haematobium; 350–1250 m/z), followed by 0.05-s product ion scans from 100 to 1500 m/z on the 50 most intense 2–5 charged ions. Peak list files were generated by Proteome discoverer v2.2 (ThermoFisher) (S. mansoni) or Protein Pilot v4.5 (Applied Biosystems, Foster City, USA) (S. japonicum/S. haematobium) using default parameters.
2.4. Database searching
MS/MS samples were analysed using Sequest HT on Proteome discoverer v2.2 (ThermoFisher) (S. mansoni) or MaxQuant v1.6.6 (S. japonicum/S. haematobium) set up to search databases comprised of proteins identified within the S. mansoni, S. japonicum and S. haematobium genomes (obtained from WormBase Parasite; S. mansoni WBPS10, accession PRJEA36577, 11774 entries; S. japonicum WBPS10, accession PRJEA34885, 12738 entries; S. haematobium WBPS10, accession PRJNA78265, 11140 entries) assuming strict trypsin digestion with 2 missed cleavages permitted. Sequest HT was searched with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 20.0 ppm. MaxQuant was searched with a fragment ion mass tolerance of 0.060 Da and a parent ion tolerance of 10.0 ppm. Carbamidomethyl of cysteine was specified in MaxQuant as a fixed modification. Gln->pyro-Glu of the N-terminus, deamidation of asparagine and glutamine, oxidation of methionine, dioxidation of methionine and acetyl of the N-terminus were specified in MaxQuant as variable modifications. Searches were also carried out against proteome databases of the host animals Mus musculus (Uniprot ID: UP000000589, 55,462 entries for S. mansoni and S. japonicum) and Mesocricetus auratus (Uniprot ID: UP000189706, 31,693 entries for S. haematobium) using the same settings. Detected host proteins that share resemblance with schistosome proteins are reported (Supplementary Tables 1–3).
2.5. Criteria for protein identification
Proteome discoverer v2.2 (ThermoFisher) (S. mansoni) or Scaffold (version Scaffold_4.8.9, Proteome Software Inc., Portland, USA) (S. japonicum/S. haematobium) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Local FDR algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.
2.6. Bioinformatic analysis
Identified proteins were assigned functional annotation through Blast2GO [22] software against the entire non-redundant (nr) NCBI database using default settings, supplemented with additional annotation from Annotation Expander (ANNEX) and gene ontology (GO) terms obtained from InterPro databases. Gene ontology annotations were also obtained for all proteins recorded in the NCBI database for each of the three species. Comparisons were made between secretome lists by utilising BLASTp (Basic Local Alignment Search Tool protein); any protein that had an 80 % or greater sequence identity with a protein from another species was considered an orthologue of that protein (e-value 10−5). Functional domains within selected proteins were identified using the InterPro database through UniProt. Alignments of these domains were carried out using BLASTp through NCBI using default settings. The SecretomeP v2.0 algorithm [23] was used to predict the method of secretion for each protein. Proteins that were assigned a neural network (NN) value >0.5 were assumed to be secreted. Secretory proteins that were predicted to possess a signal peptide were assumed to be secreted via classical pathways, while those which did not possess a signal peptide were assumed to be secreted via non-classical pathways. Those with a NN value ≤0.5 were assumed to be non-secretory.
3. Results
3.1. S. mansoni egg secretome
LC–MS/MS analysis identified 266 proteins within the S. mansoni egg secretome, including 36 uncharacterised proteins, and we confirmed the presence of IPSE/α−1 (IL-4-inducing protein), omega-1 and P40 (HSP20) (Table 1, Supplementary Table 1). Numerous additional proteins with immunomodulatory properties were noted, including glutathione S-transferase, phosphoenolpyruvate carboxykinase (SmPEPCK), SmVAL9, and peroxiredoxin (Prx1). Several other S. mansoni ESPs identified also share similarity with immunomodulatory proteins from other parasitic species, including calreticulin, released by Haemonchus contortus and Necator americanus, and serpin, produced by Brugia malayi [24–26].
Table 1.
Top 20 most abundant protein components of S. mansoni egg ESPs. The method of protein secretion as predicted by SecretomeP is listed; classically secreted (Y), non-classically secreted (A) or non-secreted (N). Proteins are ranked in order of the highest total unique matched peptides taken from three technical replicates.
| Accession Number | Protein ID | # Unique peptides | % Coverage | SecretomeP |
|---|---|---|---|---|
| G4V5U5 | Putative macroglobulin/complement | 49 | 26.7 | N |
| G4V9B9 | Putative heat shock protein | 27 | 40.0 | N |
| G4VPH5 | Glucose-6-phosphate isomerase | 20 | 48.0 | A |
| G4LW91 | Thimet oligopeptidase | 19 | 31.7 | N |
| G4V721 | Putative phosphoenolpyruvate carboxykinase | 18 | 40.7 | N |
| G4LYU6 | Serpin, putative | 18 | 52.7 | A |
| G4V855 | Aminopeptidase PILS | 17 | 21.0 | A |
| G4VFC2 | Protein disulfide-isomerase | 16 | 37.3 | Y |
| G4VJT9 | Fructose-bisphosphate aldolase | 16 | 57.7 | N |
| G4V9U9 | Putative major vault protein | 16 | 25.3 | N |
| G4V8L4 | Putative heat shock protein 70 | 15 | 33.7 | N |
| G4V8C5 | Putative alpha-glucosidase | 15 | 20.3 | A |
| C4Q5I7 | Calreticulin autoantigen homolog, putative | 13 | 34.7 | Y |
| G4M057 | WD-repeat protein, putative | 12 | 26.3 | A |
| G4LYC3 | Protein disulfide-isomerase | 12 | 30.0 | Y |
| G4VG40 | Dipeptidyl peptidase 3 | 12 | 21.3 | A |
| P09792 | Glutathione S-transferase class-mu 28 kDa isozyme | 12 | 51.3 | N |
| G4LVU8 | Filamin | 11 | 7.3 | N |
| G4VQ41 | Low-density lipoprotein receptor | 11 | 14.7 | Y |
| G4V8X7 | Putative heat shock protein-HSP20 | 11 | 36.7 | A |
We also observed the presence of several putative vaccine candidates, including Sm14FABP (fatty acid-binding protein) [27,28], peptidylglycine alpha hydroxylating mono-oxygenase (SmPHM) [29], 14-3-3 protein [30], Sm21.7 [31,32] and triosephosphate isomerase [33]. Many ubiquitous protein components involved in energy production or metabolism (fructose-bisphosphate aldolase, phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase), DNA/RNA synthesis (uridine phosphorylase, hypoxanthine phosphoribosyltransferase), amino acid synthesis (ornithine-oxo-acid transaminase), nutrition (Sm22.6, cathepsins B and D, leucine aminopeptidase) and detoxification (superoxide dismutase, thioredoxin) were also identified. Furthermore, proteins such as phosphopyruvate hydratase (enolase), heat shock protein 70 (HSP70), elongation factor 1-α/γ [34–36], thimet oligopeptidase [35], ferritin, as well as several histones and structural proteins (actin, tubulin, filamin, spectrin-β), which are characteristic of vesicle components [37,38], were noted. The presence of these latter proteins suggests that a portion of the proteins identified are packaged and released within extracellular vesicles (EVs) in vivo.
We compared the 266 proteins identified in our study with those described by others in both the secretome of adult male S. mansoni worms and the previously published egg stage secretome. The adult male S. mansoni secretome has been previously reported to comprise 111 proteins [39], and of these we found 70 proteins that had ≥80 % sequence identity with our data set (Supplementary Table 1). This result suggests that there are significant similarities in ESPs released in these two life cycle stages. The previously published S. mansoni egg secretome comprised 188 proteins [19], and of these we found 122 proteins sharing ≥80 % sequence identity with proteins in our own dataset (Supplementary Table 1).
3.2. S. japonicum egg secretome
Ninety S. japonicum proteins were identified within the egg secretome, including 28 uncharacterised proteins (Table 2, Supplementary Table 2). We confirmed the presence of P40, in addition to 3 ribonucleases (T2, Oy and X25) that share sequence identity with the S. mansoni protein omega-1. SjE16.7 (16 kDa calcium-binding protein) and Sj97 (paramyosin) were also present. In addition, glutathione S-transferase, serpin and calreticulin, are three proteins previously shown to have immunoregulatory activity in multiple helminths (see Section 3.1 above).
Table 2.
Top 20 most abundant protein components of S. japonicum egg ESPs. The method of protein secretion as predicted by SecretomeP is listed; classically secreted (Y), non-classically secreted (A) or non-secreted (N). Proteins are ranked in order of the highest total unique matched peptides taken from three technical replicates.
| Accession Number | Protein ID | # Unique Peptides | % Coverage | SecretomeP |
|---|---|---|---|---|
| Q5DET3 | Histone H4 | 19 | 71.8 | N |
| Q5DFZ8 | Fructose-bisphosphate aldolase | 16 | 55.8 | N |
| Q86G46 | Polyubiquitin | 16 | 32.1 | N |
| C1L4Z7 | Actin 5C | 13 | 33.2 | N |
| Q5DHF8 | Histone H3 | 12 | 64.5 | A |
| C1LFC4 | Aminopeptidase | 10 | 18.2 | A |
| C1L9B2 | Calreticulin | 8 | 19.8 | Y |
| Q5DDU9 | Histone H2B | 7 | 38.0 | N |
| C1LEJ4 | Histone H2A | 7 | 46.4 | A |
| Q86EU4 | Cytochrome c proximal | 7 | 50.8 | A |
| C1LQJ9 | Uncharacterized protein | 6 | 23.3 | A |
| C1LQT3 | Thioredoxin | 6 | 53.5 | A |
| Q5D8W0 | SJCHGC06710 protein | 6 | 40.8 | Y |
| P33676 | Enolase | 6 | 16.0 | A |
| Q5D9T1 | Malate dehydrogenase | 6 | 27.1 | A |
| C1LJG7 | NADH:ubiquinone oxidoreductase | 5 | 22.0 | A |
| C1LYI9 | Calcium-binding EF-hand protein | 5 | 58.7 | A |
| C1LNR0 | Protein disulfide-isomerase | 5 | 14.5 | Y |
| C1LV44 | Tryparedoxin peroxidase | 5 | 28.1 | A |
| C1LNY3 | Superoxide dismutase [Cu-Zn] | 5 | 62.6 | A |
Many ubiquitous protein components involved in energy production (fructose-bisphosphate aldolase, malate dehydrogenase, nucleoside diphosphate kinase), DNA synthesis (purine nucleoside phosphorylase, transaldolase), nutrition (aminopeptidase) and detoxification (superoxide dismutase, tryparedoxin peroxidase) were also identified. Again, we noted the presence of characteristic vesicle components including enolase, HSP70, elongation factor 1-α, histones, ferritin, and structural proteins (actin, tubulin) indicating that our data includes proteins packaged within EVs released from S. japonicum eggs.
The 90 newly identified proteins were then compared with previously described datasets of the secretome of adult S. japonicum worms and the previously published egg stage secretome. The adult S. japonicum secretome consists of 101 proteins [40], and of these only 27 proteins had ≥80 % sequence identity with proteins in our own dataset (Supplementary Table 2), suggesting that there are significantly different ESPs being produced between the two life cycle stages. It is noteworthy, however, that only 23 proteins from the previously published S. japonicum egg stage secretome, comprising 95 proteins [20], shared ≥80 % sequence identity with proteins in our dataset (Supplementary Table 2).
3.3. S. haematobium egg secretome
Fifty proteins were identified within the egg secretome of S. haematobium, including 7 uncharacterised proteins (Table 3, Supplementary Table 3). We noted the presence of Sh-IPSE/α−1, which shares sequence identity with Sm-IPSE/α−1. The components of S. haematobium egg ESPs are poorly characterised compared to those of S. mansoni or S. japonicum, which makes assigning their potential functions problematical. However, we did confirm the presence of proteins such as ShE16 (16 kDa calcium-binding protein) and neuroserpin, which are similar to other proteins which have documented immunoregulatory roles in both S. japonicum ESPs, and in other helminths as considered above.
Table 3.
Top 20 most abundant protein components of S. haematobium egg ESPs. The method of protein secretion as predicted by SecretomeP is listed; classically secreted (Y), non-classically secreted (A) or non-secreted (N). Proteins are ranked in order of the highest total unique matched peptides taken from three technical replicates.
| Accession Number | Protein ID | # Unique Peptides | % Coverage | SecretomeP |
|---|---|---|---|---|
| A0A094ZWN9 | Histone H4 | 11 | 61.5 | N |
| A0A095B131 | Polyubiquitin-C | 8 | 30.2 | N |
| A0A094ZDN2 | Thioredoxin peroxidase 2 | 8 | 43.6 | A |
| A0A095AIQ5 | Cytochrome c | 8 | 55.6 | N |
| A0A095AKM1 | Fructose-bisphosphate aldolase | 8 | 27.4 | N |
| A0A094ZX17 | GLIPR1-like protein 1 | 7 | 39.6 | Y |
| A0A095A2M4 | Thioredoxin | 7 | 61.6 | A |
| A0A095ANZ3 | Histone H3 | 5 | 33.3 | A |
| A0A095A3Q8 | Alpha-galactosidase | 5 | 19.2 | N |
| A5A6F5 | 14 kDa fatty acid-binding protein | 5 | 32.3 | A |
| A0A095A0L7 | Superoxide dismutase [Cu-Zn] | 4 | 37.5 | A |
| A0A095A463 | Uncharacterized protein | 3 | 13.5 | N |
| A0A095B4U1 | Histone H2B | 3 | 27.9 | N |
| A0A094ZS57 | 16 kDa calcium-binding protein | 3 | 25.2 | A |
| A0A094ZZE3 | Basement membrane proteoglycan | 3 | 4.8 | N |
| A0A095CF43 | Alpha-galactosidase | 3 | 12.5 | A |
| A0A094ZS46 | Spectrin alpha chain | 3 | 1.0 | N |
| A0A094ZVR4 | Peptidase inhibitor 16 | 3 | 24.5 | Y |
| A0A095CGL8 | 78 kDa glucose-regulated protein | 3 | 6.8 | Y |
| A0A094ZD40 | Actin-2 | 2 | 13.8 | A |
Several ubiquitous proteins involved in energy production (fructose-bisphosphate aldolase, alpha-galactosidase), DNA synthesis (purine nucleoside phosphorylase) and detoxification (superoxide dismutase) were once again identified, as were several proteins associated with vesicle packaging, including enolase, 78 kDa glucose-regulated protein (HSP70), histones, structural components (actin, filamin, fibrocystin, spectrin) and ferritin. Taken together, this indicates that S. haematobium EV-related protein components are also included in this dataset.
The S. haematobium egg secretome has previously been described as comprising 138 proteins [21]. Despite the difference in the number of proteins, we compared the two datasets and found that, of the 50 proteins we identified, 23 shared ≥80 % sequence identity with proteins in the previously published dataset (Supplementary Table 3).
3.4. Comparison of the three egg secretomes
BLAST was used to compare the proteins making up the three egg secretomes according to sequence identity given the difficulty in comparing proteins from the different schistosome species (different naming conventions, accession numbers etc). Proteins that shared ≥80 % sequence identity with an e-value cut off of 10−5 were considered orthologues (Fig. 1, Tables 4 and 5). When several proteins matched against the same prospective orthologue, the match with the highest % sequence identity was accepted.
Fig. 1.
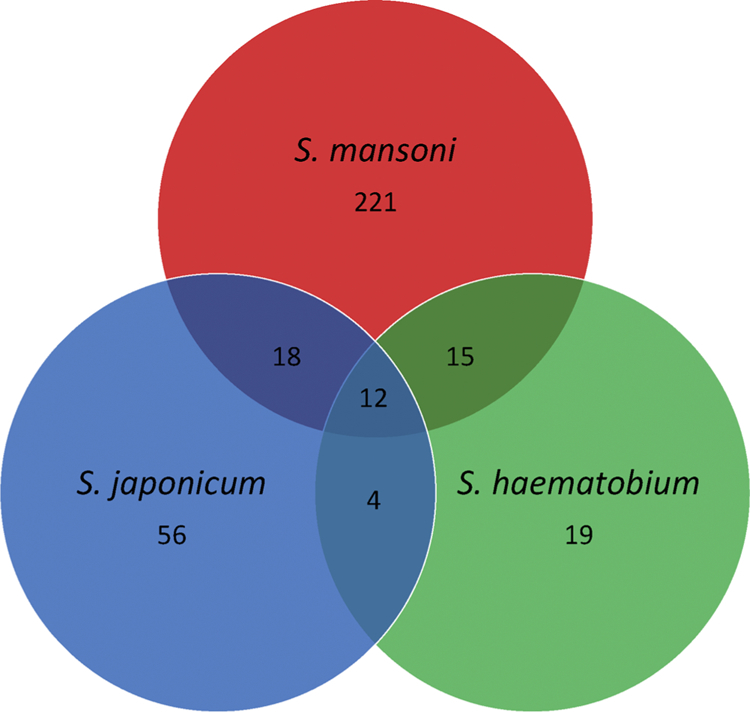
The number of shared egg stage ESPs between S. mansoni, S. japonicum and S. haematobium.
Table 4.
Twelve (12) predicted orthologues shared between S. mansoni (Sm), S. japonicum (Sj) and S. haematobium (Sh) egg excretory/secretory products.
|
S. mansoni |
S. japonicum |
S. haematobium |
% identity |
|||||
|---|---|---|---|---|---|---|---|---|
| Acc. No. | Protein ID | Acc. No. | Protein ID | Acc. No. | Protein ID | Sm-Sj | Sj-Sh | Sm-Sh |
| C4QBN1 | Histone H4 | Q5DET3 | Histone H4 | A0A094ZWN9 | Histone H4 | 100 | 100 | 100 |
| G4VLW2 | Putative actin-1 | C1L4Z7 | Actin 5C | A0A094ZD40 | Actin-2 | 98 | 97 | 96 |
| G4VJT9 | Fructose-bisphosphate aldolase | Q5DFZ8 | Fructose-bisphosphate aldolase | A0A095AKM1 | Fructose-bisphosphate aldolase | 96 | 97 | 98 |
| G4V6E9 | Histone H2B | Q5DDU9 | Histone H2B | A0A095B4U1 | Histone H2B | 95 | 100 | 95 |
| G4VT44 | Putative calmodulin | Q5DA21 | Calmodulin 3b | A0A094ZPX2 | Calmodulin | 90 | 97 | 98 |
| G4M131 | Fatty acid binding protein | O45035 | Fatty acid-binding protein | A5A6F5 | 14 kDa fatty acid-binding protein | 86 | 86 | 99 |
| Q15EU2 | Cytochrome c-like protein | Q86EU4 | Cytochrome c proximal | A0A095AIQ5 | Cytochrome c | 90 | 88 | 93 |
| G4VE60 | Thioredoxin, Trx M | Q5DA40 | SJCHGC03107 protein | A0A095CEH3 | Thioredoxin, mitochondrial | 89 | 84 | 94 |
| G4VQ58 | Phosphopyruvate hydratase | P33676 | Enolase | A0A095AJN4 | Enolase | 85 | 85 | 93 |
| G4VKD9 | Peroxiredoxin, Prx1 | C1LV44 | Tryparedoxin peroxidase | A0A094ZDN2 | Thioredoxin peroxidase 2 | 82 | 85 | 93 |
| G4V910 | Putative heat shock protein 70 | O45038 | HSP70 | A0A095CGL8 | 78 kDa glucose-regulated protein | 83 | 81 | 95 |
| G4M0D0 | Superoxide dismutase [Cu-Zn] | C1LNY3 | Superoxide dismutase [Cu-Zn] | A0A095A0L7 | Superoxide dismutase [Cu-Zn] | 81 | 80 | 95 |
Table 5.
Predicted orthologues between paired comparisons of S. mansoni, S. japonicum and S. haematobium egg excretory/secretory products.
| Acc. No. | Protein ID | Acc. No. | Protein ID | % identity |
|---|---|---|---|---|
| S. mansoni | S. japonicum | |||
| G4VG19 | Phosphoglycerate kinase | B3W661 | Phosphoglycerate kinase | 100 |
| G4VHK8 | Tubulin beta chain | C1LVD4 | Tubulin beta chain | 99 |
| G4VMF9 | Histone H2A | Q5DE19 | Histone H2A | 99 |
| G4V865 | Tubulin alpha chain | Q5D9M8 | Tubulin alpha chain | 98 |
| G4VBJ0 | Malate dehydrogenase | Q5D9T1 | Malate dehydrogenase | 94 |
| G4VPH5 | Glucose-6-phosphate isomerase | C1LK30 | Glucose-6-phosphate isomerase | 93 |
| G4LYC3 | Protein disulfide-isomerase | Q5C0A7 | Uncharacterized protein | 92 |
| G4V721 | Putative phosphoenolpyruvate carboxykinase | Q5DGF3 | SJCHGC06900 protein | 91 |
| G4VCN8 | Dihydrolipoyl dehydrogenase | C1LIA4 | Dihydrolipoyl dehydrogenase | 91 |
| Q9Y0D3 | Peroxiredoxin, Prx2 | C1LMD2 | Thioredoxin peroxidase | 90 |
| G4VAD2 | Elongation factor 1-alpha | C1LWQ6 | Elongation factor 1-alpha | 89 |
| G4VRK1 | Putative glyoxalase II | Q86DW6 | Hydroxyacylglutathione hydrolase | 89 |
| G4VJY9 | Nucleoside diphosphate kinase | Q86EJ4 | Nucleoside diphosphate kinase | 88 |
| G4M0P7 | Cell division control protein 48 | Q5D9C5 | SJCHGC09453 protein | 88 |
| G4VBG6 | Ferritin | C1L7G5 | Ferritin | 87 |
| G4VFQ0 | Peptidyl-prolyl cis-trans isomerase | Q5D8J4 | Peptidyl-prolyl cis-trans isomerase | 85 |
| G4V9B9 | Putative heat shock protein | Q5D947 | SJCHGC00820 protein | 84 |
| G4VH95 | Putative acyl-CoA-binding protein | C1LJT2 | Diazepam-binding inhibitor | 84 |
| S. mansoni | S. haematobium | |||
| G4LVU8 | Filamin | A0A094ZGB5 | Filamin-A | 95 |
| G4LXS0 | Aldo-keto reductase, putative | A0A095AEB2 | Alcohol dehydrogenase [NADP(+)] A | 95 |
| G4VAG2 | Calpain | A0A094ZH52 | Calpain | 95 |
| G4VDR4 | Family C56 non-peptidase homologue | A0A095C8I8 | Protein DJ-1 | 93 |
| G4VMD1 | Venom allergen-like (VAL) 26 protein | A0A094ZX17 | GLIPR1-like protein 1 | 90 |
| Q8T9N5 | Thioredoxin | A0A095A2M4 | Thioredoxin | 90 |
| G4VSQ5 | Superoxide dismutase | A0A095BT16 | Superoxide dismutase | 88 |
| G4VP82 | Purine nucleoside phosphorylase | A0A094ZR11 | Purine nucleoside phosphorylase | 88 |
| G4VA23 | Putative 22.6 kDa tegument-associated antigen | A0A095C6E4 | Tegument antigen | 88 |
| G4VDQ3 | Uncharacterized protein | A0A094ZWF6 | Uncharacterized protein | 87 |
| G4M010 | Low-density lipoprotein receptor | A0A094ZZE3 | Basement membrane heparan sulfate proteoglycan | 86 |
| G4LUZ7 | Ferritin | A0A094ZYX2 | Ferritin | 85 |
| G4VNW4 | Putative fibrocystin l | A0A094ZQC8 | Fibrocystin-L | 85 |
| G4VFC2 | Protein disulfide-isomerase | A0A095A463 | Uncharacterized protein | 83 |
| G4VLD6 | Alpha-galactosidase | A0A095CF43 | Alpha-galactosidase | 83 |
| S. japonicum | S. haematobium | |||
| Q86G46 | Polyubiquitin | A0A095B131 | Polyubiquitin-C | 100 |
| C1LEJ4 | Histone H2A | A0A094ZQV5 | Histone H2A | 99 |
| Q5DHF8 | Histone H3 | A0A095ANZ3 | Histone H3 | 98 |
| Q5C3I8 | SJCHGC05636 protein | A0A094ZS46 | Spectrin alpha chain | 88 |
Twelve (12) proteins were common between S. mansoni, S. japonicum and S. haematobium egg ESPs. These included several proteins documented as vaccine candidates in one or more species (fatty acid-binding protein, fructose-bisphosphate aldolase), proteins generally associated with either vesicle markers or cargo proteins (histones H2B and H4, enolase, actin, HSP70), signalling (thioredoxin, calmodulin) and antioxidant (redoxin) proteins.
S. mansoni and S. japonicum egg ESPs shared 18 proteins in common, while S. mansoni and S. haematobium eggs had 15 proteins in common. Again, a large proportion of these proteins are either vaccine targets (superoxide dismutase), vesicle markers (ferritin, elongation factor 1-α, structural proteins) or various ubiquitous elements, particularly involved in energy production/metabolism and DNA/RNA synthesis (glucose-6-phosphate isomerase, alpha-galactosidase, malate dehydrogenase, nucleoside diphosphate kinase). It should be noted that S. mansoni eggs produce two isoforms of ferritin and protein disulphide-isomerase, both of which share very low sequence identity with their counterparts; hence both proteins are listed as orthologous with S. japonicum and S. haematobium proteins separately and not grouped together.
S. japonicum and S. haematobium eggs shared only 4 proteins in common: 2 histones, actin and polyubiquitin C. These are extremely ubiquitous proteins that are again likely released as part of vesicle content.
3.5. Assignment of Gene Ontology (GO) terms
Gene ontology (GO) terms were assigned to all the identified schistosome proteins according to their function (Molecular Function or MF) or role (Biological Process or BP), as seen in Table 6. GO terms were also assigned to all recorded proteins for each species available in the NCBI database (Table 6).
Table 6.
Gene Ontology (GO) term annotations and their % representation for S. mansoni (Sm), S. japonicum (Sj) and S. haematobium (Sh) excreted/secreted products (ESPs) in our dataset compared with the complete NCBI protein groups. GO terms that are higher in ESP compared to NCBI groups are shaded
| Molecular Function (MF) | Sm ESP | Sm NCBI | Sj ESP | Sj NCBI | Sh ESP | Sh NCBI | |
|---|---|---|---|---|---|---|---|
| antioxidant activity | GO:0016209 | 2.68 | 0.86 | 3.91 | 0.61 | 6.98 | 0.22 |
| binding | GO:0005488 | 46.34 | 52.08 | 50 | 46.9 | 56.98 | 55.97 |
| catalytic activity | GO:0003824 | 47.32 | 35.86 | 40.63 | 37.47 | 31.4 | 36.28 |
| molecular function regulator | GO:0098772 | 1.71 | 1.42 | 2.34 | 1.38 | 0 | 1.85 |
| structural molecule activity | GO:0005198 | 0.98 | 1.62 | 1.56 | 7.76 | 1.16 | 2.1 |
| transporter activity | GO:0005215 | 0.73 | 6.31 | 1.56 | 5.88 | 2.33 | 5.39 |
| Biological Process (BP) | |||||||
| biological regulation | GO:0065007 | 5.11 | 10.03 | 8.05 | 6.87 | 10.33 | 11.35 |
| carbon utilization | GO:0015976 | 1 | 0.12 | 0.77 | 0.26 | 0.33 | 0.11 |
| cellular component organization | GO:0071840 | 1.56 | 4.18 | 3.45 | 6.95 | 4.67 | 4.51 |
| cellular process | GO:0009987 | 37.67 | 35.07 | 39.46 | 35.06 | 31.67 | 34.69 |
| detoxification | GO:0098754 | 0.89 | 0.3 | 1.15 | 0.22 | 1.67 | 0.1 |
| developmental process | GO:0032502 | 2 | 1.11 | 0.77 | 0.96 | 3 | 1.15 |
| immune system process | GO:0002376 | 0.33 | 0.16 | 0.38 | 0.31 | 0.67 | 0.14 |
| localization | GO:0051179 | 2.22 | 7.35 | 1.92 | 9.73 | 3.67 | 7.05 |
| metabolic process | GO:0008152 | 41.22 | 30.38 | 35.25 | 33.79 | 23.33 | 29.15 |
| multicellular organismal process | GO:0032501 | 1.44 | 1.12 | 1.53 | 1.02 | 3.67 | 1.26 |
| response to stimulus | GO:0050896 | 4.11 | 5.16 | 5.36 | 2.81 | 9.33 | 5.49 |
| signalling | GO:0023052 | 0.67 | 3.58 | 1.15 | 1.6 | 3 | 4.32 |
GO annotation revealed that most proteins within the schistosome egg secretomes are involved in either binding or catalytic activities (MF) and take part in mostly cellular or metabolic processes (BP). Thus, many of the proteins enriched are enzymes; energy-producing enzymes are particularly abundant, including fructose-bisphosphate aldolase, malate dehydrogenase and alpha-galactosidase. Enzymes involved in DNA/RNA synthesis (purine nucleoside phosphorylase, uridine phosphorylase), amino acid synthesis (ornithine-oxo-acid transaminase) and various proteases (thimet oligopeptidase, aminopeptidase and calpain) were also identified. Antioxidant activity (MF) was consistently enriched across the three schistosome secretomes and appeared to be upregulated in the egg stage in comparison with the schisto-NCBI annotations. Potential antioxidant activity could be attributed to proteins such as superoxide dismutase, tryparedoxin peroxidase, glutathione S-transferase and various peroxidase enzymes. Conversely, transporter activity (MF) and localization (BP) appeared to be downregulated in comparison with schisto-NCBI annotations.
3.6. SecretomeP analysis for signal peptides
Secretory proteins generally contain an N-terminal signal peptide sequence, unless they are secreted by non-classical means such as being packaged within vesicles. Given that we expected the proteins described here to be secretory in nature due to their presence in ESP, the algorithm SecretomeP v2.0 [23] was used to analyse the protein sequences and to determine the likelihood of classical (involving a signal peptide) or non-classical (alternative pathways) secretion based on the detection of a signal peptide and a neural network (NN) value assigned by the algorithm.
Of the 266 proteins identified in S. mansoni ESP, 151 were assigned a NN value of >0.5 (56 %) and thus were assumed to be secretory (Supplementary Table 1). Forty-nine (49) of these proteins had predicted signal peptides (18 %), while 102 did not (38 %). One protein, titin, was too large for the SecretomeP algorithm to analyse; however, a select analysis of the N-terminal half of the protein indicated that it is not secreted. Of the 90 proteins identified in S. japonicum ESP, 56 were assigned a NN value of >0.5 (62 %) and therefore assumed to be secretory (Supplementary Table 2). Eighteen (18) of these proteins had predicted signal peptides (20 %), while 38 did not (42 %). Of the 50 proteins identified in S. haematobium ESP, 26 were assigned a NN value of >0.5 (52 %) and thus are assumed to be secretory (Supplementary Table 3). Eight of these proteins had predicted signal peptides (16 %), while 18 did not (36 %).
4. Discussion
In 2007, Cass et al. published the first mass spectrometry-based proteomic analysis of a schistosome egg secretome, describing 188 S. mansoni egg ESPs [19]. Since then, mass spectrometry has been used in combination with one- (1-DE) and two-dimensional gel electrophoresis (2-DE), or OFFGEL electrophoresis, to further characterize S. mansoni, S. japonicum and S. haematobium egg secretomes. Efforts were made by Curwen et al. and Mathieson and Wilson to decipher the S. mansoni egg secretome as part of studies aimed at characterising the proteomes of multiple S. mansoni life cycle stages [41,42]. In both these latter studies, the number of proteins found was significantly lower than that of Cass et al.’s original description; both studies utilized a 2-DE/MS approach, but Curwen et al. identified only 32 unique proteins whereas Mathieson and Wilson described a mixture of isoforms and variants of only 4 distinct proteins. Mathieson and Wilson attributed the disparity to differences in the methods used to obtain the S. mansoni ESP, claiming that Cass’s method of incubating eggs overnight in a cold salt solution likely resulted in the egg membrane rupturing, contaminating the ESP mixture with the cytosolic contents of dead or dying eggs. However, differences in the detection method used (shotgun MS vs MS of 2D-PAGE protein spots) and the databases interrogated with MS/MS data could also, at least in part, explain the observed disparity.
The S. japonicum egg secretome of 95 proteins was recently described using a 1-DE/MS approach [20], while the S. haematobium egg secretome, investigated using OFFGEL/MS, comprises 138 proteins [21]. In contrast to most of the earlier studies, we analysed the schistosome egg ESPs directly in solution without prior separation. Our reasoning was that, with 1-DE or 2-DE/MS, very large molecular weight proteins may not enter the gel (and will be missed from the subsequent MS analysis), whereas very small proteins/peptides will be too small to be retained. Furthermore, MS analysis may also miss proteins expressed at low levels due to incomplete recovery of tryptic peptides following in-gel digestion. Accordingly, we detected 266, 90 and 50 proteins within the egg secretomes of S. mansoni, S. japonicum and S. haematobium, respectively. We consider these proteins are indeed reflective of the live egg secretome, as in vitro egg hatch assays showed that >85 % of our egg preparations were viable. Thus, we consider that the risk of ESP contamination with cytosolic proteins from dead or dying eggs was low.
Despite finding significantly more proteins in the S. mansoni egg secretome compared to Cass et al.’s original work [19], we identified only 122 proteins that shared ≥80 % sequence identity between the two datasets. These discrepancies were anticipated, and we attribute them to the dated nature of the database used in the earlier study. Similarly, despite identifying a similar number of proteins in the S. japonicum egg secretome compared to De Marco Verissimo’s study [20], we found only 23 proteins that shared ≥80 % sequence identity. This is likely due to a variety of reasons, including the method of mass spectrometry utilised and the precise settings applied to the analysis. Differences in the protein database used for peptide searching would also largely impact the final protein list generated. The large discrepancy in the number of proteins we detected in S. haematobium ESP compared with that previously described is likely due to the difference in MS methods used. Sotillo et al. [21] used OFFGEL electrophoresis to separate crude S. haematobium ESP into 24 distinct fractions which were each subjected to MS analysis. This procedure is reported to be more sensitive than traditional proteomics approaches used for the analysis of helminth secretions [43].
4.1. Immunomodulation by the schistosome egg
As schistosome egg laying begins, the interactions that occur between host and parasite change rapidly. Juvenile and adult schistosomes adapt to hide, or otherwise avoid inciting a major response from the host immune system indefinitely [2]. Schistosome eggs have no or limited capacity for such evasion but instead rely on quickly escaping from the host and hatching to facilitate transmission to the intermediate snail host. Following egg laying, the host immune response undergoes a shift from a low level Th1 inflammatory response to an aggressive Th2 response [5]. This is considered to be brought about by immunomodulatory components within the crude egg ESP acting on host immune cells. This shift prevents host death due to systemic inflammatory shock [8], and creates conditions that promote egg survival, host exit and successful disease transmission [7].
To bring about this host immune shift, components within the crude egg ESP act on host cells to alter the balance of cytokine and chemokine expression. These modulations are promoted by inducing the release of pro-fibrotic mediators, while dampening the release of proinflammatory agents from host cells. This stratagem can be most clearly seen in S. mansoni egg ESP, as these secretions have undergone the most detailed functional characterisation. IPSE/α−1, omega-1 and major egg antigen P40 are among the most significant modulatory elements deployed by S. mansoni eggs [9,44]. IPSE/α−1 is a hepatotoxic glycoprotein that inhibits neutrophil migration to the site of a granuloma and induces basophils to degranulate and release IL-4 and IL-13. IL-4 is a known mediator of the Th2 response and IL-13 is a key component of fibrosis, facilitating collagen production [10,11]. Omega-1 is another hepatotoxic glycoprotein that functions as a T2 ribonuclease (RNase). Omega-1 is bound and internalised by dendritic cells (DCs) in which it utilises its RNase activity to degrade DC ribosomal and messenger RNA (rRNA, mRNA) to influence DC function. DCs exposed to omega-1 have been shown to express reduced levels of IL-12, a chemokine involved with developing Th1 cells [12]. In contrast, P40 has been linked with anti-fibrotic and regulatory roles. P40 stimulation of murine lymphocytes induces IL-2 and IFN-γ expression, both of which are considered Th1 cytokines [18]. Taken together, these three proteins work in tandem towards driving the controlled and measured shift from a Th1 to a Th2 dominant host immune response.
Similar to S. mansoni, S. japonicum eggs secrete several ribonucleases (T2, Oy and X25) that share sequence identity with omega-1 (31 %, 52 % and 35 % respectively) and its own variant of P40 (66 % sequence identity with Sm-P40). In murine models, ribonuclease T2 has been shown to stimulate a Th2 host immune response by affecting DC maturation, inducing macrophages to produce anti-inflammatory IL-10 and upregulating serum levels of IL-4 [14], while Sj-P40 inhibits the progression of fibrosis by inhibiting HSC activation and promoting HSC apoptosis and senescence [15–17].
The ESPs of S. haematobium are not extensively characterised; however the eggs are known to secrete a variant of IPSE/α−1 [45] which shares 67 % sequence identity with the Sm counterpart, and although it is not known if it functions in a similar manner, it has been shown to be internalised by HTB-9 bladder cells, where it migrates to the nucleus, intimating a possible role in controlling host cell gene expression [45]. A significant clinical consequence of chronic infection with S. haematobium is the development of urogenital squamous cell carcinoma [6,46–48]. The role of secreted proteins released by S. haematobium eggs in carcinogenesis has not been clearly formulated. However, IPSE/α−1 is a major component of S. haematobium secretions and has been shown to induce cell proliferation and angiogenesis [21, 48], implying a role for this protein in urogenital carcinogenesis.
All of these proteins were found in our datasets, but schistosome egg ESPs contain additional immune-regulatory elements that may also be involved in assisting the driving, regulation, and maintenance of the dominant chronic Th2 host immune response.
In our S. mansoni protein dataset we identified several glutathione S-transferase (GST) enzymes. Members of the GST family found in S. mansoni have been shown to assist in priming a host Th2 phenotype in murine models. Treatment with recombinant SmGST28 induced the upregulation of the Th2 cytokines IL-4, IL-5 and IL-13, and reduced the expression of the Th1 cytokines TNF and IL-1β, thereby assisting the development of the Th2 host response [49]. We also found SmPEPCK, a protein capable of inducing proliferation of murine CD4 + T cells and priming them to release significant levels of INF-γ, IL-4 and IL-5 to promote a mixed Th1/Th2 response [50,51]. Also detected was SmVAL9, which modulates host extracellular matrix (ECM) remodelling in mice by altering the expression profile of various matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinase (TIMP) genes. This ultimately promotes the degradation of host ECM, likely with the aim of assisting egg translocation into the intestinal lumen [52]. Furthermore, we also detected peroxiredoxin (Prx1), a protein which assists in priming a Th2-based immune response by inducing alternatively activated macrophages which, in turn, upregulate the expression of the Th-2 cytokines IL-4, IL-5 and IL-13 in naïve CD4+ cells [53]. Moreover, calreticulin and serpin were found which share similarity to known immunoregulators secreted by other helminths. Calreticulin, also released by Haemonchus contortus and Necator americanus, has been shown to inhibit the complement cascade by interacting with the complement protein 1q (C1q) [24,25]. Serpin, also produced by Brugia malayi, inhibits neutrophil-associated cathepsin G and elastase [26] to facilitate immune-evasion.
In our S. japonicum protein dataset we noted the presence of SjE16.7, a potent neutrophil attractant and inducer of inflammation [54], and Sj97 (paramyosin), capable of inhibiting the complement pathway and binding to the Fc region of human immunoglobulins [55,56]. SjE16.7 likely acts in tandem with Sj-P40 to balance and regulate the developing Th2 immune response, while Sj97 both promotes resistance to complement-mediated killing in immature and adult worms by binding complement-associated proteins, and also facilitates immune-evasion by providing the worm with a protective coating of host antigens.
We detected ShE16 In the S. haematobium egg secretome. It is not known if ShE16 possesses the same capacity to attract neutrophils and induce inflammation as its S. japonicum counterpart; however, both proteins do share strong (70 %) sequence identity. Unfortunately, the function of many of the components present in S. haematobium egg ESPs have not been thoroughly characterised; thus, we are unable to elaborate on the specific utility of many of these detected proteins. The only other potentially immunoregulatory protein we identified is neuroserpin, which shares sequence identity with both S. mansoni and S. japonicum serpins (79 % and 62 % respectively).
Our data indicate that S. mansoni egg ESPs contains a broader range of immune-regulatory elements than either S. japonicum or S. haematobium. Most of these proteins are involved in inciting a host Th2 immune response as part of the Th1 to Th2 shift that follows egg laying; however, several proteins that promote mixed or Th1 phenotypes (e.g. SmPEPCK, SjE16.7) were also identified. These proteins are likely responsible for modulating the intensity of the developing Th2 response, allowing the egg a degree of variable control over the host response. The relatively few immunomodulatory proteins detectable in S. japonicum could be considered a contributing factor to the marked disease severity this species causes compared with a S. mansoni infection [1]. Generally, this is attributed to the larger number of eggs produced per day by a mature adult female S. japonicum worm, resulting in greater hepatic inflammation and fibrosis. However, the apparent lack of a diverse range of immunomodulatory proteins present in the S. japonicum ESP could indicate that the S. japonicum egg is unable to modulate the host immune system as effectively as that of an S. mansoni egg. This would result in a less efficient Th1 to Th2 shift and a more prolonged chronic inflammatory phenotype, partly resulting in more pronounced pathology.
4.2. Shared proteins between all three species
As mentioned above, given the difficulty of accurately comparing proteins across different species, BLAST was used to compare protein sequence identity. Proteins that showed ≥80 % identity were considered orthologues.
It was not expected that the egg-stage of the three schistosome species would share many pathologically relevant proteins in common. Unsurprisingly, only 12 proteins were found to be orthologous amongst all three species. Most of these proteins are associated with the release of EVs, either as structural components of the vesicle or as cargo, and included histones (H2B and H4), enolase, actin and HSP70 [37,38]. Several redox proteins (thioredoxin, redoxin peroxidases) were also found to be common. At some point during transmission, regardless of species, the schistosome egg will likely become encapsulated within a granuloma. At this time, the immune cells within the granuloma will release reactive oxygen species (ROS) to destroy the egg through oxidative stress. In response to this, schistosome eggs are known to secrete antioxidant proteins capable of scavenging ROS, thereby providing protection [57].
The high degree of commonality in the pathology induced by S. mansoni and S. japonicum eggs, and the relatively low overall number of proteins found in S. haematobium egg ESPs, is reflected in the patterns of assumed orthologues shared between the three species. S. mansoni and S. japonicum have the most proteins in common, with S. japonicum and S. haematobium the least. S. mansoni shares 18 proteins in common with S. japonicum, and 15 proteins in common with S. haematobium. Again, these proteins are primarily ubiquitous enzymes involved in energy production/metabolism and DNA/RNA synthesis (glucose-6-phosphate isomerase, nucleoside diphosphate kinase etc.) or are vesicle markers (ferritin, elongation factor 1-α, structural proteins). Conversely, S. japonicum and S. haematobium eggs share only 4 proteins in common, with 3 (histones H2A and H3, and actin) associated with vesicles.
To explore protein identity further, we examined the functional domains of some of the more extensively characterised proteins. Sm- and Sh-IPSE/α−1 share an interleukin-4 inducing immunoglobulin-binding domain (InterPro: IPR041305). Sequence alignment showed that this domain shares 69 % sequence identity between Sm- and Sh-IPSE/α−1, a level of identity similar to that shared by the full-length proteins (67 %). Omega-1 (Sm) and ribonuclease T2 (Sj) have 31 % sequence identity and are known to share similar activity [12,14]. Both proteins also contain a ribonuclease T2-like domain (InterPro: IPR001568), and alignment shows this domain also shares 31 % sequence identity between the two proteins. In contrast, Sm-P40 and Sj-P40 both contain two alpha crystallin/hsp20 domains (InterPro: IPR002068). While the full-length proteins share 66 % sequence identity, the first sHSP domain in each protein is 81 % similar and the second is 74 % similar. These findings indicate that some egg ESPs may possess functional domains that are ≥80 % similar with those released by another schistosome species, despite a lower level of sequence identity across the whole protein which, according to our criteria, prevents their classification as orthologues.
4.3. Gene ontology analysis
The most highly enriched annotations for all three secretomes were binding and catalytic activity (MF), and cellular and metabolic processes (BP). This reflects the high proportion of ubiquitous protein elements involved in energy production, DNA regulation, amino acid synthesis, detoxification and other processes present in the egg secretomes. Antioxidant activity (MF) was also enriched and, in comparison with annotations from all schistosome proteins recorded in the NCBI database, was upregulated in each of the three schistosome egg ESPs. Several redox proteins were identified in the egg secretomes which highlights how the schistosome egg releases proteins to combat oxidative stress during prolonged periods under assault by the host immune system whilst trapped within the granuloma [57]. Conversely, transporter activity (MF) and localization (BP) appeared to be downregulated in the egg stage when compared with all schisto-NCBI annotations. This agrees with our finding that approximately 40 % of proteins across all three secretomes were predicted to be secreted non-classically, i.e. via membrane budding/vesicle cargo and would therefore not be associated with transporter proteins or localization sequences.
4.4. Identification of secretory proteins
Several methods of protein secretion are employed by a typical cell, with the two main classes being classical and non-classical secretion [58]. Classical secretion involves the packaging of a protein into membrane-bound vesicles and subsequent transport to the cell surface for export. In this case, an N-terminal signal peptide sequence marks the protein for such transport. Non-classical secretion involves the protein moving directly to the cell surface without the aid of a signal peptide sequence, where it is released via budding from, or fusing with, the membrane [58,59].
SecretomeP analysis revealed that approximately 20 % of proteins within each of the three schistosome egg secretomes are secreted via the classical pathway, while around 40 % are secreted non-classically and the remaining 40 % are not predicted to be secreted at all. One might expect, as we are investigating excretory/secretory proteins specifically, that it is unusual to have such a high proportion of proteins predicted to be non-secretory. However, the previously published S. mansoni and S. japonicum egg stage secretomes [19,20] also reported very similar ratios of classically, non-classically and non-secreted proteins. The likely explanation is that the SecretomeP algorithm is trained specifically on mammalian and bacterial protein sequences, and therefore some degree of misinterpretation would be expected.
Our finding that most proteins across the ESPs of all three schistosomes are secreted via non-classical means indicates that secretion of EVs is a major mechanism for release of proteins from the eggs. Indeed, proteins commonly associated with EVs [37,38], either structurally or as cargo components, were detected within all three ESP lists, including enolase, histones and structural proteins (actin, filamin, tubulin etc.). The presence of these proteins within our data set suggests that it reflects both the soluble and EV-associated proteins present in schistosome ESPs. The release of proteins within EVs is of interest because the vesicle itself offers protection to its cargo proteins from proteolytic digestion and facilitates a simple method of targeted delivery to a recipient cell via internalisation. By contrast, classically secreted proteins are vulnerable to digestion and would therefore have a small local sphere of influence [60]. For parasite EVs this would be an appropriate method of delivering immunomodulatory proteins [61].
It is difficult to state emphatically whether the mode of secretion of the proteins is relevant to their function, but it must be considered that for a protein to function it must reach its target, and the non-classical method of secretion within EVs may provide the best chance of this occurring. The distance between source and target must also be considered; classically secreted proteins likely have a more significant effect within the immediate local environment of their release whereas proteins within EVs are more likely to engage with targets at a systemic level.
5. Conclusions
The commencement of egg laying during a schistosome infection marks a period of abrupt change to the host-parasite dynamic. Immature and adult worms need to evade the host immune system to ensure their survival, while the egg demonstrates an ability to manipulate it in a manner that promotes host/egg survival and increases the chance of successful egg transmission. This is brought about by immunomodulatory proteins secreted by the egg. Our findings indicate that, despite the similarities in host and pathogenicity, the egg secretomes of S. mansoni, S. japonicum and S. haematobium differ significantly and may be linked to parasite niche and the severity of host pathology. Relatively few proteins displayed sufficient similarity to be considered orthologues by our criteria, and many that did reflect ubiquitous protein elements with no clear role in host-parasite interaction. We confirmed the presence of several well characterised immunomodulatory proteins, but the function of many proteins within the three secretomes described here remains undefined. Further characterisation of these proteins is necessary to identify potential mediators of host-parasite interactions as they may provide new target candidates for vaccines against schistosomiasis.
Supplementary Material
Acknowledgements
The technical support of Mary Duke and the proteomics facility at QIMR Berghofer and the Biomedical Research Institute Schistosomiasis Research Centre are gratefully acknowledged.
Funding
This work was supported by these funders. MWR was supported by a grant (BB/L019612/1) from the Biotechnology and Biological Sciences Research Council (BBSRC). JPC was supported by a scholarship from the Department of Employment and Learning (DEL) Northern Ireland. DPM acknowledges the financial support from the National Health and Medical Research Council (NHMRC) of Australia (Grant numbers: ID613671; APP1037304; APP1098244).
Footnotes
Declaration of Competing Interest
The authors have no competing interests to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.molbiopara.2020.111322.
References
- [1].Lewis F, Karunaratne LB, Lewis FA, Freitas TC, Liang Y, Schistosomiasis, Curr. Protoc. Immunol (2001). Unit 19.1. Chapter 19. [DOI] [PubMed]
- [2].Colley DG, Bustinduy AL, Secor WE, King CH, Human schistosomiasis, Lancet (Lond. Engl.) 383 (2014) 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheever EA, Macedonia JG, Mosimann JE, Cheever AW, Kinetics of Egg Production and Egg Excretion by Schistosoma mansoni and S. japonicum in Mice Infected with a Single Pair of Worms, Am. J. Trop. Med. Hyg 50 (1994) 281–295. [DOI] [PubMed] [Google Scholar]
- [4].Deelder AM, Krijger FW, Kremsner PG, Van Etten L, Day-to-Day variation of egg output and schistosome circulating antigens in urine of Schistosoma haematobium-Infected school children from Gabon and follow-up after chemotherapy, Am. J. Trop. Med. Hyg 57 (1997) 337–341. [DOI] [PubMed] [Google Scholar]
- [5].Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA, Immunopathology of schistosomiasis, Immunol. Cell Biol 85 (2007) 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barsoum RS, Urinary schistosomiasis: review, J. Adv. Res 4 (2013) 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwartz C, Fallon PG, Schistosoma “Eggs-Iting” the host: granuloma formation and egg excretion, Front. Immunol 9 (2018) 2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rutitzky LI, Hernandez HJ, Stadecker MJ, Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 13243–13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dunne DW, Jones FM, Doenhoff MJ, The purification, characterization, serological activity and hepatotoxic properties of two cationic glycoproteins (alpha 1 and omega 1) from Schistosoma mansoni eggs, Parasitology 103 (Pt 2) (1991) 225–236. [DOI] [PubMed] [Google Scholar]
- [10].Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie ANJ, Alcami A, Fallon PG, Schistosoma mansoni secretes a chemokine binding protein with anti-inflammatory activity, J. Exp. Med 202 (2005) 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J, Fuller H, Stanley RG, Chiodini PL, Haas H, Doenhoff MJ, IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs, Mol. Biochem. Parasitol 147 (2006) 9–19. [DOI] [PubMed] [Google Scholar]
- [12].Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G, Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses, J. Exp. Med 206 (2009) 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Peng W, Feng J, Zhu D, Chen J, Sun X, Lyu L, Ju S, Duan Y, Recombinant T2 RNase protein of Schistosoma japonicum inhibits expression of a-SMA in LX-2 cells, Parasitol. Res 115 (2016) 4055–4060. [DOI] [PubMed] [Google Scholar]
- [14].Ke X-D, Shen S, Song L-J, Yu C-X, Kikuchi M, Hirayama K, Gao H, Wang J, Yin X, Yao Y, Liu Q, Zhou W, Characterization of Schistosoma japonicum CP1412 protein as a novel member of the ribonuclease T2 molecule family with immune regulatory function, Parasit. Vectors 10 (2017) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun X, Zhang L, Wang J, Chen J, Zhu D, Shen P, He X, Pan J, Peng W, Duan Y, Schistosoma japonicum protein SjP40 inhibits TGF-β1-induced activation of hepatic stellate cells, Parasitol. Res 114 (2015) 4251–4257. [DOI] [PubMed] [Google Scholar]
- [16].Chen J, Xu T, Zhu D, Wang J, Huang C, Lyu L, Hu B, Sun W, Duan Y, Egg antigen p40 of Schistosoma japonicum promotes senescence in activated hepatic stellate cells by activation of the STAT3/p53/p21 pathway, Cell Death Dis 7 (2016) e2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen L, Zhou Q, Liu E, Zhang J, Duan L, Zhu D, Chen J, Duan Y, rSjp40 inhibits activated hepatic stellate cells by promoting nuclear translocation of YB1 and inducing BMP-7/Smad1/5/8 pathway, Parasit. Vectors 12 (2019) 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cai Y, Langley JG, Smith DI, Boros DL, A cloned major Schistosoma mansoni egg antigen with homologies to small heat shock proteins elicits Th1 responsiveness, Infect. Immun 64 (1996) 1750–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, Stadecker MJ, Yates JR, Williams DL, Proteomic analysis of Schistosoma mansoni egg secretions, Mol. Biochem. Parasitol 155 (2007) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Marco Verissimo C, Potriquet J, You H, McManus DP, Mulvenna J, Jones MK, Qualitative and quantitative proteomic analyses of Schistosoma japonicum eggs and egg-derived secretory-excretory proteins, Parasit. Vectors 12 (2019) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sotillo J, Pearson MS, Becker L, Mekonnen GG, Amoahid AS, van Dam G, Corstjens PLAM, Murray J, Mduluza T, Mutapi F, Loukas A, In-depth proteomic characterization of schistosoma haematobium: towards the development of new tools for elimination, PLoS Negl. Trop. Dis 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M, Blast2GO: A universal annotation and visualization tool in functional genomics research. Application note, Bioinformatics 21 (2005) 3674–3676. [DOI] [PubMed] [Google Scholar]
- [23].Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S, Feature-based prediction of non-classical and leaderless protein secretion, Protein Eng. Des. Sel 17 (2004) 349–356. [DOI] [PubMed] [Google Scholar]
- [24].Naresha S, Suryawanshi A, Agarwal M, Singh BP, Joshi P, Mapping the complement C1q binding site in Haemonchus contortus calreticulin, Mol. Biochem. Parasitol 166 (2009) 42–46. [DOI] [PubMed] [Google Scholar]
- [25].Kasper G, Brown A, Eberl M, Vallar L, Kieffer N, Berry C, Girdwood K, Eggleton P, Quinnell R, Pritchard DI, A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins, Parasite Immunol 23 (2001) 141–152. [DOI] [PubMed] [Google Scholar]
- [26].Zang X, Yazdanbakhsh M, Jiang H, Kanost MR, Maizels RM, A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases, Blood 94 (1999) 1418–1428. [PubMed] [Google Scholar]
- [27].Aly I, ELnain G, Hamad RS, Kilany M, Ghramh HA, Alshehri A, Dajem SM, Ibrahim EH, DNA vaccination using recombinant Schistosoma mansoni fatty acid binding protein (smFABP) gene, Exp. Parasitol 194 (2018) 53–59. [DOI] [PubMed] [Google Scholar]
- [28].Al-Sherbiny M, Osman A, Barakat R, El Morshedy H, Bergquist R, Olds R, In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens, Acta Trop 88 (2003) 117–130. [DOI] [PubMed] [Google Scholar]
- [29].Mair GR, Niciu MJ, Stewart MT, Brennan G, Omar H, Halton DW, Mains R, Eipper BA, Maule AG, Day TA, A functionally atypical amidating enzyme from the human parasite Schistosoma mansoni, FASEB J 18 (2004) 114–121. [DOI] [PubMed] [Google Scholar]
- [30].Schechtman D, Tarrab-Hazdai R, Arnon R, The 14–3-3 protein as a vaccine candidate against schistosomiasis, Parasite Immunol 23 (2001) 213–217. [DOI] [PubMed] [Google Scholar]
- [31].Rezende CMF, Coitinho JB, Costa M, Silva MR, Giusta M, Oliveira-Prado R, Corrêa-Oliveira R, Nagem R, Goes AM, Biochemical analysis and identification of linear B-cell epitopes from recombinant Sm21.7 antigen from Schistosoma mansoni, Mol. Immunol 101 (2018) 29–37. [DOI] [PubMed] [Google Scholar]
- [32].Francis P, Bickle Q, Cloning of a 21.7-kDa vaccine-dominant antigen gene of Schistosoma mansoni reveals an EF hand-like motif, Mol. Biochem. Parasitol 50 (1992) 215–224. [DOI] [PubMed] [Google Scholar]
- [33].Reynolds SR, Dahl CE, Harn DA, T and B epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase, J. Immunol 152 (1994) 193–200. [PubMed] [Google Scholar]
- [34].Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, Loukas A, Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates, Int. J. Parasitol 46 (2016) 1–5. [DOI] [PubMed] [Google Scholar]
- [35].Samoil V, Dagenais M, Ganapathy V, Aldridge J, Glebov A, Jardim A, Ribeiro P, Vesicle-based secretion in schistosomes: analysis of protein and microRNA (miRNA) content of exosome-like vesicles derived from Schistosoma mansoni, Sci. Rep 8 (2018) 3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Demarta-Gatsi C, Rivkin A, Di Bartolo V, Peronet R, Ding S, Commere PH, Guillonneau F, Bellalou J, Brûé S, Abou Karam P, Cohen SR, Lagache T, Janse CJ, Regev-Rudzki N, Mécheri S, Histamine releasing factor and elongation factor 1 alpha secreted via malaria parasites extracellular vesicles promote immune evasion by inhibiting specific T cell responses, Cell. Microbiol 21 (2019) e13021. [DOI] [PubMed] [Google Scholar]
- [37].Wu Z, Wang L, Li J, Wang L, Wu Z, Sun X, Extracellular vesicle-mediated communication within host-parasite interactions, Front. Immunol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marcilla A, Trelis M, Cortés A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sánchez del Pino MM, Muñz-Antoli C, Toledo R, Bernal D, Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells, PLoS One 7 (2012) e45974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Floudas A, Cluxton CD, Fahel J, Khan AR, Saunders SP, Amu S, Alcami A, Fallon PG, Composition of the Schistosoma mansoni worm secretome: identification of immune modulatory cyclophilin A, PLoS Negl. Trop. Dis 11 (2017) e0006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu F, Cui S-J, Hu W, Feng Z, Wang Z-Q, Han Z-G, Excretory/secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum, Mol. Cell Proteom 8 (2009) 1236–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Curwen RS, Ashton PD, Johnston DA, Wilson RA, The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages, Mol. Biochem. Parasitol 138 (2004) 57–66. [DOI] [PubMed] [Google Scholar]
- [42].Mathieson W, Wilson RA, A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: the miracidium, hatch fluid and secretions, Int. J. Parasitol 40 (2010) 617–628. [DOI] [PubMed] [Google Scholar]
- [43].Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ, Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum, Mol. Cell Proteom 8 (2009) 109–121. [DOI] [PubMed] [Google Scholar]
- [44].Stadecker MJ, Hernandez HJ, The immune response and immunopathology in infection with Schistosoma mansoni: a key role of major egg antigen Sm-p40, Parasite Immunol 20 (1998) 217–221. [DOI] [PubMed] [Google Scholar]
- [45].Pennington LF, Alouffi A, Mbanefo EC, Ray D, Heery DM, Jardetzky TS, Hsieh MH, Falcone FH, H-IPSE is a pathogen secreted host nucleus-infiltrating protein (infiltrin) expressed exclusively by the Schistosoma haematobium egg stage, Infect. Immun 85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].IARC working group on the evaluation of carcinogenic risks to humans, biological agents. Volume 100 B. A review of human carcinogens, IARC Monogr. Eval. Carcinog. Risks Hum 100 (2012) 1–441. [PMC free article] [PubMed] [Google Scholar]
- [47].Brindley PJ, Loukas A, Helminth infection–induced malignancy, PLoS Pathog 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ishida K, Hsieh MH, Understanding urogenital schistosomiasis-related bladder cancer: an update, Front. Med 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Driss V, El Nady M, Delbeke M, Rousseaux C, Dubuquoy C, Sarazin A, Gatault S, Dendooven A, Riveau G, Colombel JF, Desreumaux P, Dubuquoy L, Capron M, The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils, Mucosal Immunol 9 (2016) 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Asahi H, Osman A, Cook RM, LoVerde PT, Stadecker MJ, Schistosoma mansoni phosphoenolpyruvate carboxykinase, a novel egg antigen: immunological properties of the recombinant protein and identification of a T-cell epitope, Infect. Immun 68 (2000) 3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Asahi H, Hernandez HJ, Stadecker MJ, A novel 62-kilodalton egg antigen from Schistosoma mansoni induces a potent CD4+ T helper cell response in the C57BL/6 mouse, Infect. Immun 67 (1999) 1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yoshino TP, Brown M, Wu X-J, Jackson CJ, Ocadiz-Ruiz R, Chalmers IW, Kolb M, Hokke CH, Hoffmann KF, Excreted/secreted Schistosoma mansoni venom allergen-like 9 (SmVAL9) modulates host extracellular matrix remodelling gene expression, Int. J. Parasitol 44 (2014) 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP, Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages, FASEB J 22 (2008) 4022–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu C, Chen Q, Fang Y, Wu J, Han Y, Wang Y, Yang Y, Chu M, Feng Y, Tan L, Guo X, Hu W, Wang Z, Schistosoma japonicum egg specific protein SjE16.7 recruits neutrophils and induces inflammatory hepatic granuloma initiation, PLoS Negl. Trop. Dis 8 (2014) e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deng J, Gold D, LoVerde PT, Fishelson Z, Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin, Infect. Immun 71 (2003) 6402–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Loukas A, Jones MK, King LT, Brindley PJ, McManus DP, Receptor for Fc on the surfaces of schistosomes, Infect. Immun 69 (2001) 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Alger HM, Sayed AA, Stadecker MJ, Williams DL, Molecular and enzymatic characterisation of Schistosoma mansoni thioredoxin, Int. J. Parasitol 32 (2002) 1285–1292. [DOI] [PubMed] [Google Scholar]
- [58].Kim J, Gee HY, Lee MG, Unconventional protein secretion – new insights into the pathogenesis and therapeutic targets of human diseases, J. Cell. Sci 131 (2018) jcs213686. [DOI] [PubMed] [Google Scholar]
- [59].lie Ståhl A, Johansson K, Mossberg M, Kahn R, Karpman D, Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases, Pediatr. Nephrol 34 (2019) 11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Akuma P, Okagu OD, Udenigwe CC, Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds, Front. Sustain. Food Syst 3 (2019) 23. [Google Scholar]
- [61].de la Torre-Escudero E, Gerlach JQ, Bennett APS, Cwiklinski K, Jewhurst HL, Huson KM, Joshi L, Kilcoyne M, O’Neill S, Dalton JP, Robinson MW, Surface molecules of extracellular vesicles secreted by the helminth pathogen Fasciola hepatica direct their internalisation by host cells, PLoS Negl. Trop. Dis 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


