Abstract
The COVID-19 pandemic is a serious threat to global health and the global economy. The ongoing race to develop a safe and efficacious vaccine to prevent infection by SARS-CoV-2, the causative agent for COVID-19, highlights the importance of vaccination to combat infectious pathogens. The highly accessible cutaneous microenvironment is an ideal target for vaccination since the skin harbors a high density of antigen-presenting cells and immune accessory cells with broad innate immune functions. Microarray patches (MAPs) are an attractive intracutaneous biocargo delivery system that enables safe, reproducible, and controlled administration of vaccine components (antigens, with or without adjuvants) to defined skin microenvironments. This review describes the structure of the SARS-CoV-2 virus and relevant antigenic targets for vaccination, summarizes key concepts of skin immunobiology in the context of prophylactic immunization, and presents an overview of MAP-mediated cutaneous vaccine delivery. Concluding remarks on MAP-based skin immunization are provided to contribute to the rational development of safe and effective MAP-delivered vaccines against emerging infectious diseases, including COVID-19.
Keywords: COVID-19, Cutaneous immunobiology, Infectious diseases, Microarray patches, Prophylactic immunization, Skin-targeted vaccines, SARS-CoV-2, Spike protein
Graphical abstract

1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had an unprecedented impact on global health and the global economy [[1], [2], [3], [4], [5]]. Specifically, SARS-CoV-2 has spread globally, infecting approximately 105 million people with more than 2.3 million fatalities as of February 7, 2021, as reported on the World Health Organization (WHO) coronavirus disease (COVID-19) dashboard. Initial forecasts suggested that global economic growth would decline substantially in 2020 because of COVID-19, and potential future waves of SARS-CoV-2 infection could further shrink the global economy [6,7]. Rapidly expanding public health strategies help control the spread of COVID-19; however, removal or relaxation of current interventions could result in a considerable increase in the number of SARS-CoV-2 infections, highlighting an urgent unmet need for the development of more effective and broadly applicable pandemic countermeasures [[8], [9], [10], [11]].
The remarkable history of immunization against infectious pathogens underscores the importance of vaccination to combat emerging infectious diseases [[12], [13], [14], [15]]. Indeed, since the release of the genome sequence of SARS-CoV-2 in mid-January 2020, there has been unprecedented progress toward the development of a safe and effective vaccine against SARS-CoV-2 infection [[16], [17], [18]]. Prominent advances in biomedical science and technology have enabled rapid development of several COVID-19 vaccine candidates using different biotechnology platforms, such as recombinant protein, live attenuated or inactivated virus, nucleic acid, and live viral vectored antigen strategies [[18], [19], [20], [21], [22], [23]]. Although most of these vaccine candidates are still in pre-clinical stages, some are already in advanced clinical trials for safety and efficacy testing [[23], [24], [25], [26], [27], [28], [29], [30]]. Importantly, a few SARS-CoV-2 vaccines, including Pfizer-BioNTech and Moderna mRNA vaccines, and Oxford-AstraZeneca adenovirus (Ad)-based vaccine, have already been approved for emergency use in some countries, and approval is being sought for other vaccine candidates, including Janssen’s Ad-based vaccine and Novavax’s nanoparticle formulated and adjuvanted subunit vaccine. Ultimately, these vaccine efforts and others are expected to enable effective global immunization to combat the COVID-19 pandemic.
Efficacious and widespread immunization requires safe, reproducible, versatile, patient-friendly, cost-effective, and broadly deployable vaccine delivery strategies. However, the vast majority of vaccines, including the most advanced COVID-19 vaccine candidates, are currently delivered via traditional parenteral routes (e.g., subcutaneous (SC) or intramuscular (IM)) through hypodermic needle injection [31]. These methods have established limitations. The effectiveness of many traditional vaccines is susceptible to variations in temperature that can occur between production and injection, thereby necessitating a costly cold-chain for distribution and storage [32,33]. Furthermore, several require trained healthcare providers for proper administration, and can be subject to high vaccination non-compliance due to pain, needle phobia, and inconvenience [[34], [35], [36]]. These factors, combined with other potentially complicating components, such as susceptibility to needle stick injuries and disposal of biohazardous sharp waste, present formidable obstacles for mass vaccination [[32], [33], [34], [35], [36]]. To obviate these challenges, innovative vaccine delivery technologies are being developed, which may be particularly relevant for emerging pandemics [[37], [38], [39], [40], [41]]. Microarray patches (MAPs) are among the most attractive vaccine delivery systems and offer unique advantages that could improve the safety, compliance, coverage, immunogenicity, and cost of immunization by targeting vaccine components to cutaneous microenvironments [42]. Skin-targeted vaccines have the potential to be more immunogenic due to the immune-responsive nature of the skin compared to subcutaneous or intramuscular tissues targeted by prevailing vaccination routes [42]. These advantages, and the potential to obviate the cold-chain by stabilizing vaccine components in the delivery device, could enable minimally invasive delivery, self-administration, and economically advantageous refrigeration-free storage and distribution [[42], [43], [44], [45], [46]]. As such, skin-targeted vaccination using MAPs has received considerable attention, including recent efforts to develop MAP-delivered coronaviruses vaccines.
Here, we present an overview of the rationale and current status of MAP-based intracutaneous immunization against emerging infectious diseases relevant to the development of safe and effective skin-targeted vaccines capable of inducing potent and long-lived pathogen-specific protective immunity. Specifically, we briefly discuss emerging knowledge of the host-pathogen interaction of SARS-CoV-2 infection relevant to vaccination strategies, key components of skin that make it an active immune organ, and the basics of skin-targeted immunization against infectious pathogens. We then provide a synopsis of existing MAP types and associated designs, materials, and manufacturing aspects, and describe the known attributes of MAPs that could contribute to their effectiveness in harnessing the immune-responsive skin microenvironment to enable diverse immunization approaches against emerging infectious diseases, including COVID-19. Finally, we discuss our current perspective on the opportunities and challenges for future development of MAP-directed vaccination strategies against COVID-19 and future pandemics.
2. Structural properties of SARS-CoV-2
Emergence of a new infectious disease demands urgent efforts to gain a thorough understanding of the structure of the causative pathogen and host-pathogen interactions to better understand the disease pathogenesis and to enable the rational design of safe and effective intervention strategies. In this regard, significant efforts have been made to uncover the structural properties of the novel coronavirus and key mechanisms of SARS-CoV-2 infection since COVID-19 initially emerged in late 2019 [[47], [48], [49], [50]]. The knowledge gained from the two preceding coronaviruses (SARS-CoV and MERS-CoV) outbreaks and our emerging understanding of the SARS-CoV-2 virus and infection have enabled the identification of rational targets for COVID-19 vaccine development [[51], [52], [53]]. These recent efforts, combined with advances in biotechnology, have already facilitated the rapid production of several distinct COVID-19 vaccine candidates.
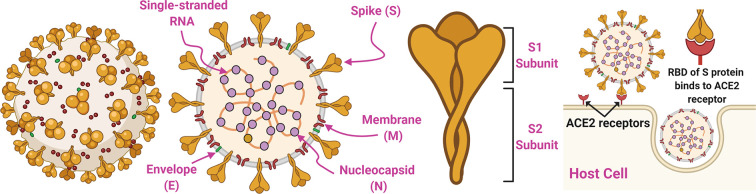
The structural properties of SARS-CoV-2 and its interactions with host cells are illustrated in Fig. 1 . SARS-CoV, MERS-CoV, and SARS-CoV-2 are Betacoronaviruses (Beta-CoVs), which are enveloped, single-stranded positive sense RNA viruses [54,55]. The nucleocapsid (N), envelope (E), membrane (M), and spike (S) proteins are important structural components of BetaCoVs [[55], [56], [57]]. The E and M proteins are critical for the formation or assembly of coronavirus particles [[58], [59], [60]]. The N protein interacts with the RNA genome of coronaviruses and participates in viral transcription and assembly of BetaCoVs [61]. The S protein of SARS-CoV-2 is a major player in the infection of host cells, and in turn, replication of SARS-CoV-2 [62]. Therefore, the S protein has received significant attention as a potential target for safe and effective intervention strategies [[63], [64], [65]]. Specifically, the SARS-CoV-2 spike glycoprotein consists of S1 and S2 subunits that mediate viral entry to host cells. The S1 subunit binds to the angiotensin-converting enzyme 2 (ACE2) receptor of host cells through its receptor-binding domain (RBD), and the S2 subunit enables viral fusion with the target cell membrane [[66], [67], [68]]. The SARS-CoV-2 S glycoprotein is cleaved by host proteases at the boundary between the S1 and S2 subunits, separating S1 from S2. This furin cleavage site is unique to SARS-CoV-2 and is not found in SARS-CoV. SARS-CoV-2 and other CoVs undergo further cleavage at a second site found within the S2 subunit, through a process that activates the S protein to promote membrane fusion [48]. The S protein is a known target of neutralizing antibodies generated in coronavirus infections, which can block the virus from binding to the ACE2 receptor [[69], [70], [71]]. Ultimately, this emerging knowledge about coronaviruses suggests that the S protein of the viral envelope is an attractive target for the rational design and development of COVID-19 vaccines [[72], [73], [74]]. This is further supported by previous efforts demonstrating the immunogenicity of the S protein of SARS-CoV and MERS-CoV [[75], [76], [77]]. To date, several vaccine candidates targeting the S protein of SARS-CoV-2 (e.g., either full S protein, S1 subunit, or RBD domain) have been developed using different antigen formats, including nucleic acid (DNA or RNA), attenuated live viral vectors (e.g., adenovirus), and recombinant protein [[78], [79], [80], [81], [82], [83], [84]]. More recently, other structural components of coronaviruses, such as the N and M proteins, have also been suggested as potential vaccine targets due to their ability to elicit virus-specific cellular immune responses that may be important for the breadth and durability of the immune response [20,85]. Thus, progressive scientific efforts on elucidation of the structure of BetaCoVs are identifying many vaccine targets against COVID-19.
Fig. 1.
Structural properties of the enveloped, positive-sense, single-stranded RNA virus SARS-CoV-2 and mechanism of SARS-CoV-2 entry into host cells. Critical viral components include Envelope (E), Membrane (M), Nucleocapsid (N), and Spike (S) proteins. The S protein consists of two subunits (S1 and S2) and plays a major role in the infection of host cells, as the receptor-binding domain (RBD) of the S protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells.
3. The skin as an immune-responsive organ
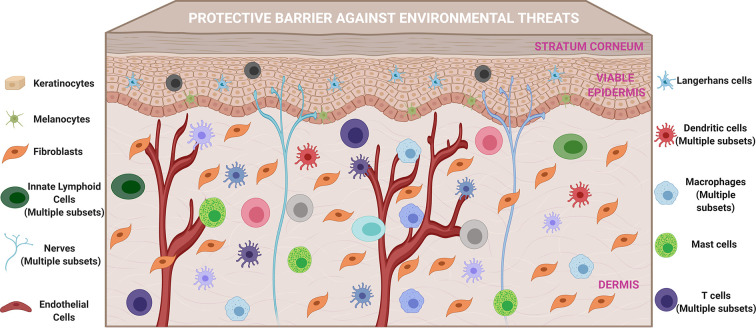
The skin is a multifunctional organ that constitutes the first line of host defense against external stressors [[86], [87], [88]]. Although the role of skin as a protective physical barrier has been broadly recognized, its unique ability to serve as an immune-responsive organ has more recently been appreciated due to advances in our understanding of skin immune mechanisms that initiate and regulate innate and adaptive immune responses against invading pathogens [[89], [90], [91], [92]]. Emerging knowledge has revealed that there is both a high density of professional antigen-presenting cells (APCs) and immune-accessory cells that form a tightly regulated immune network within the skin microenvironment [42,[91], [92], [93], [94], [95], [96]]. Our increasing understanding of the mechanisms underlying immune regulation in the skin provides novel opportunities to rationally develop effective skin-targeted vaccines based on localized immune engineering. Here, we briefly describe relevant skin-resident cells (Fig. 2 ) that are integral to immune regulation in the skin, and in turn, important contributors to pathogen-specific protective immune responses induced by skin-targeted vaccines.
Fig. 2.
The skin is a vital protective barrier against environmental threats, and an active immunological site that harbors professional antigen-presenting cells and diverse immune accessory cells that regulate cutaneous immune mechanisms and initiate and modulate adaptive immune responses to invading pathogens and skin-delivered vaccine components.
3.1. Keratinocytes
Keratinocytes are the most numerous cell population in the epidermis and play critical roles in the mechanical barrier function of the skin [[97], [98], [99]]. In addition to their structural role, keratinocytes are key innate immune cells in the skin microenvironment, where they sense injuries and pathogens, and secrete several immune mediators in response to these threats [[99], [100], [101], [102]]. Keratinocytes express pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), which recognize pathogen- or damage-associated molecular patterns (PAMPs or DAMPs), and signaling through these receptors ultimately promotes production of a myriad of immune mediators, including cytokines, chemokines, and antimicrobial peptides, to regulate immune mechanisms in the injured or infected skin [[100], [101], [102], [103]]. The immunomodulatory factors produced by keratinocytes facilitate cross-talk with other epithelial cells and cutaneous immune cells to control a wide range of immunological processes, including initiation of dendritic cell (DC) migration to the skin draining lymph nodes, activation of mast cells and innate lymphoid cells (ILCs), and recruitment of different immune cells (e.g., neutrophils, basophils, and eosinophils) to sites of infection or injury. Interestingly, a recent study demonstrated that human keratinocytes also express ACE2 at relatively higher levels than other skin cells [104], and the significance of this finding for COVID-19 infection and vaccination has yet to be determined. Similarly, another recent study showed high expression of ACE2 on epithelial cells of oral mucosa [105], which could constitute a potential route for COVID-19 infection and needs to be further studied in that context.
3.2. Fibroblasts
Fibroblasts reside in the dermis and synthesize collagen, which is essential for the formation of the connective tissue of skin, or extracellular matrix (ECM) [106,107]. Thus, fibroblasts provide mechanical support for the epidermis and contribute to the elastic nature of the skin. Like keratinocytes, fibroblasts communicate with other skin cells, express PRRs, and produce a broad range of cytokines and chemokines in response to environmental threats [91,[108], [109], [110]].
3.3. Melanocytes
Melanocytes are found in the basal layer of the epidermis and produce melanin to protect the skin against the deleterious effects of ultraviolet radiation (UVR) [111,112]. Melanocytes communicate with other skin cells to respond to invading pathogens, or to maintain cutaneous homeostasis [113]. For instance, melanocytes modulate skin immunity in response to UVR. To control innate and adaptive immune responses in the cutaneous microenvironment, melanocytes express several TLRs and secrete numerous chemokines and cytokines, such as IL-1β, IL-6, and TNF-α [[113], [114], [115], [116]].
3.4. Innate lymphoid cells
Innate lymphoid cells (ILCs) are skin-resident cells that can be classified into three major populations (ILC1, ILC2, and ILC3) based on the expression of different transcription factors (T-bet, GATA3, or RORγt, respectively) and different cytokines [[117], [118], [119]]. Specifically, ILCs imprint cytokine patterns similar to those of T helper cells [[120], [121], [122]]. For instance, ILC1s secrete type 1 cytokines (e.g., IFN-γ and TNF-α), while ILC2s produce different cytokines, such as IL-4, IL-5, IL-9, and IL-13, and ILC3s have sub-populations that secrete IL-17 and IL-22 [[117], [118], [119]]. ILCs are capable of responding to environmental threats to trigger innate immune mechanisms that facilitate the induction of adaptive immune responses [120,122]. Importantly, ILCs communicate with other skin cells, including keratinocytes, dendritic cells, and mast cells, and these interactions play critical roles in ILC survival and stimulation, as well as innate immune responses.
3.5. Mast cells
Mast cells are typically located in the dermal layer of the skin in close proximity to microvessels, nerves, and fibroblasts, and they release several immune mediators in response to external stimuli, including physical damage or invading foreign pathogens [[123], [124], [125], [126]]. In addition to their established roles in allergic diseases, mast cells are critical players in the regulation of innate and adaptive immune responses against infectious agents [[127], [128], [129], [130]]. While the activation of mast cells typically results in the production of pro-inflammatory TNF-α and Th2 cytokines (e.g., IL-4 and IL-13), mast cells also secrete neuropeptides and other cytokines, such as IL-25, IL-33, and TSLP [125,131]. Mast cell-derived signals can mobilize skin DCs toward the draining lymph nodes [132,133]. Further, under certain conditions, mast cells can function as APCs and migrate to the skin draining lymph nodes to present antigens to T and B lymphocytes [128,130,132]. Mast cells are also capable of eliciting antigen-specific CD8+ T-cell responses through MHC-I-restricted presentation of antigens [128,134]. Collectively, these features suggest that cutaneous mast cells could be strategically harnessed to improve the efficacy of skin-targeted vaccines.
3.6. Dendritic cells
The epidermal and dermal layers of human skin are rich in dendritic cells (DCs), which are professional APCs and key players in intracutaneous vaccination due to their remarkable capacity to induce adaptive immune responses [[135], [136], [137], [138]]. Cutaneous dendritic cells, such as epidermal DCs (known as Langerhans cells) and functionally distinct subsets of dermal DCs, collectively possess high plasticity, functionally adapting to microenvironmental changes in the skin and serving as important regulators of cutaneous immunity [[138], [139], [140]]. Specifically, these professional APCs, sometimes referred to as sentinel cells and named for their dendrites, can actively and efficiently sample the skin microenvironment and respond to injury or invasion by secreting a range of functionally distinct cytokines and chemokines depending on the nature of stimuli [[138], [139], [140]]. Cutaneous DCs efficiently phagocytose and process antigens, migrate to the skin draining lymph nodes, and stimulate naïve lymphocytes, and their dendritic structure helps them interact broadly with lymph node-resident cells [[138], [139], [140]]. To enable effective antigen presentation, skin DCs express MHC-I, MHC-II, T-cell adhesion, and co-stimulatory molecules essential for T-cell activation. They sense immunological cues released by other skin cells, and modulate their immunostimulatory activities based on pathogen-specific microenvironmental signals [93,138]. Skin-derived DCs have been shown to be more potent than lymph node-resident DCs in inducing antigen-specific CD8+ T-cell responses [141]. Ultimately, these unique features of skin DCs, including high functional plasticity, diversity, and ability to interact with other cells, offer exploitable and specific opportunities for vaccine development. Our emerging understanding of the roles of individual DC subsets and their communication with other skin- and lymph node-resident cells will facilitate the rational design and development of skin-targeted vaccination strategies against emerging infectious diseases.
3.7. Neurons
The skin is equipped with a sophisticated neuronal network that is capable of responding to a range of external stimuli [[142], [143], [144]]. Recent studies demonstrated that nerves in the skin regulate skin immunity through cross-talk with the cutaneous immune system [142,145,146]. Skin neurons express PRRs, including TLR3, TLR4, TLR7, and TLR9, communicate with cutaneous cells to recognize pathogens and injuries, and secrete cytokines and chemokines to modulate cutaneous immune cells [125,142,145]. Skin DCs and other cutaneous cells, such as keratinocytes and mast cells, produce mediators that stimulate the cutaneous nervous system, and activated neurons release neuropeptides that further regulate cutaneous immunity and promote adaptive immune responses to fight against environmental threats [125,146]. As such, multi-dimensional relationships exist between skin nervous, immune, and epithelial systems to control cutaneous immunity and regulate systemic immune responses. These systems can be harnessed using adjuvants to induce transient neurogenic inflammation to improve the immunogenicity of antigens targeted to the skin and/or to strategically modify systemic immune responses [147].
3.8. Macrophages
Skin-resident macrophages, found in the dermis, execute a broad range of immunological functions [[148], [149], [150]]. The vital functions of macrophages include serving as danger or pathogen sensors and presenting antigen to lymphocytes [[148], [149], [150]]. Thus, skin macrophages are equipped to recognize harmful environmental agents and to secrete different types of chemokines and cytokines, which induce a pro-inflammatory skin microenvironment and recruit different types of immune cells to the infection or injury site to eliminate pathogens, repair physical damage, and modulate adaptive immune responses [150,151]. Interestingly, skin macrophages are less potent than DCs with respect to induction of adaptive immune responses [151].
3.9. Skin-resident T cells
Cutaneous T cells play critical roles in immune responses against invading pathogens, recognizing specific foreign antigens, targeting infected cells, and producing immune mediators, such as IFN-γ and IL-17 [[152], [153], [154], [155]]. Specifically, pathogen-specific resident memory T cells (Trm), established through natural infection or vaccination, confer long-term protection. CD4+ Trm in the dermis or CD8+ Trm in the epidermis lack homing molecules and are retained in the skin [156]. Trm are poised to rapidly respond against pathogens through different mechanisms [[157], [158], [159], [160], [161]]. For instance, upon microbial invasion, cutaneous DCs can present antigen to skin-resident Trm for rapid protection, and skin-resident Trm can directly kill pathogens or infected cells [153,157]. Interestingly, skin vaccination is capable of generating Trm in distal organs (e.g., lungs), which also contribute to protective immune responses against pathogens (e.g., respiratory viruses) [162]. Ultimately, a thorough understanding of the mechanisms of induction of tissue-resident T cells via intracutaneous vaccination, as well as diverse functions of these Trm could support rational development of innovative skin-targeted immunization strategies.
3.10. Endothelial cells
Skin has a complex microvascular network that participates in controlling cutaneous immunity [91,163]. Specifically, cutaneous microvascular endothelial cells that recognize pathogens through TLR-signaling can secrete several chemokines and cytokines to regulate protective immune responses [109,163]. Further, pro-inflammatory mediators produced by other skin cells, such as IL-1β, TNF-α, CCL2, and CCL5, lead to the activation of skin endothelial cells, which increases the permeability of skin capillaries and extravasation of leukocytes from circulation into the skin [164].
3.11. Infiltrating granulocytes
The complex skin immune mechanisms are further regulated by the ingress of granulocytes (e.g., neutrophils, basophils, and eosinophils) in response to invading microbial agents [165]. Multiple immune mediators, released by the aforementioned cutaneous cells (e.g., mast cells), control the recruitment of granulocytes to the site of infection [166]. In addition to modulating innate immune responses to eliminate invading pathogens, granulocytes contribute to adaptive immune responses by regulating DC function [165]. For instance, neutrophils, the most numerous granulocytes in the circulation, quickly accumulate at sites of infection for rapid initiation of innate immune defense through different mechanisms, such as phagocytosis and production of neutrophil extracellular traps and antimicrobial peptides [132,165]. Furthermore, neutrophils influence DC function via either direct contact or secretion of cytokines and chemokines, and in turn, manipulate the magnitude and quality of ensuing pathogen-specific adaptive immune responses [165]. Thus, transient accumulation of neutrophils in the skin could be exploited to improve the efficacy of skin-targeted vaccines. Basophils and eosinophils constitute a small percentage of leukocytes in the blood and are associated with induction of Th2 immune responses [165]. Besides their critical roles in altering innate immune responses and regulating DC migration and function, granulocytes have also been reported to function as non-conventional APCs [132]. Ultimately, the influx of granulocytes to a vaccine delivery site and their impact on local immune mechanisms and adaptive immune responses should be considered for the development of safe and effective skin-targeted vaccines.
Together, it is becoming increasingly evident that the sophisticated cutaneous immune network enables the skin to respond to environmental threats through intricate, interdependent mechanisms. An improved understanding of how local skin immune mechanisms modulate systemic immunity will facilitate a paradigm-shift from empirical vaccination strategies to rationally designed intracutaneous vaccination approaches. Importantly, most of our current understanding of skin immune mechanisms is based on studies using murine models. It is well established that there are significant differences between mice and humans in terms of both skin anatomy and skin immunity, and understanding these fundamental differences will be critical for clinical translation. Moreover, in the same way that the plasticity of the skin immune system enables remarkable adaptability, it also creates somewhat of a ‘moving target’. Palpably, skin immunity is affected by a variety of factors, including age, UVR exposure, and the microbiome, and the impact of these and other variables on cutaneous immune mechanisms will need to be better understood to develop safer and more effective skin-targeted human vaccination strategies. Efforts to address these knowledge gaps should be prioritized, and will be essential to complement unprecedented ongoing vaccine development efforts.
4. Skin vaccination
The readily accessible, immunologically active skin microenvironment has long been considered an attractive target tissue for vaccination [167,168]. Specifically, the immunization campaign against smallpox that utilized intracutaneous vaccination with vaccinia virus was the first successful demonstration of a large-scale effective vaccine that induced protective immunity in human populations, and this approach depended on harnessing the skin immune network [167,168]. Since then, progress in developing skin-targeted vaccines has been relatively slow, partly because of inaccessibility created by the outermost skin barrier, the stratum corneum, and the lack of technologies capable of reproducibly introducing antigen into the skin microenvironment [169,170]. Interestingly, intramuscular (IM) and subcutaneous (SC) vaccination routes have been empirically used for the majority of vaccines without strong mechanistic evidence supporting their rationale other than reproducible delivery. Recently, there has been a substantial increase in hypothesis driven efforts to gain a fundamental understanding of the effect of immunization routes on vaccine efficacy [37,[171], [172], [173]]. Advances in skin science, coupled with evidence of successful induction of sustained humoral and cellular immune responses, even at distant organs (e.g., lungs), through skin-targeted vaccination are providing the much-needed mechanistic underpinnings for rationally designed skin vaccination strategies [162,[174], [175], [176], [177], [178], [179]]. A growing body of evidence suggests that intracutaneous immunization is mechanistically superior to traditional vaccination methods due to the more immune-responsive microenvironment present in the skin compared to subcutaneous and muscle tissues [172,[180], [181], [182]]. Further, greater efficiencies seen with skin immunization could offer dose-sparing (i.e., requiring less vaccine doses to induce protective immune responses) for both antigens and adjuvants, reducing cost and in the later instance increasing safety. Although these advantages need to be confirmed for each antigen and adjuvant candidate, they could translate into more effective global immunization programs against infectious pathogens.
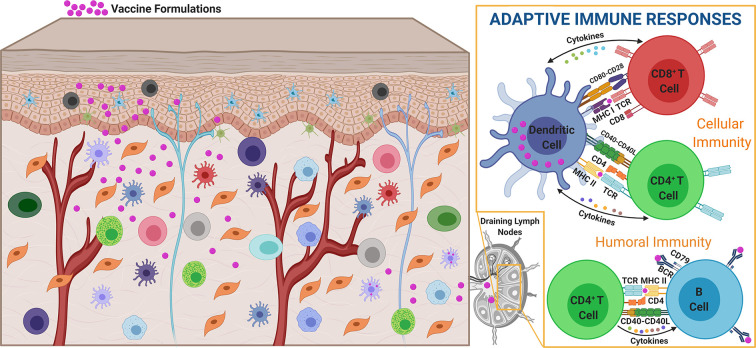
Intracutaneous vaccination utilizes innate immune mechanisms to provide environmental context (e.g., danger signals) to tune skin APC functions to promote internalization and processing of skin-delivered antigens, migration to the skin-draining lymph nodes, and presentation to adaptive immune cells [173,183]. Skin APCs can interact with lymph node-resident T cells directly, or indirectly by transferring antigens and activation signals to lymph node-resident APCs, both of which result in priming and amplification of adaptive immunity [184,185]. Further, in addition to delivering antigen to skin-resident APCs, skin-targeting could result in direct delivery of antigen to lymph node-resident APCs by way of passive diffusion through the draining lymphatics [184,186]. This rich population of APCs in the cutaneous microenvironment, their capacity to adapt their function based on environmental conditions, and their effective communication with cells in the skin-draining lymph nodes, together uniquely enable efficient priming of antigen-specific T and B cells. In turn, these regionally primed and expanded antigen-specific T and B cells can take up residence in the skin and other distal epithelial tissues to provide systemic protection. Together, these mechanisms enable skin targeted vaccines to induce efficacious systemic immunity that is both antigen specific and durable (Fig. 3 ).
Fig. 3.
Skin vaccination induces antigen-specific cellular and humoral immune responses. Introduction of vaccine components into the skin microenvironment results in antigen loading and functional skewing of skin-resident APCs capable of directly or indirectly inducing CD4+ and CD8+ T-cell responses in the draining lymph nodes. The magnitude, breadth, and longevity of these responses are influenced by vaccine dose, spatiotemporal vaccine release kinetics, and immunoregulatory signals delivered with the antigen or released by other skin cells.
Several key factors contribute to antigen presentation function of skin APCs, which impact the magnitude, quality, and longevity of vaccine-induced protective immunity. In addition to the structure and dose of antigen, spatiotemporal presence of antigen plays an important role in shaping the ensuing antigen-specific immune responses [173,187]. Importantly, microenvironmental signals (e.g., chemokines and cytokines), which are secreted by skin-resident cells and influenced by the antigen delivery method (e.g., disruption of the skin barrier), as well as antigen type, dose, and kinetics, regulate local immune mechanisms to elicit systemic protective immunity [173,[188], [189], [190]]. As such, a comprehensive understanding of these factors for different types of antigens and delivery methods is pivotal for the rational development of skin-targeted immunization strategies. Further, a thorough investigation of the correlation between local cutaneous mechanisms and resulting antigen-specific systemic adaptive immune responses is necessary for more predictable skin-targeted vaccines.
To harness the processes and mechanisms involved in generating pathogen-specific protective immune responses, immune potentiators (also known as adjuvants) that influence the quality and durability of antigen-specific adaptive immune responses induced by vaccination are being developed to induce optimal protective immunity [191,192]. Specifically, development of molecules (e.g., TLR and NLR ligands) that can activate innate immune mechanisms and/or biomaterials assisted strategies that enable spatiotemporally controlled delivery of antigens, with or without adjuvants, are two popular strategies being used to improve immunogenicity [[191], [192], [193]]. Physical disruption of the superficial skin layers has also been explored to boost immunogenicity [188,193]. Notably, despite several emerging adjuvant candidates, few adjuvants (e.g., cholera toxin, imiquimod, resiquimod, and aqueous formulation of glucopyranosyl lipid (GLA-AF)) have reached the stage of clinical trials for skin vaccination [[193], [194], [195]]. This translates into a need for substantial research to identify ideal adjuvants for skin vaccination that could function either by selective activation of accessory skin cells, or by direct action on skin APCs. Moreover, certain existing adjuvant candidates, which may be toxic with systemic exposure resulting from traditional needle injection, could be feasible via skin delivery. The identification of skin-specific adjuvants, as well as targeted delivery systems specific to different antigen formats and used as immune-potentiators, will contribute significantly to next-generation skin-targeted vaccination strategies.
Despite the theoretical advantages of the skin microenvironment targeting, there are currently only a few clinically approved skin-targeted vaccines. This is also reflected in current COVID-19 vaccine efforts in which there are only a few skin-targeted SARS-CoV-2 vaccines to date (e.g., an intradermal plasmid DNA vaccine, an intradermal mRNA vaccine, and a MAP-delivered subunit vaccine) [[196], [197], [198]]. On the other hand, the vast majority of vaccine candidates being developed against COVID-19 are delivered via traditional routes. Several knowledge gaps are now being addressed to enable the rational development of safe and effective skin-targeted vaccines against COVID-19 and other emerging infectious diseases. Local skin immune mechanisms elicited by vaccine delivery need to be elucidated, and their relationship to the nature and durability of the induced systemic adaptive immune responses must be defined. This understanding will be critical for rational selection of adjuvants and other immune modifiers to modulate skin immune circuits to control the quality and longevity of pathogen-specific systemic immune responses. For instance, antibody-dependent enhancement of natural infection and cell-mediated immunopathology resulting from sub-optimal humoral or cellular immune responses is an important safety concern for coronavirus vaccines [[199], [200], [201]]. Manipulating skin immune mechanisms to drive B-cell maturation and antigen-specific neutralizing antibody production, and skew T-cell responses toward Th1-type immunity could improve both the safety and efficacy of viral vaccines. Further, comprehensive studies are needed to compare the effects of various antigen platforms (e.g., viral vector, recombinant protein, or nucleic acid) with different antigens (e.g., S protein, N protein, or S1 subunit) and adjuvants on skin immune circuits. Finally, models capable of bridging findings from murine studies to humans will need to be developed further and deployed to reduce the empiricism of translation. Collectively, the skin offers an attractive target for the development of more effective immunization approaches against SARS-CoV-2 and other infectious pathogens. However, critical knowledge and technology gaps remain and must be addressed to realize the full potential of intracutaneous vaccination. Thus, the coming years are likely to bring an increasing number of academic and/or industrial efforts to address these gaps for the rational development of skin-targeted vaccines against a broad range of infectious diseases.
5. Microarray patches
Microarray patches (MAPs) are among the most promising skin-targeted vaccine delivery systems [42,44,202]. One of the major roadblocks to the success of intracutaneous immunization has been the lack of safe, effective, convenient, patient-friendly, and inexpensive delivery technologies that can reliably administer vaccines to targeted skin microenvironments [167,170,203]. Precise and reproducible deployment of vaccine components (e.g., antigens and adjuvants) to immunologically active skin layers (viable epidermis and dermis), which harbor APCs and other cells with diverse innate immune functions, is challenging due to the protective physical barrier, imparted by the outermost layer of skin, the stratum corneum [167,203,204]. The hydrophobic stratum corneum, which consists of several layers of dead keratinocytes embedded in an organized lipid structure, is a major obstacle to reliable delivery of hydrophilic antigens and structurally complex immunomodulators [[203], [204], [205], [206]]. Therefore, macromolecule antigens and adjuvants applied to the skin topically or via traditional transdermal patches are typically unable to penetrate the stratum corneum, as required for effective immunization. Conversely, injections with conventional hypodermic needles pierce the superficial skin layers, but delivery into the skin microenvironment is inconsistent, and they are painful and less suitable for global vaccination programs due to the need for medical expertise for proper administration, supply chain challenges, and non-compliance due to trypanophobia and inconvenience [[33], [34], [35], [36]]. Combining the simplicity of topical application or conventional transdermal patches with the delivery benefits of traditional hypodermic needle injections, while considerably improving the precision and reproducibility of the skin-targeted biocargo delivery, MAPs are a rapidly emerging technology platform for convenient, painless, and controlled vaccine delivery to the skin [[207], [208], [209]]. Over the past decade, MAPs have shown incredible promise for intracutaneous vaccination using different types of antigens, with or without adjuvants, providing an attractive alternative for the development of effective skin-targeted vaccines against SARS-CoV-2 [29,[207], [208], [209], [210]]. This section presents an overview of MAP technology and MAP-mediated immunization to contribute to the rational development of MAP-based skin-targeted vaccines.
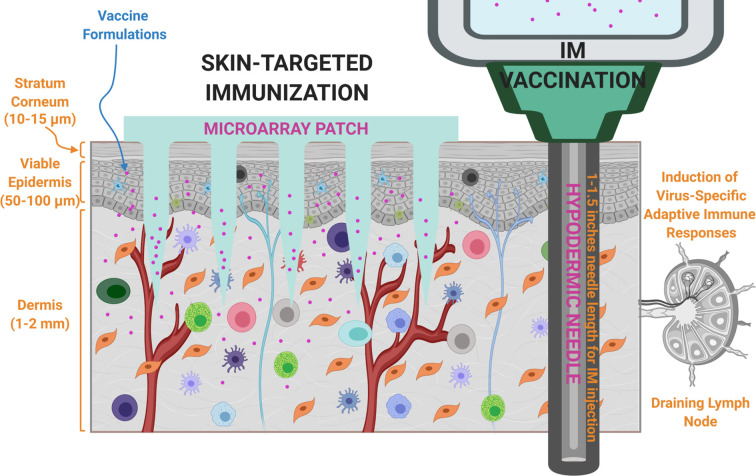
Microarray patches typically contain several micron-scale protrusions attached to a backing substrate, and these sharp-tipped microprotrusions, designed and fabricated with diverse geometries using various materials, mechanically penetrate the skin (the stratum corneum) to deliver biocargos (e.g., vaccine components) [211,212]. Unlike prevailing hypodermic needle injections, MAPs deliver vaccine components into viable skin layers that are considered more suitable immune targets for vaccination, thereby resulting in improved antigen-specific immune responses (Fig. 4 ). In addition to this mechanistic advantage, MAPs offer advantages in terms of practicality and convenience (simple and painless application and the potential for self-application), logistics (thermostable and no need for reconstitution for certain MAPs), safety (no biohazardous sharp waste generation for certain MAPs, avoiding the potential risk of disease transmission), and cost (inexpensive to manufacture and distribute or dose-sparing), which, combined with consistent skin-targeted delivery and immunogenicity benefits, render MAPs attractive for global vaccination programs [[42], [43], [44],[213], [214], [215]]. Due to these advantages, there has been great interest in the development of several types of MAPs, and in functional testing of MAPs for numerous vaccine candidates [[207], [208], [209]]. Collectively, the design, biomaterials, manufacturing, skin delivery mechanisms and efficiency, storage, acceptability, and cost of MAP systems are key factors contributing to the success of MAP-based vaccination.
Fig. 4.
Microarray patches (MAPs) enable precise, consistent, and minimally invasive administration of vaccine formulations (antigen ± adjuvant) to immunologically rich cutaneous microenvironments, whereas conventional intramuscular (IM) immunization bypasses the skin immune system, results in systemic exposure to vaccine ingredients, and causes pain.
5.1. MAP types and materials
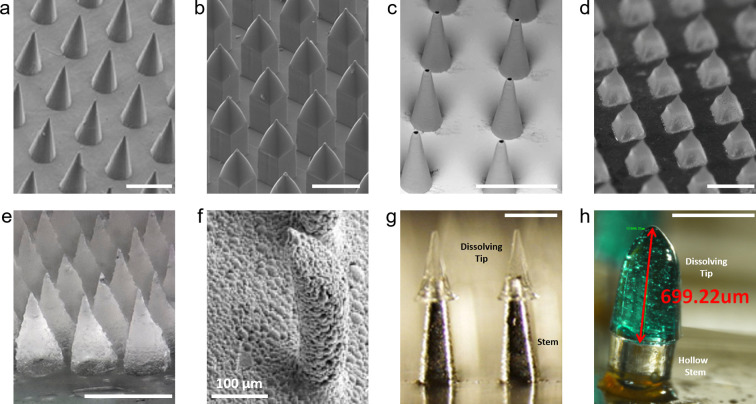
Several MAP concepts with various geometries have been designed and engineered from a broad range of materials to enable effective skin-targeted cargo delivery [[216], [217], [218]]. In addition to commonly investigated MAP types (solid without biocargo, coated, hollow, and dissolving/biodegradable), hydrogel-forming, porous, and hybrid MAPs have been explored (Fig. 5 ) [[216], [217], [218], [219], [220], [221], [222], [223], [224], [225], [226], [227]]. Besides the prevailing pyramid and conical geometries, other microarray shapes, including square and circular obelisk, negative bevel obelisk, undercut, and snake fang, have been fabricated using different materials [218,[228], [229], [230], [231], [232]]. Ultimately, each MAP design possesses different manufacturability, mechanical strength, skin insertion reliability, painlessness, vaccine dosage capacity, delivery mechanisms, and delivery efficiencies. All of these factors contribute to the convenience, efficacy, reproducibility, and cost of MAP-based skin-targeted immunization efforts.
Fig. 5.
Representative images of different MAP concepts presented in the literature. a. Solid MAPs manufactured from polylactic acid (PLA). Adapted with permission from [220]. Copyright 2010, Elsevier. b. Coated MAPs fabricated from PLA. Adapted with permission from [221] under terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0/). Copyright 2020, the Authors. c. Polymer hollow MAPs. Adapted with permission from [222] under terms of the CC-BY 4.0 license. Copyright 2019, the Authors. d. Dissolvable MAPs produced from a mixture of carboxymethylcellulose and trehalose. Adapted with permission from [223]. Copyright 2011, Wiley-VCH. e. Hydrogel-forming MAPs created from a blend of poly(methylvinylether-co-maleic acid), poly(ethylene glycol), and sodium carbonate. Adapted with permission from [224]. Copyright 2018, American Chemical Society. f. Porous MAPs fabricated from PLA. Adapted with permission from [225]. Copyright 2007, Springer Nature. g. Hybrid MAPs with stainless steel stems and dissolving poly(vinyl alcohol)/sucrose tips. Adapted with permission from [226]. Copyright 2011, Elsevier. h. Hybrid MAPs with SU-8 microtube stems and dissolving maltose tips after 3 min skin application. Adapted with permission from [227]. Copyright 2013, American Institute of Physics. Unless otherwise indicated, scale bars = 500 μm.
5.1.1. Solid MAPs (Fig. 5a)
Solid MAPs without biocargo are one of the earliest types of MAPs enabled by traditional microfabrication techniques and are used to enhance the skin permeability prior to topical application of a biocargo solution or biocargo integrating patch [[216], [217], [218]]. This concept is known as the ‘poke and patch’ strategy, as topically applied vaccine components passively diffuse through microchannels created by the application and removal of MAPs [[216], [217], [218]]. Although solid MAPs were initially created mainly from silicon due to early availability of microfabrication processes, they have been also manufactured from other materials, including ceramics, different metals (e.g., titanium, nickel, and stainless steel), and polymers, such as poly(methyl methacrylate) (PMMA), polyglycolic acid (PGA), polycarbonate, and polylactic acid (PLA) [[216], [217], [218],220,233]. The ‘poke and patch’ approach increases the permeability of skin to vaccine components, presenting proof-of-concept for MAP-based cutaneous vaccination; however, limitations including concerns related to reliability and reproducibility of delivery, poor patient adherence due to the complex application process, the need for preparation of topical vaccine formulations with different vaccine components, combined with recent advances in biomaterials science and engineering, have motivated development of other MAP types.
5.1.2. Coated MAPs (Fig. 5b)
Biocargo (vaccine) coated MAPs have been developed by complementing solid MAPs described above with favorable aqueous formulations that typically include a thickening agent or viscosity enhancer, surfactant, stabilizer, and target cargo, and with suitable coating methods [216,221,234]. This MAP concept is known as the ‘coat and poke’ approach and combines the multiple steps of the ‘poke and patch’ method into a single-unit, single-step drug/vaccine dosage form [219]. The materials used for solid MAPs have also been utilized to fabricate coated MAPs, and coating formulations containing thickening agents (e.g., carboxymethylcellulose (CMC), sodium alginate, polyethylene glycol (PEG), hyaluronic acid (HA)), surfactants (e.g., poloxamer Lutrol F68, Tween 20, and Quil-A) and stabilizers (e.g., trehalose and sucrose) facilitate reliable and reproducible coating with retained bioactivity and adhesion to the microarray surface, rapid dissolution upon skin insertion, and long-term stability of coated vaccine components [216,234]. The coating formulations can include components with adjuvant activities (e.g., Quil-A) or can be tailored for temporal release of vaccine components [[234], [235], [236]]. Although vaccine-coated MAPs have been broadly tested for skin-targeted immunization, they still pose challenges, including potential limitations on achievable vaccine doses, reductions in skin delivery efficiencies due to decreased tip sharpness because of biocargo coating, and complications associated with the coating formulations and process, which may lead to losses in the quantity of vaccine components and bioactivity of antigen. Moreover, coated MAPs could generate biohazardous sharp waste and bear the risk of disease transmission due to unsafe disposal practices. For both coated and solid MAPs fabricated with non-biodegradable materials, unwanted skin effects (e.g., granulomas or scarring) remain a potential concern due to accidental breakage of non-degradable microarrays in the skin during application. These challenges have inspired new MAP types with manufacturing, design, vaccine dose, biomaterials, safety, and application advantages.
5.1.3. Hollow MAPs (Fig. 5c)
Hollow MAPs employ the ‘poke and flow’ approach in which a liquid formulation of biocargo (e.g., vaccine) is administered through microchannels created across skin layers by microarrays [[216], [217], [218],237]. The basic version is a single needle with micron-scale dimensions (e.g., a miniaturized hypodermic needle). Hollow MAPs have been used to either directly inject liquid cargo into the skin, or provide paths or channels for cargo in a reservoir to passively diffuse into the skin [216,217]. Unlike solid MAPs that transiently improve skin permeability, hollow MAPs facilitate continuous delivery of the biocargo through their openings and can administer relatively higher doses of biocargo compared to other MAP types. Notably, hollow MAPs can deliver liquid cargo solutions without the need for dry vaccine or drug formulations, yet the formulation aspect must still be investigated to achieve effective skin concentrations with the relatively lower volume of solutions that can be delivered to the cutaneous microenvironment. Several different materials, such as metals, polymers, silicon, and glass, have been used to fabricate hollow MAPs with diverse designs [222,232,237,238]. Although hollow MAPs have been used to deliver vaccine components to skin microenvironments, the design, fabrication, and application aspects of hollow MAPs still require extra attention due to their relatively complex shapes, the potential of leakage of vaccine components, the risk of mechanical failure during skin insertion because of their weaker structure, the complicated manufacturing steps required to create hollow MAPs, and the possibility of clogging of microarray openings.
5.1.4. Dissolvable/biodegradable MAPs (Fig. 5d)
Biodissolvable/biodegradable MAPs have been widely investigated [218,223,239]. These MAPs integrating biocargos (e.g., vaccine components) are fabricated from biocompatible natural or synthetic polymers that either rapidly dissolve, or slowly degrade after skin application, thereby releasing antigens and adjuvants into the cutaneous microenvironment [218,240]. The skin residence of vaccine depends on the polymer choice, and several different polymers and sugars, such as polyvinylpyrrolidone (PVP), CMC, HA, sodium dextran, maltose, polyvinyl alcohol (PVA), PLA, cyclic-olefin copolymer, chitosan, silk, PGA, and poly(lactic-co-glycolic acid) (PLGA), have been utilized to create biodissolvable or biodegradable MAP systems [218,[239], [240], [241]]. Besides release kinetics, these materials control the manufacturability, strength and failure-free insertion ability, retention of vaccine bioactivity, skin delivery efficiency, and long-term stability of MAP-embedded vaccines. Thus, the choice of biomaterials should be considered for optimal design and production of biodissolvable/biodegradable MAPs. Similar to coated MAPs, these MAPs are single-unit, single-step, dry vaccine dosage systems that actively administer vaccine components to the skin microenvironment, but leave no biohazardous sharp waste after removal, eliminating the risk of disease transmission, since microarrays dissolve within the skin. In addition to these advantages, other benefits of dissolvable MAPs, such as simple and low-cost fabrication, convenience of storage and application, precise and consistent dosing, relatively higher vaccine dose capacity, controlled drug delivery, and ease of disposal, make them a promising alternative for effective skin-targeted vaccination. Despite these attractive attributes, the maximum vaccine doses possible without compromising the strength/reliability of skin insertion, the effect of biodissolvable/biodegradable materials on the mechanical strength of microarrays, skin delivery efficiency and residence of MAP-delivered vaccine components, the long-term stability of MAP-embedded vaccines, and the impact of implanted microarray materials on skin-resident cells and cutaneous immune mechanisms should still be thoroughly studied.
5.1.5. Hydrogel-forming MAPs (Fig. 5e)
Hydrogel-forming MAPs are a rapidly emerging MAP type for skin-targeted biomolecule delivery, and employ a ‘poke and release’ approach without depositing the microarray material to the skin [218,224,[241], [242], [243]]. These MAPs are manufactured from swellable polymers (e.g., PVA crosslinked with gelatin or poly(methylvinylether-co-maleic acid) cross-linked with PEG) that possess sufficient mechanical strength in their dry state to pierce the superficial cutaneous layers and swell upon penetrating the skin. Unlike dissolvable MAPs, hydrogel-forming microarrays remain intact within the skin microenvironment, either allowing the release of cargo embedded in the swellable polymer, or forming a membrane that adjusts the release profile based on the degree of cross-linking of the swellable polymer for controlled delivery of biocargo from the reservoir attached to microarrays [241]. Thus, hydrogel-forming MAPs could deliver relatively higher doses of biocargo compared to other dry MAP dosage forms, and theoretically leave no polymer residue within the skin microenvironment [[241], [242], [243]]. However, these MAPs must be applied for a longer period of time to deliver larger doses of biocargo. Although hydrogel-forming MAPs have gained attention for skin-targeted delivery of therapeutics, there is a limited number of vaccination studies utilizing hydrogel-forming MAPs. The effects of swellable biomaterials and cross-linking processes and agents on the bioactivity and longevity of vaccine components and their effects on the skin microenvironment will need to be investigated.
5.1.6. Porous MAPs (Fig. 5f)
Porous MAPs with a range of pore sizes and density are another MAP type that has been explored [225,[244], [245], [246], [247], [248]]. Porous MAPs are loaded with a biocargo solution that is dried into the microarray pores, and upon application, these microarrays penetrate the stratum corneum and the incorporated biocargo diffuses from the pores into the skin [217]. Porous MAPs have been created from numerous materials, such as stainless steel, titanium, ceramics, and polymers, and used for skin-targeted delivery of different biocargos [225,[247], [248], [249]]. Yet, studies with porous MAPs for vaccination are scarce. In addition, porous microarrays are typically weaker than solid microarrays due to their structure, and the risk of accidental breakage of MAPs in the skin demands biodegradable materials to be used for porous MAPs. In the future, more comprehensive studies evaluating the design, biomaterials, fabrication, and application (skin insertion ability and delivery efficiency) aspects of porous MAPs will be necessary to better understand their potential for skin-targeted vaccination.
5.1.7. Hybrid MAPs (Fig. 5g,h)
In addition to the aforementioned MAP concepts, hybrid MAPs, which assemble dissolvable or biodegradable sharp pyramid or conical regions onto non-dissolvable stems, have been proposed for skin-targeted biocargo delivery [226,227,250,251]. Rapidly separable dissolving or biodegradable polymer tips have been employed with the hybrid MAP concept to facilitate relatively shorter application times and enable bolus or sustained release of biocargos (vaccine components) embedded in the polymer [226,250,251]. Hollow MAPs with dissolvable tips have also been developed for skin penetration, followed by rapid dissolution of polymer tips to facilitate the flow of the biocargo from the openings of the microarrays [227]. Hybrid MAP systems have received less attention due to challenges associated with their scalable fabrication, as well as with reliable and user-friendly application without mechanical failure, leakage, or clogging. Thus, future efforts will be needed to develop scalable, patient-friendly, easy-to-use, and cost-effective hybrid MAPs for skin vaccination.
Together, several different MAP concepts with a wide range of designs (e.g., geometries and materials) have been developed for skin-targeted delivery of a myriad of biomolecules (e.g., vaccine components), providing an attractive alternative for intracutaneous vaccination against infectious diseases. Despite promising results obtained with these MAP systems, there are still key components of MAP-mediated skin-targeted immunization missing, which are needed to enable effective global vaccination programs for emerging infectious pathogens. A systematic comparison of MAP types for manufacturability, safety, skin insertion reliability, delivery efficiency, pharmacokinetics of MAP-delivered vaccine components, convenience, acceptability, cost, user-friendliness, and immunization efficacy will need to be performed using standard models and established performance evaluation metrics to identify and further support advancement of the most favorable MAP candidates. Indeed, the COVID-19 pandemic highlights the need for rapid development of effective vaccines, and in turn, the demand for simple, easy-to-manufacture/-store/-distribute/-apply, cost-effective, and convenient vaccine delivery systems. A growing body of evidence suggests that MAPs represent a promising intracutaneous vaccine delivery method to exploit the skin immune system, while providing additional advantages for mass vaccination. Thus, optimal MAPs could enable efficacious and widespread immunization against COVID-19 and other potential future outbreaks.
5.2. MAP fabrication techniques
Global clinical deployment of MAP-based vaccination will depend on the establishment of infrastructure for large-scale fabrication of MAPs. Thus, it is imperative to devise high-throughput, cost-effective manufacturing strategies that can enable simple, rapid, accurate, and reproducible production of MAPs. Over the past decades, numerous approaches have been developed to manufacture the aforementioned MAP types with various designs [36,216,241,[252], [253], [254], [255]]. Most MAP concepts require the creation of high-quality, solid microarray structures using inexpensive methods. These solid MAPs are either used directly for the ‘poke and patch’ approach, or coated with biocargo using different processes (e.g., dipping, spraying, gas-jet drying, layer-by-layer coating, or inkjet printing) [216,219,234,235]. Solid microarrays are also utilized as master molds to fabricate inverse or negative molds (also known as production molds) for the manufacturing of other MAP types, such as dissolvable, porous, and hybrid MAPs [216,226,245,256]. Traditional microfabrication techniques (e.g., etching and photolithography) have been broadly used to create solid MAPs; however, new approaches, such as micromachining, diamond micromilling, and most recently 3D printing, have emerged to address the material, geometry, ramp-up time, and cost limitations of cleanroom-based microfabrication strategies [216,228,229,257]. Notably, 3D printing, or additive manufacturing, approaches have drawn considerable recent attention due to their unprecedented design flexibility, which enables fabrication of more innovative microarray geometries [230,231,258,259]. For instance, 3D printing facilitated the simple preparation of hollow and undercut MAPs, which typically necessitate relatively complex, lengthy, and expensive fabrication approaches [230,231,260,261]. Thus, several manufacturing techniques have been devised to produce high-quality, solid microarray structures to enable the development of various MAP concepts, as well as to create hollow MAPs.
Additional processing steps have been integrated into MAP fabrication strategies to exploit the high-quality solid microarray structures as master molds to develop other MAP concepts. To manufacture production molds, soft-lithography using polydimethylsiloxane (PDMS) has been the most widely used approach due to its simplicity, as well as biocompatibility, flexibility, and low-cost of PDMS [229,231,241,252,256]. PDMS production molds have also been directly prepared by laser micromachining [262]. Such laser-based material removal processes pose challenges in fabricating complex microarray geometries with high-quality surfaces and sharp tips. To identify ideal materials for production molds, it is still necessary to investigate the interaction of vaccine components with PDMS and to explore other biocompatible materials for cost-effective and reliable replication of final MAPs integrating vaccine components. To create biocargo-loaded MAPs, micromolding with different drying/curing mechanisms (e.g., vacuum casting, spin-casting using a centrifuge, photopolymerization, or a combination of the aforementioned) is typically used, and can be followed by the loading of cargo solution and precision assembly of stem regions for porous or hybrid MAPs, respectively [216,226,229,248,255,256]. Recently, micromolding was also utilized to replicate high-fidelity MAP master molds, which then can be used to prepare production molds for more scalable and cost-effective MAP fabrication strategies [228,231]. In addition to commonly used approaches described above, hot embossing, injection molding, droplet-born air blowing, centrifugal lithography, and different drawing methods (e.g., thermal and magnetorheological) have been used to fabricate MAPs [261,[263], [264], [265], [266], [267], [268]]. Rapidly expanding advanced manufacturing research efforts over the past decades have led to the development of several promising MAP fabrication methods, and some of these approaches, such as dry etching and injection molding followed by a coating method for vaccine-coated MAPs, and solvent-based micromolding or casting for dissolvable MAPs integrating vaccine components, are already being used to manufacture MAPs for early-stage clinical trials.
The urgent need for rapid and effective global immunization programs against the COVID-19 pandemic clearly demonstrates the demand for inexpensive and large-scale fabrication of vaccine-loaded MAPs that can provide the aforementioned advantages by exploiting the skin immune system. Global-scale manufacturing of vaccine-loaded MAPs with regulated geometric accuracy and integrity, reproducibility, dosage uniformity, and retained bioactivity necessitates the establishment of manufacturing facilities with the ideal fabrication strategies that will follow established procedures for MAP production, quality control, and batch release. These procedures should be cost-effective and compatible with high-throughput production using industry-grade equipment and automation to enable global-scale fabrication of high-quality, sterile, vaccine-loaded MAPs. Thus, establishing the manufacturing capacity for vaccine-loaded MAPs and defining standard fabrication, quality control, packaging, and sterilization procedures will be necessary to enable MAP-based global vaccination programs for COVID-19 and future emerging infectious diseases.
5.3. Pre-clinical studies of MAP-based antiviral vaccines
Microarray patches have been used to administer skin-targeted vaccines, including recombinant live viral vector, DNA, protein, peptide, virus-like particles (VLPs), and attenuated or inactivated viruses, with or without adjuvants, and have been extensively tested in various animal models [[207], [208], [209],213,239,269]. Here, we focus on MAP-based pre-clinical immunization efforts for combatting viral infectious diseases. MAPs have been most extensively investigated for immunization against influenza in mice [208,209]. Furthermore, MAP-based vaccines for other viral infections, including hepatitis B, hepatitis C, measles, rubella, Japanese encephalitis, human immunodeficiency virus (HIV), herpes simplex virus (HSV), human papillomavirus (HPV), rabies, Zika, rotavirus, polio, chikungunya, respiratory syncytial virus (RSV), West Nile fever, dengue, Ebola, MERS, and COVID-19, have also been tested in mice and in numerous other animals, such as rats, dogs, guinea pigs, non-human primates, and pigs [198,[207], [208], [209],239,269,270]. Collectively, these pre-clinical studies suggest that MAPs are promising skin-targeted vaccine delivery systems that enable precise and reproducible administration of vaccine components to cutaneous microenvironments in different species and generally induce efficacious virus-specific humoral and cellular immune responses that are either superior or comparable to those elicited by hypodermic needle-based vaccination. Together with the simplicity of fabrication, storage, application, and disposal, specificity of delivery, and ease of integrating various vaccine components, promising pre-clinical results support further development of optimal MAP types for effective intracutaneous vaccination against emerging infectious pathogens, including respiratory viruses.
The ‘poke and patch’ approach, one of the first MAP concepts, has been tested with different vaccine types, including influenza subunit, hepatitis B DNA, and hepatitis B surface antigen (HBsAg) vaccines, using mouse models, as well as live attenuated virus vaccine against Japanese encephalitis using non-human primates [[271], [272], [273], [274]]. While skin vaccination against influenza using solid MAPs failed to elicit significant immune responses, solid MAP-based immunization against hepatitis B elicited improved antigen-specific immune responses compared to traditional hypodermic needle injection vaccination and MAP-mediated vaccination against Japanese encephalitis induced antibody responses. Although these promising results demonstrated the potential of MAP-directed skin vaccination, researchers sought alternative MAP concepts due to the aforementioned limitations of the ‘poke and patch’ approach, especially to enable more consistent and convenient delivery of vaccines to targeted skin microenvironments.
Coated MAPs have been evaluated with several different vaccine types (e.g., live attenuated, viral vector, inactivated virus, subunit, DNA, and VLPs) for numerous viral infections, such as influenza, hepatitis B, rotavirus, RSV, Ebola, dengue, measles, polio, HIV, HPV, West Nile virus, chikungunya, HSV, and hepatitis C [270,[275], [276], [277], [278], [279], [280], [281], [282], [283], [284], [285], [286], [287], [288], [289], [290], [291], [292], [293], [294], [295], [296], [297], [298], [299], [300], [301], [302], [303]]. Vaccines delivered by coated MAPs have been mainly tested in mice, but there also have been a limited number of studies in rat, guinea pig, porcine, and non-human primate models [221,291,294,296,304,305]. These studies suggest that coated MAPs are a promising platform for effective skin-targeted vaccination to induce antigen-specific protective immune responses. Although coated MAPs enable more consistent and reliable cutaneous vaccine delivery compared to the ‘poke and patch’ approach, optimal coating material and process requirements, vaccine dose limitations, risks associated with biohazardous sharp waste, along with the need for enhanced skin-targeted delivery efficiencies, have motivated researchers to continue improving coated MAPs and to develop alternative MAP types.
Hollow MAPs have been used for skin-targeted delivery of different types of vaccines, such as peptide, DNA, live attenuated virus, and inactivated virus vaccines, and have been evaluated for several viral infectious diseases, including influenza, mumps, varicella, polio, and HPV, and tested in mouse, rat, and guinea pig models [232,[306], [307], [308], [309], [310]]. Collectively, these pre-clinical studies suggest that skin vaccination using hollow MAPs is capable of eliciting robust antigen-specific adaptive immune responses. In addition to promising pre-clinical immunogenicity results, the traditional ‘medical device’ classification of hollow MAPs by regulatory agencies as opposed to the ‘combination product’ (i.e., biologic/device or drug/device) status of vaccine-loaded MAPs has facilitated the clinical development of hollow MAPs for intracutaneous immunization. However, the aforementioned complications (e.g., mechanical failure, leakage, and clogging) related to application of hollow MAPs, and remaining challenges associated with their scalable fabrication, still present barriers to widespread utilization of hollow MAPs for skin vaccination. Moreover, the long-term stability of liquid vaccine solutions used with hollow MAPs, and the possibility of self-application with hollow MAPs without the need for trained medical personnel, have yet to be addressed.
Dissolvable/biodegradable MAP types have been tested pre-clinically for intracutaneous vaccination against a wide range of viral infections, such as influenza, hepatitis B, HIV, measles, rubella, rabies, polio, dengue, Ebola, Zika, hepatitis C, rotavirus, MERS, and COVID-19, with several types of antigen platforms, including subunit, nucleic acid, VLPs, recombinant viral vector, live attenuated viruses, and inactivated viruses [198,[311], [312], [313], [314], [315], [316], [317], [318], [319], [320], [321], [322], [323], [324], [325], [326], [327], [328], [329], [330], [331], [332], [333], [334], [335], [336]]. These studies have utilized various animal models, such as mouse, rhesus macaque, dog, pig, and guinea pig [316,321,328,333,337]. Ultimately, these pre-clinical studies indicate that dissolvable or biodegradable MAPs are an attractive skin-targeted vaccine delivery system that can administer vaccine components to immunologically rich skin microenvironments to elicit pathogen-specific humoral and cellular immune responses. Moreover, these MAPs offer several manufacturing, storage, and application advantages, such as low-cost fabrication, rapid scale-up potential, easy disposal, high vaccine-dose capacity, thermostability without expensive refrigeration requirement, no biohazardous sharp waste, and no risk of disease transmission. Toward optimal skin-targeted vaccination strategies using this MAP type, the effects of dissolvable/biodegradable materials on bioactivity and long-term stability of MAP-embedded vaccine components, failure-free skin insertion reliability, and skin residence of MAP-delivered vaccines, as well as pro-inflammatory or adjuvant activities of MAP materials and immunogenicity of MAP-administered antigens are currently being investigated.
As outlined above, many studies report promising immunogenicity of viral antigens when delivered using dissolvable/biodegradable MAP systems. For instance, a comprehensive study demonstrated that MAPs can be effectively manufactured using a dissolvable polymer (PVP) to integrate an inactivated influenza virus vaccine, and the fabricated MAPs can successfully pierce the skin and dissolve upon penetration [316]. In mice, intracutaneous vaccination with these MAPs generated potent pathogen-specific immune responses that were either superior or comparable to those obtained by IM immunization. Specifically, immunization via skin using MAPs resulted in similar humoral responses, improved durable or long-lasting responses, and increased lung viral clearance compared to vaccination via IM injection. Most importantly, a single, low-dose influenza vaccine-loaded MAP was able to protect mice against intranasal lethal viral challenge. This study suggests that MAPs can provide an attractive approach for improved global vaccination programs against influenza and other infectious diseases. Indeed, driven by several published reports supporting the development of biodissolvable MAPs for increased global vaccination coverage, biodissolvable MAPs were the first MAP type exploited for pre-clinical COVID-19 vaccination [198]. In the future, more comprehensive mechanistic studies with dissolvable MAPs are expected for safer and more effective skin vaccination strategies against SARS-CoV-2 and other novel infectious pathogens.
Other types of MAPs, such as hydrogel-forming, porous, and hybrid MAPs, are relatively newer concepts and thus, they have yet to be comprehensively evaluated in animal models for skin-targeted vaccination against infectious diseases. For instance, hydrogel-forming MAPs have been evaluated for skin-targeted vaccination using a model antigen, ovalbumin (OVA) [224]. Interestingly, comparison of dissolvable and hydrogel-forming MAPs integrating OVA for skin-targeted vaccination of mice showed that both MAP types are capable of inducing antigen-specific antibody responses, but those induced by dissolvable MAPs were substantially more potent [224]. Similarly, there are a limited number of reports demonstrating intracutaneous immunization of mice with porous MAPs using OVA or influenza, which provided effective protection against viral challenge [244,246]. Hybrid MAPs have been used to implant biodegradable polymer (e.g., chitosan) microarray tips into animal skin through their rapid separation from the stems to achieve controlled release of vaccine components in the cutaneous microenvironment [250,338]. Consistent with studies demonstrating improved immunogenicity of antigens delivered in a sustained manner by daily or repeated injections, extended release of vaccine components from hybrid or other MAP types resulted in improved adaptive immune responses [250,[339], [340], [341], [342], [343], [344]]. Ultimately, hydrogel-forming, porous, and hybrid MAPs represent alternatives for skin-targeted immunization against emerging infectious diseases. However, these MAP types have yet to be fully investigated with different viral vaccine candidates and animal models to understand their mechanisms, efficacy, and safety. Importantly, whether sustained release of vaccine components via MAP-based immunization bears the risk of tolerance induction or other adverse outcomes will need to be further studied to enable more widespread utilization of sustained-release MAPs for skin vaccination.
To further improve the quality of MAP-based skin-targeted vaccination, several chemical and biological adjuvants (also known as immune-enhancers) have also been evaluated in pre-clinical studies [195,270,289,290,319,320,322,[345], [346], [347], [348], [349], [350], [351], [352]]. For instance, one study investigated the effects of two different TLR agonists (imiquimod, a TLR7 agonist, and polyinosinic:polycytidylic acid (poly(I:C)), a TLR3 agonist) on humoral and cell-mediated immune responses in mice immunized with coated MAPs loaded with influenza antigen [351]. While the effect of poly(I:C) on influenza-specific adaptive immune responses was negligible, imiquimod improved antigen-specific Th1 immunity, as evidenced by enhanced IgG2a antibodies and IFN-γ producing T cells, which provide protection against viral infectious diseases. On the other hand, a recent study evaluated the effect of poly(I:C) on immune responses elicited by skin vaccination of mice with dissolvable MAPs incorporating live adenoviral vectored antigen (OVA) and showed that poly(I:C), when co-delivered with adenovirus in the same MAP, improved antigen-specific cellular immunity [352]. Another adjuvant, monophosphoryl lipid A (MPLA), a TLR4 agonist, has been tested with coated and dissolvable MAPs against RSV and HIV, respectively, in mice [270,322]. Specifically, skin delivery of the FI-RSV vaccine by coated stainless-steel MAPs resulted in increased lung viral clearance compared to traditional IM immunization, and co-delivery of MPLA with antigen improved the immunogenicity of FI-RSV vaccine and induced protective immunity. Importantly, inclusion of MPLA decreased expression of Th2-associated cytokines and infiltrating innate inflammatory cells, while increasing Th1-type immunity in the lungs, thereby avoiding vaccine-induced immunopathology. Although the mechanisms of action of adjuvants, especially when administered via skin, have yet to be fully elucidated with different antigen types, doses, and kinetics, these studies ultimately demonstrate that adjuvants can be used effectively in MAP-based vaccination to strategically improve the magnitude and quality of pathogen-specific protective immune responses. To this end, previous efforts with adjuvanted coronaviruses vaccines could enable rational selection of ideal adjuvants to be incorporated into MAPs with optimal SARS-CoV-2 antigens for more effective COVID-19 vaccination [353].
In addition to chemical and biological adjuvants, disruption of the skin barrier and biomaterials-based nanoparticles (NPs) have been explored in pre-clinical studies as potentially safer adjuvants for MAP-delivered antigens. For instance, a comprehensive study demonstrated that application of high-density MAPs (e.g., 21,000 microprotrusions per cm2) coated with a split virion, inactivated influenza vaccine to mouse skin using a spring-based applicator device significantly increased local cell death in the skin, compared to traditional intradermal injection, and the amount of cell death was correlated with the density of microprotrusions [354]. Importantly, this local cell death increased the immunogenicity of the vaccine, as evidenced by increasing humoral responses, up to a certain level of cell death [354]. Collectively, these pre-clinical studies suggest that optimal MAP designs and application conditions can generate transient mechanical stress to induce controlled cell death and a pro-inflammatory skin microenvironment to enhance antigen-specific humoral immune responses [354,355]. However, the effects of skin cell death generated by diverse MAP designs on cutaneous immune mechanisms and adaptive immune responses have yet to be elucidated with diverse vaccine candidates. Furthermore, it will be important to determine the effect of skin cell death on cellular immune responses and whether local cell death could skew immune responses to MAP-delivered antigens. For example, Th1 immunity is deemed favorable when targeting intracellular pathogens and is relatively challenging to achieve with extracellular antigen vaccines. Importantly, these mechanisms have yet to be rationally translated to humans, in part due to differences in skin anatomy and mechanical properties, as well as differences in cutaneous immune mechanisms between murine and human skin.
Biomaterials-based NPs or nanostructures encapsulating vaccine components have been investigated pre-clinically in MAP-mediated skin immunization, and have the potential to improve immune responses by controlling spatial and temporal release of vaccine components [[356], [357], [358], [359], [360], [361], [362], [363], [364]]. Nanoparticles offer broad advantages, including enhanced stability, improved spatial targeting, better innate immunity triggering, and timed-release [[365], [366], [367]]. To exploit these advantages of NPs for intracutaneous vaccination, it is possible to use MAPs for rapid, reproducible, convenient, and effective skin-targeted delivery of vaccine-loaded NPs. Improved targeting of skin DCs or prolonged vaccine exposure to either cutaneous cells or lymph node resident DCs by NPs could enhance the strength and breadth of antigen-specific adaptive immune responses [357,360,363]. Despite promising initial results obtained with vaccine loaded NPs delivered to the skin microenvironments using MAPs, these combination systems are still in their infancy with respect to widespread use in global immunization programs. Scalable manufacturing of sterile NPs integrating vaccine components and NP-loaded MAPs with optimal vaccine loading and encapsulation efficiencies, identification of optimal MAP systems for reliable and consistent skin-targeted delivery of vaccine-loaded NPs with optimal delivery efficiencies, as well as a comprehensive mechanistic understanding of skin immunization by NP-loaded MAP systems are still needed to enable safe, inexpensive, more predictable and effective skin-targeted vaccination using these innovative systems.
Together, accumulating pre-clinical data supports the bright future of MAP-based skin immunization against emerging infectious pathogens, including SARS-CoV-2. Rationally selected MAPs can be used for various antigen formats, though specific antigen platforms can introduce unique challenges. For example, for solid MAP-assisted immunization that relies on topical application of antigen, nucleases present on the surface of the skin could potentially degrade certain types of antigen platforms, such as mRNA or plasmid DNA vaccines. On the other hand, commonly used coated, dissolvable, or hollow MAPs have been shown to be compatible with different antigen platforms, including DNA and viral vectored vaccines. Emerging hydrogel-forming, porous, and hybrid MAPs are now being evaluated with diverse antigen formats. Despite promising pre-clinical results with MAPs, there is still substantial work needed to identify and further support favorable MAP platforms to increase global immunization coverage and to more effectively respond to COVID-19 and future outbreaks. Specifically, the heterogeneity of pre-clinical studies without standard animal models, protocols, and efficacy measures makes the identification of preferred MAP concepts, designs, and materials for different vaccine candidates challenging. The lack of comprehensive understanding of local skin immune mechanisms induced by MAP-delivered antigens, with or without adjuvants, and of the precise relationship between these mechanisms and systemic adaptive responses is still an important obstacle to the shift from empirical methods to rational and predictable skin-targeted immunization strategies. Furthermore, there is still a considerable knowledge gap regarding the safety and efficacy of MAP-directed skin immunization in pre-clinical models representing higher-risk populations, such as immunocompromised and/or aged individuals. To address these knowledge gaps and to better predict the clinical safety and efficacy of vaccines delivered by MAPs, including current COVID-19 vaccine candidates, animal models with standard correlates of safety, immunogenicity, and protection must be established. Evaluating the safety and immunogenicity of MAP-delivered vaccines (e.g., SARS-CoV-2 vaccines), with or without adjuvants, in appropriate animal models and elucidating the local cutaneous immune mechanisms imparted by MAP-delivered vaccines in human skin models could provide much-needed information to drive the clinical success of MAP-mediated immunization efforts.
5.4. MAP-directed clinical vaccine trials
Well-designed clinical trials will be essential for both the mechanistic development and clinical application of MAP vaccines. Driven by the aforementioned scientific and technological progress, clinical evaluation of MAP-based skin vaccination is beginning to emerge. In line with the abundance of animal studies of MAP-directed immunization against influenza, early-phase clinical trials with MAPs have also focused on influenza vaccines. Influenza vaccines using coated, hollow, and dissolvable MAPs have been evaluated in clinical trials with promising safety, acceptability, and efficacy results [263,[368], [369], [370], [371]]. Additional clinical trials with MAP vaccines for other viral infectious diseases, such as rabies, polio, hepatitis B, and varicella-zoster virus, have also presented promising findings with regard to the safety and immunogenicity [369,[372], [373], [374], [375]]. Together, these early-phase clinical trials support advanced human clinical studies with MAP-mediated skin-targeted vaccination against a broad range of infectious diseases.
High-density MAPs coated with influenza vaccine have been evaluated in human studies with promising results [263,368]. For instance, one study reported the results of a randomized, partly-blinded, placebo-controlled, phase I trial using high-density silicon MAPs, coated with monovalent, split inactivated influenza virus vaccine and applied with a spring-loaded applicator, that suggested that MAP vaccination was safe and acceptable with no significant local or systemic adverse events [368]. Importantly, skin immunization using these influenza vaccine-coated MAPs resulted in antigen-specific humoral immune responses similar to those obtained by IM injection. A more recent study presented additional promising findings with influenza vaccine coated MAPs from a randomized, controlled phase I clinical trial with high-density polymer MAPs, coated with monovalent, split inactivated influenza virus vaccine and applied using a spring-based applicator [263]. This study concluded that the vaccine was well tolerated without any serious untoward effects, and induced antigen-specific humoral and cellular immune responses. Further, this study demonstrated potential dose-sparing advantages of MAPs over traditional IM vaccination in humans, as well as the thermostability of vaccine components at 40°C for at least 1 year [263]. Unlike earlier high-density MAPs that were produced from silicon using microfabrication techniques, these high-density polymer MAPs were manufactured using injection molding to improve the scale-up potential and reduce cost.
Hollow MAPs have also been tested in clinical vaccine trials with promising findings in terms of safety, acceptability, and immunogenicity [195,369,[372], [373], [374], [375], [376]]. For instance, the MicronJet600 has been evaluated with diverse vaccines, such as influenza, polio, and varicella-zoster virus vaccines, in human studies. MicronJet600, or its predecessor MicronJet, have also been tested in diverse populations (e.g., young and older adults, or HIV-infected individuals) [369]. Together, these vaccine trials concluded that MicronJet600 or MicronJet hollow MAPs were safe and effective for intradermal immunization of human subjects and could offer patient-adherence, dose-sparing, and immunogenicity advantages compared to traditional vaccination strategies [369]. MicronJet600 has also been evaluated in human trials for safe and effective skin-targeted delivery of an adjuvanted influenza vaccine [195]. Another hollow MAP, DebioJect, has been investigated in a phase I clinical trial for rabies vaccination, with promising findings in terms of safety, without serious adverse events, and antibody responses [372]. Driven by these findings from human clinical studies with hollow MAPs, future late-phase clinical trials with hollow MAPs are expected to further evaluate the safety and immunogenicity of hollow MAP-delivered vaccines in larger and more diverse subject populations.
Dissolvable MAPs, which have emerged to simultaneously eliminate the need for dependency on the cold chain, trained medical personnel, and biohazardous sharps waste, while offering additional immunogenicity, manufacturing, cost, and disposal advantages, have also been evaluated in human studies [370,371]. One clinical study demonstrated the safety and immunogenicity of a trivalent seasonal influenza subunit vaccine delivered by hyaluronic acid MAPs with no serious side effects and with antigen-specific adaptive immune responses equivalent to, or more effective than those induced by SC hypodermic needle vaccination [370]. For this trial, MAPs were applied using a spring-based applicator device, and participants received prime and boost vaccinations. Interestingly, this trial lacked a placebo control, data from more than half of the subjects were excluded due to failures in reliable intracutaneous delivery, and the MAP-integrated influenza vaccine lost bioactivity during storage at elevated temperatures (e.g., at 25 °C and 40 °C), suggesting the importance of MAP formulations for reproducible skin delivery and long-term stability. Another study reported comprehensive findings from a randomized, partly blinded, placebo-controlled, phase I trial with dissolvable MAPs incorporating the licensed 2014–2015 seasonal trivalent inactivated influenza vaccine (TIV, Fluvirin) [371]. This trial included a placebo control, healthcare worker-applied MAP, self-administered MAP, and IM vaccination groups with a single immunization, and concluded that dissolvable MAPs were safe for skin-targeted vaccination of human trial subjects without any serious untoward events, without generation of biohazardous sharp waste, and offering high potential for self-administration [371]. Further, the MAP-integrated vaccine was thermostable for at least 1 year without the need for refrigeration when stored in desiccated packaging. In this trial, pain was a more common reactogenicity event in the IM vaccination groups compared to the MAP vaccination groups, whereas MAP vaccination resulted in more frequent local skin reactogenicity events (e.g., mild and transient pruritus, erythema, and swelling). Importantly, the MAP-delivered vaccine was capable of generating antibody responses, with mean titers for all virus strains comparable to those elicited by IM immunization at day 28. Remarkably, vaccine delivery efficiencies and vaccine-induced humoral immune responses were comparable for MAPs that were either self-administered or applied by healthcare workers, thereby supporting the self-administration potential of MAP-based vaccines, which may offer an important advantage for global vaccination programs during pandemics. Moreover, trial subjects and healthcare workers preferred MAP-directed vaccination over IM immunization, indicating better acceptability of MAPs for immunization. These promising clinical safety, immunogenicity, and acceptability findings with dissolvable MAP influenza vaccines support further development of dissolving MAPs with advanced human studies powered or designed to reliably evaluate the immunogenicity of MAP-delivered vaccines and clinical testing of dissolvable MAP vaccines for other emerging infectious pathogens. Indeed, with encouraging data from comprehensive pre-clinical evaluation of measles and rubella (MR) vaccine-loaded dissolvable MAPs, an early-phase clinical trial with an MAP MR vaccine is expected to start soon [214].
Table 1 summarizes the published MAP-based human studies with antiviral vaccines. In addition, a commercially available microinjection system from Beckton Dickinson (BD) has been extensively evaluated for intradermal immunization and it is currently being tested in a Phase IV clinical trial to compare the efficacy of four different influenza vaccines in older adults (ClinicalTrials.gov Identifier: NCT01368796). Together, these clinical trials with MAP vaccines suggest that MAPs are safe, well-tolerated, and effective for skin-targeted vaccination, supporting further testing of MAP-based vaccines in advanced human studies and pursuit of regulatory approval and commercialization. Toward widespread use of MAP vaccines, future late-phase clinical studies will enable more comprehensive evaluation of MAP-based vaccines in diverse populations, such as those who are deemed relatively high-risk patients (e.g., elderly or immunocompromised individuals). Since the initial human studies with MAPs were designed primarily to evaluate safety and acceptability, a more thorough immunogenicity testing will be necessary to evaluate the magnitude, quality, breadth, and longevity of MAP vaccine-induced protective antibody and cellular immune responses. The self-application potential of certain MAP types, without the need for medical expertise for proper application, and without an applicator device, will need to be more extensively tested in larger and diverse populations. The lack of reliable feedback for the skin insertion of MAPs and vaccine delivery, as well as the scarcity of detailed information on vaccine delivery efficiencies of MAPs in humans must be addressed to assure consistent immunogenicity and efficacy. Based on the results from initial human clinical trials, the development of next-generation MAP concepts could be pursued to improve the skin penetration and delivery efficiencies of MAPs without compromising safety, simplicity, and convenience.
Table 1.
Summary of published clinical studies with MAP-directed antiviral vaccines. (IIV: Inactivated influenza virus, IPV: Inactivated poliovirus, VZV: Varicella-zoster virus, IRV: Inactivated rabies virus, HBV: Hepatitis B virus, Physical adjuvant: High-density MAPs applied with an applicator device. AAHS: Amorphous aluminum hydroxyphosphate sulfate, MLoS: Maximum length of studies, -: Not studied).
| MAP Type | Antigen | Adjuvant | Stability (MLoS) | Publication |
|---|---|---|---|---|
| Coated MAP [Polymer] | IIV | Physical adjuvant | 1 year at 40 °C | [263] |
| Coated MAP [Silicon] | IIV | Physical adjuvant | – | [368] |
| Hollow MAP [DebioJect™] | IRV | No | – | [372] |
| Hollow MAP [MicronJet™] | IIV | No | – | [376] |
| Hollow MAP [MicronJet™] | IIV | No | – | [377] |
| Hollow MAP [MicronJet600™] | IPV | No | – | [374] |
| Hollow MAP [MicronJet600™] | Live, attenuated VZV | No | – | [375] |
| Hollow MAP [MicronJet600™] | IPV | No | – | [378] |
| Hollow MAP [MicronJet600™] | IIV | No | – | [379] |
| Hollow MAP [MicronJet600™] | Influenza hemagglutinin virus-like particles | GLA-AF (TLR4 agonist) | – | [195] |
| Hollow MAP [VAX-ID™] | Recombinant HBV surface protein | AAHS | – | [373] |
| Hollow MAP [MicronJet™] | Influenza hemagglutinins | Virosomes | – | [380] |
| Hollow MAP [MicronJet600™] | Influenza hemagglutinins | Virosomes | – | [381] |
| Dissolvable MAP [Hyaluronic acid] | Influenza hemagglutinins | No | 6 months at 4 °C | [370] |
| Dissolvable MAP [Gelatin+sucrose] | IIV | No | 1 year at 40 °C | [371] |
5.5. Additional considerations for MAP-based vaccines
To enable the widespread utilization of MAPs with COVID-19 vaccines and other compatible vaccine components, manufacturing sites for scalable fabrication of standard MAP vaccines with dosage uniformity and high quality must be established. Further, safety, sterilization, packaging, thermostability, reliable skin application and delivery, supply chain, acceptability, disposal, regulatory approval, cost, commercialization, and return on investment will need to be fully evaluated for each MAP vaccine formulation.
The safety of MAPs is of utmost importance for widespread utilization. To this end, it will be important to elucidate the local responses induced by application of MAPs and their correlation with the level of skin barrier disruption and recovery to better understand the risk of infection through MAP-treated skin in diverse populations, including elderly and immunocompromised individuals. Due to protective abilities of the skin, the occurrence of infections at MAP-application sites is unlikely. Indeed, using in vitro models, it has been shown that the risk of cutaneous penetration of microbial agents is higher in barrier defects caused by hypodermic needle injection compared to those generated by MAP applications [382].
Microarray patches (MAPs), which will be used for skin-targeted vaccination, must be sterile to ensure that they will deliver only the intended vaccine components, without microbial agents or bioburden. MAPs could be fabricated under aseptic conditions with good manufacturing practices (GMP); however, aseptic manufacturing is complex and expensive. Alternatively, low bioburden processes and environment can be used to manufacture MAPs, followed by terminal sterilization to satisfy the requirements of regulatory authorities. Standard sterilization procedures (e.g., gamma irradiation) can be employed for MAPs when coupled with rigorous post-sterilization validation processes. For vaccine components that are incompatible with current sterilization methods, it may be necessary to create MAPs under aseptic conditions, which would considerably increase the cost of MAP vaccines. Recently, to obviate the challenges associated with sterilization of biocargo-loaded MAPs, self-sterilizing dissolving MAPs have been introduced [383]. Specifically, self-sterilizing MAPs have been prepared by integrating silver nanoparticles into CMC MAPs to prevent bacteria growth at the delivery site, and these MAPs could be an alternative to relatively complex MAP sterilization processes. Ultimately, cost-effective fabrication of sterile, high-quality MAPs, without significant effect on vaccine components and structural materials of MAPs, will be important for global vaccination programs.
The packaging, labeling, and refrigeration-free storage and transportation of MAP-based vaccines may require additional considerations for standardization and longevity. In addition to identifying ideal water-soluble biomaterials to serve as stabilizers to enable long-term viability of MAP-integrated vaccines without the need for the expensive cold-chain, utilization of desiccants and sealing methods may be required to assure long-term shelf life, which may increase the cost of MAP-vaccines. Collectively, identifying favorable MAP materials for COVID-19 vaccines and other vaccines, as well as determining the preferred packaging methods to retain the potency of vaccine components without refrigeration for an extended period of time, are important goals for effective global vaccination programs.
Reliable and consistent skin penetration and vaccine delivery is paramount to the success of MAP-based intracutaneous immunization. Depending on their design and type, MAPs could be applied either by trained healthcare personnel, or self-administered by individuals, with or without an applicator device. The variability of both applicator-assisted and applicator-free application of MAPs, with or without medical expertise, has yet to be tested in larger human studies with diverse populations. Self-administration without an applicator device would be ideal for effective global vaccination programs; however, it would be critical to confirm MAP insertion into the skin and vaccine delivery for successful immunization. To this end, feedback systems, such as simple snap-based devices and pressure-indicating films, have been incorporated into MAP systems to inform the end-users about the sufficient levels of application force required for effective skin penetration [384,385]. However, these systems still provide indirect feedback without direct validation of skin penetration and vaccine delivery, and thus, development of additional feedback mechanisms could further assure reproducible MAP application and vaccine delivery. After addressing the reproducible skin penetration and vaccine delivery concerns, MAP-mediated vaccination is expected to be preferred by the general public and healthcare workers over traditional hypodermic needle injection, which could improve patient adherence and global immunization coverage [371,386,387]. Ultimately, self-administration, without the need for medical expertise, is expected to bring many acceptability, convenience, and cost advantages, which would be especially beneficial for global vaccination efforts during pandemics.
To successfully bring MAP vaccines to market, detailed cost and return on investment analyses of MAP vaccines must be undertaken, with consideration of costs related to development and implementation of standard large-scale fabrication, quality control, sterilization, batch release, storage, distribution, application, and disposal processes. Initial cost analysis with MAP vaccines indicates that certain types of MAP-based vaccines, without the need for an applicator device, would be more cost-effective than existing vaccines, especially when the cost reductions resulting from self-application without the need for medical expertise, shelf-stability without refrigeration, no generation of biohazardous sharp waste, and more efficient usage of vaccine components are considered [214,388].
Many vaccines, including most of the current SARS-CoV-2 vaccine candidates, require booster doses to induce robust immune responses that confer durable protection, which poses substantial logistics and compliance challenges, especially in developing countries. To address these challenges, development of thermostable, single-dose, timed-release vaccines are under development [389]. Specifically, PLGA microspheres encapsulating vaccine components have been widely investigated as controlled release vaccine delivery systems via conventional hypodermic needle injection [[390], [391], [392]]. However, PLGA microparticles (MPs) encapsulating vaccines have been mainly fabricated using traditional mechanical homogenization-based emulsification solvent evaporation approaches that pose vaccine stability challenges due to the use of organic solvents and harsh stresses experienced during the process. To address these challenges, newer methods (e.g., active self-healing encapsulation (ASE) and StampEd Assembly of polymer Layers (SEAL)) have been devised to create controlled release PLGA microstructures with improved vaccine stability [393,394]. However, even these newer controlled release vaccine platforms have been administered mainly via traditional IM injections. More recently, vaccine-loaded biodegradable MPs synthesized using the ASE method have been delivered using MAPs to enable user-friendly, controlled release of vaccine components toward eliminating the need for multiple injections, a feature of considerable value particularly in developing countries [395]. To eliminate the need for return visits to healthcare providers, and in turn, to improve global vaccination coverage, composite MAPs have also been developed to enable both rapid and sustained release of vaccine components in a single dose to improve antigen-specific protective immune responses [396,397]. These innovative vaccine delivery systems, combined with potential self-application of MAPs, are expected to increase patient compliance, especially in developing countries.
6. Summary and perspective
The COVID-19 pandemic clearly demonstrates that emerging infectious pathogens pose a major global public health problem. Vaccination is the most effective strategy for reducing the burden of infectious disease, preventing millions of deaths each year, as estimated by the World Health Organization. Realizing the true potential of immunization against a novel pathogen will require: 1. identification of a rational vaccine target and production of a safe and effective vaccine, 2. determination of an ideal vaccine administration route, and 3. development of an easy-to-use, cost-effective, patient-friendly, and broadly deployable vaccine delivery system. The expanding knowledge of skin immunobiology suggests that targeting vaccines to the skin microenvironment is an attractive alternative to prevailing immunization routes (e.g., intramuscular injection) and is more suitable for mass vaccination. Advances in skin science and vaccine delivery technologies are facilitating the rational selection of safe and potent adjuvants for intracutaneous immunization, and enabling reproducible and controlled delivery of vaccine components to the skin microenvironment. MAPs are rapidly emerging skin-targeted vaccine delivery systems with several unique advantages. Their compatibility with a wide range of antigen platforms (e.g., DNA, live viral vector, subunit, and inactivated virus) and adjuvant types, as well as emerging evidence supporting their broad acceptability by general public and healthcare workers, will allow MAPs to challenge the established paradigm of hypodermic needle injections for immunization, with important implications for safer, more effective, and user-friendly vaccination against emerging infectious diseases. Evaluating effects of traditional adjuvants in MAP vaccines will be important, as both reactogenicity and beneficial immunological effects of adjuvants may depend on the route of administration. Indeed, some adjuvants deemed to be too toxic or ineffective when delivered via traditional parenteral injections, may be safe and efficacious when delivered to the targeted skin microenvironment.
Current leading SARS-CoV-2 vaccine candidates pose significant challenges for effective global immunization programs since they use traditional parenteral routes and needle injections for delivery, and require cold-chain dependent storage and distribution. Thus, effective, safe, inexpensive, needle-free, thermostable, self-administered, and broadly deployable immunization strategies are urgently needed to combat the COVID-19 pandemic and to prevent future emerging pathogen pandemics. To this end, MAP-directed skin-targeted vaccination provides an attractive solution. Rapid, focused, and collaborative efforts to better understand the mechanisms underlying MAP-mediated skin vaccination against COVID-19 using the most promising SARS-CoV-2 vaccine candidates and MAP systems will accelerate the development of effective and broadly deployable skin-targeted COVID-19 vaccines. These studies could synergize with accelerated MAP-based COVID-19 vaccine clinical trials that employ adaptive trial designs to assure safety, while enabling rapid regulatory approval. These efforts could occur simultaneously with the expansion of MAP manufacturing capacity to enable scalable fabrication of MAP vaccines in strict compliance with clinical standards. Ultimately, immunogenicity, simplicity, safety, compliance, thermostability, cost, versatility, and supply chain advantages of MAP-based vaccines make them an exciting alternative for global immunization programs. As such, we encourage scale-up of pre-clinical and clinical efforts to address current knowledge gaps and to expand capabilities through synergistic collaborations between academia, industry, and governments to effectively actualize MAP vaccine technology platforms for rapid deployment in global immunization programs.
Acknowledgements
E Korkmaz and TL Sumpter are supported by a grant from the Institute for Infection, Inflammation, and Immunity in Children (i4Kids). E Korkmaz and LD Falo Jr are supported by an NIH grant (UM1-AI106701). LD Falo Jr is supported by NIH grants (R01-AR074285 and R01-AR071277) and UPMC Enterprises (IPA 25565). The authors acknowledge BioRender for the preparation of figures.
References
- 1.Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kickbusch I., Leung G.M., Bhutta Z.A., Matsoso M.P., Ihekweazu C., Abbasi K. Covid-19: how a virus is turning the world upside down. Br. Med. J. 2020;369:m1336. doi: 10.1136/bmj.m1336. [DOI] [PubMed] [Google Scholar]
- 5.McKee M., Stuckler D. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat. Med. 2020;26:640–642. doi: 10.1038/s41591-020-0863-y. [DOI] [PubMed] [Google Scholar]
- 6.Vidya C.T., Prabheesh K.P. Implications of COVID-19 pandemic on the global trade networks. Emerg. Mark. Finance Trad. 2020;56:2408–2421. [Google Scholar]
- 7.Pak A., Adegboye O.A., Adekunle A.I., Rahman K.M., McBryde E.S., Eisen D.P. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front. Public Health. 2020;8:241. doi: 10.3389/fpubh.2020.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleta A., Martín-Corral D., Piontti A.P.Y., Ajelli M., Litvinova M., Chinazzi M., Dean N.E., Halloran M.E., Longini I.M., Jr., Merler S., Pentland A., Vespignani A., Moro E., Moreno Y. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020;4:964–971. doi: 10.1038/s41562-020-0931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdivia-Granda W.A., Richt J.A. What we need to consider during and after the SARS-CoV-2 pandemic. Vector Borne Zoon. Dis. 2020;20:477–483. doi: 10.1089/vbz.2020.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R., Chen B., Zhang T., Ren Z., Song Y., Xiao Y., Hou L., Cai J., Xu B., Li M., Chan K.K.Y., Tu Y., Yang M., Yang J., Liu Z., Shen C., Wang C., Xu L., Liu Q., Bao S., Zhang J., Bi Y., Bai Y., Deng K., Zhang W., Huang W., Whittington J.D., Stenseth N.C., Guan D., Gong P., Xu B. Global COVID-19 pandemic demands joint interventions for the suppression of future waves. Proc. Natl. Acad. Sci. U. S. A. 2020;117:26151–26157. doi: 10.1073/pnas.2012002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habersaat K.B., Betsch C., Danchin M., Sunstein C.R., Böhm R., Falk A., Brewer N.T., Omer S.B., Scherzer M., Sah S., Fischer E.F., Scheel A.E., Fancourt D., Kitayama S., Dubé E., Leask J., Dutta M., MacDonald N.E., Temkina A., Lieberoth A., Jackson M., Lewandowsky S., Seale H., Fietje N., Schmid P., Gelfand M., Korn L., Eitze S., Felgendreff L., Sprengholz P., Salvi C., Butler R. Ten considerations for effectively managing the COVID-19 transition. Nat. Hum. Behav. 2020;4:677–687. doi: 10.1038/s41562-020-0906-x. [DOI] [PubMed] [Google Scholar]
- 12.Ehreth J. The global value of vaccination. Vaccine. 2003;21:596–600. doi: 10.1016/s0264-410x(02)00623-0. [DOI] [PubMed] [Google Scholar]
- 13.Zepp F. Principles of vaccine design—lessons from nature. Vaccine. 2010;28:C14–C24. doi: 10.1016/j.vaccine.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Finco O., Rappuoli R. Designing vaccines for the twenty-first century society. Front. Immunol. 2014;5:12. doi: 10.3389/fimmu.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulmer J.B., Valley U., Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 16.Funk C.D., Laferrière C., Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 18.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 19.Poland G.A., Ovsyannikova I.G., Crooke S.N., Kennedy R.B. SARS-CoV-2 vaccine development: current status. Mayo Clin. Proc. 2020;95:2172–2188. doi: 10.1016/j.mayocp.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8:153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le T.T., Andreadakis Z., Kumar A., Román R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., II, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., Jia S.Y., Jiang H.D., Wang L., Jiang T., Hu Y., Gou J.B., Xu S.B., Xu J.J., Wang X.W., Wang W., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Lu J., Yang Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A., Yuan Z., Shen S., Guo W., Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. J. Am. Med. Assoc. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 30.Koirala A., Joo Y.J., Khatami A., Chiu C., Britton P.N. Vaccines for COVID-19: the current state of play. Paediatr. Respir. Rev. 2020;35:43–49. doi: 10.1016/j.prrv.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zafar S., Arshad M.S., Fatima S., Ali A., Zaman A., Sayed E., Chang M.W., Ahmad Z. COVID-19: current developments and further opportunities in drug delivery and therapeutics. Pharmaceutics. 2020;12:945. doi: 10.3390/pharmaceutics12100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers A.M., McCrudden M.T.C., Vincente-Perez E.M., Dubois A.V., Ingram R.J., Larrañeta E., Kissenpfennig A., Donnelly R.F. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: a proof of concept study. Int. J. Pharm. 2018;549:87–95. doi: 10.1016/j.ijpharm.2018.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallis J., Shenton D.P., Carlisle R.C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019;196:189–204. doi: 10.1111/cei.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh B., Maharjan S., Sindurakar P., Cho K.H., Choi Y.J., Cho C.S. Needle-free immunization with chitosan-based systems. Int. J. Mol. Sci. 2018;19:3639. doi: 10.3390/ijms19113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin C.I., Jeong S.D., Rejinold N.S., Kim Y.C. Microneedles for vaccine delivery: challenges and future perspectives. Ther. Deliv. 2017;8:447–460. doi: 10.4155/tde-2017-0032. [DOI] [PubMed] [Google Scholar]
- 36.Hegde N.R., Kaveri S.V., Bayry J. Recent advances in the administration of vaccines for infectious diseases: microneedles as painless delivery devices for mass vaccination. Drug Discov. Today. 2011;16:1061–1068. doi: 10.1016/j.drudis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Lambert P.H., Laurent P.E. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 38.Giudice E.L., Campbell J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Weniger B.G., Papania M.J. Vaccines. 6th ed. Saunders; Philadelphia, PA, USA: 2013. Alternative vaccine delivery methods; pp. 1200–1231. [Google Scholar]
- 40.Garg N., Aggarwal A. Advances towards painless vaccination and newer modes of vaccine delivery. Indian J. Pediatr. 2018;85:132–138. doi: 10.1007/s12098-017-2377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemoine C., Thakur A., Krajišnik D., Guyon R., Longet S., Razim A., Górska S., Pantelić I., Ilić T., Nikolić I., Lavelle E.C., Gamian A., Savić S., Milicic A. Technological approaches for improving vaccination compliance and coverage. Vaccines. 2020;8:304. doi: 10.3390/vaccines8020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korkmaz E., Balmert S.C., Carey C.D., Erdos G., Falo L.D., Jr. Emerging skin-targeted drug delivery strategies to engineer immunity: a focus on infectious diseases. Expert Opin. Drug Del. 2020 doi: 10.1080/17425247.2021.1823964. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prausnitz M.R. Engineering microneedle patches for vaccination and drug delivery to skin. Ann. Rev. Chem. Biomol. Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 44.Arya J., Prausnitz M.R. Microneedle patches for vaccination in developing countries. J. Control. Release. 2016;240:135–141. doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh H., Shin J., Kim Y.C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccine Res. 2014;3:42–49. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hettinga J., Carlisle R. Vaccination into the dermal compartment: techniques, challenges, and prospects. Vaccines. 2020;8:534. doi: 10.3390/vaccines8030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varghese P.M., Tsolaki A.G., Yasmin H., Shastri A., Ferluga J., Vatish M., Madan T., Kishore U. Host-pathogen interaction in COVID-19: pathogenesis, potential therapeutics and vaccination strategies. Immunobiology. 2020;152008 doi: 10.1016/j.imbio.2020.152008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15:630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uddin M., Mustafa F., Rizvi T.A., Loney T., Suwaidi H.A., Al-Marzouqi A.H.H., Eldin A.K., Alsabeeha N., Adrian T.E., Stefanini C., Nowotny N., Alsheikh-Ali A., Senok A.C. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12:526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J.M., Glaunsinger B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong S., Sun J., Mao Z., Wang L., Lu Y.L., Li J. A guideline for homology modeling of the proteins from newly discovered betacoronavirus, 2019 novel coronavirus (2019-nCoV) J. Med. Virol. 2020;92:1542–1548. doi: 10.1002/jmv.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16 doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satarker S., Nampoothiri M. Structural proteins in severe acute respiratory syndrome Coronavirus-2. Arch. Med. Res. 2020;51:482–491. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBride R., Van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers as a potential target for antiviral development. Antivir. Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y., Yang C., Xu X., Xu W., Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv Z., Deng Y.Q., Ye Q., Cao L., Sun C.Y., Fan C., Huang W., Sun S., Sun Y., Zhu L., Chen Q., Wang N., Chen Q., Wang N., Nie J., Cui Z., Zhu D., Shaw N., Li X.F., Li Q., Xie L., Wang Y., Rao Z., Qin C.F., Wang X. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369:1505–1509. doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alsaadi E.A.J., Jones I.M. Membrane binding proteins of coronaviruses. Futur. Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seyedpour S., Khodaei B., Loghman A.H., Seyedpour N., Kisomi M.F., Balibegloo M., Nezamabadi S.S., Gholami B., Saghazadeh A., Rezaei N. Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: a systematic review of in vitro and in vivo studies. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30032. (In Press) [DOI] [PubMed] [Google Scholar]
- 69.Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C., Fernandez-Rodriguez B., Foglierini M., Agatic G., Vanzetta F., Gopal R., Langrish C.J., Barrett N.A., Sallusto F., Baric R.S., Varani L., Zambon M., Perlman S., Lanzavecchia A. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 71.Abraham J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;20:401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salvatori G., Luberto L., Maffei M., Aurisicchio L., Roscilli G., Palombo F., Marra E. SARS-CoV-2 spike protein: an optimal immunological target for vaccines. J. Transl. Med. 2020;18:222. doi: 10.1186/s12967-020-02392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sternberg A., Naujokat C. Structural features of coronavirus SARS-CoV-2 spike protein: targets for vaccination. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samrat S.K., Tharappel A.M., Li Z., Li H. Prospect of SARS-CoV-2 spike protein: potential role in vaccine and therapeutic development. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y., Jiang S., Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines. 2018;17:677–686. doi: 10.1080/14760584.2018.1506702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai L., Zheng T., Xu K., Han Y., Xu L., Huang E., An Y., Cheng Y., Li S., Liu M., Yang M., Li Y., Cheng H., Yuan Y., Zhang W., Ke C., Wong G., Qi J., Qin C., Yan J., Gao G.F. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722–733. doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng L., Wang Q., Shan C., Yang C., Feng Y., Wu J., Liu X., Zhou Y., Jiang R., Hu P., Liu X., Zhang F., Li P., Niu X., Liu Y., Zheng X., Luo J., Sun J., Gu Y., Liu B., Xu Y., Li C., Pan W., Zhao J., Ke C., Chen X., Xu T., Zhong N., Guan S., Yuan Z., Chen L. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020;11:4207. doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., Bao L., Mo F., Li X., Huang Y., Hong W., Yang Y., Zhao Y., Ye F., Lin S., Deng W., Chen H., Lei H., Zhang Z., Luo M., Gao H., Zheng Y., Gong Y., Jiang X., Xu Y., Lv Q., Li D., Wang M., Li F., Wang S., Wang G., Yu P., Qu Y., Yang L., Deng H., Tong A., Li J., Wang Z., Yang J., Shen G., Zhao Z., Li Y., Luo J., Liu H., Yu W., Yang M., Xu J., Wang J., Li H., Wang H., Kuang D., Lin P., Hu Z., Guo W., Cheng W., He Y., Song X., Chen C., Xue Z., Yao S., Chen L., Ma X., Chen S., Gou M., Huang W., Wang Y., Fan C., Tian Z., Shi M., Wang F.S., Dai L., Wu M., Li G., Wang G., Peng Y., Qian Z., Huang C., Lau J.Y.N., Yang Z., Wei Y., Cen X., Peng X., Qin C., Zhang K., Lu G., Wei X. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 80.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., Nagata B.M., Andersen H., Martinez D.R., Noe A.T., Douek N., Donaldson M.M., Nji N.N., Alvarado G.S., Edwards D.K., Flebbe D.R., Lamb E., Doria-Rose N.A., Lin B.C., Louder M.K., O’Dell S., Schmidt S.D., Phung E., Chang L.A., Yap C., Todd J.P.M., Pessaint L., Ry A.V., Browne S., Greenhouse J., Putman-Taylor T., Strasbaugh A., Campbell T.A., Cook A., Dodson A., Steingrebe K., Shi W., Zhang Y., Abiona O.M., Wang L., Pegu A., Yang E.S., Leung K., Zhou T., Teng I.T., Widge A., Gordon I., Novik L., Gillespie R.A., Loomis R.J., Moliva J.I., Stewart-Jones G., Himansu S., Kong W.P., Nason M.C., Morabito K.M., Ruckwardt T.J., Ledgerwood J.E., Gaudinski M.R., Kwong P.D., Mascola J.R., Carfi A., Lewis M.G., Baric R.S., McDermott A., Moore I.N., Sullivan N.J., Roederer M., Seder R.A., Graham B.S. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., Manen D.V., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Huber S.K.R., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Ry A.V., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M.G., Cai Y., Chen B., Schmidt A.G., Reeves R.K., Baric R.S., Lauffenburger D.A., Alter G., Stoffels P., Mammen M., Hoof J.V., Schuitemaker H., Barouch D.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., Martinez D.R., Loos C., Atyeo C., Fischinger S., Burke J.S., Slein M.D., Chen Y., Zuiani A., Lelis F.J.N., Travers M., Habibi S., Pessaint L., Ry A.V., Brown R., Cook A., Finneyfrock B., Dodson A., Teow E., Velasco J., Zahn R., Wegmann F., Bondzie E.A., Dagotto G., Gebre M.S., He X., Jacob-Dolan C., Kirilova M., Kordana N., Lin Z., Maxfield L.F., Nampanya F., Nityanandam R., Ventura J.D., Wan H., Cai Y., Chen B., Schmidt A.G., Wesemann D.R., Baric R.S., Alter G., Andersen H., Lewis M.G., Barouch D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395:1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 85.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baroni A., Buommino E., De Gregorio V., Ruocco E., Ruocco V., Wolf R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012;30:257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Tsuruta D., Green K.J., Getsios S., Jones J.C. The barrier function of skin: how to keep a tight lid on water loss. Trends Cell Biol. 2002;12:355–357. doi: 10.1016/s0962-8924(02)02316-4. [DOI] [PubMed] [Google Scholar]
- 88.Proksch E., Brandner J.M., Jensen J.M. The skin: an indispensable barrier. Exp. Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 89.Eyerich S., Eyerich K., Traidl-Hoffmann C., Biedermann T. Cutaneous barriers and skin immunity: differentiating a connected network. Trends Immunol. 2018;39:315–327. doi: 10.1016/j.it.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Elias P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007;29:3. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 91.Quaresma J.A.S. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00034-18. e00034-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pasparakis M., Haase I., Nestle F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 93.Mathers A.R., Larregina A.T. Professional antigen-presenting cells of the skin. Immunol. Res. 2006;36:127–136. doi: 10.1385/IR:36:1:127. [DOI] [PubMed] [Google Scholar]
- 94.Kashem S.W., Haniffa M., Kaplan D.H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen A.V., Soulika A.M. The dynamics of the skin's immune system. Int. J. Mol. Sci. 2019;20:1811. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tay S.S., Roediger B., Tong P.L., Tikoo S., Weninger W. The skin-resident immune network. Curr. Dermatol. Rep. 2014;3:13–22. doi: 10.1007/s13671-013-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wickett R.R., Visscher M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006;34:S98–S110. [Google Scholar]
- 98.Nickoloff B.J. Keratinocytes regain momentum as instigators of cutaneous inflammation. Trends Mol. Med. 2006;12:102–106. doi: 10.1016/j.molmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Bitschar K., Wolz C., Krismer B., Peschel A., Schittek B. Keratinocytes as sensors and central players in the immune defense against Staphylococcus aureus in the skin. J. Dermatol. Sci. 2017;87:215–220. doi: 10.1016/j.jdermsci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 100.Pivarcsi A., Nagy I., Kemeny L. Innate immunity in the skin: how keratinocytes fight against pathogens. Curr. Immunol. Rev. 2005;1:29–42. [Google Scholar]
- 101.Dai X., Tohyama M., Murakami M., Sayama K. Epidermal keratinocytes sense dsRNA via the NLRP3 inflammasome, mediating interleukin (IL)-1β and IL-18 release. Exp. Dermatol. 2017;26:904–911. doi: 10.1111/exd.13334. [DOI] [PubMed] [Google Scholar]
- 102.Nestle F.O., Di Meglio P., Qin J.Z., Nickoloff B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olaru F., Jensen L.E. Chemokine expression by human keratinocyte cell lines after activation of toll-like receptors. Exp. Dermatol. 2010;19:e314–e316. doi: 10.1111/j.1600-0625.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xue X., Mi Z., Wang Z., Pang Z., Liu H., Zhang F. High expression of ACE2 on the keratinocytes reveals skin as a potential target for SARS-CoV-2. J. Invest. Dermatol. 2020;141:206–209. doi: 10.1016/j.jid.2020.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Intern. J. Oral Sci. 2020;12 doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sorrell J.M., Caplan A.I. Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 107.Quan T., Wang F., Shao Y., Rittié L., Xia W., Orringer J.S., Voorhees J.J., Fisher G.J. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J. Invest. Dermatol. 2013;133:658–667. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bautista-Hernández L.A., Gómez-Olivares J.L., Buentello-Volante B., Bautista-de Lucio V.M. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017;7:151–157. doi: 10.1556/1886.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller L.S., Modlin R.L. Toll-like receptors in the skin. Semin. Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 110.Tsepkolenko A., Tsepkolenko V., Dash S., Mishra A., Bader A., Melerzanov A., Giri S. The regenerative potential of skin and the immune system. Clin. Cosmet. Investig. Dermatol. 2019;12:519–532. doi: 10.2147/CCID.S196364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin J.Y., Fisher D.E. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 112.Slominski A., Paus R., Schadendorf D. Melanocytes as "sensory" and regulatory cells in the epidermis. J. Theor. Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 113.Gasque P., Jaffar-Bandjee M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015;71:413–421. doi: 10.1016/j.jinf.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 114.Tam I., Stępień K. Secretion of proinflammatory cytokines by normal human melanocytes in response to lipopolysaccharide. Acta Biochim. Pol. 2011;58:507–511. [PubMed] [Google Scholar]
- 115.Tam I., Dzierżęga-Lęcznar A., Stępień K. Differential expression of inflammatory cytokines and chemokines in lipopolysaccharide-stimulated melanocytes from lightly and darkly pigmented skin. Exp. Dermatol. 2019;28:551–560. doi: 10.1111/exd.13908. [DOI] [PubMed] [Google Scholar]
- 116.Yu N., Zhang S., Zuo F., Kang K., Guan M., Xiang L. Cultured human melanocytes express functional toll-like receptors 2–4, 7 and 9. J. Dermatol. Sci. 2009;56:113–120. doi: 10.1016/j.jdermsci.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 117.Salimi M., Ogg G. Innate lymphoid cells and the skin. BMC Dermatol. 2014;14 doi: 10.1186/1471-5945-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou S., Li Q., Wu H., Lu Q. The pathogenic role of innate lymphoid cells in autoimmune-related and inflammatory skin diseases. Cell. Mol. Immunol. 2020;17:335–346. doi: 10.1038/s41423-020-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim B.S. Innate lymphoid cells in the skin. J. Invest. Dermatol. 2015;135:673–678. doi: 10.1038/jid.2014.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kobayashi T., Ricardo-Gonzalez R.R., Moro K. Skin-resident innate lymphoid cells–cutaneous innate guardians and regulators. Trends Immunol. 2020;41:100–112. doi: 10.1016/j.it.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klose C.S.N., Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 122.Seillet C., Jacquelot N. Sensing of physiological regulators by innate lymphoid cells. Cell. Mol. Immunol. 2019;16:442–451. doi: 10.1038/s41423-019-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harvima I.T., Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm. Venereol. 2011;91:640–650. doi: 10.2340/00015555-1197. [DOI] [PubMed] [Google Scholar]
- 124.Lunderius-Andersson C., Enoksson M., Nilsson G. Mast cells respond to cell injury through the recognition of IL-33. Front. Immunol. 2012;3:82. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sumpter T.L., Balmert S.C., Kaplan D.H. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Henz B.M, Maurer M., Lippert U., Worm M., Babina M. Mast cells as initiators of immunity and host defense. Exp. Dermatol. 2001;10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x. [DOI] [PubMed] [Google Scholar]
- 127.Mantri C.K., St.John A.L. Immune synapses between mast cells and γδ T cells limit viral infection. J. Clin. Investig. 2019;129:1094–1108. doi: 10.1172/JCI122530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang Y., Xiang Z. Roles and relevance of mast cells in infection and vaccination. J. Biomed. Res. 2016;30:253–263. doi: 10.7555/JBR.30.20150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metz M., Siebenhaar F., Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008;213:251–260. doi: 10.1016/j.imbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 130.Galli S.J., Nakae S., Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 131.McLeod J.J.A., Baker B., Ryan J.J. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75:57–61. doi: 10.1016/j.cyto.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kambayashi T., Laufer T.M. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat. Rev. Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 133.Kambayashi T., Baranski J.D., Baker R.G., Zou T., Allenspach E.J., Shoag J.E., Jones P.L., Koretzky G.A. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–1496. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Komi D.E.A., Grauwet K. Role of mast cells in regulation of T cell responses in experimental and clinical settings. Clin. Rev. Allergy Immunol. 2018;54:432–445. doi: 10.1007/s12016-017-8646-z. [DOI] [PubMed] [Google Scholar]
- 135.Banchereau J., Klechevsky E., Schmitt N., Morita R., Palucka K., Ueno H. Harnessing human dendritic cell subsets to design novel vaccines. Ann. N. Y. Acad. Sci. 2009;1174:24–32. doi: 10.1111/j.1749-6632.2009.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palucka K., Banchereau J., Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sparber F., Tripp C.H., Hermann M., Romani N., Stoitzner P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: possible targets for vaccination through the skin. Immunobiology. 2010;215:770–779. doi: 10.1016/j.imbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Larregina A.T., Falo L.D. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J. Invest. Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- 139.Clausen B.E., Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front. Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palucka K., Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 141.Kitano M., Yamazaki C., Takumi A., Ikeno T., Hemmi H., Takahashi N., Shimizu K., Fraser S.E., Hoshino K., Kaisho T. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1044–1049. doi: 10.1073/pnas.1513607113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Misery L. The neuro-immuno-cutaneous system and ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2000;16:78–81. doi: 10.1034/j.1600-0781.2000.d01-8.x. [DOI] [PubMed] [Google Scholar]
- 143.Denda M., Nakatani M., Ikeyama K., Tsutsumi M., Denda S. Epidermal keratinocytes as the forefront of the sensory system. Exp. Dermatol. 2007;16:157–161. doi: 10.1111/j.1600-0625.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 144.Chu C., Artis D., Chiu I.M. Neuro-immune interactions in the tissues. Immunity. 2020;52:464–474. doi: 10.1016/j.immuni.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cohen J.A., Wu J., Kaplan D.H. Neuronal regulation of cutaneous immunity. J. Immunol. 2020;204:264–270. doi: 10.4049/jimmunol.1901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saloman J.L., Cohen J.A., Kaplan D.H. Intimate neuro-immune interactions: breaking barriers between systems to make meaningful progress. Curr. Opin. Neurobiol. 2020;62:60–67. doi: 10.1016/j.conb.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 147.Janelsins B.M., Mathers A.R., Tkacheva O.A., Erdos G., Shufesky W.J., Morelli A.E., Larregina A.T. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood. 2009;113:3017–3026. doi: 10.1182/blood-2008-06-163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malissen B., Tamoutounour S., Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 149.Kolter J., Feuerstein R., Zeis P., Hagemeyer N., Paterson N., d’Errico P., Baasch S., Amann L., Masuda T., Lösslein A., Gharun K., Meyer-Luehmann M., Waskow C., Franzke C.W., Grun D., Lammermann T., Prinz M., Henneke P. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity. 2019;50:1482–1497. doi: 10.1016/j.immuni.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 150.Delavary B.M., van der Veer W.M., van Egmond M., Niessen F.B., Beelen R.H. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 151.Mann E., Smith K., Bernardo D., Al-Hassi H., Knight S., Hart A. Review: skin and the immune system. J. Clin. Exp. Dermatol. Res. S. 2012;S2:003. [Google Scholar]
- 152.Cruz M.S., Diamond A., Russell A., Jameson J.M. Human αβ and γδ T cells in skin immunity and disease. Front. Immunol. 2018;9:1304. doi: 10.3389/fimmu.2018.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seneschal J., Clark R.A., Gehad A., Baecher-Allan C.M., Kupper T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clark R.A. Skin-resident T cells: the ups and downs of on site immunity. J. Invest. Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ho A.W., Kupper T.S. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019;19:490–502. doi: 10.1038/s41577-019-0162-3. [DOI] [PubMed] [Google Scholar]
- 156.Schreiner D., King C.G. CD4+ memory T cells at home in the tissue: mechanisms for health and disease. Front. Immunol. 2018;9:2394. doi: 10.3389/fimmu.2018.02394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Khalil S., Bardawil T., Kurban M., Abbas O. Tissue-resident memory T cells in the skin. Inflamm. Res. 2020;69:245–254. doi: 10.1007/s00011-020-01320-6. [DOI] [PubMed] [Google Scholar]
- 158.Ariotti S., Hogenbirk M.A., Dijkgraaf F.E., Visser L.L., Hoekstra M.E., Song J.Y., Jacobs H., Haanen J.B., Schumacher T.N. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 159.Park C.O., Kupper T.S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rosato P.C., Beura L.K., Masopust D. Tissue resident memory T cells and viral immunity. Curr. Opin. Virol. 2017;22:44–50. doi: 10.1016/j.coviro.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muruganandah V., Sathkumara H.D., Navarro S., Kupz A. A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front. Immunol. 2018;9:1574. doi: 10.3389/fimmu.2018.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pan Y., Liu L., Tian T., Zhao J., Park C.O., Lofftus S.Y., Stingley C.A., Mei S., Liu X., Kupper T.S. Skin delivery of modified vaccinia ankara viral vectors generates superior T cell immunity against a respiratory viral challenge. bioRxiv. 2020 doi: 10.1101/2020.1105.1106.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fitzner N., Clauberg S., Essmann F., Liebmann J., Kolb-Bachofen V. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clin. Vaccine Immunol. 2008;15:138–146. doi: 10.1128/CVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sanchez B., Li L., Dulong J., Aimond G., Lamartine J., Liu G., Sigaudo-Roussel D. Impact of human dermal microvascular endothelial cells on primary dermal fibroblasts in response to inflammatory stress, front. Cell Dev. Biol. 2019;7:44. doi: 10.3389/fcell.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Breedveld A., Groot Kormelink T., van Egmond M., de Jong E.C. Granulocytes as modulators of dendritic cell function. J. Leukoc. Biol. 2017;102:1003–1016. doi: 10.1189/jlb.4MR0217-048RR. [DOI] [PubMed] [Google Scholar]
- 166.Cardamone C., Parente R., De Feo G., Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol. Lett. 2016;178:10–14. doi: 10.1016/j.imlet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 167.Chen Z., Lv Y., Qi J., Zhu Q., Lu Y., Wu W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today. 2018;23:181–186. doi: 10.1016/j.drudis.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 168.Teunissen M.B.M., Haniffa M., Collin M.P. Insight into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design. Curr. Top. Microbiol. Immunol. 2011:25–76. doi: 10.1007/82_2011_169. Springer. [DOI] [PubMed] [Google Scholar]
- 169.Kim Y.C., Prausnitz M.R. Enabling skin vaccination using new delivery technologies. Drug Deliv. Transl. Res. 2011;1:7–12. doi: 10.1007/s13346-010-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bal S.M., Ding Z., van Riet E., Jiskoot W., Bouwstra J.A. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J. Control. Release. 2010;148:266–282. doi: 10.1016/j.jconrel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 171.Zhang L., Wang W., Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines. 2015;14:1509–1523. doi: 10.1586/14760584.2015.1081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Levin C., Perrin H., Combadiere B. Tailored immunity by skin antigen-presenting cells. Hum. Vaccin. Immunother. 2015;11:27–36. doi: 10.4161/hv.34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Combadiere B., Liard C. Transcutaneous and intradermal vaccination. Hum. Vaccin. 2011;7:811–827. doi: 10.4161/hv.7.8.16274. [DOI] [PubMed] [Google Scholar]
- 174.Falo L.D., Jr. Advances in skin science enable the development of a COVID-19 vaccine. J. Am. Acad. Dermatol. 2020;83:1226–1227. doi: 10.1016/j.jaad.2020.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu L., Zhong Q., Tian T., Dubin K., Athale S.K., Kupper T.S. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell–mediated immunity. Nat. Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kremer M., Suezer Y., Volz A., Frenz T., Majzoub M., Hanschmann K.M., Lehmann M.H., Kalinke U., Sutter G. Critical role of perforin-dependent CD8+ T cell immunity for rapid protective vaccination in a murine model for human smallpox. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koutsonanos D.G., Vassilieva E.V., Stavropoulou A., Zarnitsyn V.G., Esser E.S., Taherbhai M.T., Prausnitz M.R., Compans R.W., Skountzou I. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci. Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koutsonanos D.G., del Pilar Martin M., Zarnitsyn V.G., Jacob J., Prausnitz M.R., Compans R.W., Skountzou I. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J. Infect. Dis. 2011;204:582–591. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Quan F.S., Kim Y.C., Vunnava A., Yoo D.G., Song J.M., Prausnitz M.R., Compans R.W., Kang S.M. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Engelke L., Winter G., Hook S., Engert J. Recent insights into cutaneous immunization: how to vaccinate via the skin. Vaccine. 2015;33:4663–4674. doi: 10.1016/j.vaccine.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 181.Hickling J.K., Jones K.R., Friede M., Zehrung D., Chen D., Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull. World Health Organ. 2011;89:221–226. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kenney R.T., Frech S.A., Muenz L.R., Villar C.P., Glenn G.M. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 183.Fehres C.M., Garcia-Vallejo J.J., Unger W.W.J., Van Kooyk Y. Skin-resident antigen-presenting cells: instruction manual for vaccine development. Front. Immunol. 2013;4:157. doi: 10.3389/fimmu.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eisenbarth S.C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Allan R.S., Waithman J., Bedoui S., Jones C.M., Villadangos J.A., Zhan Y., Lew A.M., Shortman K., Heath W.R., Carbone F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 186.Watkins E.A., Hubbell J.A. Designing biofunctional immunotherapies. Nat. Rev. Mater. 2019;4:350–352. [Google Scholar]
- 187.Irvine D.J., Aung A., Silva M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Partidos C.D., Muller S. Decision-making at the surface of the intact or barrier disrupted skin: potential applications for vaccination or therapy. Cell. Mol. Life Sci. 2005;62:1418–1424. doi: 10.1007/s00018-005-4529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Joubert I.A., Kovacs D., Scheiblhofer S., Winter P., Korotchenko E., Strandt H., Weiss R. Mast cells and γδ T cells are largely dispensable for adaptive immune responses after laser-mediated epicutaneous immunization. Vaccine. 2020;38:1015–1024. doi: 10.1016/j.vaccine.2019.11.051. [DOI] [PubMed] [Google Scholar]
- 190.Ronchese F., Hilligan K.L., Mayer J.U. Dendritic cells and the skin environment. Curr. Opin. Immunol. 2020;64:56–62. doi: 10.1016/j.coi.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 191.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tom J.K., Albin T.J., Manna S., Moser B.A., Steinhardt R.C., Esser-Kahn A.P. Applications of immunomodulatory immune synergies to adjuvant discovery and vaccine development. Trends Biotechnol. 2019;37:373–388. doi: 10.1016/j.tibtech.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 193.Skountzou I., Brock N., Lelutiu N., Compans R. Immunopotentiators in Modern Vaccines. Elsevier; 2017. Adjuvants for skin vaccination; pp. 399–419. [Google Scholar]
- 194.Vasilakos J.P., Tomai M.A. The use of toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines. 2013;12:809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 195.Carter D., van Hoeven N., Baldwin S., Levin Y., Kochba E., Magill A., Charland N., Landry N., Nu K., Frevol A., Ashman J., Sagawa Z.K., Beckmann A.M., Reed S.G. The adjuvant GLA-AF enhances human intradermal vaccine responses. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aas9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E.L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M.A., Wu Y., Amante D., Park D.H., Dia Y., Ali A.R., Zaidi F.I., Generotti A., Kim K.Y., Herring T.A., Reeder S., Andrade V.M., Buttigieg K., Zhao G., Wu J.M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A.S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J.S., Muthumani K., Wang B., Carroll M.W., Kim J.J., Boyer J., Kulp D.W., Humeau L.M.P.F., Weiner D.B., Broderick K.E. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tai W., Zhang X., Drelich A., Shi J., Hsu J.C., Luchsinger L., Hillyer C.D., Tseng C.T.K., Jiang S., Du L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020;30:932–935. doi: 10.1038/s41422-020-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Jr., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peeples L. News feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc. Natl. Acad. Sci. U. S. A. 2020;117:8218–8221. doi: 10.1073/pnas.2005456117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zellweger R.M., Wartel T.A., Marks F., Song M., Kim J.H. Vaccination against SARS-CoV-2 and disease enhancement–knowns and unknowns. Expert Rev. Vaccines. 2020;19:691–698. doi: 10.1080/14760584.2020.1800463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hotez P.J., Corry D.B., Bottazzi M.E. COVID-19 vaccine design: the Janus face of immune enhancement. Nat. Rev. Immunol. 2020;20:347–348. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Badizadegan K., Goodson J.L., Rota P.A., Thompson K.M. The potential role of using vaccine patches to induce immunity: platform and pathways to innovation and commercialization. Expert Rev. Vaccines. 2020;19:175–194. doi: 10.1080/14760584.2020.1732215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gamazo C., Pastor Y., Larrañeta E., Berzosa M., Irache J.M., Donnelly R.F. Understanding the basis of transcutaneous vaccine delivery. Ther. Deliv. 2019;10:63–80. doi: 10.4155/tde-2018-0054. [DOI] [PubMed] [Google Scholar]
- 204.Li N., Peng L.H., Chen X., Nakagawa S., Gao J.Q. Transcutaneous vaccines: novel advances in technology and delivery for overcoming the barriers. Vaccine. 2011;29:6179–6190. doi: 10.1016/j.vaccine.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 205.Glenn G.M., Taylor D.N., Li X., Frankel S., Montemarano A., Alving C.R. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 2000:1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 206.Mitragotri S. Immunization without needles. Nat. Rev. Immunol. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 207.Nguyen T.T., Oh Y., Kim Y., Shin Y., Baek S.K., Park J.H. Progress in microneedle array patch (MAP) for vaccine delivery. Hum. Vaccin. Immunother. 2021;17:316–327. doi: 10.1080/21645515.2020.1767997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marshall S., Sahm L.J., Moore A.C. The success of microneedle-mediated vaccine delivery into skin. Hum. Vaccin. Immunother. 2016;12:2975–2983. doi: 10.1080/21645515.2016.1171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vrdoljak A. Review of recent literature on microneedle vaccine delivery technologies. Vaccine Dev. Therapy. 2013;3:47–55. [Google Scholar]
- 210.Abbas A.M., Fathalla W.R., Fathalla G.R., Abdelmonsef A.A. Evaluation of microneedle drug delivery system and nanoparticles use in COVID-19 patients. IJCMCR. 2020;37:5–7. [Google Scholar]
- 211.Saroja C., Lakshmi P., Bhaskaran S. Recent trends in vaccine delivery systems: a review. Int. J. Pharm. Investig. 2011;1:64–74. doi: 10.4103/2230-973X.82384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Quinn H.L., Kearney M.C., Courtenay A.J., McCrudden M.T.C., Donnelly R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014;11:1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 213.Peyraud N., Zehrung D., Jarrahian C., Frivold C., Orubu T., Giersing B. Potential use of microarray patches for vaccine delivery in low-and middle-income countries. Vaccine. 2019;37:4427–4434. doi: 10.1016/j.vaccine.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 214.Prausnitz M.R., Goodson J.L., Rota P.A., Orenstein W.A. A microneedle patch for measles and rubella vaccination: a game changer for achieving elimination. Curr. Opin. Virol. 2020;41:68–76. doi: 10.1016/j.coviro.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Prausnitz M.R., Mikszta J.A., Cormier M., Andrianov A.K. Springer; 2009. Microneedle-based Vaccines, Vaccines for Pandemic Influenza; pp. 369–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J. Control. Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 218.Fonseca D.F.S., Vilela C., Silvestre A.J.D., Freire C.S.R. A compendium of current developments on polysaccharide and protein-based microneedles. Int. J. Biol. Macromol. 2019;136:704–728. doi: 10.1016/j.ijbiomac.2019.04.163. [DOI] [PubMed] [Google Scholar]
- 219.Guillot A.J., Cordeiro A.S., Donnelly R.F., Montesinos M.C., Garrigues T.M., Melero A. Microneedle-based delivery: an overview of current applications and trends. Pharmaceutics. 2020;12:569. doi: 10.3390/pharmaceutics12060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Park J.H., Choi S.O., Seo S., Choy Y.B., Prausnitz M.R. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010;76:282–289. doi: 10.1016/j.ejpb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 221.Jeong H.R., Bae J.Y., Park J.H., Baek S.K., Kim G., Park M.S., Park J.H. Preclinical study of influenza bivalent vaccine delivered with a two compartmental microneedle array. J. Control. Release. 2020;324:280–288. doi: 10.1016/j.jconrel.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 222.Trautmann A., Roth G.L., Nujiqi B., Walther T., Hellmann R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst. Nanoeng. 2019;5:6. doi: 10.1038/s41378-019-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee J.W., Choi S.O., Felner E.I., Prausnitz M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;7:531–539. doi: 10.1002/smll.201001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Courtenay A.J., Rodgers A.M., McCrudden M.T.C., McCarthy H.O., Donnelly R.F. Novel hydrogel-forming microneedle array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol. Pharm. 2018;16:118–127. doi: 10.1021/acs.molpharmaceut.8b00895. [DOI] [PubMed] [Google Scholar]
- 225.Park J.H., Choi S.O., Kamath R., Yoon Y.K., Allen M.G., Prausnitz M.R. Polymer particle-based micromolding to fabricate novel microstructures. Biomed. Microdevices. 2007;9:223–234. doi: 10.1007/s10544-006-9024-4. [DOI] [PubMed] [Google Scholar]
- 226.Chu L.Y., Prausnitz M.R. Separable arrowhead microneedles. J. Control. Release. 2011;149:242–249. doi: 10.1016/j.jconrel.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xiang Z., Wang H., Pant A., Pastorin G., Lee C. Development of vertical SU-8 microtubes integrated with dissolvable tips for transdermal drug delivery. Biomicrofluidics. 2013;7 doi: 10.1063/1.4798471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim M.J., Park S.C., Rizal B., Guanes G., Baek S.K., Park J.H., Betz A.R., Choi S.O. Fabrication of circular obelisk-type multilayer microneedles using micro-milling and spray deposition. Front. Bioeng. Biotechnol. 2018;6:54. doi: 10.3389/fbioe.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bediz B., Korkmaz E., Khilwani R., Donahue C., Erdos G., Falo L.D., Ozdoganlar O.B. Dissolvable microneedle arrays for intradermal delivery of biologics: fabrication and application. Pharm. Res. 2014;31:117–135. doi: 10.1007/s11095-013-1137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Johnson A.R., Caudill C.L., Tumbleston J.R., Bloomquist C.J., Moga K.A., Ermoshkin A., Shirvanyants D., Mecham S.J., Luft J.C., DeSimone J.M. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Balmert S.C., Carey C.D., Falo G.D., Sethi S.K., Erdos G., Korkmaz E., Falo L.D., Jr. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J. Control. Release. 2020;317:336–346. doi: 10.1016/j.jconrel.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bae W.G., Ko H., So J.Y., Yi H., Lee C.H., Lee D.H., Ahn Y., Lee S.H., Lee K., Jun J., Kim H.H., Jeon N.L., Jung W., Song C.S., Kim T., Kim Y.C., Jeong H.E. Snake fang–inspired stamping patch for transdermal delivery of liquid formulations. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw3329. [DOI] [PubMed] [Google Scholar]
- 233.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 234.Ingrole R.S., Gill H.S. Microneedle coating methods: a review with a perspective. J. Pharmacol. Exp. Ther. 2019;370:555–569. doi: 10.1124/jpet.119.258707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Haj-Ahmad R., Khan H., Arshad M.S., Rasekh M., Hussain A., Walsh S., Li X., Chang M.W., Ahmad Z. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics. 2015;7:486–502. doi: 10.3390/pharmaceutics7040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Uppu D.S.S.M., Turvey M.E., Sharif A.R.M., Bidet K., He Y., Ho V., Tambe A.D., Lescar J., Tan E.Y., Fink K., Chen J., Hammond P.T. Temporal release of a three-component protein subunit vaccine from polymer multilayers. J. Control. Release. 2020;317:130–141. doi: 10.1016/j.jconrel.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 237.Paredes A.J., McKenna P.E., Ramöller I.K., Naser Y.A., Volpe-Zanutto F., Li M., Abbate M.T.A., Zhao L., Zhang C., Abu-Ershaid J.M., Dai X., Donnelly R.F. Microarray patches: poking a hole in the challenges faced when delivering poorly soluble drugs. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 238.Vinayakumar K.B., Kulkarni P.G., Nayak M.M., Dinesh N.S., Hegde G.M., Ramachandra S.G., Rajanna K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromech. Microeng. 2016;26 [Google Scholar]
- 239.Leone M., Mönkäre J., Bouwstra J., Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm. Res. 2017;34:2223–2240. doi: 10.1007/s11095-017-2223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Al-Japairai K.A.S., Mahmood S., Almurisi S.H., Venugopal J.R., Hilles A.R., Azmana M., Raman S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Larraneta E., Lutton R.E., Woolfson A.D., Donnelly R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater. Sci. 2016;104:1–32. [Google Scholar]
- 242.Donnelly R.F., Singh T.R.R., Garland M.J., Migalska K., Majithiya R., McCrudden C.M., Kole P.L., Mahmood T.M.T., McCarthy H.O., Woolfson A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Al-Kasasbeh R., Brady A.J., Courtenay A.J., Larrañeta E., McCrudden M.T.C., O’Kane D., Liggett S., Donnelly R.F. Evaluation of the clinical impact of repeat application of hydrogel-forming microneedle array patches. Drug Deliv. Transl. Res. 2020;10:690–705. doi: 10.1007/s13346-020-00727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Boks M.A., Unger W.W., Engels S., Ambrosini M., van Kooyk Y., Luttge R. Controlled release of a model vaccine by nanoporous ceramic microneedle arrays. Int. J. Pharm. 2015;491:375–383. doi: 10.1016/j.ijpharm.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 245.Gholami S., Mohebi M.M., Hajizadeh-Saffar E., Ghanian M.H., Zarkesh I., Baharvand H. Fabrication of microporous inorganic microneedles by centrifugal casting method for transdermal extraction and delivery. Int. J. Pharm. 2019;558:299–310. doi: 10.1016/j.ijpharm.2018.12.089. [DOI] [PubMed] [Google Scholar]
- 246.Schepens B., Vos P.J., Saelens X., van der Maaden K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses. Eur. J. Pharm. Biopharm. 2019;136:259–266. doi: 10.1016/j.ejpb.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 247.Li J., Liu B., Zhou Y., Chen Z., Jiang L., Yuan W., Liang L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.de Groot A.M., Platteel A.C.M., Kuijt N., van Kooten P.J., Vos P.J., Sijts A.J.A.M., Van Der Maaden K. Nanoporous microneedle arrays effectively induce antibody responses against diphtheria and tetanus toxoid. Front. Immunol. 2017;8:1789. doi: 10.3389/fimmu.2017.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cahill E.M., Keaveney S., Stuettgen V., Eberts P., Ramos-Luna P., Zhang N., Dangol M., O'Cearbhaill E.D. Metallic microneedles with interconnected porosity: a scalable platform for biosensing and drug delivery. Acta Biomater. 2018;80:401–411. doi: 10.1016/j.actbio.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 250.Chen M.C., Huang S.F., Lai K.Y., Ling M.H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials. 2013;34:3077–3086. doi: 10.1016/j.biomaterials.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 251.Zhu D.D., Chen B.Z., He M.C., Guo X.D. Structural optimization of rapidly separating microneedles for efficient drug delivery. J. Ind. Eng. Chem. 2017;51:178–184. [Google Scholar]
- 252.Bhatnagar S., Gadeela P.R., Thathireddy P., Venuganti V.K. Microneedle-based drug delivery: materials of construction. J. Chem. Sci. 2019;131:90. [Google Scholar]
- 253.Ali R., Mehta P., Arshad M.S., Kucuk I., Chang M.W., Ahmad Z. Transdermal microneedles—a materials perspective. AAPS PharmSciTech. 2020;21:12. doi: 10.1208/s12249-019-1560-3. [DOI] [PubMed] [Google Scholar]
- 254.He X., Sun J., Zhuang J., Xu H., Liu Y., Wu D. Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-Response. 2019;17 doi: 10.1177/1559325819878585. 1559325819878585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Indermun S., Luttge R., Choonara Y.E., Kumar P., Du Toit L.C., Modi G., Pillay V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 256.Lee J.W., Park J.H., Prausnitz M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cordeiro A.S., Tekko I.A., Jomaa M.H., Vora L., McAlister E., Volpe-Zanutto F., Nethery M., Baine P.T., Mitchell N., McNeill D.W., Donnelly R.F. Two-photon polymerisation 3D printing of microneedle array templates with versatile designs: application in the development of polymeric drug delivery systems. Pharm. Res. 2020;37 doi: 10.1007/s11095-020-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Krieger K.J., Bertollo N., Dangol M., Sheridan J.T., Lowery M.M., O’Cearbhaill E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019;5 doi: 10.1038/s41378-019-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Johnson A.R., Procopio A.T. Low cost additive manufacturing of microneedle masters. 3D Print. Med. 2019;5:2. doi: 10.1186/s41205-019-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yeung C., Chen S., King B., Lin H., King K., Akhtar F., Diaz G., Wang B., Zhu J., Sun W., Khademhosseini A., Emaminejad S. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13 doi: 10.1063/1.5127778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rad Z.F., Nordon R.E., Anthony C.J., Bilston L., Prewett P.D., Arns J.Y., Arns C.H., Zhang L., Davies G.J. High-fidelity replication of thermoplastic microneedles with open microfluidic channels. Microsyst. Nanoeng. 2017;3 doi: 10.1038/micronano.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Donnelly R.F., Majithiya R., Singh T.R.R., Morrow D.I.J., Garland M.J., Demir Y.K., Migalska K., Ryan E., Gillen D., Scott C.J. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011;28:41–57. doi: 10.1007/s11095-010-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Forster A.H., Witham K., Depelsenaire A.C.I., Veitch M., Wells J.W., Wheatley A., Pryor M., Lickliter J.D., Francis B., Rockman S., Bodle J., Treasure P., Hickling J., Fernando G.J.P. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: results from a randomized, controlled phase I clinical trial. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kim J.D., Kim M., Yang H., Lee K., Jung H. Droplet-born air blowing: novel dissolving microneedle fabrication. J. Control. Release. 2013;170:430–436. doi: 10.1016/j.jconrel.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 265.Yang H., Kim S., Kang G., Lahiji S.F., Jang M., Kim Y.M., Kim J.M., Cho S.N., Jung H. Centrifugal lithography: self-shaping of polymer microstructures encapsulating biopharmaceutics by centrifuging polymer drops. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700326. [DOI] [PubMed] [Google Scholar]
- 266.Lee K., Lee H.C., Lee D.S., Jung H. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010;22:483–486. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- 267.Chen Z., Ren L., Li J., Yao L., Chen Y., Liu B., Jiang L. Rapid fabrication of microneedles using magnetorheological drawing lithography. Acta Biomater. 2018;65:283–291. doi: 10.1016/j.actbio.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 268.Choi C.K., Lee K.J., Youn Y.N., Jang E.H., Kim W., Min B.K., Ryu W. Spatially discrete thermal drawing of biodegradable microneedles for vascular drug delivery. Eur. J. Pharm. Biopharm. 2013;83:224–233. doi: 10.1016/j.ejpb.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 269.Rodgers A.M., Cordeiro A.S., Donnelly R.F. Technology update: dissolvable microneedle patches for vaccine delivery. Med. Devices (Auckland, NZ) 2019;12:379–398. doi: 10.2147/MDER.S198220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Park S., Lee Y., Kwon Y.M., Lee Y.T., Kim K.H., Ko E.J., Jung J.H., Song M., Graham B., Prausnitz M.R., Kang S.M. Vaccination by microneedle patch with inactivated respiratory syncytial virus and monophosphoryl lipid a enhances the protective efficacy and diminishes inflammatory disease after challenge. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ding Z., Verbaan F.J., Bivas-Benita M., Bungener L., Huckriede A., van den Berg D.J., Kersten G., Bouwstra J.A. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 272.Mikszta J.A., Alarcon J.B., Brittingham J.M., Sutter D.E., Pettis R.J., Harvey N.G. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 273.Guo L., Qiu Y., Chen J., Zhang S., Xu B., Gao Y. Effective transcutaneous immunization against hepatitis B virus by a combined approach of hydrogel patch formulation and microneedle arrays. Biomed. Microdevices. 2013;15:1077–1085. doi: 10.1007/s10544-013-9799-z. [DOI] [PubMed] [Google Scholar]
- 274.Dean C.H., Alarcon J.B., Waterston A.M., Draper K., Early R., Guirakhoo F., Monath T.P., Mikszta J.A. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccines against Japanese encephalitis (ChimeriVaxTM-JE) in non-human primates. Hum. Vaccin. 2005;1:106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 275.Kim Y.C., Quan F.S., Compans R.W., Kang S.M., Prausnitz M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fernando G.J.P., Chen X., Prow T.W., Crichton M.L., Fairmaid E.J., Roberts M.S., Frazer I.H., Brown L.E., Kendall M.A.F. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010;5:e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kim Y.C., Quan F.S., Compans R.W., Kang S.M., Prausnitz M.R. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kim M.C., Lee J.W., Choi H.J., Lee Y.N., Hwang H.S., Lee J., Kim C., Lee J.S., Montemagno C., Prausnitz M.R., Kang S.M. Microneedle patch delivery to the skin of virus-like particles containing heterologous M2e extracellular domains of influenza virus induces broad heterosubtypic cross-protection. J. Control. Release. 2015;210:208–216. doi: 10.1016/j.jconrel.2015.05.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kim Y.C., Song J.M., Lipatov A.S., Choi S.O., Lee J.W., Donis R.O., Compans R.W., Kang S.M., Prausnitz M.R. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur. J. Pharm. Biopharm. 2012;81:239–247. doi: 10.1016/j.ejpb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jeong H.R., Park S., Park J.H., Bae J.Y., Kim G., Baek S.K., Park M.S., Park J.H. Preparation of H1N1 microneedles by a low-temperature process without a stabilizer. Eur. J. Pharm. Biopharm. 2019;143:1–7. doi: 10.1016/j.ejpb.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 281.Kim Y.C., Quan F.S., Yoo D.G., Compans R.W., Kang S.M., Prausnitz M.R. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Quan F.S., Kim Y.C., Compans R.W., Prausnitz M.R., Kang S.M. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J. Control. Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhu Q., Zarnitsyn V.G., Ye L., Wen Z., Gao Y., Pan L., Skountzou I., Gill H.S., Prausnitz M.R., Yang C., Compans R.W. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim Y.C., Yoo D.G., Compans R.W., Kang S.M., Prausnitz M.R. Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. J. Control. Release. 2013;172:579–588. doi: 10.1016/j.jconrel.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Quan F.S., Kim Y.C., Song J.M., Hwang H.S., Compans R.W., Prausnitz M.R., Kang S.M. Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch. Clin. Vaccine Immunol. 2013;20:1433–1439. doi: 10.1128/CVI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Weldon W.C., Martin M.P., Zarnitsyn V., Wang B., Koutsonanos D., Skountzou I., Prausnitz M.R., Compans R.W. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin. Vaccine Immunol. 2011;18:647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nguyen T.T., Choi J., Kim J.S., Park H., Yang E., Lee W.J., Baek S.K., Song M., Park J.H. Skin immunization with third-generation hepatitis B surface antigen using microneedles. Vaccine. 2019;37:5954–5961. doi: 10.1016/j.vaccine.2019.08.036. [DOI] [PubMed] [Google Scholar]
- 288.Moon S., Wang Y., Edens C., Gentsch J.R., Prausnitz M.R., Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Liu Y., Ye L., Lin F., Gomaa Y., Flyer D., Carrion R., Jr., Patterson J.L., Prausnitz M.R., Smith G., Glenn G., Compans R.W., Chinglai Y. Intradermal vaccination with adjuvanted Ebola virus soluble glycoprotein subunit vaccine by microneedle patches protects mice against lethal Ebola virus challenge. J. Infect. Dis. 2018;218:S545–S552. doi: 10.1093/infdis/jiy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Muller D.A., Depelsenaire A.C.I., Shannon A.E., Watterson D., Corrie S.R., Owens N.S., Agyei-Yeboah C., Cheung S.T.M., Zhang J., Fernando G.J., Kendall M.A.F., Young P.R. Efficient delivery of dengue virus subunit vaccines to the skin by microprojection arrays. Vaccines. 2019;7:189. doi: 10.3390/vaccines7040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Edens C., Collins M.L., Ayers J., Rota P.A., Prausnitz M.R. Measles vaccination using a microneedle patch. Vaccine. 2013;31:3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.van der Maaden K., Sekerdag E., Schipper P., Kersten G., Jiskoot W., Bouwstra J. Layer-by-layer assembly of inactivated poliovirus and N-trimethyl chitosan on pH-sensitive microneedles for dermal vaccination. Langmuir. 2015;31:8654–8660. doi: 10.1021/acs.langmuir.5b01262. [DOI] [PubMed] [Google Scholar]
- 293.Muller D.A., Pearson F.E., Fernando G.J.P., Agyei-Yeboah C., Owens N.S., Corrie S.R., Crichton M.L., Wei J.C., Weldon W.C., Oberste M.S., Young P.R., Kendall M.A.F. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci. Rep. 2016;6:22094. doi: 10.1038/srep22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.DeMuth P.C., Li A.V., Abbink P., Liu J., Li H., Stanley K.A., Smith K.M., Lavine C.L., Seaman M.S., Kramer J.A., Miller A.D., Abraham W., Suh H., Elkhader J., Hammond P.T., Barouch D.H., Irvine D.J. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat. Biotechnol. 2013;31:1082–1085. doi: 10.1038/nbt.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.DeMuth P.C., Min Y., Huang B., Kramer J.A., Miller A.D., Barouch D.H., Hammond P.T., Irvine D.J. Polymer multilayer tattooing for enhanced DNA vaccination. Nat. Mater. 2013;12:367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Andrianov A.K., DeCollibus D.P., Gillis H.A., Kha H.H., Marin A., Prausnitz M.R., Babiuk L.A., Townsend H., Mutwiri G. Poly [di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Corbett H.J., Fernando G.J.P., Chen X., Frazer I.H., Kendall M.A.F. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5:e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kines R.C., Zarnitsyn V., Johnson T.R., Pang Y.Y.S., Corbett K.S., Nicewonger J.D., Gangopadhyay A., Chen M., Liu J., Prausnitz M.R., Schiller J.T., Graham B.S. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Prow T.W., Chen X., Prow N.A., Fernando G.J.P., Tan C.S., Raphael A.P., Chang D., Ruutu M.P., Jenkins D.W., Pyke A., Crichton M.L., Raphaelli K., Goh L.Y.H., Frazer I.H., Roberts M.S., Gardner J., Khromykh A.A., Suhrbier A., Hall R.A., Kendall M.A.F. Nanopatch-targeted skin vaccination against West Nile virus and chikungunya virus in mice. Small. 2010;6:1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 300.Chen X., Kask A.S., Crichton M.L., McNeilly C., Yukiko S., Dong L., Marshak J.O., Jarrahian C., Fernando G.J.P., Chen D., Koelle D.M., Kendall M.A.F. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Control. Release. 2010;148:327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 301.Kask A.S., Chen X., Marshak J.O., Dong L., Saracino M., Chen D., Jarrahian C., Kendall M.A., Koelle D.M. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010;28:7483–7491. doi: 10.1016/j.vaccine.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 302.Gill H.S., Söderholm J., Prausnitz M.R., Sällberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Liu Y., Ye L., Lin F., Gomaa Y., Flyer D., Carrion R., Patterson J.L., Prausnitz M.R., Smith G., Glenn G., Wu H., Compans R.W., Yang C. Intradermal immunization by Ebola virus GP subunit vaccines using microneedle patches protects mice against lethal EBOV challenge. Sci. Rep. 2018;8:11193. doi: 10.1038/s41598-018-29135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kommareddy S., Baudner B.C., Bonificio A., Gallorini S., Palladino G., Determan A.S., Dohmeier D.M., Kroells K.D., Sternjohn J.R., Singh M., Dormitzer P.R., Hansenc K.J., O’Hagan D.T. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in Guinea pigs. Vaccine. 2013;31:3435–3441. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 305.Shin J.H., Park J.K., Lee D.H., Quan F.S., Song C.S., Kim Y.C. Microneedle vaccination elicits superior protection and antibody response over intranasal vaccination against swine-origin influenza a (H1N1) in mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ogai N., Nonaka I., Toda Y., Ono T., Minegishi S., Inou A., Hachiya M., Fukamizu H. Enhanced immunity in intradermal vaccination by novel hollow microneedles. Skin Res. Technol. 2018;24:630–635. doi: 10.1111/srt.12576. [DOI] [PubMed] [Google Scholar]
- 307.van der Maaden K., Heuts J., Camps M., Pontier M., van Scheltinga A.T., Jiskoot W., Ossendorp F., Bouwstra J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release. 2018;269:347–354. doi: 10.1016/j.jconrel.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 308.van der Maaden K., Trietsch S.J., Kraan H., Varypataki E.M., Romeijn S., Zwier R., van der Linden H.J., Kersten G., Hankemeier T., Jiskoot W., Bouwstra J. Novel hollow microneedle technology for depth-controlled microinjection-mediated dermal vaccination: a study with polio vaccine in rats. Pharm. Res. 2014;31:1846–1854. doi: 10.1007/s11095-013-1288-9. [DOI] [PubMed] [Google Scholar]
- 309.Schipper P., van der Maaden K., Romeijn S., Oomens C., Kersten G., Jiskoot W., Bouwstra J. Repeated fractional intradermal dosing of an inactivated polio vaccine by a single hollow microneedle leads to superior immune responses. J. Control. Release. 2016;242:141–147. doi: 10.1016/j.jconrel.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 310.Pamornpathomkul B., Niyomtham N., Yingyongnarongkul B.E., Prasitpuriprecha C., Rojanarata T., Ngawhirunpat T., Opanasopit P. Cationic niosomes for enhanced skin immunization of plasmid DNA-encoding ovalbumin via hollow microneedles. AAPS PharmSciTech. 2018;19:481–488. doi: 10.1208/s12249-017-0855-5. [DOI] [PubMed] [Google Scholar]
- 311.Nakatsukasa A., Kuruma K., Okamatsu M., Hiono T., Suzuki M., Matsuno K., Kida H., Oyamada T., Sakoda Y. Potency of whole virus particle and split virion vaccines using dissolving microneedle against challenges of H1N1 and H5N1 influenza viruses in mice. Vaccine. 2017;35:2855–2861. doi: 10.1016/j.vaccine.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 312.Raphael A.P., Prow T.W., Crichton M.L., Chen X., Fernando G.J.P., Kendall M.A.F. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6:1785–1793. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 313.Zhu W., Pewin W., Wang C., Luo Y., Gonzalez G.X., Mohan T., Prausnitz M.R., Wang B.Z. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J. Control. Release. 2017;261:1–9. doi: 10.1016/j.jconrel.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Vassilieva E.V., Kalluri H., McAllister D., Taherbhai M.T., Esser E.S., Pewin W.P., Pulit-Penaloza J.A., Prausnitz M.R., Compans R.W., Skountzou I. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv. Transl. Res. 2015;5:360–371. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wang J., Li B., Wu M.X. Effective and lesion-free cutaneous influenza vaccination. Proc. Natl. Acad. Sci. U. S. A. 2015;112:5005–5010. doi: 10.1073/pnas.1500408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sullivan S.P., Koutsonanos D.G., del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Vrdoljak A., Allen E.A., Ferrara F., Temperton N.J., Crean A.M., Moore A.C. Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods. J. Control. Release. 2016;225:192–204. doi: 10.1016/j.jconrel.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 318.Kommareddy S., Baudner B.C., Oh S., Kwon S.Y., Singh M., O’hagan D.T. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J. Pharm. Sci. 2012;101:1021–1027. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 319.Poirier D., Renaud F., Dewar V., Strodiot L., Wauters F., Janimak J., Shimada T., Nomura T., Kabata K., Kuruma K., Kusano T., Sakai M., Nagasaki H., Oyamada T. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials. 2017;145:256–265. doi: 10.1016/j.biomaterials.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 320.Qiu Y., Guo L., Zhang S., Xu B., Gao Y., Hu Y., Hou J., Bai B., Shen H., Mao P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. 2016;23:2391–2398. doi: 10.3109/10717544.2014.992497. [DOI] [PubMed] [Google Scholar]
- 321.Perez-Cuevas M.B., Kodani M., Choi Y., Joyce J., O’Connor S.M., Kamili S., Prausnitz M.R. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng. Transl. Med. 2018;3:186–196. doi: 10.1002/btm2.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pattani A., McKay P.F., Garland M.J., Curran R.M., Migalska K., Cassidy C.M., Malcolm R.K., Shattock R.J., McCarthy H.O., Donnelly R.F. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J. Control. Release. 2012;162:529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bachy V., Hervouet C., Becker P.D., Chorro L., Carlin L.M., Herath S., Papagatsias T., Barbaroux J.B., Oh S.J., Benlahrech A., Athanasopoulos T., Dickson G., Patterson S., Kwon S.Y., Geissmann F., Klavinskis L.S. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3041–3046. doi: 10.1073/pnas.1214449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zaric M., Becker P.D., Hervouet C., Kalcheva P., Yus B.I., Cocita C., O’Neill L.A., Kwon S.Y., Klavinskis L.S. Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored microneedle arrays. J. Control. Release. 2017;268:166–175. doi: 10.1016/j.jconrel.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zaric M., Becker P.D., Hervouet C., Kalcheva P., Doszpoly A., Blattman N., O’Neill L.A., Yus B.I., Cocita C., Kwon S.Y., Baker A.H., Lord G.M., Klavinskis L.S. Skin immunisation activates an innate lymphoid cell-monocyte axis regulating CD8+ effector recruitment to mucosal tissues. Nat. Commun. 2019;10:2214. doi: 10.1038/s41467-019-09969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Edens C., Collins M.L., Goodson J.L., Rota P.A., Prausnitz M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33:4712–4718. doi: 10.1016/j.vaccine.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Joyce J.C., Carroll T.D., Collins M.L., Chen M.H., Fritts L., Dutra J.C., Rourke T.L., Goodson J.L., McChesney M.B., Prausnitz M.R., Rota P.A. A microneedle patch for measles and rubella vaccination is immunogenic and protective in infant rhesus macaques. J. Infect. Dis. 2018;218:124–132. doi: 10.1093/infdis/jiy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Arya J.M., Dewitt K., Scott-Garrard M., Chiang Y.W., Prausnitz M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control. Release. 2016;239:19–26. doi: 10.1016/j.jconrel.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 329.Edens C., Dybdahl-Sissoko N.C., Weldon W.C., Oberste M.S., Prausnitz M.R. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine. 2015;33:4683–4690. doi: 10.1016/j.vaccine.2015.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Donadei A., Kraan H., Ophorst O., Flynn O., O'Mahony C., Soema P.C., Moore A.C. Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. J. Control. Release. 2019;311:96–103. doi: 10.1016/j.jconrel.2019.08.039. [DOI] [PubMed] [Google Scholar]
- 331.Turvey M.E., Uppu D.S.S.M., Mohamed Sharif A.R., Bidet K., Alonso S., Ooi E.E., Hammond P.T. Microneedle-based intradermal delivery of stabilized dengue virus. Bioeng. Transl. Med. 2019;4 doi: 10.1002/btm2.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yang H.W., Ye L., Guo X.D., Yang C., Compans R.W., Prausnitz M.R. Ebola vaccination using a DNA vaccine coated on PLGA-PLL/γPGA nanoparticles administered using a microneedle patch. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201600750. [DOI] [PubMed] [Google Scholar]
- 333.Christiansen D., Earnest-Silveira L., Grubor-Bauk B., Wijesundara D.K., Boo I., Ramsland P.A., Vincan E., Drummer H.E., Gowans E.J., Torresi J. Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-45461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Resch T.K., Wang Y., Moon S.S., Joyce J., Li S., Prausnitz M., Jiang B. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. Sci. Rep. 2018;8 doi: 10.1038/s41598-017-18973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kim E., Erdos G., Huang S., Kenniston T., Falo L.D., Jr., Gambotto A. Preventative vaccines for Zika virus outbreak: preliminary evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Beaver J.T., Mills L.K., Swieboda D., Lelutiu N., Esser E.S., Antao O.Q., Scountzou E., Williams D.T., Papaioannou N., Littauer E.Q., Romanyuk A., Compans R.W., Prausnitz M.R., Skountzou I. Cutaneous vaccination ameliorates Zika virus-induced neuro-ocular pathology via reduction of anti-ganglioside antibodies. Hum. Vaccin. Immunother. 2020;16:2072–2091. doi: 10.1080/21645515.2020.1775460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Bonificio A., Ghartey-Tagoe E., Gallorini S., Baudner B., Chen G., Singh P., O’Hagan D.T., Kommareddy S. Fabrication of cell culture-derived influenza vaccine dissolvable microstructures and evaluation of immunogenicity in guinea pigs. Vaccine. 2015;33:2930–2938. doi: 10.1016/j.vaccine.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 338.Choi I.J., Kang A., Ahn M.H., Jun H., Baek S.K., Park J.H., Na W., Choi S.O. Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J. Control. Release. 2018;286:460–466. doi: 10.1016/j.jconrel.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 339.Joyce J.C., Sella H.E., Jost H., Mistilis M.J., Esser E.S., Pradhan P., Toy R., Collins M.L., Rota P.A., Roy K., Skountzou I., Compans R.W., Oberste M.S., Weldon W.C., Norman J.J., Prausnitz M.R. Extended delivery of vaccines to the skin improves immune responses. J. Control. Release. 2019;304:135–145. doi: 10.1016/j.jconrel.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Tam H.H., Melo M.B., Kang M., Pelet J.M., Ruda V.M., Foley M.H., Hu J.K., Kumari S., Crampton J., Baldeon A.D., Sanders R.W., Moore J.P., Crotty S., Langer R., Anderson D.G., Chakraborty A.K., Irvine D.J. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Boopathy A.V., Mandal A., Kulp D.W., Menis S., Bennett N.R., Watkins H.C., Wang W., Martin J.T., Thai N.T., He Y., Schief W.R., Hammond P.T., Irvine D.J. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. U. S. A. 2019;116:16473–16478. doi: 10.1073/pnas.1902179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chen Y.H., Lai K.Y., Chiu Y.H., Wu Y.W., Shiau A.L., Chen M.C. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019;97:230–238. doi: 10.1016/j.actbio.2019.07.048. [DOI] [PubMed] [Google Scholar]
- 343.Chen M.C., Lai K.Y., Ling M.H., Lin C.W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018;65:66–75. doi: 10.1016/j.actbio.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 344.DeMuth P.C., Min Y., Irvine D.J., Hammond P.T. Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Adv. Healthc. Mater. 2014;3:47–58. doi: 10.1002/adhm.201300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Hirschberg H., van Kuijk S., Loch J., Jiskoot W., Bouwstra J., Kersten G., Amorij J.P. A combined approach of vesicle formulations and microneedle arrays for transcutaneous immunization against hepatitis B virus. Eur. J. Pharm. Sci. 2012;46:1–7. doi: 10.1016/j.ejps.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 346.Vreman S., McCaffrey J., Popma-de Graaf D.J., Nauwynck H., Savelkoul H.F.J., Moore A., Rebel J.M.J., Stockhofe-Zurwieden N. Toll-like receptor agonists as adjuvants for inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccine. Vet. Immunol. Immunopathol. 2019;212:27–37. doi: 10.1016/j.vetimm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 347.Zhou Q., Wang F., Yang F., Wang Y., Zhang X., Sun S. Augmented humoral and cellular immune response of hepatitis B virus DNA vaccine by micro-needle vaccination using Flt3L as an adjuvant. Vaccine. 2010;28:1357–1362. doi: 10.1016/j.vaccine.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 348.Shin J.H., Lee J.H., Jeong S.D., Noh J.Y., Lee H.W., Song C.S., Kim Y.C. C-di-GMP with influenza vaccine showed enhanced and shifted immune responses in microneedle vaccination in the skin. Drug Deliv. Transl. Res. 2020;10:815–825. doi: 10.1007/s13346-020-00728-1. [DOI] [PubMed] [Google Scholar]
- 349.Zhao J.H., Zhang Q.B., Liu B., Piao X.H., Yan Y.L., Hu X.G., Zhou K., Zhang Y.T., Feng N.P. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int. J. Nanomedicine. 2017;12:4763–4772. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Fernando G.J.P., Chen X., Primiero C.A., Yukiko S.R., Fairmaid E.J., Corbett H.J., Frazer I.H., Brown L.E., Kendall M.A.F. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control. Release. 2012;159:215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 351.Weldon W.C., Zarnitsyn V.G., Esser E.S., Taherbhai M.T., Koutsonanos D.G., Vassilieva E.V., Skountzou I., Prausnitz M.R., Compans R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Erdos G., Balmert S.C., Carey C.D., Falo G.D., Patel N.A., Zhang J., Gambotto A., Korkmaz E., Falo L.D., Jr. Improved cutaneous genetic immunization by microneedle array delivery of an adjuvanted adenovirus vaccine. J. Invest. Dermatol. 2020;140:2528–2531. doi: 10.1016/j.jid.2020.03.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Depelsenaire A.C.I., Meliga S.C., McNeilly C.L., Pearson F.E., Coffey J.W., Haigh O.L., Flaim C.J., Frazer I.H., Kendall M.A.F. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J. Invest. Dermatol. 2014;134:2361–2370. doi: 10.1038/jid.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ng H.I., Tuong Z.K., Fernando G.J.P., Depelsenaire A.C.I., Meliga S.C., Frazer I.H., Kendall M.A.F. Microprojection arrays applied to skin generate mechanical stress, induce an inflammatory transcriptome and cell death, and improve vaccine-induced immune responses. NPJ Vaccines. 2019;4:41. doi: 10.1038/s41541-019-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Deng L., Chang T.Z., Wang Y., Li S., Wang S., Matsuyama S., Yu G., Compans R.W., Li J.D., Prausnitz M.R., Champion J.A., Wang B.Z. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E7758–E7767. doi: 10.1073/pnas.1805713115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zaric M., Lyubomska O., Poux C., Hanna M.L., McCrudden M.T., Malissen B., Ingram R.J., Power U.F., Scott C.J., Donnelly R.F., Kissenpfennig A. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J. Invest. Dermatol. 2015;135:425–434. doi: 10.1038/jid.2014.415. [DOI] [PubMed] [Google Scholar]
- 358.Li Z., He Y., Deng L., Zhang Z.R., Lin Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice. J. Mater. Chem. B. 2020;8:216–225. doi: 10.1039/c9tb02061f. [DOI] [PubMed] [Google Scholar]
- 359.Niu L., Chu L.Y., Burton S.A., Hansen K.J., Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 360.Rodgers A.M., Cordeiro A.S., Kissenpfennig A., Donnelly R.F. Microneedle arrays for vaccine delivery: the possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin. Drug Del. 2018;15:851–867. doi: 10.1080/17425247.2018.1505860. [DOI] [PubMed] [Google Scholar]
- 361.Du G., Hathout R.M., Nasr M., Nejadnik M.R., Tu J., Koning R.I., Koster A.J., Slütter B., Kros A., Jiskoot W., Bouwstra J.A., Mönkäre J. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 362.DeMuth P.C., Moon J.J., Suh H., Hammond P.T., Irvine D.J. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zaric M., Lyubomska O., Touzelet O., Poux C., Al-Zahrani S., Fay F., Wallace L., Terhorst D., Malissen B., Henri S., Power U.F., Scott C.J., Donnelly R.F., Kissenpfennig A. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7:2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Siddhapura K., Harde H., Jain S. Immunostimulatory effect of tetanus toxoid loaded chitosan nanoparticles following microneedles assisted immunization. Nanomedicine. 2016;12:213–222. doi: 10.1016/j.nano.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 365.Sahdev P., Ochyl L.J., Moon J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014;31:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Irvine D.J., Hanson M.C., Rakhra K., Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Medhi R., Srinoi P., Ngo N., Tran H.V., Lee T.R. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020;3:8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 368.Fernando G.J.P., Hickling J., Flores C.M.J., Griffin P., Anderson C.D., Skinner S.R., Davies C., Witham K., Pryor M., Bodle J., Rockman S., Frazer I.H., Forster A.H. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (NanopatchTM) Vaccine. 2018;36:3779–3788. doi: 10.1016/j.vaccine.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 369.Levin Y., Kochba E., Hung I., Kenney R. Intradermal vaccination using the novel microneedle device MicronJet600: past, present, and future. Hum. Vaccin. Immunother. 2015;11:991–997. doi: 10.1080/21645515.2015.1010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Hirobe S., Azukizawa H., Hanafusa T., Matsuo K., Quan Y.S., Kamiyama F., Katayama I., Okada N., Nakagawa S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–58. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 371.Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., Pewin W., Frew P.M., Yu T., Thornburg N.J., Kabbani S., Lai L., Vassilieva E.V., Skountzou I., Compans R.W., Mulligan M.J., Prausnitz M.R. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390:649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Vescovo P., Rettby N., Ramaniraka N., Liberman J., Hart K., Cachemaille A., Piveteau L.D., Zanoni R., Bart P.A., Pantaleo G. Safety, tolerability and efficacy of intradermal rabies immunization with DebioJectTM. Vaccine. 2017;35:1782–1788. doi: 10.1016/j.vaccine.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 373.Van Mulder T., Withanage K., Beyers K., Vankerckhoven V., Theeten H., Van Damme P. Immunogenicity and safety of intradermal delivery of hepatitis B booster vaccine using the novel drug delivery device VAX-ID™. Vaccine. 2019;37:581–586. doi: 10.1016/j.vaccine.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 374.Anand A., Zaman K., Estívariz C.F., Yunus M., Gary H.E., Weldon W.C., Bari T.I., Oberste M.S., Wassilak S.G., Luby S.P., Heffelfinger J.D., Pallansch M.A. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine. 2015;33:6816–6822. doi: 10.1016/j.vaccine.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Beals C.R., Railkar R.A., Schaeffer A.K., Levin Y., Kochba E., Meyer B.K., Evans R.K., Sheldon E.A., Lasseter K., Lang N., Weinberg A., Canniff J., Levin M.J. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella-zoster virus vaccine: An exploratory, randomised, partly blinded trial. Lancet Infect. Dis. 2016;16:915–922. doi: 10.1016/S1473-3099(16)00133-X. [DOI] [PubMed] [Google Scholar]
- 376.Della Cioppa G., Nicolay U., Lindert K., Leroux-Roels G., Clement F., Castellino F., Galli C., Groth N., Levin Y., Del Giudice G. A dose-ranging study in older adults to compare the safety and immunogenicity profiles of MF59®-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum. Vaccin. Immunother. 2014;10:1701–1710. doi: 10.4161/hv.28618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Van Damme P., Oosterhuis-Kafeja F., Van der Wielen M., Almagor Y., Sharon O., Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 378.Troy S.B., Kouiavskaia D., Siik J., Kochba E., Beydoun H., Mirochnitchenko O., Levin Y., Khardori N., Chumakov K., Maldonado Y. Comparison of the immunogenicity of various booster doses of inactivated polio vaccine delivered intradermally versus intramuscularly to HIV-infected adults. J. Infect. Dis. 2015;211:1969–1976. doi: 10.1093/infdis/jiu841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hung I.F., Levin Y., K.K. To, Chan K.H., Zhang A.J., Li P., Li C., Xu T., Wong T.Y., Yuen K.Y. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine. 2012;30:6427–6435. doi: 10.1016/j.vaccine.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 380.Levin Y., Kochba E., Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine. 2014;32:4249–4252. doi: 10.1016/j.vaccine.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 381.Levin Y., Kochba E., Shukarev G., Rusch S., Herrera-Taracena G., van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine. 2016;34:5262–5272. doi: 10.1016/j.vaccine.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 382.Donnelly R.F., Singh T.R.R., Tunney M.M., Morrow D.I.J., McCarron P.A., O’Mahony C., Woolfson A.D. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009;26:2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.García L.E.G., MacGregor M.N., Visalakshan R.M., Ninan N., Cavallaro A.A., Trinidad A.D., Zhao Y., Hayball A.J.D., Vasilev K. Self-sterilizing antibacterial silver-loaded microneedles. ChemComm. 2019;55:171–174. doi: 10.1039/c8cc06035e. [DOI] [PubMed] [Google Scholar]
- 384.Norman J.J., Arya J.M., McClain M.A., Frew P.M., Meltzer M.I., Prausnitz M.R. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Vicente-Pérez E.M., Quinn H.L., McAlister E., O’Neill S., Hanna L.A., Barry J.G., Donnelly R.F. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm. Res. 2016;33:3072–3080. doi: 10.1007/s11095-016-2032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Arya J., Henry S., Kalluri H., McAllister D.V., Pewin W.P., Prausnitz M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Frew P.M., Paine M.B., Rouphael N., Schamel J., Chung Y., Mulligan M.J., Prausnitz M.R. Acceptability of an inactivated influenza vaccine delivered by microneedle patch: results from a phase I clinical trial of safety, reactogenicity, and immunogenicity. Vaccine. 2020;38:7175–7181. doi: 10.1016/j.vaccine.2020.07.064. [DOI] [PubMed] [Google Scholar]
- 388.Adhikari B.B., Goodson J.L., Chu S.Y., Rota P.A., Meltzer M.I. Assessing the potential cost-effectiveness of microneedle patches in childhood measles vaccination programs: the case for further research and development. Drugs R&D. 2016;16:327–338. doi: 10.1007/s40268-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Walters A.A., Krastev C., Hill A.V.S., Milicic A. Next generation vaccines: single-dose encapsulated vaccines for improved global immunisation coverage and efficacy. J. Pharm. Pharmacol. 2015;67:400–408. doi: 10.1111/jphp.12367. [DOI] [PubMed] [Google Scholar]
- 390.Tzeng S.Y., Guarecuco R., McHugh K.J., Rose S., Rosenberg E.M., Zeng Y., Langer R., Jaklenec A. Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release. J. Control. Release. 2016;233:101–113. doi: 10.1016/j.jconrel.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Guarecuco R., Lu J., McHugh K.J., Norman J.J., Thapa L.S., Lydon E., Langer R., Jaklenec A. Immunogenicity of pulsatile-release PLGA microspheres for single-injection vaccination. Vaccine. 2018;36:3161–3168. doi: 10.1016/j.vaccine.2017.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Tzeng S.Y., McHugh K.J., Behrens A.M., Rose S., Sugarman J.L., Ferber S., Langer R., Jaklenec A. Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E5269–E5278. doi: 10.1073/pnas.1720970115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.McHugh K.J., Nguyen T.D., Linehan A.R., Yang D., Behrens A.M., Rose S., Tochka Z.L., Tzeng S.Y., Norman J.J., Anselmo A.C., Xu X., Tomasic S., Taylor M.A., Lu J., Guarecuco R., Langer R., Jaklenec A. Fabrication of fillable microparticles and other complex 3D microstructures. Science. 2017;357:1138–1142. doi: 10.1126/science.aaf7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bailey B.A., Ochyl L.J., Schwendeman S.P., Moon J.J. Toward a single-dose vaccination strategy with self-encapsulating PLGA microspheres. Adv. Healthc. Mater. 2017;6:1601418. doi: 10.1002/adhm.201601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mazzara J.M., Ochyl L.J., Hong J.K., Moon J.J., Prausnitz M.R., Schwendeman S.P. Self-healing encapsulation and controlled release of vaccine antigens from PLGA microparticles delivered by microneedle patches. Bioeng. Transl. Med. 2019;4:116–128. doi: 10.1002/btm2.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Chiu Y.H., Chen M.C., Wan S.W. Sodium hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system. Biomacromolecules. 2018;19:2278–2285. doi: 10.1021/acs.biomac.8b00441. [DOI] [PubMed] [Google Scholar]
- 397.Kim J.S., Choi J., Kim J.C., Park H., Yang E., Park J.S., Song M., Park J.H. Microneedles with dual release pattern for improved immunological efficacy of hepatitis B vaccine. Int. J. Pharm. 2020;591:119928. doi: 10.1016/j.ijpharm.2020.119928. [DOI] [PubMed] [Google Scholar]