Abstract
Sorafenib, an oral chemotherapeutic agent used in the treatment of solid tumors, is associated with a variety of adverse cutaneous drug reactions in up to 90% of patients. Infrequently, delayed-type hypersensitivity reactions such as erythema multiforme occur. This case describes a child treated with sorafenib for a retrosternal desmoid tumor who developed widespread erythema multiforme across his extremities, trunk, face, and mucosal membranes.
Keywords: Drug reaction, pharmacology, sorafenib
Sorafenib is an oral multikinase inhibitor used as chemotherapy in the treatment of solid tumors.1 From 70% to 90% of patients on sorafenib experience cutaneous adverse reactions, which consist of rash (18%–66%), hand-foot skin reaction (25%–62%), alopecia (18%–53%), and xerosis (11%–23%).2,3 It is important to distinguish nonallergic drug toxicities from allergic delayed-type cutaneous hypersensitivity reactions considering the latter’s potential for inducing severe cutaneous adverse reactions, namely erythema multiforme (EM) major, Stevens-Johnson syndrome, toxic epidermal necrolysis, and anaphylactic shock.4
CASE DESCRIPTION
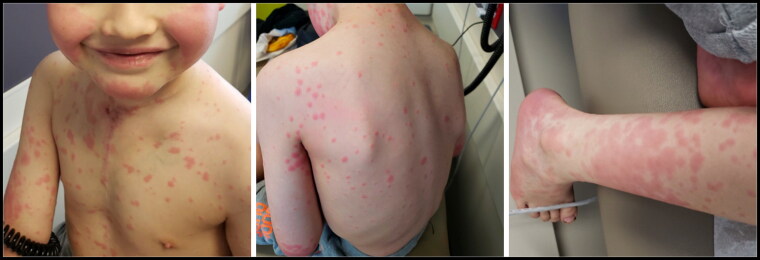
A 7-year-old white boy presented because of a widespread pruritic, erythematous rash across his face, trunk, and extremities for 5 days. He denied any symptoms of recent illness. He started the chemotherapy medication sorafenib 9 days before developing the rash. He was not on other medications and had no known drug allergies. His past medical history included a mediastinal desmoid tumor previously treated with neoadjuvant chemotherapy followed by surgical resection with multiple positive margins. On examination, he appeared well, and his vital signs were within normal limits. There were edematous, erythematous papules and plaques on his face, chest, trunk, glans and shaft of penis, scrotum, and bilateral upper and lower extremities, including both dorsal and volar surfaces of the hands and feet (Figure 1). A number of the papules had an atypical targetoid appearance with dusky centers. He also had two aphthous ulcers along the hard palate. A complete blood count was normal. A biopsy was offered and declined. The patient was clinically diagnosed with EM given the timing and appearance of the rash and mucosal involvement. He was advised to discontinue sorafenib and prescribed prednisolone 0.5 mg/kg/day with a 3-week taper. The rash slowly faded without desquamation and resolved within a few weeks.
Figure 1.
Widespread erythematous edematous papules and plaques across face, trunk, extremities, and back.
DISCUSSION
EM is an acute immune-mediated reaction, commonly in response to viral infections, that can present with targetoid cutaneous and/or mucosal lesions.5 Drug-induced EM makes up <10% of cases. The incidence of sorafenib-induced EM is variable in the literature, ranging from 0.1% to <1% in postmarketing surveillance and 1.5% and 2.4% in Japanese phase 2 and phase 3 target studies.6,7 However, in a 2012 prospective Japanese study on sorafenib’s adverse cutaneous reactions, 9 out of 36 patients (25%) presented with EM.8 It is possible that sorafenib-induced EM is more common in Japanese populations.
With an immune-mediated etiology underlying the pathogenesis of EM, drug re-exposure is generally regarded to carry an unacceptable risk and is largely avoided. Multiple reports indicate that re-exposure to the medication is most commonly met with recurrence of a more severe and faster-onset cutaneous reaction or anaphylaxis, even when started at a low dose, and immediate discontinuation is required.3,9–11 In one case, a 63-year old patient restarted sorafenib 1 month after his initial treatment led to EM and experienced anaphylaxis 3 hours after ingesting a single dose.10 Although steroid therapy in combination with sorafenib re-exposure was reportedly tolerated as a last therapeutic resort in two adult patients with hepatocellular carcinoma who had developed EM, further research is needed to determine safety.12 In our case, the patient was advised to discontinue sorafenib given his extensive eruption and mucosal involvement due to concerns for his risk of developing a life-threatening severe cutaneous adverse reaction or anaphylaxis upon repeat exposure.
A unique aspect of this patient’s case is that sorafenib-induced EM in a pediatric patient has not been reported in the literature, likely due to rare drug exposure. Adult and pediatric patients should be educated on the possibility of cutaneous drug reactions upon initiation of sorafenib treatment.
References
- 1.Escudier B, Worden F, Kudo M.. Sorafenib: key lessons from over 10 years of experience. Expert Rev Anticancer Ther. 2019;19(2):177–189. doi: 10.1080/14737140.2019.1559058. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B.. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60(2):299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor JL, Silvers DN, Grossman ME, Sherman WH.. Sorafenib-induced erythema multiforme. J Am Acad Dermatol. 2007;56(3):527–528. doi: 10.1016/j.jaad.2006.10.981. [DOI] [PubMed] [Google Scholar]
- 4.Smith W. Adverse drug reactions—allergy? side-effect? intolerance? Aust Fam Physician. 2013;42(1-2):12–16. [PubMed] [Google Scholar]
- 5.Sokumbi O, Wetter DA.. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51(8):889–902. doi: 10.1111/j.1365-4632.2011.05348.x. [DOI] [PubMed] [Google Scholar]
- 6.Akaza H, Tsukamoto T, Murai M, Nakajima K, Naito S.. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol. 2007;37(10):755–762. doi: 10.1093/jjco/hym095. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda M, Fujita T, Mii S, et al. Erythema multiforme induced by sorafenib for metastatic renal cell carcinoma. Jpn J Clin Oncol. 2012;42(9):820–824. doi: 10.1093/jjco/hys103. [DOI] [PubMed] [Google Scholar]
- 9.Sohn K, Oh S, Lim K-W, Kim M-Y, Lee S-Y, Kang H-R.. Sorafenib induces delayed-onset cutaneous hypersensitivity: a case series. Allergy Asthma Immunol Res. 2015;7(3):304–307. doi: 10.4168/aair.2015.7.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Álvares-Da-Silva MR.. Anaphylaxis preceded by erythema multiforme with sorafenib: first case report. Ann Hepatol. 2019;18(5):777–779. doi: 10.1016/j.aohep.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Kodaira M, Takahashi S, Takeuchi K, et al. Sorafenib-induced erythema multiforme for metastatic renal cell carcinoma. Ann Oncol. 2010;21(7):1563–1565. doi: 10.1093/annonc/mdq299. [DOI] [PubMed] [Google Scholar]
- 12.Shioya M, Nishimura T, Nishida A, et al. Two cases of successful sorafenib retreatment with the addition of steroid therapy following sorafenib-induced erythema multiforme in two patients with hepatocellular carcinoma. Nihon Shokakibyo Gakkai Zasshi. 2014;111(7):1424–1432. [PubMed] [Google Scholar]