Abstract
Artificial sweeteners are posing a new threat to the environment. The water ecosystem is the primary recipient of these emerging contaminants. Once ingested, sufficient amount of these artificial sweeteners escape unchanged from the human body and are added to the environment. However, some are added in the form of their breakdown products through excretion. Artificial sweeteners are resistant to wastewater treatment processes and are therefore continuously introduced into the water environments. However, the environmental behavior, fate, and long-term ecotoxicological contributions of artificial sweeteners in our water resources still remain largely unknown. Some artificial sweeteners like saccharin are used as a food additive in animal feeds. It also forms the degradation product of the sulfonylurea herbicides. All artificial sweeteners enter into the wastewater treatment plants from the industries and households. From the effluents, they finally reside into the receiving environmental bodies including wastewaters, groundwaters, and surface waters. The global production of these sweeteners is several hundred tons annually and is continuously being added into the environment.
1. Introduction
Artificial sweeteners (ASs) are the food additives used in thousands of food products throughout the world. Among many food products containing ASs are food and beverages, desserts, chewing gums, pastries, and breads especially for diabetic and/or obese people. Besides, these are also used in other personal care and pharmaceutical products [1–4] such as toothpastes and cough syrups. The most popular ASs exhibiting countless food chemistry applications are saccharin (SAC), cyclamate (CYC), aspartame (ASP), acesulfame (ACE), sucralose (SUC), alitame (ALT), neotame (NEO), and neohesperidine dihydrochalcone (NSDH) [5–7]. The use of ASs in drugs and sanitary products has also been confirmed [4, 8].
ASs are primarily used for the processing of low-calorie foods without sugar in the food industry. These sweeteners are widely used in human diet as they do not induce any glycemic effect/insulin reaction once ingested. Besides, they do not release any calories and do not have any adverse effect on the dental plaque microflora, unlike sugar [6]. CYC is currently the most developed artificial sweetener among sulfamates, followed closely by SAC [8]. In diet soft drinks, ASP and ACE-K-k are the leading brands, while SUC has been the leader in the main market for tabletop sweeteners. A class of pollutants that have entered the environment for years and have not been thoroughly studied so far in terms of occurrence, environmental fate, and toxicity assessment and/or protected by current worldwide regulations are classified as “emerging pollutants” or “emerging contaminants” [9–11]. ASs are a newly found class of environmental contaminant because of their prolonged existence and universal occurrence in various aquatic ecosystems.
It is believed that the number of emerging pollutants is more than 700 that are being added to the environment on daily basis [12]. In view of the growing pollution stress, these emerging pollutants should be explored and addressed in a systemic manner to understand their potential risk to the environment and human health [11]. The global consumption of ASs is reported to be more than 159,000 metric tons. This amounts to the market value of USD $2 billion. China is currently the leading country consuming the most ASs (32%), followed by Asia/Oceania (23%), United States (23%), Europe (12%), and Africa (7%) [13]. Global use of nonnutritive ASs in food and beverage products indicated ASP has the highest use with 18.5 thousand metric tons, followed by SAC (9.7 thousand metric tons), ACE-K (6.8 thousand metric tons), and SUC (3.3 thousand metric tons) [14]. An extremely high concentration of ASP is used in the food industry (as much as 5602 mg/kg) in Korea [15]. ASP represents the largest artificial sweetener product segment globally; it is the most popular artificial sweetener in the U.S. and is used in more than 6000 food products [16].
Although they are found in very low concentrations, emerging contaminants have a great potential to trigger adverse human and the environmental effects as reported by some researchers in in vivo and/or in vitro laboratory studies at very low concentrations. Since they are found in very low concentrations (micrograms or milligrams per kgdw) in the environment, emerging contaminants are also known as trace pollutants [17, 18]. Therefore, these contaminants are the focus of several studies due to their potential environmental impacts attributed to their toxicity for aquatic and terrestrial organisms [18–21]. A significant proportion of SAC, CYC, ACE-K-k, and SUC move from the human body in an unchanged form following ingestion [22, 23], while ASP, ALT, NEO, and NSHD are removed to a greater degree [24]. The analysis of the ASs in wastewater influents and effluents was detected only in five (ACE-K, CYC, SAC, and SUC) out of seven ASs positively. Wastewater samples (downstream of wastewater effluents) from three different locations were analysed for the presence of ASP, SAC, and SUC in USA. Of the five water samples analysed, detectable concentrations of SUC were found in all samples at 0.8 and 1.8 μg/L. However, SAC was detected at one location only at a concentration of 5 μg/L [25, 26]. Estimated 30% of the samples had detectable amounts of one or more ASs, suggesting the presence of water derived from septic system effluent. Likewise, 2.0 to 4.7 percent of groundwater seeps had a septic effluent contribution of 1% or more [27]. A remarkable influence is exhibited by the type of eluents and the pH on the ASs recovery using solid-phase extraction cartridge (SPE) [4].
Maximum limit of the artificial sweeteners as defined by the Ministry of Health and family Welfare, Food Safety and Standards Authority of India, [28] is given below (Table 1).
Table 1.
Maximum permissible limit of various artificial sweeteners approved by FSSAI.
| Artificial sweetener | Food article | Maximum limit (ppm) |
|---|---|---|
| Sodium saccharin | Carbonated water | 100 |
| Soft drink concentrate | 100 | |
|
| ||
| Aspartame | Carbonated water | 700 |
| Soft drink concentrate | 700 | |
|
| ||
| Sucralose | Carbonated water | 300 |
| Soft drink concentrate | 300 | |
|
| ||
| Acesulfame-K | Carbonated water | 300 |
| Soft drink concentrate | 300 | |
|
| ||
| Neotame | Carbonated water | 33 |
| Soft drink concentrate | 33 | |
Source. Ministry of Health and Family Welfare, Food Standards Authority of India; Notification No. 01 of August 2011.
2. Studies Search and Selection Criteria
Literature search was performed by searching databases like PubMed, PubMed Central, Research Gate, Google Scholar, Medline, and Science Direct. The research articles based on the studies are related with nonnutritive ASs and their impact in environment. The search terms which were used during the literature search include the presence, source, route of transfer, and environmental impact and estimation techniques of the ASs. Studies including the detection techniques and regional studies were also included in the present review. The focus of the review is ASs as emerging contaminants.
2.1. Environmental Impact of the ASs
ASs have been reported to have negative health effects in both humans and aquatic organisms. They were previously unrecognized in terms of their health effects due to lack of standards and guidelines for their environmental monitoring and have only recently been identified as potential environmental contaminants [29]. The presence of ASs was initially detected in water and soil [30]. A considerable amount of these ASs were reported to remain unchanged and poorly absorbed [31]. The discharged ASs from different sources make their way to the wastewater treatment plants (WWTPs). However, most of the ASs escape even the most efficient WWTPs. Meanwhile, treated effluents from these WWTPs are believed to be major point sources of ASs which ultimately find their way into the environment [32]. The concentrations of ASs in wastewater effluents were found to have no effect of the wastewater treatment even by using nanograms per liter [32, 33].
ASP is hydrolyzed to aspartylphenylalanine below the pH of 3 and above the pH of 6, and it gets transformed into 5-benzyl-3,6-dioxo-2 piperazine acetic acid [34, 35]. ALT is soluble in water (approx. 13.1% w/v at 25°C) and is relatively stable to heat because of its unique amide group. Upon hydrolysis, ALT is converted to alanine amide, aspartic acid, and b-aspartic isomer, and in the human body, the N-glucuronide is the major metabolic product [36]. NEO is an N-substituted ASP derivative [37], with its major degradation product being de-esterified NEO (WHO Food Additive Series 52: NEO (http://www.inchem.org/documents). Neohesperidin dihydrochalcone is converted by humans anoxically to 3-(3-hydroxy-4-methoxyphenyl)propionic acid or 3-(3,4-dihydroxyphenyl)propionic acid [38].
Thus, the major metabolites of ASP, NEO, ALT, and NSHD should be expected to be present in the aquatic environment instead of their parent compounds. SAC is one of the oldest ASs. Its commercial production started in 1878. It is not metabolized by the body and is excreted unchanged in the urine once ingested [39], and the unabsorbed portion is excreted with faeces [40]. SAC is found in groundwater due to application of fertilizers in agriculture, degradation of sulfonylurea herbicides, old landfills, irrigation, soil water management, use of sludge as a fertilizer, and leaks in the ducts. Municipal wastewater and sewage are also found to contain SAC. SAC is used as a galvanic brightener in industry in smaller amounts [40]. SUC has been detected in coastal and sea waters. It was further reported that concentrations up to 0.21 μg/l of SAC were found in surface waters in China [41]. ACE-K-k, SAC, SUC, and CYC which are used as sugar substitutes are the most detected food additives/sweeteners in soils and groundwater [17, 42].
However, many ASs are not degradable and could also be introduced into the soil environment [43]. ASP is a dipeptide methyl ester of l-aspartyl-l-phenylalanine. For certain foods which are stored for longer durations, such as carbonated and still beverages, the stability of ASP in aqueous media is not satisfactory. As a dipeptide ester, ASP undergoes both hydrolysis and cyclization reactions. Under acidic conditions, hydrolysis of the ester and amide bonds is favored, resulting in the formation of its constituent amino acids with a concomitant loss of sweetness. More neutral and alkaline conditions are favorable for the cyclization of ASP to the corresponding diketopiperazine [44]. The physical and chemical properties and molecular structure of some of the ASs are given in Tables 2 and 3 respectively.
Table 2.
Physical and chemical characteristics of most widely used artificial sweeteners and their main markets worldwide.
| Artificial sweetener | Brand/trade name | Main market | Chemical formula | ADI (g/kg/day) | Sweetening potential | Uses | Molar mass (g/mol) | Density (g/cm3) | Year of approval | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| ASP | NutraSweet Equal | North America, Europe, Asia | C14H18N2O5 | 40 | 200 | Foods and beverages, pharmaceuticals, etc. | 294.3 | 1.347 | 1981 | Whitehouse et al., 2008 [45]; Fenge et al., 2013 [46]; Abu-Reidah [47] |
|
| ||||||||||
| SAC | Sweet'N Low, Sweet Twin, Necta Sweet | America, Europe, Asia | C7H5NO3 | 5 | 300 | Soft drinks, tabletop sweeteners, and desserts | 183.2 | 0.83 | 1985 | Walter [46]; Feng et al., 2013 [48]; BeMiller [49] |
|
| ||||||||||
| ACE | Sunett Sweet One |
North America, Europe, Asia | C4H4KNO4S | 15 | 200 | Table-top sweeteners, beverages, dairy products, confectionery, oral hygiene products, and pharmaceuticals | 163.15 | 1.83 | 1988 | Kuhn [50]; Lange et al. [51]; Zeece [52] |
|
| ||||||||||
| NEO | Neotame | America | C20H24N2O5 | 0.3 | 7000 | Carbonated soft drinks, yogurts, cakes, drink powders, etc. | 378.46 | 1.13 | 2002 | Nabors [54]; Lange et al. [51]; Tiefenb-acher [53] |
|
| ||||||||||
| SUC | Splenda | North America | C12H19Cl3O8 | 5 | 6000 | Diet foods and beverages | 397.64 | 1.69 | 1998 | Spillane [47]; Feng et al., 2013 [48]; Hughes and Dean [56] |
|
| ||||||||||
| ALT | Aclame | Australia, Mexico, New Zealand, China | C14H25N3O4S | 1 | 2000 | Bakery products, snack foods, candies and confectionery, ice cream, and frozen dairy products | 331.43 | Not approved by FDA, thus not used in USA and EU | Feng 2016 et al., 2013 [48]; Tiefenb-acher [53]; BeMiller [49] | |
|
| ||||||||||
| CYC | Twin Sweet | Europe, Asia | C6H12NNaO3S | 7 | 30 | Baked goods, confections, desserts, soft drinks, preserves, and salad dressings | 201.22 | 0.7 | 1984 banned in USA since 1970 | Lange et al., 2012 [51]; Chakrab-orty and Das, 2016 [57] |
|
| ||||||||||
| NHDC | Europe, Japan | C28H36O15 | 5 | 1900 | Flavoring agent or adjuvant, foods including condiments and seasonings, beverage, and pharmaceuticals | 612.6 | 1.6 | 1994 | Lange et al. [51]; Ashurst et al. [58] | |
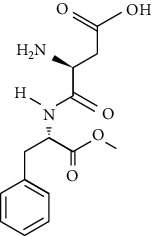
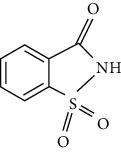
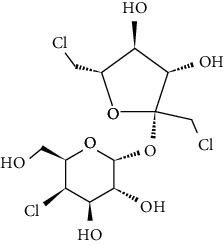
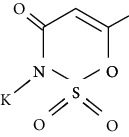
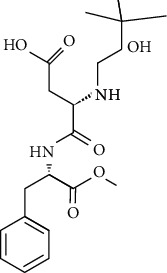
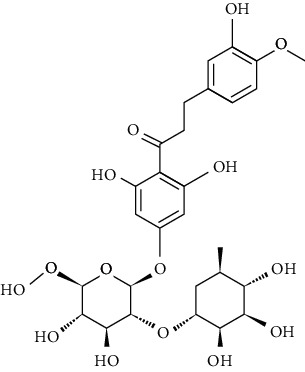
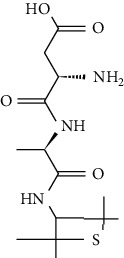
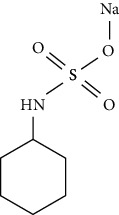
Table 3.
Molecular structures of the most widely used artificial sweeteners and a new class of emerging pollutants.
|
|
|
|
|
|
|
|
|
Molecular structures were redrawn using Chemdraw®.
ASs are recognized as a new class of environmental contaminants due to their extreme persistence and ubiquitous nature. The continuous introduction of ASs into the aquatic environments is attributed to their resistant behavior to wastewater treatment processes. However, behavior, fate, and long-term ecotoxicological contribution of ASs in water resources is at a large still unknown. The study of geographical/seasonal/hydrological interactions of ACE-K, CYC, SAC, and SUC in an open coast system at an estuarine/marine junction revealed higher occurrence of ACE-K (seasonal average: 0.22 μg L (−1)) and SUC (0.05 μg L (−1)) was noted in summer, while SAC (0.11 μg L (−1)) and CYC (0.10 μg L (−1)) were predominantly detected in winter, suggesting a strong connection with the variable chemical resistance among different sweeteners [30, 51]. The formation of new photo by-products under prolonged UV irradiation is highly viable in ACE-K and SUC compounds as were revealed by UPLC-ESI/MS degradation profile. Photodegradation results revealed that phototoxicity of ACE-K products may affect aquatic ecosystems [30, 59].
A study on estimated inputs from agriculture and households, degradation, and leaching to groundwater revealed that considerable concentration of SAC may end up in soils through liquid manure. SAC, found in piglet feed as an additive, is largely excreted. SAC may thus end up in soils in considerable quantities with manure up to a concentration of 12 mg/l and is stable for two months of storage. It was also observed that SAC is a soil metabolite of certain sulfonylurea herbicides. Meanwhile, ASs may get into the soil through irrigation with wastewater-polluted surface water and fertilization with sewage sludge (1–43 μg/L) or through leaky sewers. The soil incubation experiments revealed that CYC, SAC, ACE-K, and SUC were degraded with half-lives of 0.4–6 d, 3–12 d, 3–49 d, and 8–124 d, respectively. The study of the relative importance of entry pathways to soils, degradation, and leaching to groundwater revealed presence of SAC in groundwater (0.26 μg/L) is due to the application of manure. On the contrary, concentration of ACE-K (up to 5 μg/L) may be due to the infiltration of wastewater-polluted surface waters through stream beds [23, 42, 60].
The widespread presence of ASs in various environmental samples and their efficient recovery and accurate determination in environments depends on the various external factors including solid-phase extraction (SPE) cartridges, buffers and pH, matrix effects, and sample stability. The mainstream method of determination employed is SPE with LC-ESI-MS. The ASs, widely found in various environmental media, are ACE-K and SUC, and their investigated concentrations were found in the order of wastewater treatment plants (WWTPs) influent > WWTPs effluent > surface water > groundwater > drinking water; and atmosphere > soil. AS levels exhibit significant differences among different regions [61, 62].
ASs have been identified as emerging environmental pollutants and can be found in receiving waters, i.e., surface waters, groundwater aquifers, and drinking waters. Relative toxicity of ASs was studied by bioluminescence activity assay (using genetically modified bioluminescent bacteria from E. coli). Toxic effects were observed as the bacteria were exposed to certain concentrations of the ASs. In the bioluminescence activity assay, two toxicity response patterns were observed, namely, the induction and inhibition of the bioluminescent signal. An inhibition response pattern was observed in the response of SUC and NEO. Besides, the tested bioluminescent bacterial panel can potentially be used for detecting ASs in the environment, using a specific mode-of-action pattern [63]. SUC is increasingly being used as what scientists call a “tracer”—a substance that can help determine where contamination comes from. This capacity is essential for maintaining the quality of water, both in surface waters and in the supply of drinking water (https://www.scientificamerican.com).
Emerging pollutants are primarily of industrial origin and medical discharge and are translocated to soils, from soils to plants, and finally to consumers in the form of vegetables, fruits, etc. irrigated by treated wastewater or untreated surface water. Steroidal estrogens, bromofen, ibuprofen, caffeine, methyl dihydrojasmonate, etc. are some of the emerging pollutants other than ASs. The concentration of these pollutants in agricultural waters used for irrigation has been reported to range from 10 to 500 ng/L. The concentration of these pollutants in crops was found to be ranging from 1 to 7500 ng/kg [64]. Groundwater contamination with ASs has a number of different direct and indirect routes including percolation of treated wastewater in soil aquifer treatment, infiltration of wastewater-influenced surface waters, landfill leachate, municipal wastewater reservoirs, septic systems, and percolation of manure on agricultural land [8, 23, 65, 66].
ASs including ASP and SAC at the limit concentration of 100 mg/l was tested for their impact on model species like Lemna minor, Sinapis alba, Daphnia magna, Enchytraeus crypticus, Desmodesmus subspicatus, and Lactuca sativa. The study revealed statistically negative effects on Lemna minor, while as both ASP and SAC had negative reproductive effects on enchytraeids [67].
2.2. Source and Pathways of Emerging Contaminants
Input of contaminants into the soil and water is largely due to anthropogenic activities [68, 69] (Figure 1). Pond sources of contamination of soil, surface, and groundwater resources include discrete locations, municipal sewages, water treatment plants, accidental leaks, and landfills. Nonpoint or diffuse sources of contamination are agriculture and animal farming, industrial contaminants, exhaust gases (both industrial and vehicular), recycling, and untreated wastewater [70–75]. Emerging contaminants find their way into the soil and water environments through a source-pathway receptor model, wherein a pathway is necessary for the transport of these contaminants into these resources. Soils and water form the pathway, while living organisms (both aquatic and soil) are considered as receptors. The notable pathways include intentional disposal, landfill leachates, sewage leaks, irrigation with treated/untreated wastewaters, and animal farms [17, 74, 76–78]. Others include atmospheric air affecting plants and animals, deposition of contaminants on plant surfaces or soil, and gradual accumulation. These contaminants may persist for many years or spread through the soil profile and finally end up into the groundwater, besides incidental and structural spills and diffuse emissions [9].
Figure 1.
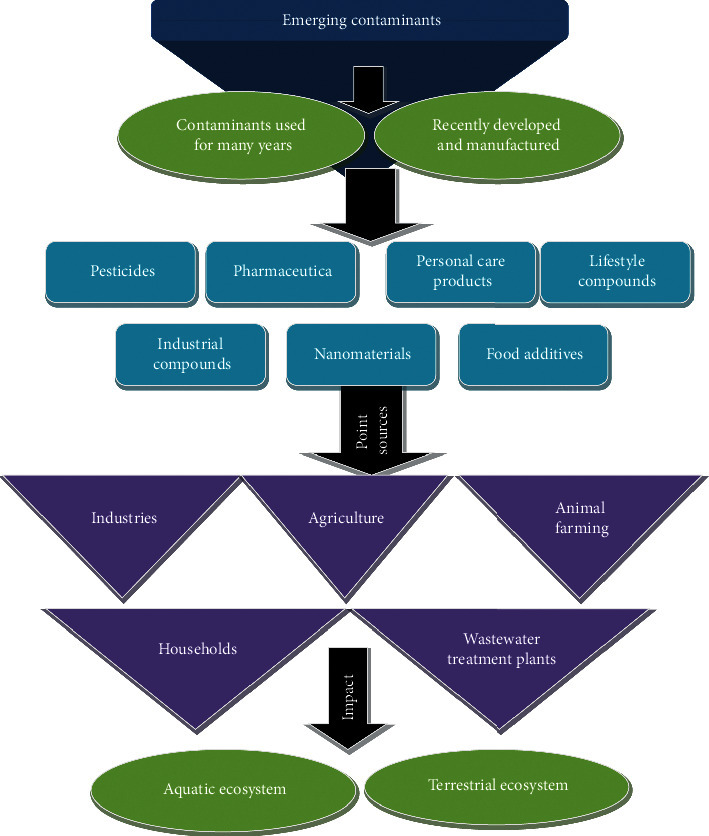
Flow chart showing emerging contaminants, sources, and their end points.
The high detection frequency of ASs in various environmental media has created great concern. Studies on ASs ecotoxicity and possible elimination routes in the environment revealed that negative impacts of ASs are more severe than expected, and the focus should be on the chronic and metabolic toxicities of ASs. Among the various ASs, CYC and SAC are easily removed, while SUC and ACE-K are generally persistent. The potential for microbial degradation of persistent ASs was reported in some regions, but clarification of the underlying mechanisms is necessary to increase the likelihood of using this approach in wide applications with a satisfactory performance [79, 80]. Emerging contaminants (pesticides and their breakdown products, pharmaceuticals, personal and house care products, lifestyle compounds, food additives, industrial products and wastes, and nanomaterials) have become a concern for the environment. Cumulative use of these products in industries, agriculture, domestic use, and healthcare services has led to their appearance in significant amounts in soils, surface, and groundwater resources with unpredictable consequences. Toxicity and bioaccumulation data of these emerging contaminants are limited [81].
The presence of various emerging contaminants including pharmaceuticals and personal care products (PPCPs) in groundwater is mainly attributed to anthropogenic activities as most of the compounds detected in the water samples are synthetic products, except caffeine [82]. ASs and PPCPs find their way into the groundwater from different sources and pathways including household PPCPs, industrial and hospital wastewater, drugs, and AS containing foods. The synthetic products or their breakdown compounds are added to landfills, septic system, STP effluent, sewer, soil, and surface water through livestock excreta, solid waste, surface water, toilets, and or sinks. These compounds are finally added to the groundwater in varied concentrations due to the addition of PPCPs containing sewage waters, leakage or managed aquifer recharge from sewer, sewage treatment plants, and septic system. A number of compounds including antibiotics, anti-inflammatories, lipid regulators, psychiatric drugs, stimulants, insect repellants, and sunscreen agents have been reported in groundwater in different concentrations [83].
A number of policies framed for the protection of the groundwater have a continuous challenge with the addition of bulk (700 approx.) emerging contaminants from various points and diffuse sources besides conventional pollutants [82].
2.3. Ecotoxicity of the ASs
The worldwide environmental distribution of ASs has prompted researchers towards the assessment of the ecotoxicological impact of these ASs. Depending on the presence, persistence, and bioaccumulation potential, ASs have varied toxic effects in the ecosystem, plant growth, and aquatic organisms [24]. SUC has been reported to affect the physiology and locomotive behavior of Daphnia magna at 0.0001–5 mg/L. Altered swimming height and increased swimming speed were observed in this planktonic crustacean. Besides, the time to reach food and shelter was found to be prolonged in Gammarid [84]. However, Daphnia magna and Mysida shrimp (Americamysis bahia) were reported to have no effect of SUC at ≤1800 mg/L and ≤93 mg/L of SUC, respectively, on their survival, growth, and reproduction [85]. Short-term effects of SUC on egg production, hatching rate, food intake, and mortality of two species of Arctic copepods at concentrations ranging from 0 to 50,000 ng/L showed nonsignificant effects on these parameters [86]. A recent study has reported that ASP (100 mg/kg) was toxic to Lemna minor, Sinapis alba, Daphnia magna, Enchytraeus crypticus, Desmodesmus subspicatus, and Lactuca sativa while both SAC and ASP (100 mg/kg) disrupted the reproduction of Enchytraeidae [67].
A study investigated the relative toxicity of the ASs using genetically modified bioluminescent bacteria from E. coli. The bioluminescent bacteria luminesce when they detect toxicants. The bacteria were exposed to varied concentrations of the ASs to study the toxic effects. In this assay, two toxicity response patterns were reported, namely, the induction and inhibition of the bioluminescent signal. An inhibition response pattern may be observed in the response of SUC in all the tested strains: TV1061 (MLIC = 1 mg/mL), DPD2544 (MLIC = 50 mg/mL), and DPD2794 (MLIC = 100 mg/mL). It is also observed in NEO in the DPD2544 (MLIC = 2 mg/mL) strain. However, the induction response pattern may be observed in its response in SAC in TV1061 (MLIndC = 5 mg/mL) and DPD2794 (MLIndC = 5 mg/mL) strains, ASP in DPD2794 (MLIndC = 4 mg/mL) strain, and ACE-K in DPD2794 (MLIndC = 10 mg/mL) strain [63].
The increased toxicity of ACE-K and SUC intermediates to living organisms following photolysis or electrolysis has also been documented in several other studies [87, 88]. The increased ecotoxicity of ACE-K after UV irradiance was found to be induced by the accumulation of OH• which caused a strong oxidative status even at a concentration of 0.1 mg/L in the liver of fish [89].
A study indicated that SUC disrupted the gene responsible for the absorption of sucrose from sugarcane, thereby inhibiting the absorption and transportation of sucrose within plants [90]. A strong positive association was observed between the concentration of SUC (0.0001–5 mg/L) and neurological and oxidative changes that could cause sublethal effects in Daphnia [91]. In addition, at environmentally appropriate concentrations (0.05 and 155 μg/L) with various exposure periods (12, 24, 48, 72, and 96 h), the possible SUC-induced toxicological hazard to Cyprinus carpio was also assessed. The results indicated that the content of lipid peroxidation, hydroperoxide, and protein carbonyl and the activity of antioxidant enzymes in the tested species especially in the gills, brain, and muscles were significantly increased [92]. SAC and CYC have also recently been found to exhibit plant cytotoxic and mutagenic (Allium cepa) effects at permitted levels [93].
In addition, it should be noted that while the parent ASs have been shown to be minimally harmful to aquatic habitats, they can potentially turn through different pathways into more toxic metabolites. The acute toxicity calculation of artificial sweetener metabolites showed that the acute inhibition effect of ACE-K-k in V. fischeri was significantly amplified to EC50 = 125.5 mg/L after photo treatment, with a measurable magnification factor of 575. Further study revealed that a calculated increase in toxicity (above 99.5 percent) was due to metabolite accumulation. This result is unusual since the enhancement factor for other persistent organic contaminants is rarely more than 20 times higher, illustrating the great concerns about ASs. Studies have found that the hazardous amplification of SUC ranged from 2670 mg/L to 156.2 mg/L with a detectable magnification factor of 16.5. (close to the “harmful” criterion of SUC of 100 mg/L). Considering the toxicity results given the more persistent and toxic by-products, ACE-K appeared to have a long-term ecological effect than SUC [30].
Furthermore, since a variety of contaminants can coexist in the environment, the toxic effects of AS mixtures with other chemicals in the environment should also be considered. In the order of classification of SAC > caffeine > ASP > SUC, ASs can affect the heart rate, eye density, and body length of the fish, and a cumulative effect combined with caffeine was observed [94]. Besides, some findings may consider that, at current environmental levels, ASs show reasonably low acute toxicity to species [86, 95].
Significant advancement in the analysis and detection has helped in discovery and quantification of emerging contaminants not only in living beings but also in diverse environmental substances. However, little data are available on the adverse effects of environmental exposure on the general population [69].
3. Conclusion
Artificial sweeteners being discussed for their positive and negative health implications are yet to be solved. A new addition into this debate is the negative impact of the ASs on the environment. As these are called as emerging contaminants, these ecotoxic compounds have added to the already extreme load of other pollutants. A two-way tackling methodology is required to contain the negative impacts of these emerging contaminants which are affecting both the human health as well as the health of the ecosystem. These observations may also indicate that the concentrations of SUC detected in the environment were well below of what is required to elicit effects in freshwater or marine invertebrates. However, it is also important to mention that, of the many ASs being used worldwide, the presence and concentration and ecotoxicity vary from one ecosystem to another. Therefore, observations made a few years back may not be the same at present as the number and bulk both increase at a very fast rate along with other environmental factors and pollutants. The growing evidence of the increased concentration and ecotoxicity of the ASs in the environment demands complete risk assessment, estimation and ecotoxicity tests, and elimination of these emerging contaminants. The review highlights the ecological impact of the ASs and their breakdown products which have recently been classified as emerging contaminants. The presence of the ASs and their degradation products in aquatic systems is a new threat to the aquatic life due to their presence in significant concentration. Besides testing, estimation techniques, sources, and transformation pathways, the review is expected to benefit the scientific community in future prospects for environment safety assessment.
Data Availability
Data are available within the article and its supplementary materials
Disclosure
No funding was received for this study
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Ab Qayoom Naik conceptualized the study and prepared the draft. Vinoy K. Shrivastava did formal analysis and reviewed and edited the manuscript. Ab Qayoom Naik and Tabassum Zafar wrote and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- 1.Cohen S. M., Arnold L. L., Emerson J. L. Safety of saccharin. Agro Food Industry Hi-Tech. 2008;19(6):p. 24. [Google Scholar]
- 2.Naik A. Q., Zafar T., Shrivastav V. K. Health implications associated with aspartame consumption: a substantial review. Pakistan Journal of Biological Sciences. 2018;21(3):127–134. doi: 10.3923/pjbs.2018.127.134. [DOI] [PubMed] [Google Scholar]
- 3.Subedi B., Kannan K. Fate of Artificial Sweeteners in Wastewater Treatment Plants in New York State, U.S.A. Environmental Science & Technology. 2014;48(23):13668–13674. doi: 10.1021/es504769c. [DOI] [PubMed] [Google Scholar]
- 4.Zygler A., Wasik A., Namieśnik J. Analytical methodologies for determination of artificial sweeteners in foodstuffs. TrAC Trends in Analytical Chemistry. 2009;28(9):1082–1102. doi: 10.1016/j.trac.2009.06.008. [DOI] [Google Scholar]
- 5.Asimakopoulos A. G., Nikoleli G. P., Thomaidis N. S., Nikolelis D. P. Methods of Analysis of Saccharin. In: Nollet L. M. L., Toldra F., editors. Handbook of Analysis of Active Compounds in Functional Foods. 3rd. Boca Raton, FL, USA: CRC Press; 2012. [Google Scholar]
- 6.Kokotou M. G., Asimakopoulos A. G., Thomaidis N. S. Sweeteners. In: Nollet L. M. L., Toldrá F., editors. Food Analysis by HPLC. Boca Raton, FL, USA: CRC Press; 2012. [Google Scholar]
- 7.Nikoleli G. P., Asimakopoulos A. G., Nikolelis D. P. Methods of Analysis of Acesulfame-K and Aspartame. In: Nollet L. M. L., Toldra F., editors. Handbook of Analysis of Active Compounds in Functional Foods. 3rd. Boca Raton: CRC Press; 2012. [Google Scholar]
- 8.Buerge I. J., Buser H.-R., Kahle M., Müller M. D., Poiger T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environmental Science & Technology. 2009;43(12):4381–4385. doi: 10.1021/es900126x. [DOI] [PubMed] [Google Scholar]
- 9.Houtman C. J. Emerging contaminants in surface waters and their relevance for the production of drinking water in Europe. Journal of Integrative Environmental Sciences. 2010;7(4):271–295. doi: 10.1080/1943815x.2010.511648. [DOI] [Google Scholar]
- 10.Lopez de Alda M. J., Díaz-Cruz S., Petrovic M., Barceló D. Liquid chromatography-(tandem) mass spectrometry of selected emerging pollutants (steroid sex hormones, drugs and alkylphenolic surfactants) in the aquatic environment. Journal of Chromatography. A. 2003;1000(1-2):503–526. doi: 10.1016/s0021-9673(03)00509-0. [DOI] [PubMed] [Google Scholar]
- 11.Naidu R., Arias Espana V. A., Liu Y., Jit J. Emerging contaminants in the environment: risk-based analysis for better management. Chemosphere. 2016a;154:350–357. doi: 10.1016/j.chemosphere.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 12.Dulio V., Von Der Ohe P. C. Working Group on Prioritisation of Emerging Substances. France: NORMAN Association, Verneuil En Halatte; 2013. NORMAN Prioritisation framework for emerging substances. [Google Scholar]
- 13.Chemical Economics Handbook. Sweeteners, High-Intensity. London, UK: IHS Markit; 2017. [Google Scholar]
- 14.Euromonitor International. Where Do Sugar and Sweeteners Stand Today. 2017. https://blog.euromonitor.com/2017. [Google Scholar]
- 15.Ha M.-S., Ha S.-D., Choi S.-H., Bae D.-H. Assessment of Korean consumer exposure to sodium saccharin, aspartame and stevioside. Food Additives & Contaminants: Part A. 2013;30(7):1238–1247. doi: 10.1080/19440049.2013.797114. [DOI] [PubMed] [Google Scholar]
- 16.Myers R. L. The 100 Most Important Chemical Compounds: A Reference Guide. Westport, CT, USA: Greenwood Publishing Group; 2007. [Google Scholar]
- 17.Lapworth D. J., Baran N., Stuart M. E., Ward R. S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environmental Pollution. 2012;163:287–303. doi: 10.1016/j.envpol.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Murray K. E., Thomas S. M., Bodour A. A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environmental Pollution. 2010;158(12):3462–3471. doi: 10.1016/j.envpol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Schriks M., Heringa M. B., van der Kooi M. M. E., de Voogt P., van Wezel A. P. Toxicological relevance of emerging contaminants for drinking water quality. Water Research. 2010;44(2):461–476. doi: 10.1016/j.watres.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Tadeo J., Sánchez-Brunete C., Albero B., García-Valcárcel A., Pérez R. Analysis of emerging organic contaminants in environmental solid samples. Open Chemistry. 2012;10(3):480–520. doi: 10.2478/s11532-011-0157-9. [DOI] [Google Scholar]
- 21.Wu X., Ernst F., Conkle J. L., Gan J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environment International. 2013;60:15–22. doi: 10.1016/j.envint.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Renwick A., Thompson J. P., OʼShaughnessy M., Walter E. J. The metabolism of cyclamate to cyclohexylamine in humans during long-term administration. Toxicology and Applied Pharmacology. 2004;196(3):367–380. doi: 10.1016/j.taap.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Scheurer M., Brauch H.-J., Lange F. T. Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT) Analytical and Bioanalytical Chemistry. 2009;394(6):1585–1594. doi: 10.1007/s00216-009-2881-y. [DOI] [PubMed] [Google Scholar]
- 24.Kokotou M. G., Asimakopoulos A. G., Thomaidis N. S. Artificial sweeteners as emerging pollutants in the environment: analytical methodologies and environmental impact. Analytical Methods. 2012;4(10):3057–3070. doi: 10.1039/c2ay05950a. [DOI] [Google Scholar]
- 25.Ferrer I., Thurman E. M. Analysis of sucralose and other sweeteners in water and beverage samples by liquid chromatography/time-of-flight mass spectrometry. Journal of Chromatography A. 2010;1217(25):4127–4134. doi: 10.1016/j.chroma.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Van Stempvoort D. R., Roy J. W., Brown S. J., Bickerton G. Artificial sweeteners as potential tracers in groundwater in urban environments. Journal of Hydrology. 2011;401(1-2):126–133. doi: 10.1016/j.jhydrol.2011.02.013. [DOI] [Google Scholar]
- 27.Spoelstra J., Senger N. D., Schiff S. L. Artificial Sweeteners Reveal Septic System Effluent in Rural Groundwater. Journal of Environmental Quality. 2017;46(6):1434–1443. doi: 10.2134/jeq2017.06.0233. [DOI] [PubMed] [Google Scholar]
- 28. Ministry of Health and Family Welfare, Food Standards Authority of India; Notification No. 01 of August 2011.
- 29.Miraji H., Othman O. C., Ngassapa F. N., Mureithi E. W. Research trends in emerging contaminants on the aquatic environments of Tanzania. Scientifica. 2016;2016:6. doi: 10.1155/2016/3769690.3769690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang Z., Jiang Y., Tsoi Y.-K., Leung K. S.-Y. Evaluating the environmental impact of artificial sweeteners: a study of their distributions, photodegradation and toxicities. Water Research. 2014;52(4):p. 260. doi: 10.1016/j.watres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Menzies I. S., Jenkins A. P., Heduan E., Catt S. D., Segal M. B., Creamer B. The effect of poorly absorbed solute on intestinal absorption. Scandinavian Journal of Gastroenterology. 1990;25(12):1257–1264. doi: 10.3109/00365529008998562. [DOI] [PubMed] [Google Scholar]
- 32.Tran N. H., Hu J., Li J., Ong S. L. Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Research. 2014;48:443–456. doi: 10.1016/j.watres.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 33.Stolte S., Steudte S., Schebb N. H., Willenberg I., Stepnowski P. Ecotoxicity of artificial sweeteners and stevioside. Environment International. 2013;60:123–127. doi: 10.1016/j.envint.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Gloria M. B. A. Intense sweeteners and synthetic colorants. In: Nollet L. M. L., editor. Food Analysis by HPLC. 2nd. New York, NY, USA: Marcer Dekker, Inc.; 1997. [Google Scholar]
- 35.Sardesai V., Waldshan T. H. Natural and synthetic intense sweeteners. The Journal of Nutritional Biochemistry. 1991;2(5):236–244. doi: 10.1016/0955-2863(91)90081-f. [DOI] [Google Scholar]
- 36.Nabors L. B. O. Alternative Sweeteners. New York, NY, USA: Marcer Dekker; 2001. [Google Scholar]
- 37.Prakash I., Bishay I., Schroeder S. Neotame: synthesis, stereochemistry and sweetness. Synthetic Communications. 1999;29(24):4461–4467. doi: 10.1080/00397919908086610. [DOI] [Google Scholar]
- 38.Braune A., Engst W., Blaut M. Degradation of neohesperidin dihydrochalcone by human intestinal bacteria. Journal of Agricultural and Food Chemistry. 2005;53(5):1782–1790. doi: 10.1021/jf0484982. [DOI] [PubMed] [Google Scholar]
- 39.Čopikova J., Moravcova J., Wimmer Z., Opletal L., Lapčik O., Drašar P. Nahradni sladidla. Chemicke Listy. 2013;107:867–874. [Google Scholar]
- 40.Velisek J., Hajslova J. Chemistry of Food in Czech. Chemie Potravin 2. OSSIS: Havličkův Brod; 2009. [Google Scholar]
- 41.Gan Z., Sun H., Feng B., Wang R., Zhang Y. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Research. 2013;47(14):4928–4937. doi: 10.1016/j.watres.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Buerge I. J., Keller M., Buser H.-R., Müller M. D., Poiger T. Saccharin and other artificial sweeteners in soils: estimated inputs from agriculture and households, degradation, and leaching to groundwater. Environmental Science & Technology. 2011;45(2):615–621. doi: 10.1021/es1031272. [DOI] [PubMed] [Google Scholar]
- 43.Smrckova S., Bindzar J. Nahradni sladidla jako poputanty vody. Chemicke Listy. 2014;108:1125–1132. [Google Scholar]
- 44.Furia T. E. CRC Handbook of Food Additives. 2nd. Boca Raton, FL, USA: CRC Press; 1980. [Google Scholar]
- 45.Whitehouse C. R., Boullata J., McCauley L. A. The potential toxicity of artificial sweeteners. Workplace Health & Safety (AAOHN) Journal. 2008;56(6):251–259. doi: 10.3928/08910162-20080601-02. [DOI] [PubMed] [Google Scholar]
- 46.Walters E. The Sweetener Book. 2013. [Google Scholar]
- 47.Spillane W. J. Optimizing Sweet Taste in Foods. Ireland: Wood Head; 2006. [Google Scholar]
- 48.Feng B. T., Gan Z. W., Hu H. W., Sun H. W. Research progress on the environmental behaviour of artificial sweeteners. Environmental Chemistry. 2013;32:1158–1166. [Google Scholar]
- 49.BeMiller J. N. James N. BeMiller. Carbohydrate Chemistry for Food Scientists. 3rd. Cambridge, MA, USA: AACC International Press; 2019. Carbohydrate and non-carbohydrate sweeteners. [Google Scholar]
- 50.Kuhn C., Bufe B., Winnig M., et al. Bitter taste receptors for Saccharin and Acesulfame K. Journal of Neuroscience. 2004;24(45):10260–10265. doi: 10.1523/jneurosci.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lange F. T., Scheurer M., Brauch H.-J. Artificial sweeteners-a recently recognized class of emerging environmental contaminants: a review. Analytical and Bioanalytical Chemistry. 2012;403(9):2503–2518. doi: 10.1007/s00216-012-5892-z. [DOI] [PubMed] [Google Scholar]
- 52.Zeece M. Michael Zeece, Introduction to the Chemistry of Food. Cambridge, MA, USA: Academic Press; 2020. Flavors. [Google Scholar]
- 53.Tiefenbacher K. F. Tiefenbacher K. F. Technology of Main Ingredients—Sweeteners and Lipids. Cambridge, MA, USA: Academic Press; 2017. [Google Scholar]
- 54.Nabors L. B. O. Sweet choices: sugar replacements for foods and beverages. Food Technology. 2002;56(45):28–32. [Google Scholar]
- 55.Abu-Reidah I. M. Carbonated beverages. In: Galanakis C. M., editor. Trends in Non-Alcoholic Beverages. Cambridge, MA, USA: Academic Press; 2020. [Google Scholar]
- 56.Hughes C. V., Dean J. A. Mechanical and chemotherapeutic home oral hygiene. In: Dean J. A., editor. McDonald and Averyʼs Dentistry for the Child and Adolescent. 10th. Maryland Heights, MO, USA: Mosby; 2016. [Google Scholar]
- 57.Das A., Chakaraborty R. Sweeteners: classification, sensory and health effects. In: Caballero B., Finglas P. M., Toldrá F., editors. Encyclopedia of Food and Health. Cambridge, MA, USA: Academic Press; 2016. [Google Scholar]
- 58.Ashurst P. .R., Hargitt R., Palmer F. Ingredients in Soft Drinks. In: Ashurst P. R., Hargitt R., Palmer F., editors. Woodhead Publishing Series in Food Science, Technology and Nutrition, Soft Drink and Fruit Juice Problems Solved. 2nd. Sawston, UK: Woodhead Publishing; 2017. [Google Scholar]
- 59.Osin O. A., Yu T., Cai X., et al. photocatalytic degradation of 4-nitrophenol by C, N-TiO2: degradation efficiency vs. embryonic toxicity of the resulting compounds. Frontiers in Chemistry. 2018;6:p. 192. doi: 10.3389/fchem.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleinsteuber S., Rohwerder T., Lohse U., Seiwert B., Reemtsma T. Sated by a zero-calorie sweetener: wastewater bacteria can feed on acesulfame. Frontiers in Microbiology. 2019;10:p. 2606. doi: 10.3389/fmicb.2019.02606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo J., Wu L., Zhang Q., et al. Review on the determination and distribution patterns of a widespread contaminant artificial sweetener in the environment. Environmental Science and Pollution Research. 2019;26(19):19078–19096. doi: 10.1007/s11356-019-05261-4. [DOI] [PubMed] [Google Scholar]
- 62.Tran N. H., Hu J., Ong S. L. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC-MS/MS and isotope dilution. Talanta. 2013;113:82–92. doi: 10.1016/j.talanta.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 63.Harpaz D., Yeo L., Cecchini F., et al. Measuring artificial sweeteners toxicity using a bioluminescent bacterial panel. Molecules. 2018;23(10):p. 2454. doi: 10.3390/molecules23102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh B. N., Hidangmayum A., Singh A., Guru A., Yashu B. R., Singh G. S. Effect of emerging contaminants on crops and mechanism of toxicity. In: Lichtfouse E., editor. Sustainable Agriculture Reviews. Cham, Switzerland: Springer; 2020. [Google Scholar]
- 65.Mead R. N., Morgan J. B., Avery G. B., et al. Occurrence of the artificial sweetener sucralose in coastal and marine waters of the United States. Marine Chemistry. 2009;116(1–4):13–17. doi: 10.1016/j.marchem.2009.09.005. [DOI] [Google Scholar]
- 66.Scheurer M., Storck F. R., Graf C., et al. Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. Journal of Environmental Monitoring. 2011;13(4):966–973. doi: 10.1039/c0em00701c. [DOI] [PubMed] [Google Scholar]
- 67.Kobeticova K., Mocova K. A., Mrhalkova L., Frycova Z., Koci V. Artificial sweeteners and the environment. Czech Journal of Food Science. 2016;34:149–153. [Google Scholar]
- 68.Fabietti G., Biasioli M., Barberis R., Ajmone-Marsan F. Soil contamination by organic and inorganic pollutants at the regional scale: the case of Piedmont, Italy. Journal of Soils and Sediments. 2009;10(10):290–300. doi: 10.1007/s11368-009-0114-9. [DOI] [Google Scholar]
- 69.Lei M., Zhang L., Lei J., et al. Overview of emerging contaminants and associated human health effects. BioMed Research International. 2015;40:p. 4796. doi: 10.1155/2015/404796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balderacchi M., Benoit P., Cambier P., et al. Groundwater pollution and quality monitoring approaches at the European level. Critical Reviews in Environmental Science and Technology. 2013;43(4):323–408. doi: 10.1080/10643389.2011.604259. [DOI] [Google Scholar]
- 71.Bone J., Head M., Barraclough D., et al. Soil quality assessment under emerging regulatory requirements. Environment International. 2010;36(6):609–622. doi: 10.1016/j.envint.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 72.EUGRIS. Diffuse Pollution. 2011. Portal for soil and water management in Europe. http://www.eugris.info/FurtherDescription. [Google Scholar]
- 73.Jurado A., Vàzquez-Suñé E., Carrera J., López de Alda de Alda M., Pujades E., Barceló D. Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Science of the Total Environment. 2012;440:82–94. doi: 10.1016/j.scitotenv.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 74.Ongley E. D., Xiaolan Z., Tao Y. Current status of agricultural and rural non-point source pollution assessment in China. Environmental Pollution. 2010;158(5):1159–1168. doi: 10.1016/j.envpol.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 75.Stuart M., Lapworth D., Crane E., Hart A. Review of risk from potential emerging contaminants in UK groundwater. Science of the Total Environment. 2012;416:1–21. doi: 10.1016/j.scitotenv.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 76.Borylo A., Skwarzec B., Olszewski G. The radiochemical contamination (210Po and 238U) of zone around phosphogypsum waste heap in Wislinka (northern Poland) Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering. 2012;47(5):675–687. doi: 10.1080/10934529.2012.660052. [DOI] [PubMed] [Google Scholar]
- 77.Macherius A., Eggen T., Lorenz W. G., Reemtsma T., Winkler U., Moeder M. Uptake of galaxolide, tonalide, and triclosan by carrot, barley, and meadow fescue plants. Journal of Agricultural and Food Chemistry. 2012;60(32):7785–7791. doi: 10.1021/jf301917q. [DOI] [PubMed] [Google Scholar]
- 78.Mahmoud E., Abd El-Kader N. Heavy metal immobilization in contaminated soils using phosphogypsum and rice straw compost. Land Degradation & Development. 2015;26(8):819–824. doi: 10.1002/ldr.2288. [DOI] [Google Scholar]
- 79.Cantwell M. G., Katz D. R., Sullivan J., Kuhn A. Evaluation of the artificial sweetener sucralose as a sanitary wastewater tracer in Narragansett Bay, Rhode Island, USA. Marine Pollution Bulletin. 2019;146:711–717. doi: 10.1016/j.marpolbul.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo J., Zhang Q., Cao M., et al. Ecotoxicity and environmental fates of newly recognized contaminants-artificial sweeteners: A review. Science of the Total Environment. 2019;653:1149–1160. doi: 10.1016/j.scitotenv.2018.10.445. [DOI] [PubMed] [Google Scholar]
- 81.Gomes A. R., Justino C., Rocha-Santos T., Freitas A. C., Duarte A. C., Pereira R. Review of the ecotoxicological effects of emerging contaminants to soil biota. Journal of Environmental Science and Health, Part A. 2017;52(10):992–1007. doi: 10.1080/10934529.2017.1328946. [DOI] [PubMed] [Google Scholar]
- 82.Knee K. L., Gossett R., Boehm A. B., Paytan A. Caffeine and agricultural pesticide concentrations in surface water and groundwater on the north shore of Kauai (Hawaii, USA) Marine Pollution Bulletin. 2010;60(8):1376–1382. doi: 10.1016/j.marpolbul.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Sui. 2013. [Google Scholar]
- 84.Wiklund A.-K. E., Breitholtz M., Bengtsson B.-E., Adolfsson-Erici M. Sucralose-an ecotoxicological challenger? Chemosphere. 2012;86(1):50–55. doi: 10.1016/j.chemosphere.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 85.Huggett D. B., Stoddard K. I. Effects of the artificial sweetener sucralose on Daphnia magna and Americamysis bahia survival, growth and reproduction. Food and Chemical Toxicology. 2011;49(10):2575–2579. doi: 10.1016/j.fct.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 86.Hjorth M., Hansen J. H., Camus L. Short-term effects of sucralose on Calanus finmarchicus and Calanus glacialisin Disko Bay, Greenland. Chemistry and Ecology. 2010;26(5):385–393. doi: 10.1080/02757540.2010.504672. [DOI] [Google Scholar]
- 87.Calza P., Sakkas V. A., Medana C., Vlachou A. D., Dal Bello F., Albanis T. A. Chemometric assessment and investigation of mechanism involved in photo-Fenton and TiO2 photocatalytic degradation of the artificial sweetener sucralose in aqueous media2 photocatalytic degradation of the artificial sweetener sucralose in aqueous media. Applied Catalysis B: Environmental. 2013;129(2):71–79. doi: 10.1016/j.apcatb.2012.08.043. [DOI] [Google Scholar]
- 88.Lin H., Wu J., Oturan N., Zhang H., Oturan M. A. Degradation of artificial sweetener saccharin in aqueous medium by electrochemically generated hydroxyl radicals. Environmental Science and Pollution Research. 2015;23(5):1–12. doi: 10.1007/s11356-015-5633-x. [DOI] [PubMed] [Google Scholar]
- 89.Ren Y., Geng J., Li F., Ren H., Ding L., Xu K. The oxidative stress in the liver of Carassius auratus exposed to acesulfame and its UV irradiance products. Science of the Total Environment. 2016;571:755–762. doi: 10.1016/j.scitotenv.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 90.Reinders A., Sivitz A. B., Hsi A., Grof C. P. L., Perroux J. M., Ward J. M. Sugarcane ShSUT1: analysis of sucrose transport activity and inhibition by sucralose. Plant, Cell and Environment. 2006;29(10):1871–1880. doi: 10.1111/j.1365-3040.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 91.Eriksson Wiklund A. K., Adolfsson-Erici M., Liewenborg B., Gorokhova E. Sucralose induces biochemical responses in Daphnia magna. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0092771.e92771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saucedo-Vence K., Elizalde-Velázquez A., Dublán-García O., et al. Toxicological hazard induced by sucralose to environmentally relevant concentrations in common carp (Cyprinus carpio) Science of the Total Environment. 2017;575:347–357. doi: 10.1016/j.scitotenv.2016.09.230. [DOI] [PubMed] [Google Scholar]
- 93.Oliveira V. A. D., Oliveira V. M. A. D., Oliveira T. W. N. D., et al. Evaluation of Cytotoxic and Mutagenic Effects of Two Artificial Sweeteners Using Eukaryotic Test Systems. African Journal of Biotechnology. 2017;16(11) [Google Scholar]
- 94.Lee W., Wang Y. C. Assessing developmental toxicity of caffeine and sweeteners in medaka (Oryzias latipes) Springerplus. 2015;4(1):1–10. doi: 10.1186/s40064-015-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tollefsen K. E., Nizzetto L., Huggett D. B. Presence, fate and effects of the intense sweetener sucralose in the aquatic environment. Science of the Total Environment. 2012;438(3):510–516. doi: 10.1016/j.scitotenv.2012.08.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available within the article and its supplementary materials


