Abstract
Molluscs are among the most ancient, diverse, and important of all animal taxa. Even so, no individual mollusc species has emerged as a broadly applied model system in biology. We here make the case that both perceptual and methodological barriers have played a role in the relative neglect of molluscs as research organisms. We then summarize the current application and potential of molluscs and their genomes to address important questions in animal biology, and the state of the field when it comes to the availability of resources such as genome assemblies, cell lines, and other key elements necessary to mobilising the development of molluscan model systems. We conclude by contending that a cohesive research community that works together to elevate multiple molluscan systems to ‘model’ status will create new opportunities in addressing basic and applied biological problems, including general features of animal evolution.
This article is part of the Theo Murphy meeting issue ‘Molluscan genomics: broad insights and future directions for a neglected phylum’.
Keywords: mollusc, evolution, model organism, genomics
1. Introduction
Molluscs are globally important as sources of food, calcium, and pearls, and as vectors of human disease. From an evolutionary perspective, molluscs are notable for their remarkable diversity: originating over 500 MA, there are over 70 000 extant mollusc species [1], with molluscs present in virtually every ecosystem. However, despite their biological, ecological (e.g. invasive species), economic (e.g. fisheries), and medical importance (e.g. schistosomiasis vector), critical steps towards understanding molluscan biology have been prevented by both general challenges associated with working with molluscs and specific challenges in genome sequencing and assembly. This has been compounded by the long-standing presumption that molluscs and related phyla (figure 1) are not sufficiently important or of high-enough profile to be worthy of intense research focus, relative to studies on vertebrates or other invertebrates. Molluscs also often have large, highly repetitive, and heterozygous genomes [3,4]. Together, these multiple challenges mean that as animal genome sequencing has increased in pace, well-assembled molluscan genomes (and associated resources like genome browsers; table 1) have remained scarce, at least until very recently.
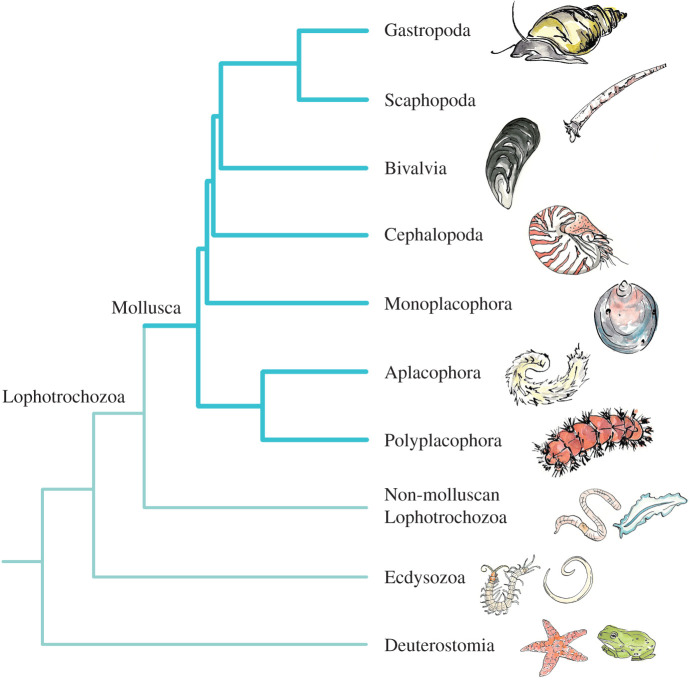
Figure 1.
Relationships among the major lineages of Mollusca, relative to other Lophotrochozoa, and Ecdysozoa and Deuterostomia outgroups. The structure of the phylogeny is based on that presented by Kocot et al. [2], using a phylogenomic dataset. Representative organismal images provided by Emily Jalinsky. (Online version in colour.)
Table 1.
An overview of the some of the most well-developed molluscan model systems. All featured taxa have a completed genome assembly, albeit of varying assembly quality. Other resources (e.g. genome browser, tools for transgenesis) are comparatively rare, as are taxa that are suited to laboratory culture. The table is not comprehensive, instead featuring a set of diverse mollusc taxa with the potential for broader applicability as ‘models’.
| class | common name | speciesa | habitat | genome | laboratory | inbred | immortal | CRISPR-Cas9 | publicationsc | publicationsd (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| browser | life-cycleb | lines available | cell line | transgenesis | 2010–2020 | genetics/genomics | ||||
| Polyplacophora | West Indian fuzzy chiton | Acanthopleura granulata | marine | 34 | 3 | |||||
| Monoplacophora | Laevipilina hyalina | marine | 8 | 50 | ||||||
| Gastropoda | Apple snail | Pomacea canaliculata | freshwater | yes | 553 | 12 | ||||
| Bloodfluke planorb | Biomphalaria glabrata | freshwater | yes | yes | yes | yes | 1068 | 21 | ||
| Great pond snail | Lymnaea stagnalis | freshwater | yese | yes | yes | yes | 1180 | 14 | ||
| New Zealand mud snail | Potamopyrgus antipodarum | freshwater | yes | yes | 339 | 14 | ||||
| Periwinkle | Littorina saxatilis | intertidal | 652 | 19 | ||||||
| Abalone | Haliotis spp. | marine | yes | 1743 | 28 | |||||
| Cone snail | Conus spp. | marine | 364 | 26 | ||||||
| Eastern mudsnail | Tritia (Ilyanassa) obsoleta | marine | 175 | 15 | ||||||
| Owl limpet | Lottia gigantea | marine | yes | 100 | 36 | |||||
| Scaly-foot snail | Chrysomallon squamiferum | marine | 13 | 46 | ||||||
| Sea hare | Aplysia californica | marine | yes | yes | partial | 1226 | 18 | |||
| Slipper snail/black-foot snail | Crepidula spp. | marine | yes | yes | 317 | 10 | ||||
| Grove snail | Cepaea nemoralis | terrestrial | yes | 171 | 20 | |||||
| Giant African land snail | Achatina fulica | terrestrial | yes | 289 | 14 | |||||
| Garden snail | Cornu aspersum | terrestrial | 138 | 10 | ||||||
| Cephalopoda | Dwarf cuttlefish | Sepia bandensis | marine | 1171 | 9 | |||||
| Longfin inshore squid | Doryteuthis pealeii | marine | 178 | 8 | ||||||
| Octopus | Octopus spp. | marine | yes | 2874 | 10 | |||||
| Squid | Euprymna spp. | marine | yes | yes | 243 | 50 | ||||
| Bivalviaf | Manila clam | Ruditapes phillipinarum | marine | 1593 | 19 | |||||
| Mussel | Mytilus spp. | marine | 8230 | 14 | ||||||
| Pacific oyster | Crassostrea gigas | marine | yes | yes | 6292 | 24 | ||||
| Pearl oyster | Pinctada fucata | marine | 1008 | 36 | ||||||
| Scallop | Argopecten | marine | 577 | 29 | ||||||
| Scallop | Patinopecten | marine | 290 | 40 | ||||||
| Scallop | Chlamys | marine | 791 | 46 | ||||||
| Scallop | Pecten | marine | 695 | 11 | ||||||
| Softshell clam | Mya arenaria | marine | 410 | 16 |
aGenus only is shown if several closely related species are in use.
bAlthough many species can be induced to spawn in the laboratory, few are amenable to culture through the whole life cycle and over repeated generations; excludes single reports.
cAssessed by searching Web of Knowledge 2010–2020 using the genus name as a search term; for Conus it was also necessary to add ‘snail*’ as a search term, for ‘Mya’ and ‘Cornu’ it was necessary to add the species name.
dAssessed by searching Web of Knowledge 2010–2020 using the genus name as a search term AND ‘gene’ OR ‘genes’ OR ‘genomic’ or ‘genomics’.
eNot yet publically available.
fMany bivalves can be grown in farms, sometimes over generations, but the conditions could not be described as ‘laboratory’; likewise, inbreeding is possible in many species (e.g. oyster), but they cannot be described as ‘inbred lines’ and are not generally available.
The relative neglect of research on molluscs, including the absence of targeted funds for molluscan genome sequencing, has been problematic for the collective effort. This challenge is especially evident now that highly contiguous genome assemblies are a starting point for much of modern biology. Of course, this is a very recent development; until the past decade or so, the genome assembly of the most commonly used research organisms was usually the last to be developed, well after laboratory culture was established, and tools such as transgenesis, cell-lineage tracing, etc., were made available.
Now, in an era when it is straightforward to sequence and assemble a genome, there is a risk that the genome is viewed as the end goal, and the corollary, that a genome on its own is sufficient to raise an animal to ‘model’ status. Like others, we would argue that a stricter definition of ‘model’ is more useful: a model organism is a species that is convenient for the study of a particular biological process, and for which there is sufficient infrastructure, and appropriate resources, to enable investigations [5–7]. Ideally, a model species should have a well characterized ecology (although this is rarely the case, and often comes last), be easily collected and amenable to laboratory culture (figure 2). As the system develops and grows, the associated scientific community should adapt appropriate technologies and methods, especially CRISPR-Cas9, and benefit from access to resources such as biological databases (e.g. multiple-species genome browsers) and cell lines.
Figure 2.
Idealized ‘ELCTR’ criteria for the making of a model mollusc. Images from top: Cepaea nemoralis at the University of Nottingham (Daniel Ramos Gonzalez); Potamopyrgus antipodarum laboratory culture at the University of Iowa (Justin Torner); attendees at the Royal Society ‘Pearls of Wisdom’ molluscan genome meeting 2019 (Liam Helm); CRISPR-Cas9 cartoon (National Human Genome Research Institute, CC-BY-2.0); investigators using the MolluscDB [8] database (Chelsea Higgins). (Online version in colour.)
Regardless of how we or others define model taxa, breakthroughs in DNA sequencing technology and assembly approaches are finally allowing scientists to produce the first cost-effective assemblies of notable molluscan genomes, such as octopus [9], giant African land snail [10], and the deep-sea scaly foot snail [11]. As technology overcomes these challenges, molluscan models and genomes will be used to address broad questions in biology. A deepening of the basic knowledge of molluscs and their genomes will also provide the resources and tools needed to take key steps towards harnessing the unique biology of molluscs for human benefit and preserving important biodiversity.
Accordingly, our aim here is to provide a brief overview of the role that model molluscs (aka ‘research organisms’ [12], or even ‘non-model model organisms' [5]) and their genomes have to play in molluscan biology, and animal biology in general. We illustrate some of the benefits but also address some of the most important issues that continue to limit further study and, ultimately, prevent human benefit. As there is insufficient space to comprehensively cover each member of this large set of concepts and taxa, we reference authoritative reviews on individual topics or species where appropriate [e.g. 3,13–15].
2. Making a model mollusc
Scientists in the pre-genomic era of molecular biology came together to study a handful of more-or-less prescribed model animals, mainly vertebrates (e.g. chick, mouse) in the Deuterostomia and the nematode and fruitfly in the Ecdysozoa [7,16]. These efforts led to the development of tools and resources specifically for these organisms, including infrastructure such as databases and strain collections, in addition to molecular toolkits, and extensive collections of reproducible techniques and methods [5]. In comparison, the third main animal group, the Lophotrochozoa (figure 1), which includes molluscs and other diverse spiralian lineages such as the annelids, has been more or less ignored, especially with respect to tools, infrastructure, and resources.
To this day, the Lophotrochozoa does not contain a taxon around which a large research community has formed, with the possible exception of some platyhelminthes, especially the Schistosoma parasite (which ironically has to be maintained by vectoring through the snail intermediate host Biomphalaria glabrata [17]), and some other flatworms [12,18]). This is unfortunate because lophotrochozoans are especially valuable in providing a powerful comparative framework to study animal evolution, because of their ancient divergence from the other groups (approx. 500–700 MA), their diverse body plans and in light of evidence for retention of an ancestral bilaterian gene repertoire relative to traditional Ecdysozoan models like flies and nematodes [19–21].
The consequent absence of appropriate tools and resources, including genomes and methods for transgenesis, has hindered progress in understanding molluscs relative to other groups. In parallel, while there are many meetings and conferences devoted to single model animals, there are few equivalent resources for molluscs.
While no single model species can address all possible biological questions, this fundamental limitation did not prevent the development of organisms like the fruitfly, nematode, yeast, and mouse into model systems to be applied to broad questions across biology. Why has no model mollusc taken its place among the other textbook model organisms? In our opinion, this is probably a historical contingency, in that yeast, flies, worms, etc. were relatively ‘easy’ to find and convenient to maintain. In comparison, many molluscs are more difficult to raise in laboratory conditions and have a relatively long or complex life cycle.
Whichever the explanation, the consequence is that scientists who study molluscs have typically taken a piecemeal approach, applying one or several related mollusc species in the context of a targeted question. For instance, octopus and other cephalopods are often used for studies on intelligence and consciousness [22,23]. A much wider range of molluscs is used to study more general neurobiological questions. Another example comes from the focussed use of Biomphalaria glabrata in the context of research into snail-vectored trematode diseases like schistosomiasis [e.g. 24,25]. Biomineralization is also an active topic for which a variety of taxa are used, from marine species such as abalone [26] and oyster [27] to freshwater Lymnaea stagnalis pond snails [28] or Cepaea land snails [29]. Indeed, many mollusc species have been put forward as ‘models’ in the niche sense of the concept, to answer a limited range of biological questions [30–38], although these model prospects frequently lack infrastructure and appropriate resources to enable in-depth investigations [5–7].
Molluscs have thus lagged behind relative to other animal phyla, even when the former are being used to ask the same types of questions. The absence of genetic and genomic resources is also likely an important—and self-reinforcing—part of the problem. For example, studies on the shell polymorphism of the snail Cepaea were a crucial element towards establishing the role of natural selection in maintaining morphological variation, with the genus becoming a pre-eminent model for ecological genetics [39,40]. However, the absence of a high-quality genome assembly and genetic tools means that the genes that drive the ‘exuberant’ variety of shell colours and patterns remain unknown in Cepaea, preventing further progress. By contrast, the precise mutation that defines a famous colour polymorphism in the peppered moth has been identified [41]. More broadly, the use of genomics to characterize Lepidopteran wing colour genes has led to an array of different research avenues [42] and many training opportunities for junior scientists. A similar example of a prominent mollusc held back by the absence of genomic resources is provided by the freshwater New Zealand snail Potamopyrgus antipodarum, a textbook model system for the evolution of sexual reproduction and host–parasite coevolution [43], for which a genomic assembly is nearly complete.
While model molluscs are individually valuable, the community of researchers associated with each system is generally too small to provide the resources or impetus needed to develop that system into a more broadly applicable model. The accompanying resources and tools are also generally missing (table 1). This challenge even extends to the biomedically important snail vectors of human disease (including B. glabrata), for which the range of tools, resources and applications is insignificant in comparison to the economic damage caused by the diseases. All of these problems are often compounded by the large and repetitive genomes that characterize many molluscan species (but not all, e.g. Lottia gigantea [44]).
There are nonetheless some mollusc species (or groups of related species) for which it has been argued that there is a critical mass of persons and resources to study a range of questions. In particular, Fodor et al. [15] make the case for the pond snail L. stagnalis. This species has long been a model for neurobiology, but in more recent years, the genus has been used to study ecotoxicology [45], sexual selection [46], biomineralization [28], parasitology [47,48], and development [49]. However, while we do not doubt the relatively wide-ranging existing utility of the pond snail, development of further key resources would allow L. stagnalis to be applied powerfully to an even wider range of research questions. Equally, we would argue that it is better to strive to ensure that any tools and resources are effective or useful in a range of species (e.g. both L. stagnalis and B. glabrata), rather than groups competing for pre-eminence of their ‘own’ model system. These different groups can also come together and exchange knowledge and resources. A good example is provided by the newly formed ‘Spiraliabase’, whose stated aim is to grow the community, incorporating laboratories from around the world into an interactive and cohesive group that can plan future meetings, grants, and education efforts [50].
The take-home message is that model species, including but not limited to molluscs, should continue to be selected according to the biological question. At the same time, we should push for permanent and reusable data, resources and web tools [8,51], including high-quality contiguous genomes [9–11], portable genome browsers [52], and pipelines that can be used for other taxa [2].
3. The potential for genomics in molluscs
Molluscan models may be used to make inferences about other taxa that have not been studied in the same manner or are so seemingly unique (e.g. the scaly-foot snail [11]; transmissable cancers in bilvalves [53]) that they are thought to confer especially distinctive insights. There are also considerable potential commercial benefits, especially in aquaculture [54,55]. More generally, there is a compelling argument that broad insights into the evolution of the Bilateria requires representatives from each of its three main groups, the Deuterostomia, Ecdysozoa and Lophotrochozoa [44,56,57]. The importance of adequate representation is exemplified by the presumption in the early 2000s that the signalling gene nodal was a deuterostome innovation because it was absent in the ecdysozoan fruitfly and nematode. This inference turned out to be premature as it was made in the near absence of genomic resources for any lophotrochozoan taxon. In 2009, nodal was reported in the limpet Lottia as well as B. glabrata [58], and subsequently in other lophotrochozoan phyla. The gene had evidently been lost during the evolution of the Ecdysozoa, with the lack of data for the Lophotrochozoa incurring a misleading interpretation.
We here provide a few key examples (table 1 and figure 3) from some especially high-profile and potentially powerful systems, ranging from single species to diverse molluscan groups. Rather than trying to summarize the main research findings (for which directed reviews are a better source), our aim is to illustrate important research questions to which molluscs can be usefully applied and how the study of model molluscs and their genomes may impact upon on our understanding of this phylum and the much wider group of animal life. Despite recent progress, there is still very little genomic research on molluscs, with broader implications for (mis)understanding animal biology and missed opportunities regarding human benefits.
Figure 3.
Representative molluscs used in research and highlighted in this work. The classes Gastropoda (including pond snail Lymnaea stagnalis, bloodfluke planorb Biomphalaria glabrata, New Zealand mud snail Potamopyrgus antipodarum, slipper snail Crepidula fornicata, cone snails Conus spp.), Bivalvia (including various clams, mussels, scallops) and Cephalopodia (including Octopus) all include several model species. In comparison, there are no species that could be described as models in the other classes and groups, including the Scaphopoda, Monoplacophora, Aplacophora and Polyplacophora. Image credit: Emily Jalinsky. (Online version in colour.)
4. Blood-fluke planorb Biomphalaria glabrata: disease prevention
Snails are an important vector of human disease. The most well known of these diseases is schistosomiasis, which sickens hundreds of millions and kills thousands of people every year [59]. Snails also are the source of several other food- and water-borne diseases that are biomedically or agriculturally significant, such as opisthorchiasis and fascioliasis [60,61]. To date, the majority of relevant research on these diseases has tended to focus on the parasites and their interactions with humans. In comparison, the snails have received comparatively little attention despite a growing body of evidence that controlling the snail vectors is perhaps the most effective means to reduce the incidence of the disease [62].
Most disease-focused work to date has involved the snail B. glabrata, intermediate host to the Schistosoma mansoni parasite. This snail is amenable to laboratory culture and is easy to raise. The B. glabrata resources available include a genome assembly [63,64], a linkage map [65], several long-standing laboratory lines (some inbred [66]), and the only molluscan (and lophotrochozoan) immortal cell line [67,68].
Much of the research on B. glabrata has aimed to characterize the snail immune system [69–71], with a long-term view to identify biological targets that may lead to the development of methods to block or prevent schistosome infection. Another avenue has been in identifying loci that confer resistance to infection [24,25,63,64], albeit usually partial, so that individuals might be bred that are wholly refractory to infection or transmission. This might be via conventional breeding and an understanding of Mendelian genetics, but it seems more likely (barring ethical issues [72]) that in the future, new technologies [73] such as CRISPR/Cas9 gene editing and gene drive techniques will provide a faster and more effective means of inserting and driving a resistance component through the population [64,74].
Gene finding and mapping and genetic manipulation all require, or are benefited by, a well-assembled genome. While this resource is in place for B. glabrata and is leading to scientific advances [25,64], the community lacks a chromosomal-level assembly, a set of re-sequenced B. glabrata laboratory lines, or wild isolates. A further important issue is that transgenic methods have not yet been applied to B. glabrata. Finally, there is little knowledge of the other snail species that are important intermediate vectors for schistosomes and other trematodes [61].
5. Great pond-snail Lymnaea stagnalis: development, biomineralization, neurobiology, eco-toxicology, sexual selection
Pond snails have long been used to study molluscan development in general, and development of the shell in particular. More recently, pond snails have come to the fore in studies of biomineralization [28], neurobiology [75], eco-toxicology [45], and sexual selection [46]. In our own work, we have used inherited variation in the chirality of the body and the shell to understand the establishment of left–right asymmetry in snails and the conserved role of the formin gene in establishing chirality in bilaterians [49,76–79].
Most of the recent advances have been made in the absence of a mature genome assembly. For example, although a fragmented genome assembly has been available since 2016 [49], and a well-assembled and annotated genome assembly is in progress (consortium led by Marie-Agnes Coutellec and funded by Genoscope), we mainly relied upon traditional BAC sequencing and linkage mapping [76] to identify the chirality gene, albeit aided by high-throughput sequencing methods. Others have used L. stagnalis transcriptome data (rather than genomic) to identify horizontal gene transfer between invertebrates and vertebrates, likely facilitated by host–parasite interactions [47]. Similarly, in the absence of a genome, peptide sequencing of seminal fluid was used to identify ovipostatin, a protein that suppresses egg mass production [80]. Subsequently, the genome sequence was used to identify the complete gene sequence of ovipostatin; gene expression data were used to understand the role of ovipostatin in reproduction [81].
One clear benefit of working with L. stagnalis is that the snails are straightforward to keep in the laboratory and can be raised in the thousands, either from controlled crosses or by self-fertilization. In our work, we undertook repeated rounds of self-fertilization and full-sib mating to create highly inbred lines. One of these lines was then used to create the in-progress genome assembly and is also freely available to other labs.
Another advantage of using pond snails is that proof-of-principle experiments have shown that CRISPR/Cas9 methods are an efficient means to knock-down gene function in early embryos. In recent work, Abe & Kuroda [82] injected early L. stagnalis embryos with a CRISPR/Cas9 knock-down construct and then raised these embryos to hatching in glass capillaries, using the knock-down to provide definitive proof that a mutation in the formin gene is causative of changes in chirality ([83], but see commentary [84]). It is nevertheless unclear if this method will achieve wide uptake. A key issue, in addition to the skill and equipment needed for microinjection, is that L. stagnalis embryos do not readily develop outside of the egg capsule. This problem of embryo viability is likely general to many molluscs. Thus, like B. glabrata, L. stagnalis stands as a promising model mollusc that nevertheless faces substantial hurdles alongside a general lack of resources and methods.
6. New Zealand freshwater snail Potamopyrgus antipodarum: host–parasite coevolution, evolution of sex
The tiny prosobranch snail Potamopyrgus antipodarum, unusual in the frequent natural coexistence between obligately sexual and obligately asexual individuals, is a textbook model for host–parasite coevolution and the evolution of sex. John Maynard Smith [85] first promoted the system as one with perhaps uniquely high potential to apply to the study of sex. Curt Lively and collaborators discovered an important role for host–parasite coevolution in the maintenance of sex in at least in some P. antipodarum populations [e.g. 86,87]. This work also raised a host of follow-on and still unanswered questions, including but not limited to the mechanisms driving the origin of new asexual lineages, whether factors like a low rate of origin of new asexual lineages explain the handful of high-sex populations in which coevolving parasites are relatively scare, and how asexual reproduction influences genomes and phenotypes.
Answering these questions in P. antipodarum requires genomic resources, which is what spurred us to start generating transcriptomes nearly 10 years ago [88]. There now exist dozens of transcriptomes [89–91] and a forthcoming high-quality draft genome assembly (table 1) as well as dozens of resequenced genomes [e.g. 92] representing the diversity of the species in its native range. These resources have been used to, for example, demonstrate evidence for accelerated mutation accumulation in the genomes of asexual P. antipodarum [92] and reconstruct the invasion route of destructive P. antipodarum populations that have colonized North America and Europe [93]. These new genomic resources have also revealed a very recent genome duplication in Potamopyrgus that has complicated genome assembly [94]. In the future, comparative analysis of patterns of nucleotide and structural evolution in these genomes could be used to assess support for a host of major hypotheses for sex and host-parasite coevolution. Like most other molluscan systems, however, other important genomic tools (e.g. transgenesis, cell lines) await development.
7. Various bivalves and gastropods: biomineralization
Molluscs are a powerful system in which to study the evolution and mechanics of biomineralization because of their high diversity and their highly complex, robust and often patterned shells [13,14,95]. In this respect, bivalves, in particular, are also a subject of relatively intense study, both because of their important commercial applications and because a few bivalve species are highly invasive [54].
The general finding of biomineralization studies to date is that the majority of the proteins that are involved in making the shell are unique to each separate group, with only a low proportion shared across, for example, bivalves and gastropods [13,26]. Accordingly, no single mollusc species has come to dominate the subject area. A diversity of models will ultimately be required to draw general conclusions and identify common patterns.
An early survey of genes involved in molluscan shell formation was based on an analysis of the oyster genome [27]. Because some of these shell proteins constitute important components of the extracellular matrix across metazoans, Zhang et al. [27] suggested that the organic matrix of the shell might share key similarities—but nevertheless still harbours some major differences—with the connective tissue of other animals. Wollesen et al. [96] made an analogous point with respect to the fact that characteristic brain regionalization genes in other animal lineages are expressed in the mantle of molluscs during development, suggesting that brain regionalization genes might have been co-opted into the shell patterning in molluscs. Other important findings include the fact that a large proportion of secreted proteins contain simple repetitive motifs, which might further promote the evolvability of the mantle secretome [13].
Nonetheless, despite substantial progress, considerable caution is required in making general inferences on biomineralization. In particular, most of the genomic studies on biomineralization to date have involved either bivalves (Crassostrea, Pinctada oysters, and Mytilus mussels) or gastropods (Haliotis, Lymnaea), comprising just two of the eight major lineages of the Mollusca (figure 1). As there is only one exception, using chitons [97], broad conclusions set in an evolutionary framework are premature.
A key aim for the future must be to understand the regulatory networks that lie at the core of shell formation and whether there is a conserved genetic ‘toolbox’ [14]. Prior gene expression studies in a range of species have revealed several conserved genes expressed in discrete zones within and around the developing shell, hinting at a conserved network (see references in [13,14]). Broader sampling should include, for example, studies of the embryonic shells of a variety of taxa as well as polyplacophoran shell plates and test whether these structures have independent evolutionary or developmental origins. Only then may it be possible to make progress in understanding the means by which gene interactions and deviations from regulatory networks contribute to the diversity of the shell patterning phenotype.
8. Slipper shell Crepidula: early development
The early development of molluscs is characterized by a spiral cleavage pattern, a form of development that unites several lophotrochozoan groups, including annelids, some flatworms and most molluscs, but excluding cephalopods [98,99]. Spiralian development has relatively few cell divisions before gastrulation, meaning that it is possible (in theory) to map the fate of each cell in the blastula.
The traditional focus of this area of research has aimed to understand the developmental process and the means by which diverse larval and adult body plans are produced. Most studies have been carried out in a relatively small group of model systems, frequently gastropods and bivalves (e.g. [38,99], but not always; see [100]), that were selected for reasons such as ease of production of embryos and an ability to access and manipulate them during early development.
The slipper snail Crepidula fornicata is perhaps the most high-profile species in the context of the study of molluscan development [37] (alongside Tritia (Ilyanassa) obsoleta, table 1). While the original research on the species was used to create the cell lineage nomenclature of spiral cleavage [101], recent work has produced high-resolution cell lineage fate maps, described the morphogenetic events during gastrulation, and provided important insight into the molecular basis of early development [102–104]. Notably, C. fornicata was also the first molluscan species in which CRISPR/Cas9 genome editing was demonstrated [105]. Most recently, the sister taxon the black-foot snail Crepidula atrasolea has come to the fore as a complementary model because it has a short life cycle and is easy to rear through successive generations in closed aquaria [106,107]. A further benefit is that the rearing methods that are being used are open source and economical and may thus be easily applied to other species [106]. Even so, it is not clear at this point whether C. atrasolea will come to be used by many research groups; whether this happens is likely dependent upon ease of culture and the tools that are made available.
9. Octopus and other cephalopods: intelligence, adaptive camouflage, vision, development
Cephalopods show several remarkable features that distinguish them from other molluscs, including camera eyes, high intelligence, and the absence of the stereotyped spiral cleavage pattern. As the ‘first intelligent beings on the planet’ (Brenner, quoted in [23]), cephalopod models therefore have the potential to offer special insight into an especially wide range of biological questions [108]. Cephalopod genome assemblies and functional genomic methods that have been developed in parallel offer a powerful means to study a wide range of sophisticated adaptations that evolved in cephalopods, independently of similar traits in vertebrates.
The first cephalopod genome assembly [9] revealed that while the octopus developmental and neuronal gene set is roughly the same as that found across other invertebrates, there have been large expansions in two gene families: the protocadherins and a zinc-finger transcription factor family. Both of these gene families also independently expanded in vertebrates. The same study also showed that messenger RNA editing plays a major role in generating diversity in proteins involved in neural function. Broadly similar results have since been reported from other cephalopods [109]. Most recently of all, CRISPR/Cas9 gene editing was used to knock out a pigmentation gene in the longfin inshore squid (Doryteuthis pealeii) [110].
Nonetheless, much of the potential in co-opting genomics into the study of cephalopods otherwise remains largely unrealized. For instance, while molluscs have perhaps the greatest diversity in eye structure of all animals [111], there are few genomic comparative studies that span the wide diversity of molluscs/lophotrochozoans [112] and their light-sensing systems (e.g. in scallops [113]). Instead, even recent studies continue to use a gene-by-gene approach [114,115] rather than taking advantage of genome-era resources.
A potential challenge facing cephalopod researchers is an apparent disconnect between the genomics and behaviour-focussed studies. As an example, a key recent work hypothesised that intelligence in cephalopods and some vertebrates might have evolved through similar processes, yet there was no discussion of ‘genomics’ in the work [22]. Perhaps the barrier is a lack of interdisciplinarity, alongside challenges in devising methods that use genomics to experimentally test hypothesized genotype–phenotype relationships with respect to intelligence, consciousness, etc. A transcriptome atlas of the brain would provide a powerful starting point [116]. Subsequent work could be modelled on studies in vertebrates, promoting a comparative approach to understand the cellular and genetic innovations that underpin cephalopod brain expansion [117]. More broadly, a framework that compares cephalopods to vertebrates, and also to other molluscs (e.g. Aplysia, Lymnaea) with relatively simple neuronal systems could provide important steps towards our understanding of the evolution of complex cognition.
A final issue is that experiments involving cephalopods may require more guidelines and permissions than typical for other invertebrates (e.g. [118]). These additional restrictions are obviously appropriate for the welfare of these cephalopod models, but will also likely incur extra costs and time, as well as potentially limiting the nature of experimentation.
10. Cone snails: bioactive compounds for human benefit
Cone snails have long attracted scientific interest because of the potency of the venom that they use to immobilise their prey. The requirement for cone snail venom peptides to be simultaneously potent and specific to their molecular targets means that these peptides are both of interest to physiologists and are bioactive compounds that may be used for medical benefit. The remarkable diversity of conotoxins means that prospecting studies will certainly lead to new therapeutic applications [119,120].
To date, proteomic and transcriptomic studies have revealed that individual cone snail species use hundreds of different peptides (e.g. [121–123]), with the total conotoxin repertoire across all approximately 10 000 venomous species in the Conoidea estimated at around 100 000 distinct molecules [124]. Although significant progress has been made—including the first commercially available conoidean venom peptide drug used to treat chronic pain [125]—the study of conotoxins, or ‘venomics’, is underdeveloped relative to the potential academic and commercial gains. Despite profound medical and thus commercial potential, the majority of the genomic resources for cone snails are limited to transcriptomes, alongside some partial genomes [126,127]. The existing resources have been adequate from a basic bioprospecting perspective, but more refined studies will benefit from well-assembled genomes and the development of a common model species.
One example of an advance that can come from the availability of these resources is with respect to a better understanding of conopeptide post-translational modifications, which are carried out by enzymes that are themselves of biomedical interest [121]. Characterizing these interactions will be facilitated by the development of a model species with a genome assembly and associated resources. The high diversity of conopeptides also demands that scientists prospect across an unusually wide range of species, providing a strong case for generating genomic data and other tools from a wide variety of species.
11. What's missing from molluscan models?
Now that molluscan genome assemblies are relatively straightforward to produce and becoming commonplace, it is worthwhile to consider the barriers that remain towards using these genomic resources to understand the biology of molluscs and beyond. Some of these barriers are taxon-specific or new, while other challenges are long-standing and of much wider relevance. For the latter, solutions might be especially likely to be transformative in enabling molluscan research.
12. Accessible transgenesis
In most phyla outside of the Lophotrochozoa, the advent of gene editing, particularly via the CRISPR/Cas9 system, has transformed our ability to study the basics of animal biology. Gene editing has also provided powerful means to implement solutions to important human problems [105] such as biological control of disease vectors [72,128]. By contrast, CRISPR/Cas9 has been used on only a few occasions in molluscs over 5 years [82,105,129,130]. Similarly, RNA interference has seen some successes (e.g. [131]), especially in bivalves, but has still not received wide take-up [54,55,132,133].
These tools might be scarce at least in part because of the continuing lack of research focus and funds directed towards molluscs. However, it is also the case that technical challenges play a part, especially in terms of vector delivery and successful culture of genetically modified embryos. In the current iteration, gene editing is ‘efficient’ in molluscs (e.g. [110]), but only once the vector is successfully delivered into the embryo. In our view, the continuing problem with using these methods in many molluscan species is that the delivery tends to be technical and highly skilled and thus low throughput.
We believe that these barriers can be overcome with a few relatively straightforward solutions. For example, the mollusc community could invest in the development of viral vectors (e.g. virus pseudotyped with the Vesicular Stomatitis Virus G protein, with suitable promoters and polyadenylation sequences) that are able to directly deliver constructs to molluscan cells or embryos, obviating the requirement for low through-put injection or electroporation, and the removal of the embryo from any protective membranes. Packaged lentiviral vectors are able to deliver transgenes efficiently across a broad host range [134–136], have been widely used in gene therapy for the past twenty years or so, and are optimized to give high levels of expression and integration.
Presently, we are not aware of experiments that have established that the same vectors will also infect snail cells. The VSVg protein binds to the LDL receptor and to other members of this family [137], which is widespread in metazoan organisms including snails. It is, therefore, reasonable to suppose that VSVg pseudotyped viruses will transfect snail cells. An alternative would be a targeted method such as ‘ReMOT Control’ CRISPR [138]. The technology already exists; it just needs to be adapted for use in molluscs.
Transgenic methods are a necessary complement to genomics; there are limited means to gain proof of gene function if there is no quick and straightforward method to up- or downregulate the expression of a gene. Gene editing is of course just one of the tools available—but it is probably the most powerful available, especially given the absence of other technologies.
A persuasive example of the applied benefits that could come from the development of gene-editing and gene-drive methods is the possibility of engineering B. glabrata to make the snail resistant to infection by schistosomes. The delivery vector could be engineered so that gene drive would push the resistance trait through the population. Similar methods might be used to control crop pests, by first screening for genes that define susceptibility to agrochemicals, or that induce avoidance behaviours (e.g. to crops). This knowledge could then be used for the development of novel chemicals that may be used as targeted molluscicides or to insert constructs that are then driven through the population. Likewise, for the benefit of developmental biology, individual genes could be labelled with fluorescent markers and then used to trace molecules and cells through development, such as has been applied in other model organisms (e.g. [139]). In our own work on left–right asymmetry, such developments are necessary to trace the molecular dynamics of the cytoskeleton during chiral cleavage [140].
13. Cell culture and immortal cell lines
Molluscan cell lines are an essential complement for in vitro assays of gene function, proof-of-principle studies of viral transfection and electroporation, as well as testing of vaccines or molluscides. However, while primary culture cells have a role, there is only a single immortal molluscan cell line, Bge, derived in the 1970s with considerable effort from B. glabrata [67,68]. By contrast, there are over 500 lines derived from insects [67], but only the single B. glabrata line to represent the whole of the Lophotrochozoa.
The Bge line shows considerable karyotype variation from native snails [141], but still retains haemocyte-like morphology and behaviour, including encapsulation of schistosome sporocysts [142,143]. To date, the Bge line has been mainly used to understand interactions between the snail-derived cells and schistosome larval stages, including immune resistance [144]. Most recently, the genome of the Bge cell line was sequenced, highlighting variation in genes that may have contributed to immortalization, especially genes involved in regulation of the cell cycle, including apoptosis and transcriptional regulation [144].
There is further considerable benefit that could be gained from deriving more lines from B. glabrata, as well as new lines from other species. Given the difficulties involved, one strategy could be to identify the genetic changes that make molluscan cells immortal. A starting point could be the application of genomics towards understanding the genetic changes that produce transmissible cancers in bivalves [53]. This knowledge could then be used construct new lines in a relatively straightforward manner—as well as also useful in bringing together a pan-metazoan view of cancer biology. It would also make any mollusc accessible to cell culture, and ultimately, enable e.g. organoid experiments that could be used to understand molluscan developmental pathways and cell signalling [145]. Unfortunately, it is not currently possible to derive long-term cultures directly from transmissible cancer cells (Stephen Goff, Michael Metzger pers. comm.).
14. Improved extraction of high-molecular weight DNA
Extracting high-molecular weight and contaminant-free DNA is a continuing problem for molluscan research, recently brought into focus by new DNA sequencing methods that require long, contiguous, and break-free DNA, ideally from a single individual. Co-extracting contaminants such as mucopolysaccharides and polyphenols are often noted as a potential problem in that they may hinder library preparation and/or sequencing steps (although the reality is that the precise cause is rarely known or investigated). The usual mitigation strategy is to try a variety of extraction methods [146–148], which frequently involve a reagent such as CTAB [149], and then to select the most appropriate approach for the chosen organism.
It is a continuing problem that there is no high-molecular weight extraction method that routinely works for all molluscan taxa, exacerbated by the different but ill-defined demands of the various sequencing platforms. In our experience, a further problem is that there is considerable variation in DNA quality from individuals extracted at the same time, using the same method.
Improving both DNA preservation and extraction techniques will be important with respect to a suite of new directives (e.g. Earth Biogenome project [150]) that aim to sequence the genomes of all known eukaryotic taxa. For example, in the UK, the Sanger Tree of Life project has committed to sequencing the genomes of all native species within a few years [151]. In this case, the pinch-points are likely to be associated with technical issues such as DNA extraction or specimen collection and identification, rather than the sequencing itself.
There is also considerable potential in using the extraction of environmental DNA to monitor and/or identify molluscs [152–155]. The ‘ancient’ DNA that remains in molluscan shells and other material, including subfossil and museum species, also has the potential to transform our study of the past ecological and evolutionary dynamics. In this latter respect, several new studies have provided successful proof-of-principle [156–158]. While the methods for the extraction of ancient DNA have been available for some time, improved and cheaper sequencing technologies have enabled substantial recent progress in the insights that can be generated from this DNA. Access to a high-quality genome assembly for each species will provide another qualitative step forward, by enabling subtractive bioinformatic methods when sequencing degraded material.
15. Greater diversity in molluscan models
Despite our opening argument for transformative benefits associated with the availability of a diverse set of molluscan models, the reality is that the majority of molluscan research, including genomics, has mainly been restricted to the gastropods and bivalves, with a select group working on cephalopods. There are relatively few genomic studies and model species that represent the other classes, including the Scaphopoda (tusk shells), Monoplacophora, Polyplacophora (chitons), and the Aplacophora (Caudofoveata and Solenogastres). The notable exceptions are a growing resource of broad phylogenomic studies that have aimed to understand molluscan phylogeny and origins, mainly using transcriptome sequences [159,160] but recently also genome data using strategically selected genomes [2,161].
The lack of representative species from these other groups, mainly linked to difficulties in obtaining specimens, their sometimes small size and their (lack of) maintenance in a laboratory, is a continuing problem. The rapid improvement in technology and costs associated with methods for genomic resource development provides at least a partial solution. Even if a particular taxon is difficult to collect and study while alive, it is nevertheless now straightforward to develop genomic resources, which may then be used in comparative studies. Thus, in this respect, the first chiton [97] and monoplacophoran [2] genome assemblies are important because they will advance the study of molluscan and animal evolution.
16. Concluding remarks
While molluscan genome assemblies are becoming commonplace, the absence of a fully ‘mobilized’ model mollusc means that there remain substantial challenges in devising methods to interrogate molluscan biology. However, as others have acknowledged [14], a single species is unlikely to ever meet all of the requirements. It remains the case that the biological question should dictate the species used.
We acknowledge that structural issues with respect to the study of molluscs (e.g. transgenesis, DNA extraction, cell culture, large and repetitive genomes) will be difficult to overcome. But by making the case that there are considerable benefits to studying molluscs, both from the perspective of general biology and for human health and well-being, we believe that major progress is still possible, by striving for a cohesive research community [50] that will ensure that this important phylum, and the wider grouping of Spiralia and Lophotrochozoa to which it belongs, is no longer neglected.
Acknowledgements
This review was stimulated by the many conversations that we had with fellow scientists during the Theo Murphy international scientific meeting ‘Pearls of wisdom: synergising leadership and expertise in molluscan genomics', which was held at the Chicheley Hall 16th–17th September 2019. We are grateful to all who participated. Thanks also to Joris Koene, Yumi Nakadera, and an anonymous referee for their helpful comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
Both A.D. and M.N. conceived and wrote the paper together.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Rosenberg G. 2014. A new critical estimate of named species-level diversity of the recent Mollusca. Am. Malacological Bull. 32, 308-322. ( 10.4003/006.032.0204) [DOI] [Google Scholar]
- 2.Kocot KM, Poustka AJ, Stöger I, Halanych KM, Schrödl M. 2020. New data from Monoplacophora and a carefully-curated dataset resolve molluscan relationships. Sci. Rep. 10, 101. ( 10.1038/s41598-019-56728-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes-dos-Santos A, Lopes-Lima M, Castro LFC, Froufe E. 2020. Molluscan genomics: the road so far and the way forward. Hydrobiologia 847, 1705-1726. ( 10.1007/s10750-019-04111-1) [DOI] [Google Scholar]
- 4.Yang Z, Zhang L, Hu J, Wang J, Bao Z, Wang S. 2020. The evo-devo of molluscs: insights from a genomic perspective. Evol. Dev. 22, e12336. ( 10.1111/ede.12336) [DOI] [PubMed] [Google Scholar]
- 5.Russell JJ, et al. 2017. Non-model model organisms. BMC Biol. 15, 55. ( 10.1186/s12915-017-0391-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz PS. 2016. ‘Model organisms’ in the light of evolution. Curr. Biol. 26, R649-R650. ( 10.1016/j.cub.2016.05.071) [DOI] [PubMed] [Google Scholar]
- 7.Dietrich MR, Ankeny RA, Chen PM. 2014. Publication trends in model organism research. Genet. 198, 787-794. ( 10.1534/genetics.114.169714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caurcel C, Laetsch DR, Challis R, Kumar S, Gharbi K, Blaxter M. 2021. MolluscDB: a genome and transcriptome database for molluscs. Phil. Trans. R. Soc. B 376, 20200157. ( 10.1098/rstb.2020.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS. 2015. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524, 220-224. ( 10.1038/nature14668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, et al. 2019. A chromosomal-level genome assembly for the giant African snail Achatina fulica. GigaScience 8, giz124. ( 10.1093/gigascience/giz124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, et al. 2020. The Scaly-foot Snail genome and implications for the origins of biomineralised armour. Nat. Commun. 11, 1657. ( 10.1038/s41467-020-15522-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez AA. 2018. To solve old problems, study new research organisms. Dev. Biol. 433, 111-114. ( 10.1016/j.ydbio.2017.09.018) [DOI] [PubMed] [Google Scholar]
- 13.Kocot KM, Aguilera F, McDougall C, Jackson DJ, Degnan BM. 2016. Sea shell diversity and rapidly evolving secretomes: insights into the evolution of biomineralization. Front. Zool. 13, 23. ( 10.1186/s12983-016-0155-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark MS, et al. 2020. Deciphering mollusc shell production: the roles of genetic mechanisms through to ecology, aquaculture and biomimetics. Biol. Rev. 95, 1812-1837. ( 10.1111/brv.12640) [DOI] [PubMed] [Google Scholar]
- 15.Fodor I, Hussein AAA, Benjamin PR, Koene JM, Pirger Z. 2020. The unlimited potential of the great pond snail, Lymnaea stagnalis. eLife 9, e56962. ( 10.7554/eLife.56962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews BJ, Vosshall LB. 2020. How to turn an organism into a model organism in 10 ‘easy’ steps. J. Exp. Biol. 223, jeb218198. ( 10.1242/jeb.218198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eveland LK, Haseeb MA. 2011. Laboratory rearing of Biomphalaria glabrata snails and maintenance of larval schistosomes in vivo and in vitro. In Biomphalaria snails and larval trematodes (eds Toledo R, Fried B), pp. 33-55. New York, NY: Springer. [Google Scholar]
- 18.Mouton S, Wudarski J, Grudniewska M, Berezikov E. 2018. The regenerative flatworm Macrostomum lignano, a model organism with high experimental potential. Int. J. Dev. Biol. 62, 551-558. ( 10.1387/ijdb.180077eb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, McDougall C, Troscianko J, Chen W-C, Jayaraman-Nagarajan A, Shimeld SM, Ferrier DEK. 2009. An EST screen from the annelid Pomatoceros lamarcki ireveals patterns of gene loss and gain in animals. BMC Evol. Biol. 9, 240. ( 10.1186/1471-2148-9-240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y-J, Kanda M, Koyanagi R, Hisata K, Akiyama T, Sakamoto H, Sakamoto T, Satoh N. 2018. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nat. Ecol. Evol. 2, 141-151. ( 10.1038/s41559-017-0389-y) [DOI] [PubMed] [Google Scholar]
- 21.Guijarro-Clarke C, Holland PWH, Paps J. 2020. Widespread patterns of gene loss in the evolution of the animal kingdom. Nat. Ecol. Evol. 4, 519-523. ( 10.1038/s41559-020-1129-2) [DOI] [PubMed] [Google Scholar]
- 22.Amodio P, Boeckle M, Schnell AK, Ostojíc L, Fiorito G, Clayton NS. 2019. Grow smart and die young: why did cephalopods evolve intelligence? Trends Ecol. Evol. 34, 45-56. ( 10.1016/j.tree.2018.10.010) [DOI] [PubMed] [Google Scholar]
- 23.Gross M. 2015. Intelligent life without bones. Curr. Biol. 25, R775-R777. ( 10.1016/j.cub.2015.08.061) [DOI] [PubMed] [Google Scholar]
- 24.Allan ERO, Tennessen JA, Bollmann SR, Hanington PC, Bayne CJ, Blouin MS. 2017. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Neglected Trop. Dis. 11, e0005362. ( 10.1371/journal.pntd.0005362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo MG, Humphries JE, Mourao MM, Marquez J, Gonzalez A, Montelongo CE. 2020. Biomphalaria glabrata immunity: post-genome advances. Dev. Comp. Immunol. 104, 103557. ( 10.1016/j.dci.2019.103557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, McDougall C, Green K, Simpson F, Worheide G, Degnan BM. 2006. A rapidly evolving secretome builds and patterns a sea shell. BMC Biol. 4, 40. ( 10.1186/1741-7007-4-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, et al. 2012. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490, 49-54. ( 10.1038/nature11413) [DOI] [PubMed] [Google Scholar]
- 28.Herlitze I, Marie B, Marin F, Jackson DJ. 2018. Molecular modularity and asymmetry of the molluscan mantle revealed by a gene expression atlas. Gigascience 7, giy056. ( 10.1093/gigascience/giy056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann K, Jackson DJ. 2014. Characterization of the pigmented shell-forming proteome of the common grove snail Cepaea nemoralis. BMC Genomics 15, 249. ( 10.1186/1471-2164-15-249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bridger JM, Brindley PJ, Knight M. 2018. The snail Biomphalaria glabrata as a model to interrogate the molecular basis of complex human diseases. PLoS Neglected Trop. Dis. 12, e0006552. ( 10.1371/journal.pntd.0006552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez S, Sanchez-Marin P, Bellas J, Vinas L, Besada V, Fernandez N. 2019. Limpets (Patella spp. Mollusca, Gastropoda) as model organisms for biomonitoring environmental quality. Ecol. Indic. 101, 150-162. ( 10.1016/j.ecolind.2019.01.016) [DOI] [Google Scholar]
- 32.Robledo JAF, et al. 2019. From the raw bar to the bench: bivalves as models for human health. Dev. Comp. Immunol. 92, 260-282. ( 10.1016/j.dci.2018.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tascedda F, Malagoli D, Accorsi A, Rigillo G, Blom J.MC, Ottaviani E. 2015. Molluscs as models for translational medicine. Med. Sci. Monit. Basic Res. 21, 96-99. ( 10.12659/MSMBR.894221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malagoli D. 2018. Going beyond a static picture: the apple snail Pomacea canaliculata can tell us the life history of molluscan hemocytes. ISJ-Invert. Surviv. J. 15, 61-65. ( 10.25431/1824-307X/isj.v15i1.61-65) [DOI] [Google Scholar]
- 35.Carls-Diamante S. 2017. The octopus and the unity of consciousness. Biol. Phil. 32, 1269-1287. ( 10.1007/s10539-017-9604-0) [DOI] [Google Scholar]
- 36.Hayes KA, Cowie RH, Jørgensen A, Schultheiß R, Albrecht C, Thiengo SC. 2009. Molluscan models in evolutionary biology: apple snails (Gastropoda: Ampullariidae) as a system for addressing fundamental questions. Am. Malacological Bull. 27, 47-58. ( 10.4003/006.027.0204) [DOI] [Google Scholar]
- 37.Henry JQ, Lyons DC. 2016. Molluscan models: Crepidula fornicata. Curr. Opin. Genet. Dev. 39, 138-148. ( 10.1016/j.gde.2016.05.021) [DOI] [PubMed] [Google Scholar]
- 38.Goulding MQ, Lambert JD. 2016. Mollusc models I. The snail Ilyanassa. Curr. Opin. Genet. Dev. 39, 168-174. ( 10.1016/j.gde.2016.07.007) [DOI] [PubMed] [Google Scholar]
- 39.Jones JS, Leith BH, Rawlings P. 1977. Polymorphism in Cepaea: a problem with too many solutions? Annu. Rev. Ecol. Syst. 8, 109-143. ( 10.1146/annurev.es.08.110177.000545) [DOI] [Google Scholar]
- 40.Cain AJ, Sheppard PM. 1954. Natural selection in Cepaea. Genetics 39, 89-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van't Hof AE, Campagne P, Rigden DJ, Yung CJ, Lingley J, Quail MA, Hall N, Darby AC, Saccheri IJ. 2016. The industrial melanism mutation in British peppered moths is a transposable element. Nature 534, 102-105. ( 10.1038/nature17951) [DOI] [PubMed] [Google Scholar]
- 42.Merrill RM, et al. 2015. The diversification of Heliconius butterflies: what have we learned in 150 years? J. Evol. Biol. 28, 1417-1438. ( 10.1111/jeb.12672) [DOI] [PubMed] [Google Scholar]
- 43.Emlen DJ, Zimmer C. 2020. Evolution: making sense of life, 3rd edn. New York, NY: Macmillan. [Google Scholar]
- 44.Simakov O, et al. 2013. Insights into bilaterian evolution from three spiralian genomes. Nature 493, 526-531. ( 10.1038/nature11696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouetard A, Besnard AL, Vassaux D, Lagadic L, Coutellec MA. 2013. Impact of the redox-cycling herbicide diquat on transcript expression and antioxidant enzymatic activities of the freshwater snail Lymnaea stagnalis. Aquat. Toxicol. 126, 256-265. ( 10.1016/j.aquatox.2012.11.013) [DOI] [PubMed] [Google Scholar]
- 46.Nakadera Y, Smith AT, Daupagne L, Coutellec M-A, Koene JM, Ramm SA. 2020. Divergence of seminal fluid gene expression and function among natural snail populations. J. Evol. Biol. 33, 1440-1451. ( 10.1111/jeb.13683) [DOI] [PubMed] [Google Scholar]
- 47.Gilbert C, Schaack S, Pace JK, Brindley PJ, Feschotte C. 2010. A role for host–parasite interactions in the horizontal transfer of transposons across phyla. Nature 464, 1347-1350. ( 10.1038/nature08939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skala V, Walker AJ, Horak P. 2020. Snail defence responses to parasite infection: the Lymnaea stagnalis–Trichobilharzia szidati model. Dev. Comp. Immunol. 102, 103464. ( 10.1016/j.dci.2019.103464) [DOI] [PubMed] [Google Scholar]
- 49.Davison A, et al. 2016. Formin is associated with left–right asymmetry in the pond snail and the frog. Curr. Biol. 26, 654-660. ( 10.1016/j.cub.2015.12.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anonymous. 2020. Spiraliabase. 22/09/2020. See https://www.spiraliabase.org/.
- 51.Anonymous. 2020. MolluscaBase 22/09/2020. See http://www.molluscabase.org.
- 52.Challis RJ, Kumar S, Stevens L, Blaxter M. 2017. GenomeHubs: simple containerized setup of a custom Ensembl database and web server for any species. Database 2017, bax039. ( 10.1093/database/bax039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metzger MJ, Villalba A, Carballal MJ, Iglesias D, Sherry J, Reinisch C, Muttray AF, Baldwin SA, Goff SP. 2016. Widespread transmission of independent cancer lineages within multiple bivalve species. Nature 534, 705-709. ( 10.1038/nature18599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potts R, Gutierrez AP, Penaloza CS, Regan T, Bean TP, Houston RD. 2021. Potential of genomic technologies to improve disease resistance in molluscan aquaculture. Phil. Trans. R. Soc. B 376, 20200168. ( 10.1098/rstb.2020.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollenbeck CM, Johnston IA. 2018. Genomic tools and selective breeding in molluscs. Front. Genet. 9, 253. ( 10.3389/fgene.2018.00253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davison A. 2020. Flipping shells: unwinding LR asymmetry in mirror-image molluscs. Trends Genet. 36, 189-202. ( 10.1016/j.tig.2019.12.003) [DOI] [PubMed] [Google Scholar]
- 57.Wang S, et al. 2017. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat. Ecol. Evol. 1, 120. ( 10.1038/s41559-017-0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grande C, Patel NH. 2009. Nodal signalling is involved in left–right asymmetry in snails. Nature 405, 1007-1011. ( 10.1038/nature07603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotez PJ, et al. 2014. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Neglected Trop. Dis. 8, e2865. ( 10.1371/journal.pntd.0002865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. 2012. The tumorigenic liver fluke Opisthorchis viverrini – multiple pathways to cancer. Trends Parasitol. 28, 395-407. ( 10.1016/j.pt.2012.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu X-T, Gu Q-Y, Limpanont Y, Song L-G, Wu Z-D, Okanurak K, Lv Z-Y. 2018. Snail-borne parasitic diseases: an update on global epidemiological distribution, transmission interruption and control methods. Infect. Dis. Poverty 7, 28. ( 10.1186/s40249-018-0414-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokolow SH, et al. 2016. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Neglected Trop. Dis. 10, e0004794. ( 10.1371/journal.pntd.0004794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adema CM, et al. 2017. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 8, 14451. ( 10.1038/ncomms15451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tennessen JA, Bollmann SR, Peremyslova E, Kronmiller BA, Sergi C, Hamali B, Blouin MS. 2020. Clusters of polymorphic transmembrane genes control resistance to schistosomes in snail vectors. eLife 9, e59395. ( 10.7554/eLife.59395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tennessen JA, Bollmann SR, Blouin MS. 2017. A targeted capture linkage map anchors the genome of the schistosomiasis vector snail, Biomphalaria glabrata. G3-Genes Genomes Genetics 7, 2353-2361. ( 10.1534/g3.117.041319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larson MK, Bender RC, Bayne CJ. 2014. Resistance of Biomphalaria glabrata 13–16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int. J. Parasitol. 44, 343-353. ( 10.1016/j.ijpara.2013.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshino TP, Bickham U, Bayne CJ. 2013. Molluscan cells in culture: primary cell cultures and cell lines. Can. J. Zool. 91, 391-404. ( 10.1139/cjz-2012-0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansen EL. 1976. A cell line from embryos of Biomphalaria glabrata (Pulmonata): establishment and characteristics. In Invertebrate tissue culture research applications (ed. Maramorosch K), pp. 75-99. New York, NY: Academic Press. [Google Scholar]
- 69.Pila EA, Li HY, Hambrook JR, Wu XZ, Hanington PC. 2017. Schistosomiasis from a snail's perspective; advances in snail immunity. Trends Parasitol. 33, 845-857. ( 10.1016/j.pt.2017.07.006) [DOI] [PubMed] [Google Scholar]
- 70.Adema CM, Loker ES. 2015. Digenean-gastropod host associations inform on aspects of specific immunity in snails. Dev. Comp. Immunol. 48, 275-283. ( 10.1016/j.dci.2014.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang SM, Adema CM, Kepler TB, Loker ES. 2004. Diversification of Ig superfamily genes in an invertebrate. Science 305, 251-254. ( 10.1126/science.1088069) [DOI] [PubMed] [Google Scholar]
- 72.Brossard D, Belluck P, Gould F, Wirz CD. 2019. Promises and perils of gene drives: navigating the communication of complex, post-normal science. Proc. Natl Acad. Sci. USA 116, 7692-7697. ( 10.1073/pnas.1805874115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hotez PJ, Pecoul B, Rijal S, Boehme C, Aksoy S, Malecela M, Tapia-Conyer R, Reeder JC. 2016. Eliminating the neglected tropical diseases: translational science and new technologies. PLoS Neglected Trop. Dis. 10, e0003895. ( 10.1371/journal.pntd.0003895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sokolow SH, Wood CL, Jones IJ, Lafferty KD, Kuris AM, Hsieh MH, De Leo GA.. 2018. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends Parasitol. 34, 23-40. ( 10.1016/j.pt.2017.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fodor I, Urban P, Kemenes G, Koene JM, Pirger Z. 2020. Aging and disease-relevant gene products in the neuronal transcriptome of the great pond snail (Lymnaea stagnalis): a potential model of aging, age-related memory loss, and neurodegenerative diseases. Invert. Neurosci. 20, 9. ( 10.1007/s10158-020-00242-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu MM, Davey JW, Banerjee R, Han J, Yang F, Aboobaker A, Blaxter ML, Davison A. 2013. Fine mapping of the pond snail left-right asymmetry (chirality) locus using RAD-Seq and fibre-fish. PLoS ONE 8, e71067. ( 10.1371/journal.pone.0071067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davison A, Frend HT, Moray C, Wheatley H, Searle LJ, Eichhorn MP. 2009. Mating behaviour in Lymnaea stagnalis pond snails is a maternally inherited, lateralized trait. Biol. Lett. 5, 20-22. ( 10.1098/rsbl.2008.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davison A, Barton NH, Clarke B. 2009. The effect of coil phenotypes and genotypes on the fecundity and viability of Partula suturalis and Lymnaea stagnalis: implications for the evolution of sinistral snails. J. Evol. Biol. 22, 1624-1635. ( 10.1111/j.1420-9101.2009.01770.x) [DOI] [PubMed] [Google Scholar]
- 79.Johnson HF, Davison A. 2019. A new set of endogenous control genes for use in quantitative real-time PCR experiments show that formin Ldia2dex transcripts are enriched in the early embryo of the pond snail Lymnaea stagnalis (Panpulmonata). J. Molluscan Stud. 85, 389-397. ( 10.1101/660381) [DOI] [Google Scholar]
- 80.Koene JM, Sloot W, Montagne-Wajer K, Cummins SF, Degnan BM, Smith JS, Nagle GT, ter Maat A. 2010. Male accessory gland protein reduces egg laying in a simultaneous hermaphrodite. PLoS ONE 5, e0010117. ( 10.1371/journal.pone.0010117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swart EM, Davison A, Ellers J, Filangieri RR, Jackson DJ, Marien J, van der Ouderaa IBC, Roelofs D, Koene JM.. 2019. Temporal expression profile of an accessory-gland protein that is transferred via the seminal fluid of the simultaneous hermaphrodite Lymnaea stagnalis. J. Molluscan Stud. 85, 177-183. ( 10.1093/mollus/eyz005) [DOI] [Google Scholar]
- 82.Abe M, Kuroda R. 2019. The development of CRISPR for a mollusc establishes the formin Lsdia1 as the long-sought gene for snail dextral/sinistral coiling. Development 146, dev.175976. ( 10.1242/dev.175976) [DOI] [PubMed] [Google Scholar]
- 83.Kuroda R, Abe M. 2020. Response to ‘Formin, an opinion’. Development 147, dev187435. ( 10.1242/dev.187435) [DOI] [PubMed] [Google Scholar]
- 84.Davison A, McDowell GS, Holden JM, Johnson HF, Wade CM, Chiba S, Jackson DJ, Levin M, Blaxter ML. 2020. Formin, an opinion. Development 147, dev187427. ( 10.1242/dev.187427) [DOI] [PubMed] [Google Scholar]
- 85.Maynard SJ. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 86.Gibson AK, Delph LF, Vergara D, Lively CM. 2018. Periodic, parasite-mediated aelection for and against sex. Am. Nat. 192, 537-551. ( 10.1086/699829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lively CM. 1987. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328, 519-521. ( 10.1038/328519a0) [DOI] [Google Scholar]
- 88.Wilton PR, Sloan DB, Logsdon JM, Doddapaneni H, Neiman M. 2013. Characterization of transcriptomes from sexual and asexual lineages of a New Zealand snail (Potamopyrgus antipodarum). Mole. Ecol. Res. 13, 289-294. ( 10.1111/1755-0998.12051) [DOI] [PubMed] [Google Scholar]
- 89.Bankers L, Fields P, McElroy KE, Boore JL, Logsdon JM, Neiman M. 2017. Genomic evidence for population-specific responses to co-evolving parasites in a New Zealand freshwater snail. Mol. Ecol. 26, 3663-3675. ( 10.1111/mec.14146) [DOI] [PubMed] [Google Scholar]
- 90.Paczesniak D, Jokela J, Larkin K, Neiman M. 2013. Discordance between nuclear and mitochondrial genomes in sexual and asexual lineages of the freshwater snail Potamopyrgus antipodarum. Mol. Ecol. 22, 4695-4710. ( 10.1111/mec.12422) [DOI] [PubMed] [Google Scholar]
- 91.Jalinsky J, Logsdon JM, Neiman M. 2020. Male phenotypes in a female framework: evidence for degeneration in sperm produced by male snails from asexual lineages. J. Evol. Biol. 33, 1050-1059. ( 10.1111/jeb.13632) [DOI] [PubMed] [Google Scholar]
- 92.Sharbrough J, Luse M, Boore JL, Logsdon JM, Neiman M. 2018. Radical amino acid mutations persist longer in the absence of sex. Evolution 72, 808-824. ( 10.1111/evo.13465) [DOI] [PubMed] [Google Scholar]
- 93.Donne C, Neiman M, Woodell JD, Haase M, Verhaegen G. 2020. A layover in Europe: reconstructing the invasion route of asexual lineages of a New Zealand snail to North America. Mol. Ecol. 29, 3446-3465. ( 10.1111/mec.15569) [DOI] [PubMed] [Google Scholar]
- 94.Logsdon JM, Neiman M, Boore J, Sharbrough J, Bankers L, McElroy K, Jalinsky J, Fields P, Wilton P. 2017. A very recent whole genome duplication in Potamopyrgus antipodarum predates multiple origins of asexuality & associated polyploidy. Peerj Preprints 5, e3046v3041. ( 10.7287/peerj.preprints.3046v1) [DOI] [Google Scholar]
- 95.Clark MS. 2020. Molecular mechanisms of biomineralization in marine invertebrates. J. Exp. Biol. 223, jeb206961. ( 10.1242/jeb.206961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wollesen T, Scherholz M, Rodríguez Monje SV, Redl E, Todt C, Wanninger A. 2017. Brain regionalization genes are co-opted into shell field patterning in Mollusca. Sci. Rep. 7, 5486. ( 10.1038/s41598-017-05605-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varney RM, Speiser DI, McDougall C, Degnan BM, Kocot KM. 2021. The iron-responsive genome of the chiton Acanthopleura granulata. Genome Biol. Evol. 13, evaa263. ( 10.1093/gbe/evaa263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lambert JD. 2010. Developmental patterns in spiralian embryos. Curr. Biol. 20, R72-R77. ( 10.1016/j.cub.2009.11.041) [DOI] [PubMed] [Google Scholar]
- 99.Henry JQ. 2014. Spiralian model systems. Int. J. Dev. Biol. 58, 389-401. ( 10.1387/ijdb.140127jh) [DOI] [PubMed] [Google Scholar]
- 100.Henry JQ, Okusu A, Martindale MQ. 2004. The cell lineage of the polyplacophoran, Chaetopleura apiculata: variation in the spiralian program and implications for molluscan evolution. Dev. Biol. 272, 145-160. ( 10.1016/j.ydbio.2004.04.027) [DOI] [PubMed] [Google Scholar]
- 101.Conklin EG. 1897. The embryology of Crepidula, a contribution to the cell lineage and early development of some marine gasteropods. J. Morphol. 13, 1-226. ( 10.1002/jmor.1050130102) [DOI] [Google Scholar]
- 102.Osborne CC, Perry KJ, Shankland M, Henry JQ. 2018. Ectomesoderm and epithelial–mesenchymal transition-related genes in spiralian development. Dev. Dyn. 247, 1097-1120. ( 10.1002/dvdy.24667) [DOI] [PubMed] [Google Scholar]
- 103.Perry KJ, Lyons DC, Truchado-Garcia M, Fischer AHL, Helfrich LW, Johansson KB, Diamond JC, Grande C, Henry JQ. 2015. Deployment of regulatory genes during gastrulation and germ layer specification in a model spiralian mollusc Crepidula. Dev. Dyn. 244, 1215-1248. ( 10.1002/dvdy.24308) [DOI] [PubMed] [Google Scholar]
- 104.Henry JJ, Perry KJ, Fukui L, Alvi N. 2010. Differential localization of mRNAs during early development in the mollusc, Crepidula fornicata. Integr. Comp. Biol. 50, 720-733. ( 10.1093/icb/icq088) [DOI] [PubMed] [Google Scholar]
- 105.Perry KJ, Henry JQ. 2015. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis 53, 237-244. ( 10.1002/dvg.22843) [DOI] [PubMed] [Google Scholar]
- 106.Henry JQ, Lesoway MP, Perry KJ. 2020. An automated aquatic rack system for rearing marine invertebrates. BMC Biol. 18, 46. ( 10.1186/s12915-020-00772-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henry JQ, Lesoway MP, Perry KJ, Osborne CC, Shankland M, Lyons DC. 2017. Beyond the sea: Crepidula atrasolea as a spiralian model system. Int. J. Dev. Biol. 61, 479-493. ( 10.1387/ijdb.170110jh) [DOI] [PubMed] [Google Scholar]
- 108.O'Brien CE, Roumbedakis K, Winkelmann IE. 2018. The current state of cephalopod science and perspectives on the most critical challenges ahead from three early-career researchers. Front. Physiol. 9, 700. ( 10.3389/fphys.2018.00700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.da Fonseca RR, et al. 2020. A draft genome sequence of the elusive giant squid, Architeuthis dux. GigaScience 9, giz152. ( 10.1093/gigascience/giz152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crawford K, Diaz Quiroz JF, Koenig KM, Ahuja N, Albertin CB, Rosenthal JJC. 2020. Highly efficient knockout of a squid pigmentation gene. Curr. Biol. 30, 3484-3490. ( 10.1016/j.cub.2020.06.099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serb JM, Eernisse DJ. 2008. Charting evolution's trajectory: using molluscan eye diversity to understand parallel and convergent evolution. Evol. Educ. Outreach 1, 439-447. ( 10.1007/s12052-008-0084-1) [DOI] [Google Scholar]
- 112.Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH. 2016. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol. Evol. 8, 3640-3652. ( 10.1093/gbe/evw248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y, et al. 2017. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 8, 1721. ( 10.1038/s41467-017-01927-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Imarazene B, Andouche A, Bassaglia Y, Lopez P-J, Bonnaud-Ponticelli L. 2017. Eye development in Sepia officinalis embryo: what the uncommon gene expression profiles tell us about eye evolution. Front. Physiol. 8, 613. ( 10.3389/fphys.2017.00613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Navet S, Buresi A, Baratte S, Andouche A, Bonnaud-Ponticelli L, Bassaglia Y. 2017. The Pax gene family: highlights from cephalopods. PLoS ONE 12, e0172719. ( 10.1371/journal.pone.0172719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung S-H, Song HY, Hyun YS, Kim Y-C, Whang I, Choi T-Y, Jo S. 2018. A brain atlas of the long arm octopus, Octopus minor. Exp. Neurobiol. 27, 257-266. ( 10.5607/en.2018.27.4.257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deryckere A, Seuntjens E. 2018. The cephalopod large brain enigma: are conserved mechanisms of stem cell expansion the key? Front. Physiol. 9, 1160. ( 10.3389/fphys.2018.01160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anonymous. 2010. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Official J. Eur. Union 53, 33-79. [Google Scholar]
- 119.Gorson J, Holford M. 2016. Small packages, big returns: uncovering the venom diversity of small invertebrate conoidean snails. Integr. Comp. Biol. 56, 962-972. ( 10.1093/icb/icw063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lewis RJ, Dutertre S, Vetter I, Christie MJ. 2012. Conus venom peptide pharmacology. Pharmacol. Rev. 64, 259-298. ( 10.1124/pr.111.005322) [DOI] [PubMed] [Google Scholar]
- 121.Dutertre S, Jin AH, Kaas Q, Jones A, Alewood PF, Lewis RJ. 2013. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell. Proteomics 12, 312-329. ( 10.1074/mcp.M112.021469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu AP, et al. 2020. Transcriptomic profiling reveals extraordinary diversity of venom peptides in unexplored predatory gastropods of the genus Clavus. Genome Biol. Evol. 12, 684-700. ( 10.1093/gbe/evaa083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu H, Bandyopadhyay PK, Olivera BM, Yandell M. 2011. Characterization of the Conus bullatus genome and its venom-duct transcriptome. BMC Genomics 12, 60. ( 10.1186/1471-2164-12-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tayo LL, Lu B, Cruz LJ, Yates JR 3rd. 2010. Proteomic analysis provides insights on venom processing in Conus textile. J. Proteome Res. 9, 2292-2301. ( 10.1021/pr901032r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmidtko A, Lötsch J, Freynhagen R, Geisslinger G. 2010. Ziconotide for treatment of severe chronic pain. The Lancet 375, 1569-1577. ( 10.1016/S0140-6736(10)60354-6) [DOI] [PubMed] [Google Scholar]
- 126.Andreson R, Roosaare M, Kaplinski L, Laht S, Kõressaar T, Lepamets M, Brauer A, Kukuškina V, Remm M. 2019. Gene content of the fish-hunting cone snail Conus consors. bioRxiv, 590695. ( 10.1101/590695) [DOI]
- 127.Barghi N, Concepcion GP, Olivera BM, Lluisma AO. 2016. Structural features of conopeptide genes inferred from partial sequences of the Conus tribblei genome. Mol. Genet. Genomics 291, 411-422. ( 10.1007/s00438-015-1119-2) [DOI] [PubMed] [Google Scholar]
- 128.Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. 2018. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 36, 1062-1066. ( 10.1038/nbt.4245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang JF, You WW, Xu ZW, Yan QN, Shi CG, Tang B, Luo X, Li G, Ke CH. 2019. An effective microinjection method and TALEN-mediated genome editing in Pacific abalone. Mar. Biotechnol. 21, 441-447. ( 10.1007/s10126-019-09901-1) [DOI] [PubMed] [Google Scholar]
- 130.Yu H, Li HJ, Li Q, Xu R, Yue CY, Du SJ.. 2019. Targeted gene disruption in pacific oyster based on CRISPR/Cas9 ribonucleoprotein complexes. Mar. Biotechnol. 21, 301-309. ( 10.1007/s10126-019-09885-y) [DOI] [PubMed] [Google Scholar]
- 131.Pila EA, Tarrabain M, Kabore AL, Hanington PC. 2016. A novel toll-like receptor (TLR) influences compatibility between the gastropod Biomphalaria glabrata, and the digenean trematode Schistosoma mansoni. PLoS Pathog. 12, e1005513. ( 10.1371/journal.ppat.1005513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suzuki M, Saruwatari K, Kogure T, Yamamoto Y, Nishimura T, Kato T, Nagasawa H. 2009. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 325, 1388-1390. ( 10.1126/science.1173793) [DOI] [PubMed] [Google Scholar]
- 133.Fang D, Xu GR, Hu YL, Pan C, Xie LP, Zhang RQ. 2011. Identification of genes directly involved in shell formation and their functions in pearl oyster, Pinctada fucata. PLoS ONE 6, e021860. ( 10.1371/journal.pone.0021860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kines KJ, Mann VH, Morales ME, Shelby BD, Kalinna BH, Gobert GN, Chirgwin SR, Brindley PJ. 2006. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Exp. Parasitol. 112, 209-220. ( 10.1016/j.exppara.2006.02.003) [DOI] [PubMed] [Google Scholar]
- 135.Wilkins C, Dishongh R, Moore SC, Whitt MA, Chow M, Machaca K. 2005. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 436, 1044-1047. ( 10.1038/nature03957) [DOI] [PubMed] [Google Scholar]
- 136.Letchworth GJ, Rodriguez LL, Barrera JDC. 1999. Vesicular stomatitis. Vet. J. 157, 239-260. ( 10.1053/tvjl.1998.0303) [DOI] [PubMed] [Google Scholar]
- 137.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. 2013. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 110, 7306-7311. ( 10.1073/pnas.1214441110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaverra-Rodriguez D, Macias VM, Hughes GL, Pujhari S, Suzuki Y, Peterson DR, Kim D, McKeand S, Rasgon JL. 2018. Targeted delivery of CRISPR–Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nature Comm. 9, 3008. ( 10.1038/s41467-018-05425-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Robin FB, McFadden WM, Yao B, Munro EM. 2014. Single-molecule analysis of cell surface dynamics in Caenorhabditis elegans embryos. Nat. Methods 11, 677. ( 10.1038/nmeth.2928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shibazaki Y, Shimizu M, Kuroda R. 2004. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr. Biol. 14, 1462-1467. ( 10.1016/j.cub.2004.08.018) [DOI] [PubMed] [Google Scholar]
- 141.Rinaldi G, Yan HB, Nacif-Pimenta R, Matchimakul P, Bridger J, Mann VH, Smout MJ, Brindley PJ, Knight M. 2015. Cytometric analysis, genetic manipulation and antibiotic selection of the snail embryonic cell line Bge from Biomphalaria glabrata, the intermediate host of Schistosoma mansoni. Int. J. Parasitol. 45, 527-535. ( 10.1016/j.ijpara.2015.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshino TP, Coustau C, Modat S, Castillo MG. 1999. The Biomphalaria glabrata embryonic (Bge) molluscan cell line: Establishment of an in vitro cellular model for the study of snail host-parasite interactions. Malacologia 41, 331-343. [Google Scholar]
- 143.Yoshino TP, Laursen JR. 1995. Production of Schistosoma mansoni daughter sporocysts from mother sporocysts maintained in synxenic culture with Biomphalaria glabrata embryonic (Bge) cells. J. Parasitol. 81, 714-722. ( 10.2307/3283960) [DOI] [PubMed] [Google Scholar]
- 144.Wheeler NJ, Dinguirard N, Marquez J, Gonzalez A, Zamanian M, Yoshino TP, Castillo MG. 2018. Sequence and structural variation in the genome of the Biomphalaria glabrata embryonic (Bge) cell line. Parasites Vectors 11, 496. ( 10.1186/s13071-018-3059-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A. 2017. The hope and the hype of organoid research. Development 144, 938-941. ( 10.1242/dev.150201) [DOI] [PubMed] [Google Scholar]
- 146.Panova M, et al. 2016. DNA extraction protocols for whole-genome sequencing in marine organisms. In Marine genomics: methods and protocols (ed. Bourlat SJ), pp. 13-44. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 147.Galindo LA, Puillandre N, Strong EE, Bouchet P. 2014. Using microwaves to prepare gastropods for DNA barcoding. Mole. Ecol. Res. 14, 700-705. ( 10.1111/1755-0998.12231) [DOI] [PubMed] [Google Scholar]
- 148.Jaksch K, Eschner A, Rintelen TV, Haring E. 2016. DNA analysis of molluscs from a museum wet collection: a comparison of different extraction methods. BMC Res. Notes 9, 348. ( 10.1186/s13104-016-2147-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arseneau J-R, Steeves R, Laflamme M. 2017. Modified low-salt CTAB extraction of high-quality DNA from contaminant-rich tissues. Mole. Ecol. Resources 17, 686-693. ( 10.1111/1755-0998.12616) [DOI] [PubMed] [Google Scholar]
- 150.Lewin HA, et al. 2018. Earth biogenome project: sequencing life for the future of life. Proc. Natl Acad. Sci. USA 115, 4325-4333. ( 10.1073/pnas.1720115115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anonymous. 2020 04/08/2020. See https://www.sanger.ac.uk/collaboration/darwin-tree-life-project/.
- 152.West KM, Stat M, Harvey ES, Skepper CL, DiBattista JD, Richards ZT, Travers MJ, Newman SJ, Bunce M. 2020. eDNA metabarcoding survey reveals fine-scale coral reef community variation across a remote, tropical island ecosystem. Mol. Ecol. 29, 1069-1086. ( 10.1111/mec.15382) [DOI] [PubMed] [Google Scholar]
- 153.Goldberg CS, Sepulveda A, Ray A, Baumgardt J, Waits LP. 2013. Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum). Freshwater Sci. 32, 792-800. ( 10.1899/13-046.1) [DOI] [Google Scholar]
- 154.Clusa L, Miralles L, Basanta A, Escot C, Garcia-Vazquez E. 2017. eDNA for detection of five highly invasive molluscs. A case study in urban rivers from the Iberian Peninsula. PLoS ONE 12, e0188126. ( 10.1371/journal.pone.0188126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woodell JD, Neiman M, Levri EP. 2020. Matching a snail's pace: successful use of environmental DNA techniques to detect early stages of invasion by the destructive New Zealand mud snail. Biol. Invasions ( 10.1101/2020.08.12.248948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Der Sarkissian C, Möller P, Hofman CA, Ilsøe P, Rick TC, Schiøtte T, Sørensen MV, Dalén L, Orlando L.. 2020. Unveiling the ecological applications of ancient DNA from mollusk shells. Front. Ecol. Evol. 8, 37. ( 10.3389/fevo.2020.00037) [DOI] [Google Scholar]
- 157.Der Sarkissian C, et al. 2017. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Mole. Ecol. Resources 17, 835-853. ( 10.1111/1755-0998.12679) [DOI] [PubMed] [Google Scholar]
- 158.Villanea FA, Parent CE, Kemp BM. 2016. Reviving Galápagos snails: ancient DNA extraction and amplification from shells of probably extinct endemic land snails. J. Molluscan Stud. 82, 449-456. ( 10.1093/mollus/eyw011) [DOI] [Google Scholar]
- 159.Kocot KM, et al. 2011. Phylogenomics reveals deep molluscan relationships. Nature 477, 452-456. ( 10.1038/nature10382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kocot KM, Todt C, Mikkelsen NT, Halanych KM. 2019. Phylogenomics of Aplacophora (Mollusca, Aculifera) and a solenogaster without a foot. Proc. R. Soc. B 286, 20190115. ( 10.1098/rspb.2019.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sigwart JD, Lindberg DR, Chen C, Sun J. 2021. Molluscan phylogenomics requires strategically selected genomes. Phil. Trans. R. Soc. B 376, 20200161. ( 10.1098/rstb.2020.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.