Abstract
Objective:
To compare outcomes after bariatric surgery between Medicaid and non-Medicaid patients and assess whether differences in social determinants of health were associated with postoperative weight loss.
Background:
The literature remains mixed on weight loss outcomes and healthcare utilization for Medicaid patients after bariatric surgery. It is unclear if social determinants of health geocoded at the neighborhood level are associated with outcomes.
Methods:
Patients who underwent laparoscopic sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) from 2008 to 2017 and had ≥1 year of follow-up within a large health system were included. Baseline characteristics, 90-day and 1-year outcomes, and weight loss were compared between Medicaid and non-Medicaid patients. Area deprivation index (ADI), urbanicity, and walkability were analyzed at the neighborhood level. Median regression with percent total body weight (TBW) loss as the outcome was used to assess predictors of weight loss after surgery.
Results:
Six hundred forty-seven patients met study criteria (191 Medicaid and 456 non-Medicaid). Medicaid patients had a higher 90-day readmission rate compared to non-Medicaid patients (19.9% vs 12.3%, P < 0.016). Weight loss was similar between Medicaid and non-Medicaid patients (23.1% vs 21.9% TBW loss, respectively; P = 0.266) at a median follow-up of 3.1 years. In adjusted analyses, Medicaid status, ADI, urbanicity, and walkability were not associated with weight loss outcomes.
Conclusions:
Medicaid status and social determinants of health at the neighborhood level were not associated with weight loss outcomes after bariatric surgery. These findings suggest that if Medicaid patients are appropriately selected for bariatric surgery, they can achieve equivalent outcomes as non-Medicaid patients.
Keywords: Bariatric surgery, Medicaid, Obesity, Outcomes, Social determinants of health, Weight loss
Mini-abstract: The relationship among Medicaid status, social determinants of health, and bariatric surgery outcomes is unclear. Using electronic health record data and social determinant variables geocoded at the neighborhood level, we found that Medicaid status, socioeconomic deprivation, household location (rural vs urban), and neighborhood walkability were not associated with weight loss outcomes after bariatric surgery. However, Medicaid patients had higher readmission rates within 90 days of surgery.
Supplemental Digital Content is available in the text.
INTRODUCTION
Severe obesity (body mass index [BMI] ≥ 35.0 kg/m2) is a major public health threat. The current prevalence of severe obesity in the United States is currently approximately 15%1 and is projected to increase to nearly 25% within the next decade.2 The treatment of severe obesity includes both nonsurgical and surgical modalities. Medical weight management strategies, such as dietary modification, physical activity, obesity medications, and behavioral strategies are modestly effective over long-term periods, but weight regain is common.3–7 Numerous randomized controlled trials and observational studies have found that, compared to medical weight management strategies, bariatric surgery results in greater weight loss and comorbidity resolution, lengthened lifespan, and improved quality of life.8–11 In the United States, the Medicaid system pays for a significant portion of obesity care. Each year, state Medicaid programs spend nearly $8 billion on obesity care, including costs for 20,000 patients who undergo bariatric surgery.12,13
The literature remains mixed on the impact of Medicaid status on weight loss after bariatric surgery,14–16 but studies have reported that Medicaid patients who undergo bariatric surgery have higher rates of postoperative emergency department (ED) visits and readmissions.17,18 Social determinants of health, including poverty, low median household income, low education, and unemployment, have been identified as predictors of worse outcomes in vascular, surgical oncology, and general surgery patients.19,20 A 2016 time-series analysis reported that more “walkable” neighborhoods were associated with a lower prevalence of obesity.21 It is unknown if neighborhood characteristics, such as neighborhood socioeconomic deprivation and walkability, are associated with outcome differences in patients undergoing bariatric surgery.
The objectives of this study were to compare outcomes after bariatric surgery for Medicaid and non-Medicaid patients and to assess whether differences in neighborhood social determinants of health were associated with weight loss after surgery. The 3 social determinant variables analyzed were area deprivation index (ADI), urbanicity, and neighborhood walkability. We hypothesized that social determinants, rather than Medicaid status, would be associated with differences in weight loss outcomes.
METHODS
Data Sources
We used the University of Wisconsin Hospital and Clinics (UW) institutional bariatric surgery registry (BSR) to identify our study cohort. The BSR includes all patients who undergo bariatric surgery at UW and contains information on baseline patient demographics, comorbidities and postoperative resolution, and surgical outcomes. A trained data extractor not involved in patient care reviewed all inpatient and outpatient visits, diagnostic studies, and lab values recorded in the UW electronic health record (EHR) to populate the BSR. Additional height and weight data beyond the 1-year follow-up reported in the BSR were extracted from the EHR.
Three geocoded databases were used to analyze social determinant variables at the neighborhood level: (1) ADI for neighborhood socioeconomic deprivation22,23; (2) rural-urban commuting area (RUCA) database for neighborhood urbanicity24; and (3) walkability index for neighborhood walkability.25
We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines within the Enhancing the QUAlity and Transparency Of health Research network in the methodology and reporting of this study.26
Study Population
All patients 18 years of age or older who underwent a primary laparoscopic sleeve gastrectomy (SG) or laparoscopic Roux-en-Y gastric bypass (RYGB) between 2008 and 2017 were included. This study period was chosen to ensure that all patients had at least one year of postoperative follow-up at the time the analysis was performed.
Patient Characteristics
Age (at the time of surgery), sex (male or female), race/ethnicity (white non-Hispanic or non-white [Black, Asian, Native American, Hispanic, and other/unspecified]), preoperative BMI (BMI on the day of surgery), and preoperative diagnosis of coronary artery disease, gastroesophageal reflux disease, hyperlipidemia, hypertension, obstructive sleep apnea, and type 2 diabetes mellitus (T2DM) were identified from the BSR. Smoking status at the time of surgery was identified from the BSR and categorized as: never smoker, quit <6 months or ≥6 months before surgery, and current smoker. The criteria for these patient characteristics and comorbidities were extracted from clinic notes, imaging studies, and laboratory values, which we have previously described.14,15 Preoperative diagnosis of anxiety or depression were identified using ICD-9 or -10 codes in the EHR at any time before surgery (see Supplemental Digital Content 1 http://links.lww.com/AOSO/A12, which contains the full list of ICD-9 and -10 codes for anxiety and depression).
Insurance type was obtained from the BSR and was defined as the insurance held by the patient at the time of surgery for commercial and Medicare patients or within 3 years of surgery for Medicaid patients (given that low income is a Medicaid eligibility criterion and patients with low income have low socioeconomic mobility27,28).
Neighborhood Characteristics
Patient household location (latitude/longitude) was extracted from the EHR and used to analyze 3 geocoded social determinant variables at the census block group (eg, neighborhood) level: (1) ADI is a validated measure of neighborhood socioeconomic deprivation composed of 17 indicators from 2015 US Census data such as unemployment rate, household income, and education level.22,23 ADI is a continuous variable from 1 to 100, with 1 representing the least deprived neighborhood and 100 representing the most deprived neighborhood. (2) RUCA is a measure of urbanization using work-commuting data from the 2000 US Census.24 RUCA codes of 1–3 indicate urban or metropolitan neighborhoods; 4–6 indicate suburban or micropolitan neighborhoods; and 7–10 indicate rural neighborhoods. (3) Walkability index is a measure of how walkable a census block group is based on 2010 US Census data. It is a composite measure that includes intersection density, proximity to transit stops, and land use.25 Walkability is a continuous variable, with 1 being the least walkable and 20 being the most walkable.
90-Day and One-Year Postoperative Outcomes
We applied previously described definitions of 90-day and one-year postoperative outcomes from the BSR.14,15 Postoperative outcomes within 90 days of surgery included all bariatric surgery-related ED visits, readmissions, reoperations, endoscopic dilations, anastomotic/staple line leaks, anastomotic stricture/sleeve stenosis, and marginal ulcers. Wound complications included any wound infections or intra-abdominal abscesses. Hemorrhage was defined as any bleeding event requiring a blood transfusion. A composite “other complication” variable included acute renal failure, cerebral vascular accident, deep vein thrombosis, myocardial ischemia, pneumonia, pulmonary embolism, and urinary tract infection.
Postoperative outcomes within 1 year of surgery included RYGB anastomotic strictures, sleeve stenosis, revisional operations, and resolution of comorbidities (gastroesophageal reflux disease, hyperlipidemia, hypertension, obstructive sleep apnea, and T2DM). A comorbidity was considered resolved if patients with a preoperative diagnosis did not have the diagnosis 1 year after surgery, as determined by inpatient and outpatient notes, the absence of the diagnosis on active problem lists, and/or the absence of prescribed medication for the comorbidity.
Postoperative Weight Outcomes
Postoperative BMI, absolute BMI change, and percent total body weight (TBW) loss were identified through the EHR and calculated based on the BMI at each patient’s most recent clinical encounter. Length of follow-up was defined as the time between surgery and the most recent clinical encounter within the EHR. Using a modified version of an algorithm proposed by Cheng et al29 and used by our group previously,30 height and weight data from the EHR were “cleaned” to minimize inclusion of incorrect heights and weights due to data entry errors. All weights below 55 pounds or above 1,000 pounds were removed. Clinical encounters with missing weight data were excluded. For encounters with missing height data, height was imputed from the most recent height available in the BSR. BMIs were calculated using imputed or available height and weight data. Biologically implausible BMIs (BMIs <7.5 kg/m2 or >108.8 kg/m2) were excluded.29,30 No patients were excluded from the study cohort during data imputation and cleaning.
Statistical Analysis
Baseline characteristics and outcomes were compared between Medicaid and non-Medicaid patients using 2-sample t tests for continuous variables and χ2-test for categorical variables.
We used median regression with 3 different models to evaluate the effect of Medicaid status and other variables on %TBW loss. Median regression was selected, rather than linear regression, to mitigate the influence of outliers. Model 1 included Medicaid status as the only independent variable, with % TBW loss as the outcome. Model 2 included Medicaid status and patient characteristics (age, sex, race/ethnicity, preoperative diagnosis of T2DM, surgery type, and follow-up time). These predictors were included a priori based on hypotheses that they may be associated with postoperative weight loss. Model 3 included Medicaid status, patient characteristics, and the 3 social determinant variables (ADI, urbanicity, and walkability). We also performed median regression analysis with %TBW loss as the outcome for the Medicaid and non-Medicaid cohorts, adjusting for age, sex, race/ethnicity, surgery type, follow-up time, preoperative diagnosis with T2DM, ADI, urbanicity, and walkability.
In a subgroup analyses, we compared postoperative outcomes between Medicaid and non-Medicaid patients who had follow-up intervals of 3 years or more using 2-sample t tests for continuous variables and χ2-test for categorical variables. Median regression analysis with %TBW loss as the outcome using Model 1, Model 2, and Model 3 was performed with patients who had 3 years of follow-up or more.
R version 3.6.3 software was used for the statistical analysis. This study was approved by the University of Wisconsin Minimal Risk Institutional Review Board (#2017-0443), and the need for informed consent was waived.
RESULTS
Patient Characteristics
We identified 798 patients who underwent a primary laparoscopic bariatric procedure during the study period. After applying exclusion criteria, 647 patients were included in the final study population (Fig. 1). Of these patients, 456 (70.5%) were non-Medicaid and 191 (29.5%) were Medicaid. Within the Medicaid cohort, 155 patients (81.2%) had Medicaid at the time of surgery; 30 (15.7%) and 6 (3.1%) patients had Medicare and commercial insurance, respectively.
FIGURE 1.
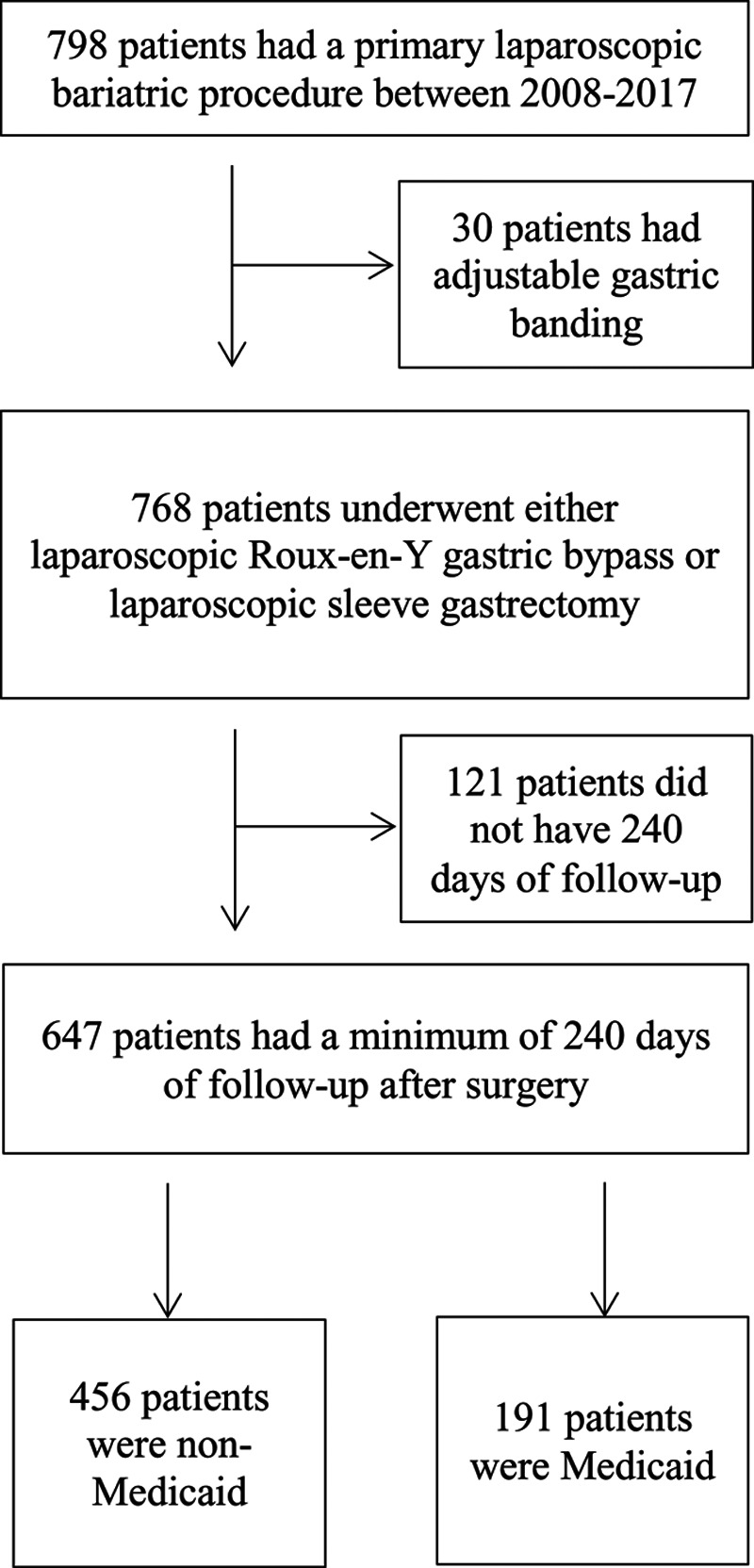
Study cohort creation: STROBE diagram.
Medicaid patients were younger than non-Medicaid patients (mean age 44.8 vs 50.8 years, P < 0.001) and were more likely to be non-white (19.9% non-white vs 7.9%) (Table 1). Medicaid patients had higher preoperative BMIs (48.3 vs 45.9 kg/m2, P < 0.001), a higher prevalence of T2DM (57.6% vs 41.7%, P < 0.001), and a higher prevalence of smoking history (54.5% vs 45.9%, P = 0.006) compared to non-Medicaid patients. There were similar distributions of RYGB and SG between Medicaid and non-Medicaid patients (66.5% of Medicaid vs 65.1% of non-Medicaid patients underwent RYGB; 33.5% of Medicaid patients vs 34.9% of non-Medicaid patients underwent SG; P = 0.809).
Table 1.
Characteristics of Medicaid and Non-Medicaid Patients
| Non-Medicaid (n = 456) | Medicaid (n = 191) | P | |
|---|---|---|---|
| Age (y), mean (SD) | 50.8 (12.2) | 44.8 (10.9) | <0.001 |
| Sex (n, %) | |||
| Male | 110 (24.1) | 37 (19.4) | 0.225 |
| Female | 346 (75.9) | 154 (80.6) | |
| Race/ethnicity (n, %) | |||
| White, non-Hispanic | 420 (92.1) | 153 (80.1) | <0.001 |
| Other (non-White) | 36 (7.9) | 38 (19.9) | |
| Preoperative BMI (kg/m2), mean (SD) | 45.9 (7.8) | 48.3 (8.3) | 0.001 |
| Preoperative comorbidities (n, %) | |||
| Anxiety | 68 (14.9) | 41 (21.5) | 0.055 |
| Coronary artery disease | 47 (10.3) | 16 (8.4) | 0.542 |
| Depression | 119 (26.1) | 59 (30.9) | 0.251 |
| Gastroesophageal reflux disease | 241 (52.9) | 116 (60.7) | 0.08 |
| Hyperlipidemia | 218 (47.8) | 90 (47.1) | 0.942 |
| Hypertension | 318 (69.7) | 135 (70.7) | 0.885 |
| Obstructive sleep apnea | 324 (71.1) | 135 (70.7) | 1.000 |
| Type 2 diabetes mellitus | 190 (41.7) | 110 (57.6) | <0.001 |
| Preoperative smoking status (n, %) | |||
| Never smoked | 247 (54.2) | 87 (45.5) | 0.006 |
| Quit < 6 mo before surgery | 9 (2.0) | 12 (6.3) | |
| Quit ≥ 6 mo before surgery | 200 (43.9) | 92 (48.2) | |
| Current smoker | 0 (0.0) | 0 (0.0) | |
| Surgery type (n, %) | |||
| Sleeve gastrectomy | 159 (34.9) | 64 (33.5) | 0.809 |
| Roux-en-Y gastric bypass | 297 (65.1) | 127 (66.5) | |
| Insurance type (n, %) | |||
| Commercial | 314 (68.9) | 0 (0.0) | N/A |
| Medicare | 142 (31.1) | 0 (0.0) | |
| Area deprivation index mean (SD) | 43.4 (19.7) | 53.0 (19.7) | <0.001 |
| Urbanicity (RUCA) (n, %) | |||
| Metropolitan | 333 (73.0) | 118 (61.8) | 0.033 |
| Micropolitan | 50 (11.0) | 29 (15.2) | |
| Rural | 72 (15.8) | 44 (23.0) | |
| Walkability (walkability index), mean (SD) | 8.1 (3.7) | 8.1 (3.9) | 0.93 |
NA indicates not applicable.
Medicaid patients were more likely to reside in socioeconomically deprived neighborhoods (ADI 53.0 vs 43.4, P < 0.001) and in rural areas (23.0% vs 15.8%, P = 0.033) (Table 1). There were no differences in neighborhood walkability between Medicaid and non-Medicaid patients (8.1 vs 8.1, P = 0.93).
90-Day and One-Year Postoperative Outcomes
Within 90 days of surgery, Medicaid patients had a higher readmission rate (19.9% vs 12.3%, P < 0.016) compared to non-Medicaid patients. Medicaid patients also visited the ED more frequently than non-Medicaid patients, although the difference was not statistically significant (30.4% vs 22.6%, P = 0.66) (Table 2). We found no differences in the rates of anastomotic/staple line leaks or other complications between Medicaid and non-Medicaid patients.
Table 2.
Postoperative Outcomes
| Non-Medicaid | Medicaid | P | |
|---|---|---|---|
| 90-d outcomes (n, %) | |||
| Emergency department visits | 103 (22.6) | 58 (30.4) | 0.066 |
| Readmissions | 56 (12.3) | 38 (19.9) | 0.021 |
| Reoperations | 14 (3.1) | 4 (2.1) | 0.416 |
| Dilations | 34 (7.5) | 16 (8.4) | 0.907 |
| Anastomotic/staple line leak | 5 (1.1) | 1 (0.5) | 0.416 |
| Hemorrhage | 7 (1.5) | 4 (2.1) | 0.471 |
| Marginal ulcer | 8 (1.8) | 5 (2.6) | 0.761 |
| Wound complications | 14 (3.1) | 9 (4.7) | 0.318 |
| Other | 27 (5.9) | 13 (6.8) | 0.322 |
| 1-y comorbidity resolution (n, %) | |||
| Gastroesophageal reflux disease | 93 (42.1) | 43 (39.5) | 0.722 |
| Hyperlipidemia | 58 (29.4) | 22 (27.2) | 0.771 |
| Hypertension | 100 (34.7) | 45 (39.2) | 0.652 |
| Obstructive sleep apnea | 105 (35.7) | 50 (41.7) | 0.265 |
| Type 2 diabetes mellitus | 107 (59.4) | 46 (46.9) | 0.058 |
| 1-y outcomes (n, %) | |||
| Revisions | 14 (3.1) | 5 (2.6) | 0.952 |
| Anastomotic strictures | 18 (6.1) | 10 (7.9) | 0.612 |
| Sleeve stenosis | 2 (1.3) | 3 (4.7) | 0.272 |
At 1-year postoperatively, we found no differences in comorbidity resolution or revisional surgery rates between Medicaid and non-Medicaid patients (Table 2). There were no differences in the rates of operative revisions, anastomotic strictures, or sleeve stenosis between Medicaid and non-Medicaid patients.
Postoperative Follow-Up
Medicaid and non-Medicaid patients had median follow-ups of 3.6 and 3.0 years, respectively. For the Medicaid cohort, 5.2%, 36.7%, and 58.1% had 1, 2 to 3, or >3 years of follow-up data, respectively. For the non-Medicaid cohort, 8.3%, 41.5%, and 50.2% had 1, 2 to 3, or >3 years of follow-up data, respectively.
Postoperative Weight Outcomes
There were no differences in postoperative %TBW loss between Medicaid and non-Medicaid patients (23.1% vs 21.9%, P = 0.266) (Table 3) at median follow-ups of 3.6 and 3.0 years, respectively. Medicaid and non-Medicaid patients also had similar absolute BMI changes (11.1 vs 10.2 kg/m2, P = 0.088).
Table 3.
Weight Loss After Surgery
| Non-Medicaid (n = 456) | Medicaid (n = 191) | P | |
|---|---|---|---|
| Length of follow-up (y), median (IQR) | 3.0 (3.3) | 3.6 (3.7) | 0.033 |
| Postoperative BMI at most follow-up (kg/m2), mean (SD) | 35.7 (8.1) | 37.1 (9.4) | 0.053 |
| Absolute BMI change, mean (SD) | 10.2 (6.5) | 11.1 (6.7) | 0.088 |
| % total body weight loss at most recent follow-up, mean (SD) | 21.9 (12.9) | 23.1 (13.0) | 0.266 |
Predictors of Weight Loss After Bariatric Surgery
In median regression with %TBW loss as the outcome and Medicaid status as the only independent variable, Medicaid patients had a median TBW loss that was 2.0% (95% CI, −0.1 to 4.2) more than non-Medicaid patients, but this was not statistically significant (Table 4). In the fully adjusted model (Model 3), patients who underwent SG lost 10.5% (95% CI, 8.1 to 12.9) TBW less than those who underwent RYGB (Table 4). Patients with preoperative T2DM lost 2.3% (95% CI, 0.5 to 4.4) TBW less than those without T2DM. Patients with greater neighborhood socioeconomic deprivation had slightly more weight loss after surgery; for each 10 unit increase in ADI, patients lost 0.6% (95% CI, 0.2 to 1.1) TBW more after surgery. Urbanicity and walkability were not associated with weight loss after surgery.
Table 4.
Median Regression With %TBW Loss as the Outcome
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Variable | Median % TBW Loss (95% CI) | Median % TBW Loss (95% CI) | Median % TBW Loss (95% CI) |
| Medicaid status | |||
| Non-Medicaid | Ref. | Ref. | Ref. |
| Medicaid | 2.04 (−0.10, 4.21) | 1.59 (−0.19, 2.98) | 0.97 (−0.70, 2.63) |
| Age (+5 y) | — | −0.24 (−0.57, 0.17) | −0.28 (−0.68, 0.25) |
| Sex | |||
| Female | — | Ref. | Ref. |
| Male | — | 0.05 (−1.57, 1.61) | −0.49 (−2.27, 1.47) |
| Race/ethnicity | |||
| Non-White | — | Ref. | Ref. |
| White, non-Hispanic | — | 0.62 (−2.79, 3.01) | 1.08 (−2.54, 4.01) |
| Surgery type | |||
| Roux-en-Y gastric bypass | — | Ref. | Ref. |
| Sleeve gastrectomy | — | −10.80 (−12.76, −8.56) | −10.51 (−12.92, −8.06) |
| Follow-up time (+1 y) | — | −1.02 (−1.35, −0.52) | −1.03 (−1.37, −0.40) |
| Preoperative diagnosis of type 2 diabetes | — | −2.34 (−4.24, −0.78) | −2.34 (−4.44, −0.49) |
| Area deprivation index (+10 units) | — | — | 0.58 (0.17, 1.06) |
| Urbanicity (RUCA) | |||
| Urban | — | — | Ref. |
| Micropolitan | — | — | −1.76 (−4.12, 2.91) |
| Rural | — | — | −1.92 (−4.41, 0.77) |
| Walkability (walkability index) (+1 unit) | — | — | −0.01 (−0.30, 0.40) |
In adjusted analyses restricted to the Medicaid cohort, patients who underwent SG lost 7.8% (95% CI, 4.8 to 13.7) TBW less than those who underwent RYGB. Patients with preoperative T2DM lost 5.5% (95% CI, 0.5 to 7.4) TBW less than those without diabetes (see Supplemental Digital Content 2, http://links.lww.com/AOSO/A13, which contains the full table of the subgroup analysis using median regression with %TBW loss as the outcome). Neighborhood socioeconomic deprivation, urbanicity, and walkability were not associated with postoperative weight loss for Medicaid patients.
In the non-Medicaid cohort, those who underwent SG lost 11.0% (95% CI, 7.6 to 12.4) TBW less than those who underwent RYGB (see Supplemental Digital Content 2, http://links.lww.com/AOSO/A13, which contains the full table of the subgroup analysis using median regression with %TBW loss as the outcome). Patients with greater neighborhood socioeconomic deprivation lost slightly more weight; for each 10 unit increase in ADI, patients lost 0.7% (95% CI, 0.1 to 1.1) TBW more after surgery.
Subgroup Analysis: Patients With at Least 3 Years of Follow-Up
Among patients with at least 3 years of follow-up, there were no differences in the rates of 90-day complications, 1-year outcomes, or weight loss outcomes between Medicaid and non-Medicaid patients (see Supplemental Digital Content 3, http://links.lww.com/AOSO/A14, which contains the full table of 90-day and one-year postoperative outcomes between Medicaid and non-Medicaid patients who had 3 years of follow-up or more; see Supplemental Digital Content 4, http://links.lww.com/AOSO/A15, which contains the full table of weight loss after surgery for Medicaid and non-Medicaid patients who had 3 years of follow-up or more).
In median regression with %TBW loss as the outcome for patients who had 3 years of follow-up or more, Medicaid status was not associated with weight loss after surgery. Patients who underwent SG or had preoperative T2DM loss less weight after surgery than those who underwent RYGB or did not have preoperative T2DM, respectively (see Supplemental Digital Content 5, http://links.lww.com/AOSO/A16, which contains the full table of the median regression with %TBW loss as the outcome for patients with 3 years of follow-up or more).
DISCUSSION
Our findings suggest that neighborhood-level social determinants of health, including socioeconomic deprivation, urban versus rural living environment, and walkability, were not associated with weight loss after bariatric surgery. We found that Medicaid patients experienced similar weight loss but higher healthcare utilization compared with non-Medicaid patients following bariatric surgery. We rejected our hypothesis that social determinants, rather than Medicaid status, were associated with differences in weight loss outcomes.
At the neighborhood level, social determinants of health were not associated with weight loss outcomes after bariatric surgery. Although individual patient income is the most commonly used surrogate marker for socioeconomic status, we used ADI because we were interested in the relationships between neighborhood characteristics and patient outcomes. ADI was created by Singh31 as a multidimensional characterization of neighborhood socioeconomic disparity that was initially used to measure neighborhood inequities in mortality rates. Kind et al23 subsequently found that among Medicare patients hospitalized with congestive heart failure, pneumonia, or myocardial infarction, the most socioeconomically deprived 15% of patients experienced higher readmission rates. To our knowledge, the relationship between ADI and health outcomes has only been reported for one other surgical condition—pancreas cancer. In Power’s retrospective cohort analysis of 1,552 pancreatic adenocarcinoma patients undergoing curative-intent surgery, ADI was not associated with surgical complications or survival.32
We found that urban versus rural status and neighborhood walkability were not associated with weight loss after bariatric surgery. The literature describing the relevance of these variables on bariatric surgery outcomes is limited. Bergmann’s retrospective study of 122 bariatric surgery patients in West Virginia found that patients who lived in rural settings lost a similar amount of weight compared with their urban counterparts at 12 months of follow-up.33 Reid’s single-institution study of Canadian adults who underwent RYGB reported that patients who lived in more walkable neighborhoods did not have higher physical activity levels.34 Weight loss outcomes were not explored but the authors suggested that neighborhood walkability may not impact weight loss after bariatric surgery. Studies of nonbariatric surgery patients have reported that increased neighborhood walkability was associated with lower rates of overweight/obesity.21,35 Given these disparate results, the importance of a patient’s neighborhood and its influence on bariatric surgery outcomes remains unclear. Further evaluation with qualitative studies involving interviews with bariatric surgery patients would provide more granular data on the relationships between individual factors, neighborhood characteristics, and weight loss after surgery.
Medicaid and non-Medicaid patients in our study experienced similar weight loss 3 years postoperatively. These findings contrast with single institution studies of RYGB patients from 2014 and 2016, in which Medicaid patients lost less weight that non-Medicaid patients (approximately 50% excess body weight loss vs 65%, respectively).14,15 However, our study findings are supported by a 2018 systematic review that found no differences in weight loss up to 2 years after surgery between Medicaid and non-Medicaid cohorts. Two single institution retrospective studies that evaluated over 2,500 RYGB patients also found similar weight loss outcomes between Medicaid and non-Medicaid patients within 3 years of surgery.36,37 Regardless of Medicaid status, SG patients in our study cohort lost less weight than RYGB patients, a finding that is well described in the literature.38
Medicaid patients in our analysis had higher healthcare utilization with 90 days of surgery, specifically readmissions, which is consistent with previous studies. Takemoto’s systematic review reported higher rates of readmissions (OR 2.8, 95% CI, 1.2 to 7.0) and ED visits (OR 3.2, 95% CI, 1.1 to 9.1) for Medicaid patients within 90 days of surgery.16 A single institution study of patients who underwent laparoscopic RYGB reported that Medicaid patients had more than double the rate of readmissions (37.0% vs 14.7%; P = 0.01) and ED visits (48.2% vs 27.4%, P = 0.06) over 90 days postoperatively.14 Relationships between Medicaid status and healthcare utilization have also been reported in other surgical fields. Claflin’s analysis of nearly 140,000 adults undergoing general, vascular, or gynecological surgery using a clinical registry in Michigan reported that Medicaid patients had higher rates of ED visits and readmissions.39 A study of over 440,000 patients undergoing colectomy in the United States concluded Medicaid insurance was an independent predictor of hospital readmission.40
Higher healthcare utilization in the Medicaid cohort suggests that there may be a need for increased postoperative surveillance in the Medicaid population. The Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program introduced a quality improvement project in 2013 called the Decreasing Readmissions through Opportunities Provided (DROP), which aimed to decrease 30-day readmissions.41 The program included increased patient education, protocolized discharge checklists, and frequent phone and clinic follow-ups. One single institution study found that DROP decreased readmission rates from 8% to 2.5% over 18 months.41 Dissemination and implementation of this program at all accredited bariatric centers, with a focus on Medicaid patients, may be beneficial.
These study findings have implications for access to bariatric surgery care for Medicaid patients, who have historically had a difficult time accessing bariatric surgery due to stringent criteria for bariatric surgery approval. For example, to qualify for bariatric surgery in Wisconsin, patients with type 2 diabetes are required to have prescriptions for at least 2 diabetes medications,42 whereas Medicare and most commercial insurers simply accept a diagnosis of diabetes. Some Medicaid programs require that all patients participate in a medically supervised weight loss program for 6 months,43 despite the American Society for Metabolic and Bariatric Surgery position that insurance-mandated preoperative weight loss limits access to bariatric care.44 Our results suggest that Medicaid patients can achieve similar weight loss outcomes at 3 years compared to non-Medicaid patients following both SG and RYGB. If these findings are supported using longitudinal, national data, some state Medicaid programs may need to reevaluate their bariatric surgery approval criteria. Furthermore, given that bariatric surgery programs consist of multidisciplinary teams of health psychologists, dietitians, and bariatric surgeons, the data suggest they can function effectively as the gatekeepers to bariatric surgery.
Our study has several limitations. First, the social determinant variables were analyzed at the neighborhood level, rather than the patient level. We were interested in exploring how one’s neighborhood was associated with weight loss, but values for the social determinant variables may not be applicable for every patient within a particular neighborhood. Second, the walkability index may not accurately reflect how walkable all neighborhoods are given that the variables that comprise it are static (eg, intersection density). Real-time cellular phone data using the global positioning system may be a better indicator of walkability, but they are not widely available for research. Third, we defined Medicaid patients as those who had Medicaid within 3 years of surgery. Some Medicaid patients could have improved their SES at the time of surgery. However, our Medicaid cohort had higher ADI scores compared to non-Medicaid patients. Moreover, only 6 Medicaid patients had commercial insurance at the time of surgery. Fourth, Medicaid-specific qualifications for bariatric surgery vary between states, so our findings may not be generalizable to all Medicaid patients. Fifth, we did not specifically identify preoperative functional status, which may impact weight loss outcomes after bariatric surgery. Finally, this is a retrospective observational study, so there may be unmeasured confounding.
In conclusion, Medicaid patients had similar weight loss outcomes after bariatric surgery compared to non-Medicaid patients, but had higher rates of readmissions. Social determinants of health at the neighborhood level were not associated with weight loss outcomes. These findings suggest that if Medicaid patients are appropriately selected and medically optimized for bariatric surgery, they can achieve equivalent outcomes as non-Medicaid patients. Additional qualitative research is needed to better understand the role that one’s neighborhood has on achieving weight loss after bariatric surgery.
Acknowledgments
L.M.F., N.L., M.V., B.M.H., A.M., M.K.J., and L.P.H. contributed to study design. N.L., M.V., M.K.J., and B.M.H. contributed to data collection and analysis. N.L., M.V., B.M.H., A.M., L.P.H., and L.M.F. contributed to manuscript composition. All co-authors participated in the data interpretation and manuscript revisions. All co-authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Supplementary Material
Footnotes
Disclosure: All authors have completed the ICMJE uniform disclosure form and declare: no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. Effort on this study and manuscript were made possible by an NIH R-21 (NIMHD) awarded to Dr. Funk. This study was also funded by the American College of Surgeons George H.A. Clowes Career Development Award and a VA Career Development Award to Dr. Funk (CDA 015-060). Further funding was through the NIH T32 Surgical Oncology Research Training Program (grant T32 CA090217-17). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the DVA, or the US government.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014; 311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019; 381:2440–2450 [DOI] [PubMed] [Google Scholar]
- 3.Golzarand M, Toolabi K, Farid R.The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017; 31:4331–4345 [DOI] [PubMed] [Google Scholar]
- 4.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016; 151:1046–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018; 320:1172–1191 [DOI] [PubMed] [Google Scholar]
- 6.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016; 315:2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maciejewski ML, Shepherd-Banigan M, Raffa SD, et al. Systematic review of behavioral weight management program MOVE! for veterans. Am J Prev Med. 2018; 54:704–714 [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014; 149:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017; 376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström L.Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013; 273:219–234 [DOI] [PubMed] [Google Scholar]
- 11.Sundbom M, Hedberg J, Marsk R, et al. ; Scandinavian Obesity Surgery Registry Study Group. Substantial decrease in comorbidity 5 years after gastric bypass: a population-based study from the Scandinavian obesity surgery registry. Ann Surg. 2017; 265:1166–1171 [DOI] [PubMed] [Google Scholar]
- 12.Wang YC, Pamplin J, Long MW, et al. Severe obesity in adults cost state Medicaid programs nearly $8 billion n 2013. Health Aff. 2015; 34:1923–1931 [DOI] [PubMed] [Google Scholar]
- 13.Abraham A, Ikramuddin S, Jahansouz C, et al. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016; 26:1371–1377 [DOI] [PubMed] [Google Scholar]
- 14.Chen EY, Fox BT, Suzo A, et al. One-year surgical outcomes and costs for medicaid versus non-medicaid patients undergoing laparoscopic Roux-en-Y gastric bypass: a single-center study. Surg Laparosc Endosc Percutan Tech. 2016; 26:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk LM, Suzo A, Mikami DJ, et al. Two-year outcomes for medicaid patients undergoing laparoscopic Roux-en-Y gastric bypass: a case-control study. Obes Surg. 2014; 24:1679–1685 [DOI] [PubMed] [Google Scholar]
- 16.Takemoto E, Andrea SB, Wolfe BM, et al. Weighing in on bariatric surgery: effectiveness among Medicaid beneficiaries - limited evidence and future research needs. Obesity. 2018; 26:463–473 [DOI] [PubMed] [Google Scholar]
- 17.Mora-Pinzon MC, Henkel D, Miller RE, et al. Emergency department visits and readmissions within 1 year of bariatric surgery: a statewide analysis using hospital discharge records. Surgery. 2017; 162:1155–1162 [DOI] [PubMed] [Google Scholar]
- 18.Dallal RM, Datta T, Braitman LE.Medicare and Medicaid status predicts prolonged length of stay after bariatric surgery. Surg Obes Relat Dis. 2007; 3:592–596 [DOI] [PubMed] [Google Scholar]
- 19.Bennett KM, Scarborough JE, Pappas TN, et al. Patient socioeconomic status is an independent predictor of operative mortality. Ann Surg. 2010; 252:552–557; discussion 557 [DOI] [PubMed] [Google Scholar]
- 20.Mehaffey JH, Hawkins RB, Charles EJ, et al. Socioeconomic “Distressed Communities Index” improves surgical risk-adjustment. Ann Surg. 2020; 271:470–474 [DOI] [PubMed] [Google Scholar]
- 21.Creatore MI, Glazier RH, Moineddin R, et al. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA. 2016; 315:2211–2220 [DOI] [PubMed] [Google Scholar]
- 22.Knighton AJ, Belnap T, Stephenson B, et al. Introduction of an Area Deprivation Index measuring patient socio-economic status in an integrated health system: Implications for population health. eGEMS. 2016; 4:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014; 161:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Department of Agriculture Economic Research Service. Rural-Urban Commuting Area codes. Available at: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed August 20, 2019
- 25.United States Environmental Protection Agency. Walkability Index. Available at: https://catalog.data.gov/dataset/walkability-index. Accessed August 20, 2019
- 26.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008; 61:344–349 [DOI] [PubMed] [Google Scholar]
- 27.McMurrer DP, Sawhill I V.How much do Americans move up and down the economic ladder. Oppor Am. 1996; 3:1–4 [Google Scholar]
- 28.Wisconsin Department of Health Services. BadgerCare Plus. Available at: https://www.dhs.wisconsin.gov/badgercareplus/index.htm. Accessed August 20, 2019
- 29.Cheng FW, Gao X, Mitchell DC, et al. Body mass index and all-cause mortality among older adults. Obesity (Silver Spring). 2016; 24:2232–2239 [DOI] [PubMed] [Google Scholar]
- 30.Liu N, Birstler J, Venkatesh M, et al. Weight loss for patients with obesity: an analysis of long-term electronic health record data. Med Care. 2020; 58:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh GK.Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003; 93:1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers BD, Fulp W, Dhahri A, et al. The impact of socioeconomic deprivation on clinical outcomes for pancreatic adenocarcinoma at a high-volume cancer center: a retrospective cohort analysis Ann Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergmann KL, Cox SJ, Tabone LE.Influence of a rural environment on patient access and outcomes for bariatric surgery. Surg Obes Relat Dis. 2017; 13:632–636 [DOI] [PubMed] [Google Scholar]
- 34.Reid RER, Carver TE, Reid TGR, et al. Effects of neighborhood walkability on physical activity and sedentary behavior long-term post-bariatric surgery. Obes Surg. 2017; 27:1589–1594 [DOI] [PubMed] [Google Scholar]
- 35.James P, Kioumourtzoglou MA, Hart JE, et al. Interrelationships between walkability, air pollution, greenness, and body mass index. Epidemiology. 2017; 28:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes S, Napolitano MA, Lent MR, et al. The effect of insurance status on pre- and post-operative bariatric surgery outcomes. Obes Surg. 2015; 25:191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen-Otsu E, Ward EK, Mitchell B, et al. The effect of Medicaid status on weight loss, hospital length of stay, and 30-day readmission after laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg. 2015; 25:295–301 [DOI] [PubMed] [Google Scholar]
- 38.McTigue KM, Wellman R, Nauman E, et al. ; PCORnet Bariatric Study Collaborative. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: The National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA Surg. 2020; 155:e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claflin J, Dimick JB, Campbell DA, et al. Understanding disparities in surgical outcomes for medicaid beneficiaries. World J Surg. 2019; 43:981–987 [DOI] [PubMed] [Google Scholar]
- 40.Sastow DL, White RS, Mauer E, et al. The disparity of care and outcomes for medicaid patients undergoing colectomy. J Surg Res. 2019; 235:190–201 [DOI] [PubMed] [Google Scholar]
- 41.Morton J.The first metabolic and bariatric surgery accreditation and quality improvement program quality initiative: decreasing readmissions through opportunities provided. Surg Obes Relat Dis. 2014; 10:377–378 [DOI] [PubMed] [Google Scholar]
- 42.BadgerCare Plus and Medicaid.. Bariatric surgery. Available at: https://www.forwardhealth.wi.gov/WIPortal/Subsystem/KW/Print.aspx?ia=1&p=1&sa=50&s=3&c=11&nt=Bariatric+Surgery. Accessed August 22, 2019
- 43.Obesity Coverage.. Medicaid’s criteria for weight loss surgery coverage. Available at: https://www.obesitycoverage.com/insurance-and-costs/am-i-covered/check-my-insurance/item/medicaid-s-criteria-for-weight-loss-surgery-coverage. Accessed August 22, 2019
- 44.Kim JJ, Rogers AM, Ballem N, et al. ; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS updated position statement on insurance mandated preoperative weight loss requirements. Surg Obes Relat Dis. 2016; 12:955–959 [DOI] [PubMed] [Google Scholar]


