Abstract
Purpose of review
To outline recent discoveries in epigenetic regulatory mechanisms that have potential implications in the development of renal fibrosis following kidney transplantation.
Recent findings
The characterization of renal fibrosis following kidney transplantation has shown TGFβ/Smad signaling to play a major role in the progression to chronic allograft dysfunction. The onset of unregulated proinflammatory pathways are only exacerbated by the decline in regulatory mechanisms lost with progressive patient age and comorbidities such as hypertension and diabetes. However, significant developments in the recognition of epigenetic regulatory markers upstream of aberrant TGFβ-signaling has significant clinical potential to provide therapeutic targets for the treatment of renal fibrosis. In addition, discoveries in extracellular vesicles and the characterization of their cargo has laid new framework for the potential to evaluate patient outcomes independent of invasive biopsies.
Summary
This review summarizes the main findings in epigenetic machinery specific to the development of renal fibrosis and highlights therapeutic options that have significant potential to translate into clinical practice.
Keywords: renal fibrosis, chronic allograft dysfunction, DNA methylation, lncRNA, miRNA, extracellular vesicles
Introduction
End-stage-renal disease (ESRD) continues to be a major world health problem and has been projected to increase by 29–68% in the next 10 years [1]. While dialysis and supportive care can prolong ESRD, kidney transplantation remains the best alternative to improve patient wellbeing and quality of life. However, complications of surgery or of the donor organ has reinstated many patients back to ESRD management or awaiting a secondary transplantation. These outcomes partially originate from the lack of criteria for evaluating the donor organ and the current limitation to predict long-term graft survival. Presently, clinical outcomes in kidney transplant patients are evaluated not only on clinical parameters (elevated systolic blood pressure, depressed glomerular filtration rate, albumin-to-creatinine ratio in urine) but also by the progressive development of interstitial fibrosis and tubular atrophy (IFTA) in successive post-transplant biopsies [2]. Developments in functional MRI have improved non-invasive diagnostics but have yet to replace the need for biopsies [3]. Instead, in an attempt to non-invasively monitor the kidney graft, transplant researchers now search for alternative methods of evaluating the graft and have partially turned toward the assessment of molecular biomarkers present in urine, plasma, and serum. Although molecular studies still, to some degree, rely upon graft biopsies, the improved evaluation of circulating markers can have a profound impact on reducing the number of biopsies done [4]. These studies, which are further strengthened by the advancement of new methodological advances such as single-cell RNAseq and the increased awareness of epigenetic fluctuations specific to both the donor organ and the recipient, aim to reframe how clinicians predict clinical outcomes [5]. In this review, we outline the major recent developments in renal fibrosis and characterize the extensive epigenetic machinery specific to fibrosis development in kidney transplant grafts, highlighting the current understanding of molecular biomarkers and the potential impact of such on clinical practice.
Pathogenesis of chronic allograft dysfunction to renal fibrosis
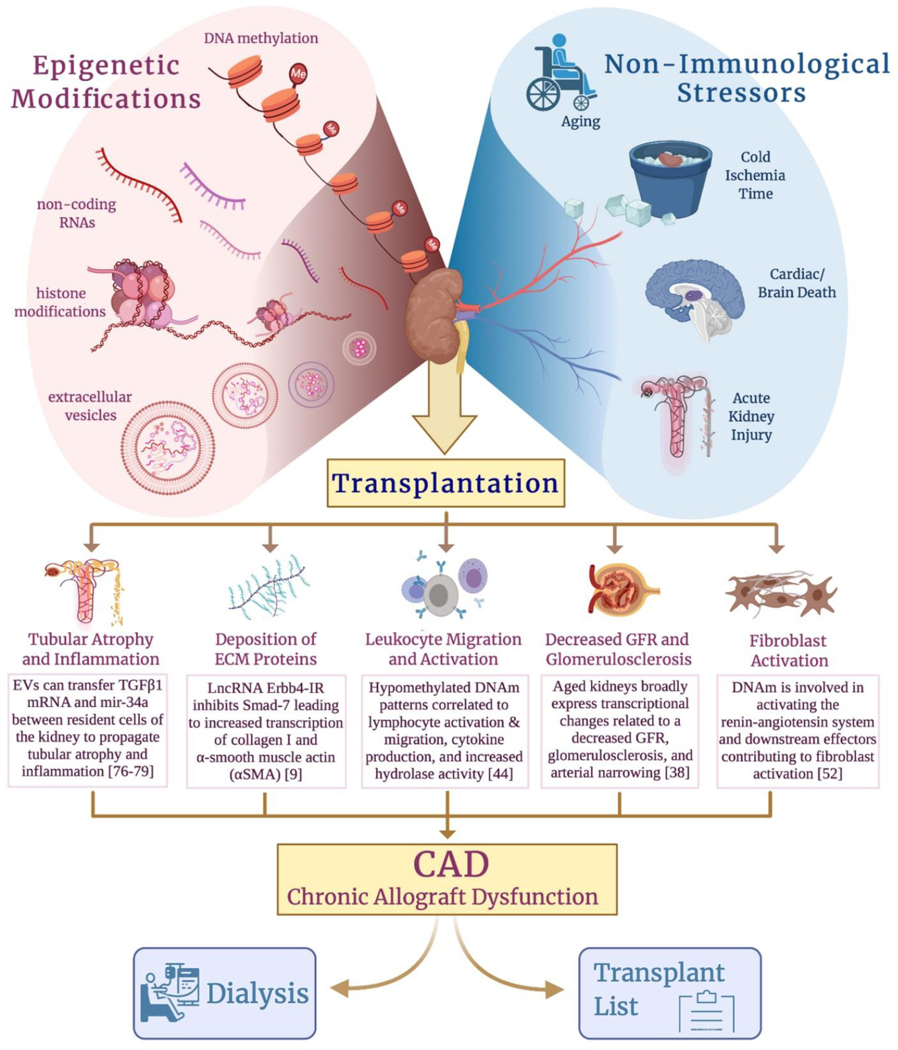
Late graft loss continues to be a major problem after kidney transplantation (KT), mainly as consequence of death with a functioning graft and intrinsic allograft failure (or chronic allograft dysfunction (CAD)). CAD remains a major cause of allograft attrition over time, resulting in reinstitution of end-stage renal disease care. CAD is considered by many to be a variant of chronic kidney disease (CKD), with both immune and non-immune mechanisms, contributing to the development of IFTA and progressive loss of graft function. Early development of graft fibrosis is predictive of late graft function. (Figure 1)
Figure 1.
Kidney transplantation remains one of the best options to restore patient wellbeing and quality of life following the induction of kidney disease. However, non-immunological stressors such as progressive age, prolonged cold ischemia time, donation after cardiac/brain death, or procurement of a kidney which suffered from a previous AKI can prompt epigenetic modifications that instigate dysfunctional recovery mechanisms following transplantation. These mechanisms then propagate towards the migration/activation of leukocytes, fibroblast activation, and deposition of ECM, ultimately culminating in the development of IFTA and decreased GFR through glomerulosclerosis. After the initiation of IFTA and decrease in GFR, the kidney graft will eventually progress to CAD and the patient will need to be returned to ESRD-management or re-enlisted on the transplant waiting list.
Specifically, renal fibrosis following transplantation is a multifactorial injury which originates from not only the damage incurred through the transplantation process but also via emerging mechanisms associated with endothelial-to-mesenchymal transition (EMT), cell cycle arrest in the G2/M phase, and metabolic disorders [6,7,8]. Upstream of these aberrant mechanisms are a milieu of dysregulated signaling pathways. Two of which, the TGFβ/Smad [9,10] and the Wnt/β-Catenin signaling axis [11,12], encompass most downstream effectors that are specific to kidney transplantation. It has been described that the degree of renal fibrosis and the associated risk to progress to CAD correlates to the length of cold ischemia time [13,14,15]. This would suggest that the severity of the ischemia-reperfusion injury (IRI) associated with the transplantation procedure instigates many of the dysregulated signaling pathways lending to dysfunctional recovery mechanisms and lays the foundation for the fibrotic scar.
IRI is associated with damage to the tubular epithelial cells characterized by the loss of cell-adhesion molecules and a denuded basement membrane, further contributing to damage of the proximal tubular cells and cast formation [16]. In ideal conditions, the activation of hypoxia-inducible factor-1-α (HIF1α) in concert with the ischemic damage to the proximal tubular cells would result in an upregulation of miRNA-23 from epithelial cells that function as a promoter of neutrophil and monocyte infiltration and induction of tubulointerstitial inflammation [17,18]. Resolution of macrophages from a pro-inflammatory phenotype (M1) to pro-repair (M2) signals the initiation of the re-epithelialization process [8]. However, secondary to prolonged cold ischemia or transplantation from marginal or so-called non-ideal organ donors (i.e., donation after cardiac/brain death, donor of advanced age) can trigger dysfunctional repair mechanisms. Ischemic damage, specifically, can upregulate the expression of long-noncoding RNA (lncRNA) Erbb4-IR which directly binds and inhibits Smad-7, a crucial regulator of TGFβ1’s inflammatory effects, leading to increased transcription of collagen I and α-smooth muscle actin (αSMA) [9]. With a currently ageing population, the number of donor organs from older recipients has increased during last years and is expected to continue, representing an important percentage of the available donor organ pool for kidney transplantation. In aged donors (from donors older than 65 yo.), aberrant TGFβ1 signaling is only exacerbated by the unregulated Wnt9a activity that is accompanied with progressive age [19].
Historically, therapeutic developments have targeted the TGFβ1/Smad signaling axis as a potential to halt the progression to fibrosis. More recently, it was found that TGFβ1 signaling could be redirected from profibrotic to anti-inflammatory through the delivery of T-cell factor, which enhanced the interaction between β-catenin and FoxO, thereby bolstering the differentiation of TGFβ1-induced regulatory T-cells (iTregs) [20*]. However, aberrant TGFβ1 signaling could be downstream to dysfunctional epigenetic regulatory mechanisms. In a study by Zhou et al., TGFβ1-induced EMT and the epithelial cell cycle arrest in the G2/M phase that are characteristic of progressive renal fibrosis were suppressed through the silencing of enhancer of zeste homolog-2 (EZH2), a methyltransferase that induces the trimethylation of histone H3 lysine 27 (H3K27m3). In fact, suppression of EZH2 led to the downregulation of key fibrotic genes, Snail-1 and Twist-1, suggesting that EZH2 might be upstream of multiple signaling pathways and could be a therapeutic target for treatment of renal fibrosis [21*]. (Figure 2)
Figure 2.
Prolonged cold ischemia time is directly correlated with the progression to IFTA and CAD in a transplanted kidney. Hypermethylation of the graft can induce dysregulated TGFβ-signaling which upregulates downstream effectors Smad 1–4, thereby contributing to the initiation of EndMT, cell cycle arrest in G2/M phase, and a host of metabolic disorders. This in concert with upregulated Wnt/β-Catenin signaling secondary to progressive age or co-morbidities, such as hypertension and diabetes, can further magnify TGFβ aberrant mechanisms through an increase in macrophage infiltration and shift polarization toward a pro-inflammatory M1 phenotype. However, recent therapeutic developments in the use of T-cell factor or the downregulation of EZH2 can shift TGFβ-signaling towards the anti-inflammatory Smad-7 and the increase differentiation of iTregs.
Epigenetics: new insights into chronic allograft dysfunction
A major shift in understanding fibrosis in renal grafts has been the recognition that epigenetic markers, regulatory processes which control gene expression without changing DNA sequence, integrate the various intrinsic and extrinsic regulatory mechanisms which trigger fibrogenesis. The epigenome represents the merging point between genetics and environment and, in the case of transplantation, the environment shifts from donor to recipient thereby providing a clear alteration in epigenomic processes, including DNA methylation (DNAm) patterns, histone modifications, and the action of non-coding RNAs (ncRNA), with microRNAs (miRNA) being the more studied in this last group.
DNA methylation in the kidney graft
DNA methylation (DNAm) occurs by the addition of a methyl group to the 5-carbon position of the cytosine ring in genomic DNA, thereby creating a 5-methylcytosine (5mC), by methyltransferase enzymes (DNMTs). Typically, these enzymes target areas rich in cytosine-guanine repeats, termed ‘CpG islands’ [22]. CpG islands are associated with the promoter regions of genes and are present in two states: Heterochromatin, highly methylated DNA with a multitude of histone repressive modifications, or Euchromatin, unmethylated and transcriptionally permissive regions [23]. Still more intergenic methylation can be present at enhancer or insulator elements at adjacent sites termed ‘CpG island shores’. CpG island shores present more variable DNAm patterns as compared to CpG islands and are highly correlated with variations in gene expression [24–27].
DNAm represents a relatively stable and reversible process. Its removal can be initiated by ten-11 translocation (TET) enzymes, which convert 5mC to 5-hydroxymethylcytosine (5hmC) [28,29]. These epigenetic processes thus shift with the environment from donor to recipient and in turn to the exacerbation of oxidative and inflammatory stress. Fluctuations in DNAm are represented in a variety of pathologies which could negatively affect graft outcomes. Aging has specifically been found to be associated with a loss of kidney integrity and expressed transcriptional changes secondary to aberrant structural remodeling, decreased blood flow, and a progressive decrease in glomerular filtration [30, 31, 32*]. Heylen et al. investigated the role of age-related changes in DNA methylation patterns in relation to post-transplant renal fibrosis. In the study, the authors found that TET demethylation enzymes were downregulated thereby contributing to age-associated hypermethylation. In addition, 11.5% of the assessed CpG islands and shores were found to be significantly hypermethylated. In the same study, Heylen et al. reported that loss of TET-activity, independent of cold ischemia time, contributed to the accumulation of oxidative stress and the accompanied loss of DNA hydroxymethylation. Accumulation of these changes contributed to the downregulation of key inhibitors (specifically Dkk1 and Dkk2) of the Wnt/β-Catenin signaling pathway [30], thereby stimulating the progression of interstitial fibrosis, glomerulosclerosis, and CAD (33–37).
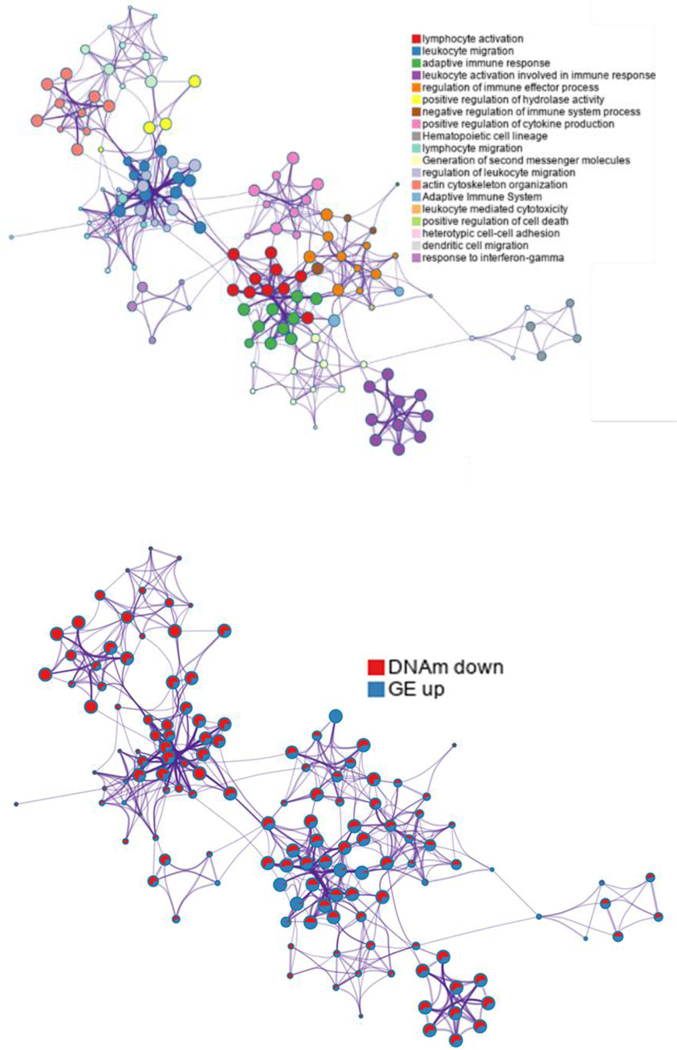
Unraveling the cause and effect of DNAm on graft survival independent of other confounding variables presented a major limitation in recognizing biomarkers. To this end, Bontha et al. investigated the use of a multidimensional systems approach that focused not only on global kidney graft DNAm, but also the downstream gene expression and variations in miRNA in pre- and post-transplant biopsies with progressive IFTA and declining graft function [38*]. Many of the mechanisms elucidated showed DNAm of CpG island shores to be the root cause for upregulating immune-mediated injury while suppressing metabolic mechanisms necessary for graft function. Further evaluations of data from our studies have shown significantly differentially hypomethylated DNAm patterns in kidney graft biopsies from transplant patients with histological evidence of IFTA after 24 months post-transplantation. When compared to gene expression patterns, the same biopsies showed lymphocyte activation, leukocyte migration, adaptive immune response, leukocyte activation involved in immune response, regulation of immune effector processes, positive regulation of hydrolase activity, positive regulation of cytokine production, lymphocyte migration, and regulation of leukocyte migration after evaluating gene enrichment functions (Figure 3). Otherwise, hypermethylated CpG genes related to decreased gene expression of metabolism-related genes (i.e. carbohydrate metabolic processes, cellular modified amino acid metabolic process, among other). These results strongly support a role of DNA methylation and regulation of downstream effectors during kidney graft fibrogenesis. (Figure 3)
Figure 3.
Network of enriched terms when using significantly differentially hypomethylated DNAm patterns and over-expressed genes identified from same kidney graft biopsies from transplant patients with histological evidence of IFTA after 24 months post-transplantation (a) colored by cluster ID, where nodes that share the same cluster ID are typically close to each other, (b) network of enriched terms presented as pie charts, where pies are color-coded based on the identities of the gene lists. DNAm: DNA methylation; IFTA: Interstitial Fibrosis and Tubular Atrophy
Micro RNAs and graft fibrosis
As mentioned above, recently miRNAs have come into focus as powerful epigenetic regulators of gene expression. MiRNAs are small, non-coding RNAs that cause the repression of target genes through the posttranscriptional degradation of mRNA and/or translational inhibition of protein expression [39]. Numerous miRNAs have been identified to reinforce the pathogenesis of interstitial fibrosis. Studies demonstrate that mir-155–5p [40,41], mir-21 [41–45], miR-103a-3p [46], mir-142–3p [44], mir-153–3p [47], mir-196b-5p [48], and mir-184 [49] promote renal fibrogenesis, while the mir-29 family [42,50], the mir-30 family [42], mir-342–3p [51], mir-133a [41], mir-200b [41], and mir-148a [52] inhibit apoptosis and fibrosis through a myriad of interconnected mechanisms.
A complex epigenetic network exists whereby even miRNA expression is under epigenetic control. In our previous study, integrative approaches were used to further explore whether DNAm could regulate gene expression indirectly via the regulation of miRNA. Through gene expression analyses of paired kidney allograft biopsies with IFTA, genes were identified that were regulated by hypomethylated miRNAs (located in the TSS region). Most of these genes were related to metabolomic processes and were notably downregulated [37].
Several fibrosis-associated miRNAs have recently been identified in kidney disease. In a recent study, Schauerte et al., using a murine model of allogenic kidney transplantation with CAD, identified fibrosis-associated miR-21a-5p by whole miRNAome expression analysis to be among the most highly upregulated miRNAs. In renal fibroblasts cultured in vitro, miR-21a-5p was transcriptionally activated by interleukin 6–induced signal transducer and activator of transcription 3. Co-culture of LPS-activated macrophages with renal fibroblasts increased expression levels of miR-21a-5p and markers of fibrosis and inflammation. In addition, mature miR-21a-5p was secreted by macrophages in small vesicles, which were internalized by renal fibroblasts, thereby promoting profibrotic and proinflammatory effects. Notch2 receptor was identified as a potential target of miR-21a-5p and validated by luciferase gene reporter assays. Therapeutic silencing of miR-21a-5p in mice after allogenic kidney transplantation resulted in an amelioration of CAD, as indicated by a reduction in fibrosis development, inflammatory cell influx, tissue injury and BANFF lesion scoring. The results support an antagonistic role of miR-21a-5p having beneficial effects on kidney function. Further evaluations of potential miR-21a-5p silencing may therefore be a viable therapeutic option in the treatment of patients following kidney transplantation to avoid/decrease the development of CAD [53].
Long-noncoding RNAs and graft fibrosis
It has been recently demonstrated that long-noncoding RNAs and miRNAs are exceptionally interconnected, thus, many studies are currently attempting to define the intricate relationship between these circulating biomarkers. For instance, Lnc-1700020I14Rik was discovered to downregulate mir-34a [54], which was previously found to induce fibrosis through the TGF-β pathway [42]. Further, Chen et al. found that under fibrotic renal conditions, lncRNA LINC00667 expression was decreased and tightly regulated by mir-19–3p [55]. Similarly, lncRNA Erbb4-IR was revealed to promote fibrotic renal injury in diabetic mice by targeting miR-29b [50]. Erbb4-IR is further reported to be highly expressed in TGF-β/Smad3-mediated renal fibrosis [56]. Alternatively, increased expression of lnc-TSI [58], lnc-MEG3 [54], and lnc-1700020I14Rik [54] resulted in the downregulation of the TGF-β/Smad3 pathway, demonstrating a potential therapeutic strategy for decreasing renal injury. Although promising, the study of lncRNAs is still in its infancy. For instance, MALAT1 was once proposed to play a role in ischemia-reperfusion injury and AKI [59], however others were not able to confirm its significance in vivo [60]. Still, there is great potential for future clinical interventions involving noninvasive circulating biomarkers.
Extracellular vesicles in the progression of renal fibrosis
Extracellular vesicles (EVs) are involved in cell–to–cell communication and they can pass from the systemic circulation into endothelial cells and tubular epithelial kidney cells and into the urine. RNA can be detected in body fluids in a nuclease resistant form, mainly as part of EVs, making extracellular RNAs (exRNAs) excellent non-invasive markers for disease detection, risk prediction, and therapeutic intervention (61, 62, 63). The content of EVs include a multiplicity of proteins, miRNAs, lncRNA, mRNAs, and lipids [64,65]. MiRNAs are the most studied among the different classes of exRNAs and recent evidence suggest that c-miRNAs, not only function as disease biomarkers, but also as critical regulators of cellular crosstalk [66–69]. Previous studies have shown that EVs can transfer TGFβ1 mRNA and miRNA-34a between different resident cells of the kidney to propagate pro-fibrotic signaling through fibroblast activation, inflammation, and tubular atrophy [70–73]. In addition, urinary EVs are constitutively released and the selection of their cargo is likely to be representative of the intracellular state, thereby allowing EVs to potentially be used as biomarkers of renal injury [74]. Sonoda et al. explored the implication of acute kidney injury on the repertoire of cargo associated with urinary EVs. Using an acute ischemic model of injury, miR-16–5p, miR-24–3p, miR-200c-3p were increased at one day post-reperfusion. The target mRNA expression levels of these three miRNAs were collectively increased in the medulla suggesting that the EV cargo correlated with the intracellular state. However, three days following reperfusion, the assortment of miRNA expression shifted to favor those involved in TGFβ1 signaling mechanisms, such as the miR200-family, miR-148a-3p, and miR-9a-5p [71].
Herein, we also propose to evaluate tRNA fragments. These ncRNAs are mainly classified in groups: tRNA halves (30–40nt), tRF-3s or tRF-5s (18–22nt) or tRF-1s (variable length) from 3’ trailer sequence of tRNA [75] tRNA halves were described as associated with response to cellular stress [76–78]. Furthermore, tRNA halves are highly expressed in lymphoid tissues, acting as signaling molecules in immune responses [79].
Further studies characterizing the composition of EV cargo between pre- and post-transplant patients who have variable presentations of renal fibrosis can provide a deeper understanding of the cell-to-cell communication which is suspected to be driving fluctuations in epigenetic regulation and downstream progression of CAD.
Conclusions
Key discoveries regarding the shift in epigenetic regulatory mechanisms following kidney transplantation has significant implication to redefine the prediction of ultimate graft outcomes. In addition, recently reports about EVs and their potential to promote shifts in paracrine signaling secondary to cellular stress mechanisms has profound translational potential as new methods to capture and quantify EV cargo in circulation and urine can provide biomarkers specific to the transplanted kidney before the onset of renal fibrosis and the progression to CKD.
Key points:
Renal fibrosis and the progression to chronic allograft dysfunction continues to return patients to managing end-stage-renal disease or awaiting transplantation.
TGFβ/Smad signaling seems to be at the center of the onset of fibrosis and is further exacerbated by dysfunctional Wnt/β-catenin signaling that accompanies progressive age and comorbidities such as hypertension and diabetes.
Significant progress has been made in recognizing the key DNAm, miRNA, and lncRNA fluctuations which accompany the onset of renal fibrosis.
The characterization of EVs and their cargo has significant implications to expand our ability to assess graft outcomes independent of invasive procedures.
Acknowledgements
Financial support and sponsorship
The research results included in this report were supported by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants, R01DK080074 (PI=Mas, V), R01 DK109581 (Co-PIs= Mas V/Archer K), and RO1DK122682 (PI= Mas V)
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading:
- 1).McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD Incidence and Prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127–135. doi: 10.1681/ASN.2018050531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate [published correction appears in Kidney Int. 2018 Nov;94(5):1025–1026]. Kidney Int. 2018;93(6):1442–1451. doi: 10.1016/j.kint.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Wang W, Yu Y, Wen J, et al. Combination of Functional Magnetic Resonance Imaging and Histopathologic Analysis to Evaluate Interstitial Fibrosis in Kidney Allografts. Clin J Am Soc Nephrol. 2019;14(9):1372–1380. doi: 10.2215/CJN.00020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Zhou LT, Qiu S, Lv LL, et al. Integrative Bioinformatics Analysis Provides Insight into the Molecular Mechanisms of Chronic Kidney Disease. Kidney Blood Press Res. 2018;43(2):568–581. doi: 10.1159/000488830 [DOI] [PubMed] [Google Scholar]
- 5).Wu H, Humphreys BD. The promise of single-cell RNA sequencing for kidney disease investigation. Kidney Int. 2017;92(6):1334–1342. doi: 10.1016/j.kint.2017.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93(3):568–579. doi: 10.1016/j.kint.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 7).Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–107. doi: 10.1016/j.diff.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 8).Han HI, Skvarca LB, Espiritu EB, Davidson AJ, Hukriede NA. The role of macrophages during acute kidney injury: destruction and repair. Pediatr Nephrol. 2019;34(4):561–569. doi: 10.1007/s00467-017-3883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Feng M, Tang PM, Huang XR, et al. TGF-β Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol Ther. 2018;26(1):148–161. doi: 10.1016/j.ymthe.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 11).Feng Y, Liang Y, Ren J, Dai C. Canonical Wnt Signaling Promotes Macrophage Proliferation during Kidney Fibrosis. Kidney Dis (Basel). 2018;4(2):95–103. doi: 10.1159/000488984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Feng Y, Ren J, Gui Y, et al. Wnt/β-Catenin-Promoted Macrophage Alternative Activation Contributes to Kidney Fibrosis. J Am Soc Nephrol. 2018;29(1):182–193. doi: 10.1681/ASN.2017040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011 [DOI] [PubMed] [Google Scholar]
- 14).Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65(2):713–718. doi: 10.1111/j.1523-1755.2004.00416.x [DOI] [PubMed] [Google Scholar]
- 15).Yilmaz S, McLaughlin K, Paavonen T, et al. Clinical predictors of renal allograft histopathology: a comparative study of single-lesion histology versus a composite, quantitative scoring system. Transplantation. 2007;83(6):671–676. doi: 10.1097/01.tp.0000262015.77625.90 [DOI] [PubMed] [Google Scholar]
- 16).Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–1353. doi: 10.1002/cphy.c110041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Li ZL, Lv LL, Tang TT, et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95(2):388–404. doi: 10.1016/j.kint.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 18).Hewitson TD, Holt SG, Smith ER. Progression of Tubulointerstitial Fibrosis and the Chronic Kidney Disease Phenotype - Role of Risk Factors and Epigenetics. Front Pharmacol. 2017;8:520. Published 2017 Aug 8. doi: 10.3389/fphar.2017.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Luo C, Zhou S, Zhou Z, et al. Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J Am Soc Nephrol. 2018;29(4):1238–1256. doi: 10.1681/ASN.2017050574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Qiao X, Rao P, Zhang Y, et al. Redirecting TGF-β Signaling through the β-Catenin/Foxo Complex Prevents Kidney Fibrosis. J Am Soc Nephrol. 2018;29(2):557–570. doi: 10.1681/ASN.2016121362*Presents a novel signaling mechanism that alleviates TGFβ-induced renal fibrosis while simultaneously upregulating differentiation of iTregs.
- 21).Zhou X, Xiong C, Tolbert E, Zhao TC, Bayliss G, Zhuang S. Targeting histone methyltransferase enhancer of zeste homolog-2 inhibits renal epithelial-mesenchymal transition and attenuates renal fibrosis. FASEB J. 2018;32(11):fj201800237R. doi: 10.1096/fj.201800237R*Describes EZH2 as an upstream modulator of multiple pathways specific to fibrosis development in the kidney. Has overarching implications as a therapeutic target for future studies.
- 22).Ziller MJ, Gu H, Müller F, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–481. doi: 10.1038/nature12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15(2):69–81. doi: 10.1038/nrg3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Visone R, Bacalini MG, Di Franco S, et al. DNA methylation of shelf, shore and open sea CpG positions distinguish high microsatellite instability from low or stable microsatellite status colon cancer stem cells. Epigenomics. 2019;11(6):587–604. doi: 10.2217/epi-2018-0153 [DOI] [PubMed] [Google Scholar]
- 25).Muse ME, Titus AJ, Salas LA, et al. Enrichment of CpG island shore region hypermethylation in epigenetic breast field cancerization. Epigenetics. 2020;15(10):1093–1106. doi: 10.1080/15592294.2020.1747748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Sun N, Zhang J, Zhang C, et al. Using Illumina Infinium Human Methylation 450K Bead Chip to explore genome‑wide DNA methylation profiles in a human hepatocellular carcinoma cell line. Mol Med Rep. 2018;18(5):4446–4456. doi: 10.3892/mmr.2018.9441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Choi H, Joe S, Nam H. Development of Tissue-Specific Age Predictors Using DNA Methylation Data. Genes (Basel). 2019;10(11):888. Published 2019 Nov 4. doi: 10.3390/genes10110888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Bachman M, Uribe-Lewis S, Yang X, Williams M, Murrell A, Balasubramanian S. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat Chem. 2014;6(12):1049–1055. doi: 10.1038/nchem.2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Williams K, Christensen J, Pedersen MT, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473(7347):343–348. doi: 10.1038/nature10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Heylen L, Thienpont B, Busschaert P, et al. Age-related changes in DNA methylation affect renal histology and post-transplant fibrosis. Kidney Int. 2019;96(5):1195–1204. doi: 10.1016/j.kint.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 31).Puelles VG, Cullen-McEwen LA, Taylor GE, et al. Human podocyte depletion in association with older age and hypertension. Am J Physiol Renal Physiol. 2016;310(7):F656–F668. doi: 10.1152/ajprenal.00497.2015 [DOI] [PubMed] [Google Scholar]
- 32).Rowland J, Akbarov A, Eales J, et al. Uncovering genetic mechanisms of kidney aging through transcriptomics, genomics, and epigenomics. Kidney Int. 2019;95(3):624–635. doi: 10.1016/j.kint.2018.10.029*Review of molecular changes associated with progressive age in the kidney.
- 33).Zuo Y, Liu Y. New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis. Nephrology (Carlton). 2018;23 Suppl 4:38–43. doi: 10.1111/nep.13472 [DOI] [PubMed] [Google Scholar]
- 34).Huffstater T, Merryman WD, Gewin LS. Wnt/β-Catenin in Acute Kidney Injury and Progression to Chronic Kidney Disease. Semin Nephrol. 2020;40(2):126–137. doi: 10.1016/j.semnephrol.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair [published online ahead of print, 2020 Sep 28]. Nat Rev Nephrol. 2020; 10.1038/s41581-020-00343-w. doi: 10.1038/s41581-020-00343-w [DOI] [PubMed] [Google Scholar]
- 36).Granata S, Benedetti C, Gambaro G, Zaza G. Kidney allograft fibrosis: what we learned from latest translational research studies [published online ahead of print, 2020 Mar 19]. J Nephrol. 2020; 10.1007/s40620-020-00726-z. doi: 10.1007/s40620-020-00726-z [DOI] [PubMed] [Google Scholar]
- 37).Meng P, Zhu M, Ling X, Zhou L. Wnt signaling in kidney: the initiator or terminator? [published online ahead of print, 2020 Sep 17]. J Mol Med (Berl). 2020; 10.1007/s00109-020-01978-9. doi: 10.1007/s00109-020-01978-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Bontha SV, Maluf DG, Archer KJ, et al. Effects of DNA Methylation on Progression to Interstitial Fibrosis and Tubular Atrophy in Renal Allograft Biopsies: A Multi-Omics Approach. Am J Transplant. 2017;17(12):3060–3075. doi: 10.1111/ajt.14372*Utilized a systems approach to evaluate DNA methylation and fluctuations in miRNA specific to the onset of interstitial fibrosis between kidney pre- and post-transplant biopsies.
- 39).Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews. Genetics. 2010. September;11(9):597–610. DOI: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 40).Zhang W, Li X, Tang Y, Chen C, Jing R, Liu T. miR-155–5p Implicates in the Pathogenesis of Renal Fibrosis via Targeting SOCS1 and SOCS6. Oxid Med Cell Longev. 2020;2020:6263921. Published 2020 Jun 6. doi: 10.1155/2020/6263921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Glover EK, Jordan N, Sheerin NS, Ali S. Regulation of Endothelial-to-Mesenchymal Transition by MicroRNAs in Chronic Allograft Dysfunction. Transplantation. 2019;103(4):e64–e73. doi: 10.1097/TP.0000000000002589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Liu Z, Wang Y, Shu S, Cai J, Tang C, Dong Z. Non-coding RNAs in kidney injury and repair. Am J Physiol Cell Physiol. 2019;317(2):C177–C188. doi: 10.1152/ajpcell.00048.2019 [DOI] [PubMed] [Google Scholar]
- 43).Solé C, Moliné T, Vidal M, Ordi-Ros J, Cortés-Hernández J. An Exosomal Urinary miRNA Signature for Early Diagnosis of Renal Fibrosis in Lupus Nephritis. Cells. 2019;8(8):773. Published 2019 Jul 25. doi: 10.3390/cells8080773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Zununi Vahed S, Poursadegh Zonouzi A, Ghanbarian H, et al. Differential expression of circulating miR-21, miR-142–3p and miR-155 in renal transplant recipients with impaired graft function. Int Urol Nephrol. 2017;49(9):1681–1689. doi: 10.1007/s11255-017-1602-2 [DOI] [PubMed] [Google Scholar]
- 45).Gniewkiewicz MS, Paszkowska I, Gozdowska J, et al. Urinary MicroRNA-21–5p as Potential Biomarker of Interstitial Fibrosis and Tubular Atrophy (IFTA) in Kidney Transplant Recipients. Diagnostics (Basel). 2020;10(2):113. Published 2020 Feb 19. doi: 10.3390/diagnostics10020113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Lu Q, Ma Z, Ding Y, et al. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis [published correction appears in Nat Commun. 2019 Aug 6;10(1):3628]. Nat Commun. 2019;10(1):2145. Published 2019 May 13. doi: 10.1038/s41467-019-10116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Zhang XF, Yang Y, Zhang J, Cao W. Microvesicle-containing miRNA-153–3p induces the apoptosis of proximal tubular epithelial cells and participates in renal interstitial fibrosis. Eur Rev Med Pharmacol Sci. 2019;23(22):10065–10071. doi: 10.26355/eurrev_201911_19574 [DOI] [PubMed] [Google Scholar]
- 48).Hu R, Li X, Peng C, et al. miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001101. doi: 10.1136/bmjdrc-2019-001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Chen B The miRNA-184 drives renal fibrosis by targeting HIF1AN in vitro and in vivo. Int Urol Nephrol. 2019;51(3):543–550. doi: 10.1007/s11255-018-2025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Sun SF, Tang PMK, Feng M, et al. Novel lncRNA Erbb4-IR Promotes Diabetic Kidney Injury in db/db Mice by Targeting miR-29b. Diabetes. 2018;67(4):731–744. doi: 10.2337/db17-0816 [DOI] [PubMed] [Google Scholar]
- 51).Jiang ZH, Tang YZ, Song HN, Yang M, Li B, Ni CL. miRNA‑342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med. 2020;45(1):45–52. doi: 10.3892/ijmm.2019.4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Nariman-Saleh-Fam Z, Bastami M, Ardalan M, Sharifi S, Hosseinian Khatib SM, Zununi Vahed S. Cell-free microRNA-148a is associated with renal allograft dysfunction: Implication for biomarker discovery. J Cell Biochem. 2019;120(4):5737–5746. doi: 10.1002/jcb.27860 [DOI] [PubMed] [Google Scholar]
- 53).Schauerte C, Hübner A, Rong S, et al. Antagonism of profibrotic microRNA-21 improves outcome of murine chronic renal allograft dysfunction. Kidney Int. 2017;92(3):646–656. doi: 10.1016/j.kint.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 54).Xue R, Li Y, Li X, Ma J, An C, Ma Z. miR-185 affected the EMT, cell viability, and proliferation via DNMT1/MEG3 pathway in TGF-β1-induced renal fibrosis. Cell Biol Int. 2019;43(10):1152–1162. doi: 10.1002/cbin.11046 [DOI] [PubMed] [Google Scholar]
- 55).Chen W, Zhou ZQ, Ren YQ, et al. Effects of long non-coding RNA LINC00667 on renal tubular epithelial cell proliferation, apoptosis and renal fibrosis via the miR-19b-3p/LINC00667/CTGF signaling pathway in chronic renal failure [published correction appears in Cell Signal. 2019 May;57:1]. Cell Signal. 2019;54:102–114. doi: 10.1016/j.cellsig.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 56).Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. doi: 10.1016/j.mam.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 57).Huang S, Xu Y, Ge X, et al. Long noncoding RNA NEAT1 accelerates the proliferation and fibrosis in diabetic nephropathy through activating Akt/mTOR signaling pathway. J Cell Physiol. 2019;234(7):11200–11207. doi: 10.1002/jcp.27770 [DOI] [PubMed] [Google Scholar]
- 58).Wang P, Luo ML, Song E, et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway. Sci Transl Med. 2018;10(462):eaat2039. doi: 10.1126/scitranslmed.aat2039 [DOI] [PubMed] [Google Scholar]
- 59).Tian H, Wu M, Zhou P, Huang C, Ye C, Wang L. The long non-coding RNA MALAT1 is increased in renal ischemia-reperfusion injury and inhibits hypoxia-induced inflammation. Ren Fail. 2018;40(1):527–533. doi: 10.1080/0886022X.2018.1487863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Kölling M, Genschel C, Kaucsar T, et al. Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Sci Rep. 2018;8(1):3438. Published 2018 Feb 21. doi: 10.1038/s41598-018-21720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol. 2016;311(5):F844–F851. doi: 10.1152/ajprenal.00429.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).De Palma G, Sallustio F, Schena FP. Clinical Application of Human Urinary Extracellular Vesicles in Kidney and Urologic Diseases. Int J Mol Sci. 2016;17(7):1043. Published 2016 Jun 30. doi: 10.3390/ijms17071043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Musante L, Bontha SV, La Salvia S, et al. Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet - a neglected source for uEVs. Sci Rep. 2020;10(1):3701. Published 2020 Feb 28. doi: 10.1038/s41598-020-60619-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Turchinovich A, Drapkina O, Tonevitsky A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front Immunol. 2019;10:202. Published 2019 Feb 28. doi: 10.3389/fimmu.2019.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379(10):958–966. doi: 10.1056/NEJMra1704286 [DOI] [PubMed] [Google Scholar]
- 66).Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7):727. Published 2019 Jul 15. doi: 10.3390/cells8070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Ståhl AL, Johansson K, Mossberg M, Kahn R, Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34(1):11–30. doi: 10.1007/s00467-017-3816-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Farzamfar S, Hasanpour A, Nazeri N, et al. Extracellular micro/nanovesicles rescue kidney from ischemia-reperfusion injury. J Cell Physiol. 2019;234(8):12290–12300. doi: 10.1002/jcp.27998 [DOI] [PubMed] [Google Scholar]
- 69).Gomzikova MO, James V, Rizvanov AA. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front Immunol. 2019;10:2663. Published 2019 Nov 15. doi: 10.3389/fimmu.2019.02663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Zhou Y, Xiong M, Niu J, et al. Secreted fibroblast-derived miR-34a induces tubular cell apoptosis in fibrotic kidney. J Cell Sci. 2014;127(Pt 20):4494–4506. doi: 10.1242/jcs.155523 [DOI] [PubMed] [Google Scholar]
- 71).Lv LL, Feng Y, Wen Y, et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J Am Soc Nephrol. 2018;29(3):919–935. doi: 10.1681/ASN.2017050523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Borges FT, Melo SA, Özdemir BC, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24(3):385–392. doi: 10.1681/ASN.2012101031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Liu X, Miao J, Wang C, et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 2020;97(6):1181–1195. doi: 10.1016/j.kint.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 74).Sonoda H, Lee BR, Park KH, et al. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci Rep. 2019;9(1):4692. Published 2019 Mar 18. doi: 10.1038/s41598-019-40747-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Kumar P, Kuscu C, Dutta A. Biogenesis and Function of Transfer RNA-Related Fragments (tRFs). Trends Biochem Sci. 2016;41(8):679–689. doi: 10.1016/j.tibs.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Torrent M, Chalancon G, de Groot NS, Wuster A, Madan Babu M. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci Signal. 2018;11(546):eaat6409. Published 2018 Sep 4. doi: 10.1126/scisignal.aat6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Shen Y, Yu X, Zhu L, Li T, Yan Z, Guo J. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J Mol Med (Berl). 2018;96(11):1167–1176. doi: 10.1007/s00109-018-1693-y [DOI] [PubMed] [Google Scholar]
- 78).Li Q, Hu B, Hu GW, et al. tRNA-Derived Small Non-Coding RNAs in Response to Ischemia Inhibit Angiogenesis. Sci Rep. 2016;6:20850. Published 2016 Feb 11. doi: 10.1038/srep20850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Kuscu C, Kumar P, Kiran M, Su Z, Malik A, Dutta A. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA. 2018;24(8):1093–1105. doi: 10.1261/rna.066126.118 [DOI] [PMC free article] [PubMed] [Google Scholar]