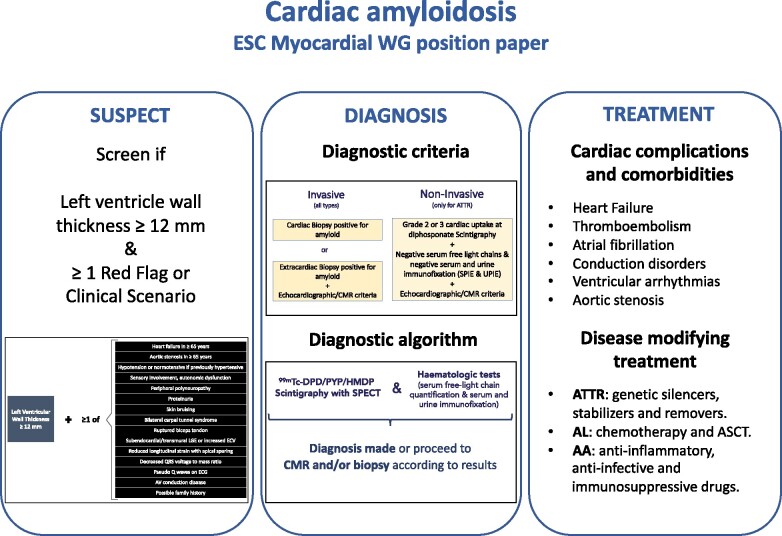
Abstract
Cardiac amyloidosis is a serious and progressive infiltrative disease that is caused by the deposition of amyloid fibrils at the cardiac level. It can be due to rare genetic variants in the hereditary forms or as a consequence of acquired conditions. Thanks to advances in imaging techniques and the possibility of achieving a non-invasive diagnosis, we now know that cardiac amyloidosis is a more frequent disease than traditionally considered. In this position paper the Working Group on Myocardial and Pericardial Disease proposes an invasive and non-invasive definition of cardiac amyloidosis, addresses clinical scenarios and situations to suspect the condition and proposes a diagnostic algorithm to aid diagnosis. Furthermore, we also review how to monitor and treat cardiac amyloidosis, in an attempt to bridge the gap between the latest advances in the field and clinical practice.
Keywords: Amyloidosis, Cardiac amyloidosis, Diagnosis, Treatment, AL, TTR, Transthyretin
Graphical Abstract
Cardiac amyloidosis
Introduction
Cardiac amyloidosis is characterized by the extracellular deposition of mis-folded proteins in the heart with the pathognomonic histological property of green birefringence when viewed under cross polarized light after staining with Congo red.1
Although considered a rare disease, recent data suggest that cardiac amyloidosis is underappreciated as a cause of common cardiac diseases or syndromes.2 Recent advances in cardiac imaging, diagnostic strategies, and therapies have improved the recognition and treatment of cardiac amyloidosis.1 , 2
The aim of this position paper by the European Society of 'Cardiology (ESC) Working Group on Myocardial and Pericardial Diseases is to help cardiologists and other physicians in recognizing, diagnosing, and treating patients with cardiac amyloidosis.
Definitions and classifications
Types of cardiac amyloidosis
While more than 30 proteins are known to be capable of aggregating as amyloid in vivo, only nine amyloidogenic proteins accumulate in the myocardium to cause significant cardiac disease.3
Nevertheless, some forms (AApoAI, AApoAII, AApoAIV, Aβ2M, AFib, AGel) are very rare and cardiac amyloidosis secondary to chronic inflammatory and infectious diseases (AA), although still encountered, is now much less frequent. Accordingly, >98% of currently diagnosed cardiac amyloidosis results from fibrils composed of monoclonal immunoglobulin light chains (AL) or transthyretin (ATTR), either in its hereditary (ATTRv) or acquired (ATTRwt) form. Table 1 describes the main characteristics of each type of cardiac amyloidosis.
Table 1.
Amyloidosis subtypes that affect the heart
| Amyloidosis type | Protein | Hereditary | Frequency of heart involvement | Median survival from diagnosis (months) | Usual extracardiac signs |
|---|---|---|---|---|---|
| AL | Immunoglobulin light chain | No | 70% |
24 6 (if HF at diagnosis and not treated) |
Nephropathy, proteinuria, autonomic dysfunction, polyneuropathy, macroglossia, spontaneous bruising, liver involvement |
| ATTRwt | Transthyretin | No | 100% | 57 | CTS, LSS, ruptured biceps tendon |
| ATTRv | Transthyretin | Yes |
30–100% Depending on the mutation |
31 (Val142Ile) 69 (non-Val142Ile) |
Polyneuropathy, orthostatic hypotension, vitreous opacities, gastrointestinal problems |
| AA | Serum amyloid A | No | 5% | 133 | Renal impairment (95%), proteinuria, hepatomegaly, gastrointestinal problems |
| AFib | Fibrinogen α | Yes | Rare | 180 | Renal impairment, proteinuria |
| AApoAI | Apolipoprotein A-I | Yes |
Rare Depending on the mutation |
No data. Probably >120 |
Primarily renal impairment, proteinuria, hepatosplenomegaly, adrenal insufficiency, dysphonia due to laryngeal involvement |
| AApoAII | Apolipoprotein A-II | Yes |
Rare Depending on the mutation |
No data | Primarily renal impairment, proteinuria |
| AApoAIV | Apolipoprotein A-IV | No | Unknown | 79 | Primarily renal impairment |
| Aβ2M | β2-microglobulin | No | 80% | No data | Long-term dialysis, CTS, joint problems |
| AGel | Gelsolin | Yes |
5% Primarily conduction disease |
Near normal life expectancy | Corneal lattice dystrophy, cutis laxa, drooping eyelids, paresthaesia, proteinuria (rare) |
AA, serum amyloid A amyloidosis; AApoAI, apolipoprotein AI amyloidosis; AApoAII, apolipoprotein AII amyloidosis; AApoAIV, apolipoprotein A-IV amyloidosis; Aβ2M, β2-microglobulin amyloidosis; AFib, fibrinogen amyloidosis; AGel, gelsolin amyloidosis; AL, light-chain amyloidosis; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CTS, carpal tunnel syndrome; HF, heart failure; LSS, lumbar spinal stenosis.
Definition of cardiac amyloidosis: diagnostic criteria
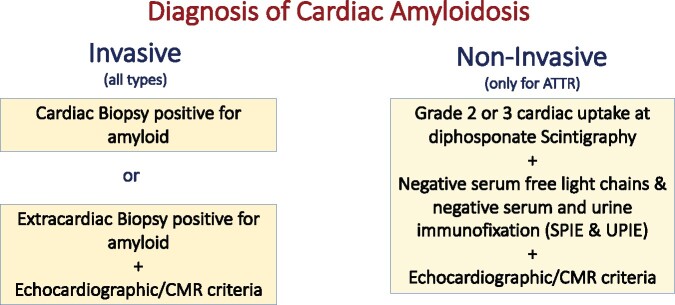
Cardiac amyloidosis is diagnosed when amyloid fibrils are found within cardiac tissue. Both invasive and non-invasive diagnostic criteria have been proposed. Invasive diagnostic criteria apply to all forms of cardiac amyloidosis whereas non-invasive criteria are accepted only for ATTR (Figure 1).
Figure 1.
Invasive and non-invasive diagnosis of cardiac amyloidosis. ATTR, transthyretin amyloidosis; CMR, cardiac magnetic resonance; SPIE, serum protein electrophoresis with immunofixation; UPIE, urine protein electrophoresis with immunofixation.
Invasive diagnostic criteria
Cardiac amyloidosis is confirmed when an endomyocardial biopsy demonstrates amyloid deposits after Congo red staining irrespective of the degree of left ventricular (LV) wall thickness. Identification of amyloid should be followed by classification of the amyloid fibril protein. Although the gold standard for defining the type of amyloid remains mass spectrometry, immunohistochemistry, or immunoelectron microscopy are routinely employed for amyloid typing in specialized centres.4
Diagnosis is also confirmed if amyloid deposits within an extracardiac biopsy are accompanied either by characteristic features of cardiac amyloidosis by echocardiography, in the absence of an alternative cause for increased LV wall thickness, or by characteristic features on cardiac magnetic resonance (CMR) (Table 2).
Table 2.
Echocardiographic and cardiac magnetic resonance criteria for non-invasive and invasive (with extracardiac biopsy-proven amyloidosis) diagnosis of cardiac amyloidosis
| Echocardiography |
| Unexplained LV thickness (≥12 mm) plus 1 or 2: |
|
| CMR |
Characteristic CMR findings (a and b have to be present):
|
CMR, cardiac magnetic resonance; ECV, extracellular volume; IVS, interventricular septum; LGE, late gadolinium enhancement; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; PWT, posterior wall thickness; TAPSE, tricuspid annular plane systolic excursion.
Abnormal gadolinium kinetics: myocardial nulling preceding or coinciding with the blood pool.
A recent multicentre study has proposed an echocardiographic score to facilitate echocardiographic diagnosis of AL or ATTR amyloidosis in the presence of increased LV wall thickness.5 Although not yet externally validated, a score ≥8 points in the presence of LV wall thickness ≥12 mm in combination with amyloid deposits in an extracardiac biopsy could also be considered diagnostic of cardiac amyloidosis (Table 2).
Non-invasive diagnostic criteria
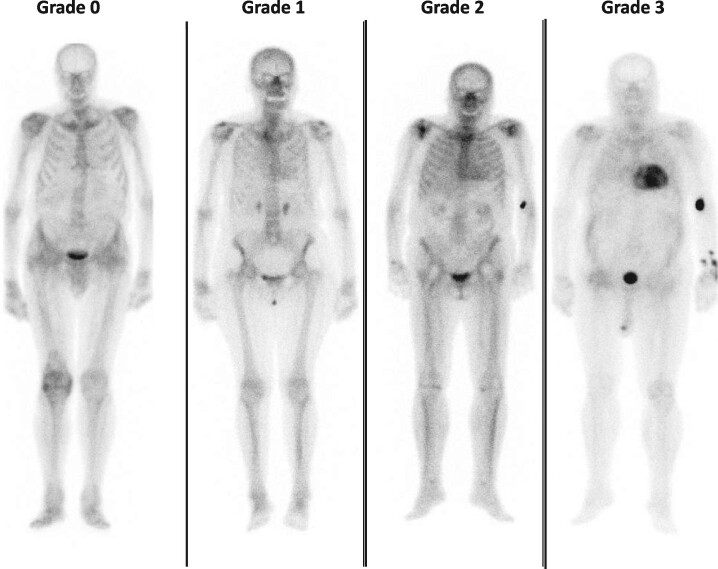
Cardiac ATTR amyloidosis can be diagnosed in the absence of histology in the setting of typical echocardiographic/CMR findings when 99mTc-pyrophosphate (PYP), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) or 99mTc-hydroxymethylene diphosphonate (HMDP) scintigraphy shows Grade 2 or 3 myocardial uptake of radiotracer (Figure 2) and clonal dyscrasia is excluded by all the following tests: serum free light chain (FLC) assay, serum (SPIE), and urine (UPIE) protein electrophoresis with immunofixation.6 The combination of SPIE, UPIE, and quantification of serum FLC has a sensitivity of 99% for identifying abnormal pro-amyloidotic precursor in AL amyloidosis.7 It is important to stress that serum and urine protein electrophoresis should always be performed with immunofixation to increase the sensitivity of the assays for detecting monoclonal proteins (Table 3). Interpretation of low-level monoclonal protein or mild elevations in the kappa: lambda ratio (FLC ratio) could be challenging. These findings can be encountered in patients with chronic kidney disease (CKD) or with monoclonal gammopathy of undetermined significance (MGUS). In patients with CKD, as the glomerular filtration rate (GFR) declines, the renal clearance of polyclonal FLC decreases and serum concentrations rise.8 The FLC ratio also varies as the GFR declines, but this depends on the FLC assay available [Freelite assay (Binding Site) or N Latex assay (Siemens)].8 With the N Latex assay, the FLC ratio decreases as GFR declines but no reference range in CKD has yet been proposed. In contrast, with the most frequently used Freelite assay, FLC ratio increases as GFR declines and a ratio of 0.37–3.1 has been proposed to be normal in patients with CKD. No reference values are available according to the severity of CKD, but in patients with moderate CKD (estimated GFR <45 mL/min/1.73 m2 by CKD-EPI formula) in the setting of a normal SPIE/UPIE, a FLC ratio up to 2.0 (or 3.1 if in dialysis) can typically be considered normal (Table 3). Otherwise, consultation with a haematologist is warranted.
Figure 2.
Cardiac uptake grading in bisphosphonate scintigraphy. Grade 0: absence of tracer myocardial uptake and normal bone uptake; Grade 1: myocardial uptake in a lower degree than at bone level; Grade 2: similar myocardial and bone uptake; and Grade 3: myocardial uptake greater than bone with reduced/absent bone uptake.
Table 3.
Serum and urine tests to rule out light-chain amyloidosis
| Testsa | What does it detect? | Most sensitive test for: | Normal range |
|---|---|---|---|
| SPIE | Clonal immunoglobulin and/or clonal light chain | Confirming clonal immunoglobulin production | No monoclonal protein present |
| UPIE | Clonal immunoglobulin and/or clonal light chain | Confirming clonal light chain production | No monoclonal protein present |
| Serum free light-chain assay | Ratio of serum kappa: lambda light chains |
Detecting low-level clonal light chain production; clonality assumed if ratio is far from 1:1 |
Freelite: 0.26–1.65b N Latex: 0.53–1.51 |
eGFR, estimated glomerular filtration rate; SPIE, serum protein electrophoresis with immunofixation; UPIE, urine protein electrophoresis with immunofixation.
If any of these tests are abnormal, bone scintigraphy should not be used to establish the diagnosis of transthyretin amyloidosis.
In patients with kidney disease, mild elevations in the kappa: lambda ratio are frequently encountered. In the setting of a normal SPIE/UPIE, a kappa: lambda ratio up to 2.0 in subjects with eGFR ≤ 45 mL/min/1.73 m2 (up to 3.1 if in dialysis) can typically be considered normal. This correction is not applicable to Siemens N Latex assay.
In the absence of a detectable monoclonal protein and an abnormal serum FLC ratio, the specificity of Grade 2 or 3 bone scintigraphy for cardiac ATTR when the disease is suspected has been proposed to be almost 100%.6 Please note, however, that scintigraphy should always include single photon emission computed tomography to confirm that cardiac uptake corresponds to myocardium uptake and not from cardiac chambers (Table 4). Nevertheless, recent reports have shown that rare situations can also lead to positive cardiac uptake.9 These situations should always be considered when interpreting scintigraphy results (Table 4).
Table 4.
Possible false positives and false negatives of bisphosphonate scintigraphy for detecting transthyretin cardiac amyloidosis
| Situation | How to suspect and confirm? | |
|---|---|---|
| False positive | AL amyloidosis | Abnormal SPIE, UPIE or serum free light ratio. Requires histologic confirmation. |
| Hydroxychloroquine cardiac toxicity | Interrogation. Requires histologic confirmation. | |
| AApoAI and AApoAII amyloidosis | Concomitant kidney disease present. Genetic testing. | |
| ApoAIV amyloidosis | Concomitant kidney disease present. Requires histologic confirmation. | |
| Aβ2M amyloidosis | Long-term dialysis (>9 years). Requires histologic confirmation. | |
| Blood pool | Cardiac dysfunction could be present. Use SPECT to detect uptake in myocardium. Delay acquisition. | |
| Rib fractures, valvular/annular calcifications | Use SPECT to detect uptake in myocardium. | |
| Recent myocardial infarction (<4 weeks) | Interrogation. Use SPECT to detect diffuse uptake in myocardium. | |
| False negative | Phe84Leu ATTRv, Ser97Tyr ATTRv | Concomitant neuropathy. Familial disease. Genetic testing. |
| Very mild disease | Requires histologic confirmation. | |
| Delayed acquisition | Shorter acquisition time interval. | |
| Premature acquisition | Prolong acquisition time interval. |
AApoAI, apolipoprotein AI amyloidosis; AApoAII, apolipoprotein AII amyloidosis; AApoAIV, apolipoprotein A-IV amyloidosis; Aβ2M, β2-microglobulin amyloidosis; AL, light-chain amyloidosis; ATTRv, hereditary transthyretin amyloidosis; SPECT, single photon emission computed tomography; SPIE, serum protein electrophoresis with immunofixation; UPIE, urine protein electrophoresis with immunofixation.
Once cardiac ATTR amyloidosis is confirmed, genetic counselling and testing should be performed to assess for the presence of TTR mutations in order to differentiate between ATTRwt and ATTRv. Genetic testing should be performed even in elderly patients, as a significant number can have TTR mutations.10
Essential concepts
Although nine types of cardiac amyloidosis are known, AL and ATTR currently account for the vast majority of cardiac amyloidosis.
Both invasive and non-invasive diagnostic criteria are accepted to diagnose cardiac amyloidosis. While invasive diagnostic criteria apply to all forms of cardiac amyloidosis, non-invasive criteria are accepted only for ATTR.
Diagnosis of cardiac amyloidosis
Diagnosis of cardiac amyloidosis includes two critical phases: (i) suspicious phase and (ii) definite diagnosis phase. The latter phase also includes appropriate typing of the amyloid, which is critical to guide specific treatment.
When to suspect cardiac amyloidosis
Red flags
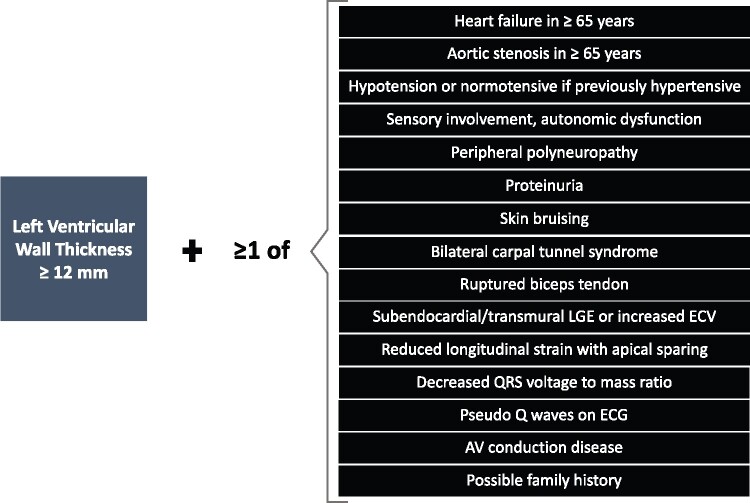
Cardiac amyloidosis typically appears within a constellation of extracardiac signs and symptoms that are extremely useful to suspect the disease in the presence of compatible cardiac imaging findings. These signs and symptoms are termed ‘red flags’ and include proteinuria (even mild), macroglossia, skin bruises, and carpal tunnel syndrome, among others (Table 5). There are also various red flags at the cardiac level, such as heart failure [including disproportionately high N-terminal pro-B-type natriuretic peptide (NT-proBNP)] that appears to be in disproportion to ‘objective’ findings on echocardiogram, ‘unexplained’ right heart failure in the presence of ostensibly ‘normal’ ventricular and valvular function, or ‘idiopathic’ pericardial effusion. Persistent troponin elevation, disproportionally low QRS voltage or early conduction system disease are also signs that could evoke cardiac amyloidosis (Table 5).
Table 5.
Cardiac and extracardiac amyloidosis red flags
| Type | Red flag | Amyloidosis where it is most frequently found |
|---|---|---|
| Extracardiac | ||
| Clinical | Polyneuropathy | ATTRv, AL, AA, AGel |
| Dysautonomia | ATTR, AL | |
| Skin bruising | AL | |
| Skin discoloration | AApoAI | |
| Cutis laxa | AGel | |
| Macroglossia | AL | |
| Deafness | ATTRwt | |
| Bilateral carpal tunnel syndrome | ATTRv, ATTRwt | |
| Ruptured biceps tendon | ATTRwt | |
| Lumbar spinal stenosis | ATTRwt | |
| Vitreous deposits | ATTRv | |
| Corneal lattice dystrophy | AGel | |
| Family history | ATTRv, AApoAI, AApoAII | |
| Laboratory | Renal insufficiency | AL, AA, AApoAI, AApoAII, AApoAIV, Aβ2M, AFib |
| Proteinuria | AL, AA, AApoAI, AApoAII, AFib | |
| Cardiac | ||
| Clinical | Hypotension or normotensive if previous hypertensive | ATTR, AL |
| ECG | Pseudoinfarct pattern | All |
| Low/decreased QRS voltage to degree of LV thickness | All | |
| AV conduction disease | All | |
| Laboratory | Disproportionally elevated NT-proBNP to degree of HF | All |
| Persisting elevated troponin levels | ATTR, AL | |
| Echocardiogram | Granular sparkling of myocardium | All |
| Increased right ventricular wall thickness | All | |
| Increased valve thickness | All | |
| Pericardial effusion | All | |
| Reduced longitudinal strain with apical sparing pattern | All | |
| CMR | Subendocardial late gadolinium enhancement | All |
| Elevated native T1 values | All | |
| Increased extracellular volume | All | |
| Abnormal gadolinium kinetics | All |
AA, serum amyloid A amyloidosis; AApoAI, apolipoprotein AI amyloidosis; AApoAII, apolipoprotein AII amyloidosis; AApoAIV, apolipoprotein A-IV amyloidosis; Aβ2M, β2-microglobulin amyloidosis; AFib, fibrinogen amyloidosis; AGel, gelsolin amyloidosis; AL, light-chain amyloidosis; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; AV, atrio-ventricular; CMR, cardiac magnetic resonance; ECG, electrocardiogram; HF, heart failure; LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Clinical scenarios
In addition to cardiac and extracardiac findings fostering the suspicion, there are several clinical situations in which cardiac amyloidosis should always be considered.
Cardiac disease in the presence of a typical systemic condition such as plasma cell dyscrasia, nephrotic syndrome, peripheral neuropathy, or a chronic systemic inflammatory condition should prompt consideration of amyloidosis, particularly if compatible cardiac imaging findings are present.
Increased wall thickness in a non-dilated left ventricle is a prominent characteristic of cardiac amyloidosis and should trigger further evaluation when found in elderly patients with common cardiac syndromes like heart failure with preserved ejection fraction, hypertrophic cardiomyopathy, or severe aortic stenosis, particularly among those undergoing transcatheter aortic valve replacement.11–13
As ATTR has been found in a significant number of patients (up to 7–19%) in the abovementioned clinical scenarios, and with the possibility of non-invasive diagnosis, we recommend ascertainment of cardiac amyloidosis in individuals with increased wall thickness with either heart failure, aortic stenosis, or red flag signs/symptoms, particularly if older than 65 years (Figure 3).
Figure 3.
Screening for cardiac amyloidosis. AV, atrio-ventricular; ECG, electrocardiogram; ECV, extracellular volume; LGE, late gadolinium enhancement.
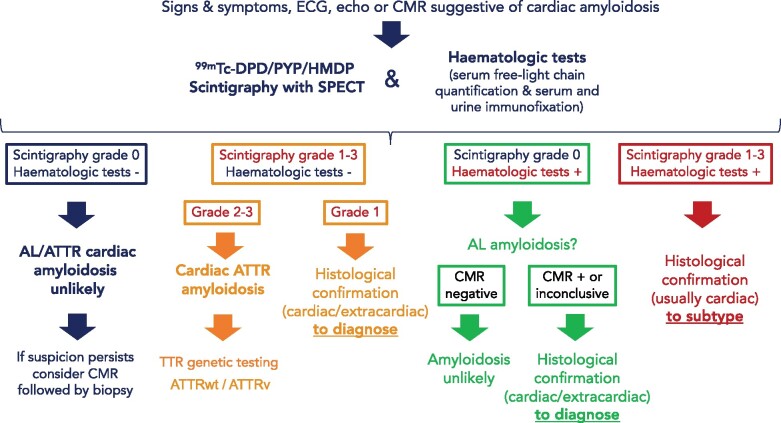
Diagnostic algorithm
Once cardiac amyloidosis is suspected, a timely, definitive diagnosis should be obtained as patient outcomes depend largely on early initiation of therapy (particularly in AL).
As the large majority of cases of cardiac amyloidosis are AL and ATTR, we propose a diagnostic algorithm focusing on identifying these subtypes by the initial use of 99mTc-PYP, DPD or HMDP scintigraphy coupled to assessment for monoclonal proteins by SPIE, UPIE, and quantification of serum FLC (Figure 4).
Figure 4.
Diagnostic algorithm for cardiac amyloidosis. AL, light-chain amyloidosis;ATTR, transthyretin amyloidosis; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CMR, cardiac magnetic resonance; ECG, electrocardiogram; SPECT, single photon emission computed tomography; TTR, transthyretin.
The results of these tests could lead to four scenarios:
Scintigraphy does not show cardiac uptake and assessments for monoclonal proteins are negative. There is a very low probability of cardiac amyloidosis and ATTR and AL amyloidosis are unlikely. An alternative diagnosis should be considered. Nevertheless, if suspicion persists, consider CMR followed by cardiac or extracardiac biopsy as bone scintigraphy could be negative in some ATTRv mutations (tracer uptake depends on TTR fibril composition) and in rare subtypes of cardiac amyloidosis (Table 5).
Scintigraphy shows cardiac uptake and assessments for monoclonal proteins are negative. If cardiac uptake is Grade 2 or 3, ATTR cardiac amyloidosis can be diagnosed. Proceed with genetic testing to differentiate between ATTRv and ATTRwt forms. In the case that cardiac uptake is Grade 1, non-invasive diagnosis is not possible and histological confirmation of amyloid deposits (could be extracardiac) is required.
Scintigraphy does not show cardiac uptake and at least one of the monoclonal protein tests is abnormal. Light-chain amyloidosis has to be ruled out promptly and CMR can be used to confirm cardiac involvement. If CMR findings do not support cardiac amyloidosis, the diagnosis is very unlikely. In the case that CMR findings are supportive or inconclusive, cardiac or extracardiac histological demonstration of amyloid deposits is required to diagnose AL cardiac amyloidosis. Cardiac or other clinically affected organ biopsy is recommended to avoid time delay to diagnosis and consultation with a haematologist is warranted.14 If CMR cannot be performed promptly, consider performing biopsy directly.
Scintigraphy shows cardiac uptake and at least one of the monoclonal protein tests is abnormal. Transthyretin amyloidosis with concomitant MGUS (or any haematological disorder that produces FLC), AL amyloidosis, or coexistence of both AL and ATTR amyloidosis are possible in this scenario. Diagnosis of cardiac amyloidosis, in this case, requires histology with amyloid typing, usually via endomyocardial biopsy.
Essential concepts
Cardiac amyloidosis should be considered in patients with increased wall thickness in the presence of cardiac or extracardiac red flags and/or in specific clinical situations.
A diagnostic algorithm based initially on the use of bone scintigraphy coupled to assessment for monoclonal proteins allows appropriate diagnosis in patients with suggestive signs/symptoms.
Outcome and prognosis
Prognosis in cardiac amyloidosis
Although different methods to prognosticate in cardiac amyloidosis have been proposed, the focus has moved to multiparametric biomarker-based prognostic scores, and biomarker-based staging systems have been developed for AL and ATTR cardiac amyloidosis (Table 6).15–19
Table 6.
Prognostic staging scores in light-chain and transthyretin amyloidoses
|
Kumar et al.
15 (Mayo)
|
Lilleness et al.
16 (BU)
|
Grogan et al.
17 (Mayo)
|
Gillmore et al.
18 (NAC)
|
Cheng et al.
19 (Columbia)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| AL | AL | ATTRwt | ATTRv and ATTRwt | ATTRv and ATTRwt | |||||
|
Staging parameters: FLC-diff ≥ 18 mg/dL Troponin T ≥ 0.025 ng/mL NT-proBNP ≥ 1800 pg/mL |
Staging parameters: Troponin I > 0.1 ng/mL BNP > 81 pg/mL |
Staging parameters: Troponin T > 0.05 ng/mL NT-proBNP > 3000 pg/mL |
Staging parameters: eGFR < 45 mL/min/1.73 m2 NT-proBNP > 3000 pg/mL |
Scoring parameters: Mayo or NAC score (0–2 points) Daily dose of furosemide or equivalent: 0 mg/kg (0 points), >0–0.5 mg/kg (1 point), >0.5–1 mg/kg (2 points), and > 1 mg/kg (3 points) NYHA class I-IV (1 to 4 points) |
|||||
|
| |||||||||
| Stage | 5-year survival | Stage | Median survival | Stage |
4-year survival/ median survival |
Stage | Median survival | Score |
Median survival |
|
| |||||||||
|
Stage I (0 parameters) |
68% |
Stage I (0 parameters) |
Not reached |
Stage I (0 parameters) |
57% 66 months |
Stage I (0 parameters) |
69.2 months | Score 1–3 | 90.5 months |
|
Stage II (1 parameter) |
60% |
Stage II (1 parameter) |
112.8 months |
Stage II (1 parameter) |
42% 40 months |
Stage II (1 parameter) |
46.7 months | Score 4–6 |
38.5 months (Mayo) 36 months (NAC) |
|
Stage III (2 parameters) |
28% |
Stage III (2 parameters) |
51.6 months |
Stage III (2 parameters) |
18% 20 months |
Stage III (2 parameters) |
24.1 months | Score 7–9 |
20.3 months (Mayo) 19.8 months (NAC) |
|
Stage IV (3 parameters) |
14% |
Stage IIIb (2 parameters and BNP > 700 pg/mL) |
12 months | ||||||
AL, light-chain amyloidosis; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; BU, Boston University School of Medicine; eGFR, estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease formula; FLC-diff, difference between involved and uninvolved free light chain; NAC, UK National Amyloidosis Centre; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Available scoring systems have been constructed using parameters obtained ‘at presentation’ and provide an initial prognostic stratification. The prognostic impact of any change of the scores during follow-up has not yet been validated, even though recent studies have shown promising results.20
Progression of cardiac amyloidosis
While there have been multiple studies delineating baseline risk factors associated with adverse outcomes (principally mortality) in AL and ATTR, and data are emerging from the placebo arm of therapeutic trials,21 there is a dearth of published data on longitudinal aspects of disease progression and none that are population-based without referral and ascertainment biases. In the era of emerging effective therapies, this is a major unmet need.
Follow-up of patients with cardiac amyloidosis
Although no studies have yet addressed the optimal follow-up scheme in patients with cardiac amyloidosis, a common scheme consists of 6-month visits with electrocardiogram (ECG) and complete blood tests (including NT-proBNP and troponin) and yearly echocardiogram and 24-h Holter ECG. A summary of recommended follow-up tests can be found in Table 7.
Table 7.
Proposed follow-up scheme in cardiac amyloidosis
| AL | ATTR | ||||
|---|---|---|---|---|---|
| Patients with cardiac amyloidosis |
Every month (during initial haematological treatment):
|
Every 6 months:
|
|||
Every 3–4 months (after completing initial haematological treatment):
|
Every 12 months:
|
||||
Every 6 months:
|
|||||
Every 12 months:
|
|||||
| ATTRv asymptomatic genetic carriers a |
Yearly:
|
Every 2 years:
|
Every 3 years or if any of above complementary tests is abnormal:
|
||
6MWD, 6-min walking distance; AL, light-chain amyloidosis; ATTR, transthyretin amyloidosis; ATTRv, hereditary transthyretin amyloidosis; CMR, cardiac magnetic resonance; ECG, electrocardiogram; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Clinical follow-up to be started 10 years before the age of disease onset of affected relatives or predicted usual onset age for the specific ATTRv mutation.
Follow-up of mutation carriers and genetic counselling
Genetic testing is recommended for relatives of patients with an inheritable form of cardiac amyloidosis. Such testing should occur along with genetic counselling of patients and their families. As all hereditary amyloidoses have an adult onset, genetic testing of minors is discouraged. Genetic testing could be offered during young adulthood if genetic information would seem useful to guide professional choices or for reproductive planning.
As age of onset, clinical penetrance, and progression depend upon the variant, assessment of penetrance in allele carriers is generally recommended to start ∼10 years prior to the age of disease onset in affected members of the family (or other individuals with the same mutation), or as soon as symptoms compatible with amyloidosis develop (Table 7).22
Essential concepts
While several staging systems are available to facilitate prognosis, there are limited data on how to assess progression. In the era of emerging effective specific therapies, this is a major unmet need.
Follow-up of patients with cardiac amyloidosis and mutation carriers should be conducted following a structured protocol.
Treatment
Treatment of cardiac amyloidosis involves two areas: (i) treatment and prevention of complications and (ii) stopping or delaying amyloid deposition by specific treatment.
Treatment of complications and comorbidities
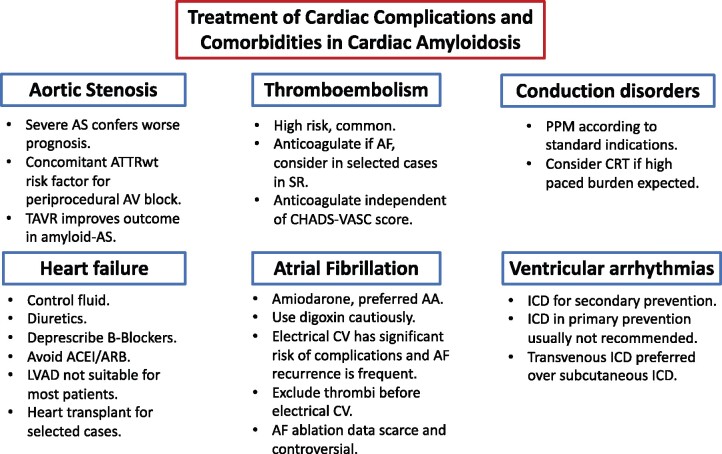
Supportive care of patients with cardiac amyloidosis encompasses different clinical aspects including treatment of heart failure, arrhythmias, conduction disturbances, thromboembolism, and concomitant presence of severe aortic stenosis (Figure 5).23–25
Figure 5.
Treatment of cardiac complications and comorbidities in cardiac amyloidosis. AA, antiarrhythmic; ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; AS, aortic stenosis; ATTRwt, wild-type transthyretin amyloidosis; AV, atrio-ventricular; CRT, cardiac resynchronization therapy; CV, cardioversion; ICD, implantable cardioverter-defibrillator; LVAD, left ventricular assist device; PPM, permanent pacemaker; SR, sinus rhythm; TAVR, transcatheter aortic valve replacement.
Specific (disease-modifying) treatment
Treating the process of amyloid deposition should target the production of amyloid precursor protein or the assembly of amyloid fibrils.
Light-chain amyloidosis
Specific treatment in cardiac AL amyloidosis should be undertaken by multidisciplinary teams involving oncohaematology and cardiology specialists and, whenever possible, patients should be referred to specialized centres.26
Patients with AL amyloidosis not only have a haematologic malignancy, but also their multiorgan involvement makes them particularly fragile and susceptible to treatment toxicity. Therapeutic approaches depend on risk assessment that is defined in many circumstances by the degree of cardiac involvement (Supplementary material online, Figure S1) and cardiac response depends also on haematological response (Supplementary material online, Table S1).23 , 24 The role of the cardiologist in the specific treatment includes: (i) cardiac assessment for initial haematologic strategies, including consideration of autologous stem cell transplantation (Supplementary material online, Table S1), (ii) heart transplant evaluation, and (iii) cardiac monitoring during chemotherapy.
Transthyretin amyloidosis
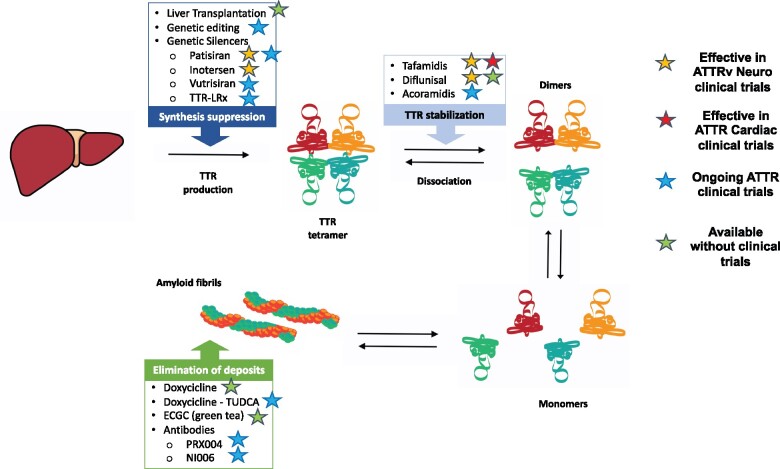
There is an increasing availability of novel, effective, targeted therapeutic options for ATTRwt and ATTRv. A prompt diagnosis is essential to enable the timely treatment of neurological, cardiac, and other systemic manifestations, as therapy is more effective in the early stages of the disease.27–29 Effective therapies reduce the production of mutated (liver transplantation) and overall TTR (genetic silencers) or stabilize circulating TTR molecule (stabilizers), preventing their dissociation or cleavage into amyloidogenic fragments (Figure 6). Several new compounds are under investigation, including agents directed to remove amyloid fibrils (Supplementary material online).
Figure 6.
Available and future disease-modifying therapies in transthyretin amyloidosis (ATTR). ATTRv, hereditary transthyretin amyloidosis; TTR, transthyretin.
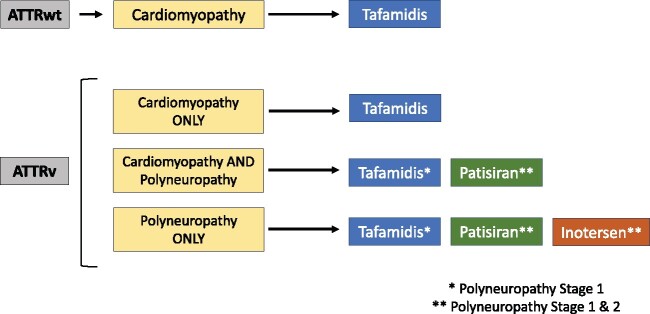
Current therapeutic alternatives distinguish between ATTRv and ATTRwt and, in the case of ATTRv, according to the presence of cardiomyopathy, polyneuropathy, or both (Figure 7). A detailed description of ATTR therapies that are either available or are being tested in phase III trials can be found in Supplementary material online. Tafamidis should be generally considered the agent of choice in ATTR cardiac patients with reasonable expected survival while patisiran could be considered in ATTRv patients with cardiac involvement in whom gene silencers are prescribed due to symptomatic neurological disease.
Figure 7.
Proposed therapeutic alternatives in transthyretin amyloidosis patients. ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis.
Essential concepts
Management of cardiac amyloidosis involves treatment and prevention of complications, and halting or delaying amyloid deposition by specific treatments.
Specific pharmacologic treatments available for ATTR amyloidosis include stabilizing molecules (tafamidis) and genetic silencers (patisiran and inotersen).
Tafamidis is currently the only drug that has shown efficacy in a randomized trial in patients with ATTRwt and ATTRv with cardiomyopathy, and should be considered in patients with reasonable expected survival.
Organization of patient care
Collaboration between centres remains essential because not all centres can perform complex diagnostic techniques (such as endomyocardial biopsy and mass spectrometry) or prescribe disease-modifying therapies, and interaction between cardiologists, haematologists, transplant surgeons, neurologists, and other specialists could be needed. The best strategy for the management of patients with amyloidosis is not the ‘hub-and-spoke’ model, but rather a network where centres can do at least some parts of the diagnostic workup, exchange opinions and information, and refer patients to regional or national referral centres for selected procedures or particularly complex decisions.
Summary and future directions
As knowledge evolves and new therapeutic alternatives to treat cardiac amyloidosis emerge, new areas of research and unsolved questions arise. Some of the grey zones and areas of active research are summarized in Table 8.
Table 8.
Areas of investigation and uncertainty in cardiac amyloidosis
| Pathophysiology |
Amyloidogenesis
|
Determinants of phenotypic heterogeneity
|
| Diagnosis |
| Populations to screen for cardiac amyloidosis and optimal screening method |
| Expanded genetic testing in the overall population |
| Identification of a plasmatic biomarkers of unfolded TTR |
| Artificial intelligence tools to facilitate diagnosis (imaging, ECG, etc.) |
| Identification of the target of bone tracers within amyloid deposits |
| Validation of PET tracers for diagnosis of cardiac amyloidosis, differential diagnosis of ATTR vs. AL, and evaluation of amyloid burden |
| Natural history |
| Disease trajectories among carriers of different mutations |
Definition and measurement of disease progression
|
| Treatment of complications |
| Initiation of anticoagulation in patients without atrial fibrillation |
| Efficacy of heart failure drugs in patients with different degrees of heart failure |
| Efficacy of beta-blockers. Identification of patients who could benefit |
| Role of invasive heart failure monitoring devices |
| Identification of patients that benefit from prophylactic pacemaker |
| Identification of subgroups that can benefit from ICD and CRT |
| Disease-modifying treatments |
| New antiplasma cell therapy in AL |
| New stabilizers in ATTR |
| New gene silencers in ATTR |
Early initiation of therapy:
|
| Comparison between diverse disease-modifying drugs in ATTR |
| Definition of disease progression despite therapy in ATTR |
| Criteria for switching from one drug to another |
| Early identification of responders/non-responders to specific therapies |
| Role of combined therapy |
| Antibodies to induce removal of tissue amyloid deposits |
| Genetic editing treatments |
AL, light-chain amyloidosis; ATTR, transthyretin amyloidosis; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; ICD, implantable cardioverter-defibrillator; PET, positron emission tomography; TTR, transthyretin.
It is expected that advances in the field will change the way we diagnose, prognosticate, and treat cardiac amyloidosis in the next few years. Meanwhile, in this paper, the ESC Working Group on Myocardial and Pericardial Diseases proposes an invasive and non-invasive definition of cardiac amyloidosis, addresses clinical scenarios and situations to suspect the condition, and proposes a diagnostic algorithm to aid diagnosis. Furthermore, we also review how to monitor and treat cardiac amyloidosis, in an attempt to bridge the gap between the latest advances in the field and clinical practice.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
P.G.-P. reports grant support from Instituto de Salud Carlos III (PI20/01379). M.F. is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447). E.G.-L. reports grant support from Instituto de Salud Carlos III (PI18/0765). M.S.M. reports grant support from National Institutes of Health [R01HL139671-01], [R21AG058348], and [K24AG036778].
Conflict of interest: M.A. reports receiving an Advisory board fee and a Research Grant from Pfizer and from Sanofi Genzyme. U.E. reports consulting fees and grant support from Pfizer and Sanofi. Institutional grants from Pfizer. M.F. reports consulting fees from Pfizer, Akcea, Ionis, Alnylam, Alexion, Sanofi, and research grant from Pfizer. P.G.P. reports speaking fees from Pfizer, Eidos, Alnylam, and Akcea. Consulting fees from Pfizer, Eidos, Neuroinmmune, Alnylam, Prothena, and Akcea. Research support to his institution from Pfizer, Eidos, and Alnylam. J.D.G. reports consulting and speaking fees from Pfizer, Akcea, Alnylam, and Eidos. E.G.-L. reports speaking fees from Pfizer and Alnylam. Consulting fees from Pfizer and Proclara. Research support to her institution from Pfizer, Eidos, and Alnylam. M.G. reports grant/clinical trial support from Alnylam, Eidos, Prothena, and Pfizer. I.K. reports speaking fees from Akcea Therapeutics Germany and Pfizer and consulting fees from Akcea Therapeutics Germany. A.V.K. reports consulting fees from Pfizer, Akcea, Alnylam, and Neurimmune as well as speaking fees from Pfizer, Akcea, and Alnylam. A.L. reports speaking fees from Pfizer. Consulting fees from Pfizer and Alnylam. M.S.M. reports consulting income from Pfizer, GSK, EIdos, Prothena, Akcea, and Alnylam, and institution received clinical trial funding from Pfizer, Prothena, Eidos, and Alnylam. C.R. reports speaking fees from Pfizer, Alnylam, and Akcea. Consulting fees from Pfizer, Alnylam, Prothena, and Akcea. Institutional Research Grants from Pfizer. A.G.R. reports honoraria for presentations from Astra-Zeneca. Other authors declare no conflict of interest.
Supplementary Material
Contributor Information
Pablo Garcia-Pavia, Heart Failure and Inherited Cardiac Diseases Unit, Department of Cardiology, Hospital Universitario Puerta de Hierro Majadahonda, CIBERCV, Manuel de Falla, 2, 28222 Madrid, Spain; Universidad Francisco de Vitoria (UFV), Pozuelo de Alarcon, Spain; European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart.
Claudio Rapezzi, Cardiologic Centre, University of Ferrara, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy.
Yehuda Adler, Leviev Heart Centre, Chaim Sheba Medical Centre (affiliated to Tel Aviv University), Israel.
Michael Arad, Heart Failure Institute, Leviev Heart Centre, Sheba Hospital and Sackler School of Medicine, Tel Aviv University, Israel.
Cristina Basso, European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart; Cardiovascular Pathology Unit, University Hospital, Padua, Italy; Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padua, Padua, Italy.
Antonio Brucato, Dipartimento di Scienze Biomediche e Cliniche, Università degli Studi d Milano, Ospedale Fatebenefratelli, Italy.
Ivana Burazor, Belgrade University School of Medicine, Cardiology, Institute for Rehabilitation, Belgrade, Serbia.
Alida L P Caforio, European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart; Cardiology, Department of Cardiac Thoracic Vascular Sciences and Public Health, University of Padova, Padova, Italy.
Thibaud Damy, European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart; French Referral Centre for Cardiac Amyloidosis, Amyloidosis Mondor Network, GRC Amyloid Research Institute, CHU Henri Mondor, Créteil, France.
Urs Eriksson, GZO—Zurich Regional Health Centre, Wetzikon & Cardioimmunology, Centre for Molecular Cardiology, University of Zurich, Switzerland.
Marianna Fontana, National Amyloidosis Centre, Division of Medicine, University College London, Royal Free Hospital, London, UK.
Julian D Gillmore, National Amyloidosis Centre, Division of Medicine, University College London, Royal Free Hospital, London, UK.
Esther Gonzalez-Lopez, Heart Failure and Inherited Cardiac Diseases Unit, Department of Cardiology, Hospital Universitario Puerta de Hierro Majadahonda, CIBERCV, Manuel de Falla, 2, 28222 Madrid, Spain; European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart.
Martha Grogan, Cardiac Amyloid Clinic, Division of Circulatory Failure, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Stephane Heymans, Department of Cardiology, Maastricht University, CARIM School for Cardiovascular Diseases, Maastricht, Netherlands; Centre for Molecular and Vascular Biology, KU Leuven, Leuven, Belgium; ICIN-Netherlands Heart Institute, Holland Heart House, Utrecht, Netherlands.
Massimo Imazio, Cardiovascular and Thoracic Department, University Cardiology, AOU Città della Salute e della Scienza di Torino, Torino, Italy.
Ingrid Kindermann, Department of Internal Medicine III (Cardiology, Angiology and Intensive Care), Saarland University Medical Centre, Saarland University, Homburg/Saar, Germany.
Arnt V Kristen, Department of Cardiology, University of Heidelberg, Germany; Cardiovascular Centre Darmstadt, Heidelberg, Germany.
Mathew S Maurer, Cardiac Amyloidosis Program, Centre for Advanced Cardiac Care, Columbia University Irving Medical Centre, New York Presbyterian Hospital, New York, NY, USA.
Giampaolo Merlini, Amyloidosis Research and Treatment Centre, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy; Department of Molecular Medicine, University of Pavia, Italy.
Antonis Pantazis, Cardiomyopathy Service, Royal Brompton Hospital, London, UK.
Sabine Pankuweit, Department Of Cardiology, Philipps-University Marburg, Marburg, Germany.
Angelos G Rigopoulos, Mid-German Heart Centre, Department of Internal Medicine III, Division of Cardiology, Angiology and Intensive Medical Care, University Hospital Halle, Martin-Luther-University Halle-Wittenberg, Halle (Saale), Germany.
Ales Linhart, 2nd Department of Medicine, Department of Cardiovascular Medicine, First Faculty of Medicine, Charles University, General University Hospital, Prague, Czech Republic.
References
- 1. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 2017;135:1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018;25:215–219. [DOI] [PubMed] [Google Scholar]
- 4. Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol 2015;24:343–350. [DOI] [PubMed] [Google Scholar]
- 5. Boldrini M, Cappelli F, Chacko L, Restrepo-Cordoba MA, Lopez-Sainz A, Giannoni A, Aimo A, Baggiano A, Martinez-Naharro A, Whelan C, Quarta C, Passino C, Castiglione V, Chubuchnyi V, Spini V, Taddei C, Vergaro G, Petrie A, Ruiz-Guerrero L, Moñivas V, Mingo-Santos S, Mirelis JG, Dominguez F, Gonzalez-Lopez E, Perlini S, Pontone G, Gillmore J, Hawkins PN, Garcia-Pavia P, Emdin M, Fontana M. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13:909–920. [DOI] [PubMed] [Google Scholar]
- 6. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 7. Palladini G, Russo P, Bosoni T, Verga L, Sarais G, Lavatelli F, Nuvolone M, Obici L, Casarini S, Donadei S, Albertini R, Righetti G, Marini M, Graziani MS, Melzi D'Eril GV, Moratti R, Merlini G. Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine. Clin Chem 2009;55:499–504. [DOI] [PubMed] [Google Scholar]
- 8. Sprangers B, Claes K, Evenepoel P, Kuypers D, Poesen K, Delforge M, Bossuyt VX, Meijers B. Comparison of 2 serum-free light-chain assays in CKD patients. Kidney Int Rep 2020;5:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Layoun ME, Desmarais J, Heitner SB, Masri A. Hot hearts on bone scintigraphy are not all amyloidosis: hydroxychloroquine-induced restrictive cardiomyopathy. Eur Heart J 2020;41:2414–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. López-Sainz Á, Hernandez-Hernandez A, Gonzalez-Lopez E, Domínguez F, Restrepo-Cordoba MA, Cobo-Marcos M, Gómez-Bueno M, Hernandez-Perez FJ, Oteo JF, Mirelis JG, Cavero MA, Moñivas V, Mingo SS, de Haro-Del Moral FJ, Krsnik I, Salas C, Bornstein B, Briceño A, López JA, Vázquez J, Alonso-Pulpón L, Segovia J., Garcia-Pavia P. Clinical profile and outcome of cardiac amyloidosis in a Spanish referral center. Rev Esp Cardiol (Engl Ed) 2021;74:149–158. [DOI] [PubMed] [Google Scholar]
- 11. González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 12. Damy T, Costes B, Hagège AA, Donal E, Eicher JC, Slama M, Guellich A, Rappeneau S, Gueffet JP, Logeart D, Planté-Bordeneuve V, Bouvaist H, Huttin O, Mulak G, Dubois-Randé JL, Goossens M, Canoui-Poitrine F, Buxbaum JN. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J 2016;37:1826–1834. [DOI] [PubMed] [Google Scholar]
- 13. Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sayago I, Krsnik I, Gómez-Bueno M, García-Pavía P, Jaramillo N, Salas C, Mingo S, Oteo JF, Alonso-Pulpón L, Segovia J. Analysis of diagnostic and therapeutic strategies in advanced cardiac light-chain amyloidosis. J Heart Lung Transplant 2016;35:995–1002. [DOI] [PubMed] [Google Scholar]
- 15. Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 2019;133:215–223. [DOI] [PubMed] [Google Scholar]
- 17. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016;68:1014–1020. [DOI] [PubMed] [Google Scholar]
- 18. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39:2799–2806. [DOI] [PubMed] [Google Scholar]
- 19. Cheng RK, Levy WC, Vasbinder A, Teruya S, De Los Santos J, Leedy D, Maurer MS. Diuretic dose and NYHA functional class are independent predictors of mortality in patients with transthyretin cardiac amyloidosis. JACC CardioOncol 2020;2:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Law S, Petrie A, Chacko L, Cohen OC, Ravichandran S, Gilbertson JA, Rowczenio D, Wechalekar A, Martinez-Naharro A, Lachmann HJ, Whelan CJ, Hutt DF, Hawkins PN, Fontana M, Gillmore JD. Disease progression in cardiac transthyretin amyloidosis is indicated by serial calculation of National Amyloidosis Centre transthyretin amyloidosis stage. ESC Heart Fail 2020;7:3942–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 22. Conceição I, Coelho T, Rapezzi C, Parman Y, Obici L, Galán L, Rousseau A. Assessment of patients with hereditary transthyretin amyloidosis—understanding the impact of management and disease progression. Amyloid 2019;26:103–111. [DOI] [PubMed] [Google Scholar]
- 23. Muchtar E, Grace L, Grogan M. The challenges in chemotherapy and stem cell transplantation for light-chain amyloidosis. Can J Cardiol 2020;36:384–395. [DOI] [PubMed] [Google Scholar]
- 24. Saith SE, Maurer MS, Patel AR. Systemic amyloidosis due to monoclonal immunoglobulins. Hematol Oncol Clin North Am 2020;34:1055–1068. [DOI] [PubMed] [Google Scholar]
- 25. Garcia-Pavia P, Domínguez F, Gonzalez-Lopez E. Transthyretin amyloid cardiomyopathy. Med Clin (Barc) 2021;156:126–134. [DOI] [PubMed] [Google Scholar]
- 26. Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood 2016;128:159–168. [DOI] [PubMed] [Google Scholar]
- 27. Rapezzi C, Elliott P, Damy T, Nativi-Nicolau J, Berk JL, Velazquez EJ, Boman K, Gundapaneni B, Patterson TA, Schwartz JH, Sultan MB, Maurer MS. Efficacy of tafamidis in patients with hereditary and wild-type transthyretin amyloid cardiomyopathy: further analyses from ATTR-ACT. JACC Heart Fail 2021;9:115–123. [DOI] [PubMed] [Google Scholar]
- 28. Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin K-P, Vita G, Attarian S, Planté-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 29. Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Planté-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH 3rd, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceição I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA, Coelho T. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.