Abstract
Objectives
This study aims to compare the treatment outcomes of periodontal intrabony defects by using platelet-rich fibrin (PRF) with other commonly utilized modalities.
Materials and methods
The eligibility criteria comprised randomized controlled trials (RCTs) comparing the clinical outcomes of PRF with that of other modalities. Studies were classified into 10 categories as follows: (1) open flap debridement (OFD) alone versus OFD/PRF; (2) OFD/bone graft (OFD/BG) versus OFD/PRF; (3) OFD/BG versus OFD/BG/PRF; (4–6) OFD/barrier membrane (BM), OFD/PRP, or OFD/enamel matrix derivative (EMD) versus OFD/PRF; (7) OFD/EMD versus OFD/EMD/PRF; (8–10) OFD/PRF versus OFD/PRF/metformin, OFD/PRF/bisphosphonates, or OFD/PRF/statins. Weighted means and forest plots were calculated for probing depth (PD), clinical attachment level (CAL), and radiographic bone fill (RBF).
Results
From 551 articles identified, 27 RCTs were included. The use of OFD/PRF statistically significantly reduced PD and improved CAL and RBF when compared to OFD. No clinically significant differences were reported when OFD/BG was compared to OFD/PRF. The addition of PRF to OFD/BG led to significant improvements in CAL and RBF. No differences were reported between any of the following groups (OFD/BM, OFD/PRP, and OFD/EMD) when compared to OFD/PRF. No improvements were also reported when PRF was added to OFD/EMD. The addition of all three of the following biomolecules (metformin, bisphosphonates, and statins) to OFD/PRF led to statistically significant improvements of PD, CAL, and RBF.
Conclusions
The use of PRF significantly improved clinical outcomes in intrabony defects when compared to OFD alone with similar levels being observed between OFD/BG and OFD/PRF. Future research geared toward better understanding potential ways to enhance the regenerative properties of PRF with various small biomolecules may prove valuable for future clinical applications. Future research investigating PRF at histological level is also needed.
Clinical relevance
The use of PRF in conjunction with OFD statistically significantly improved PD, CAL, and RBF values, yielding to comparable outcomes to OFD/BG. The combination of PRF with bone grafts or small biomolecules may offer certain clinical advantages, thus warranting further investigations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00784-021-03825-8.
Keywords: Intrabony defect, Platelet-rich fibrin, L-PRF, Advanced-PRF
Introduction
Periodontal disease is one of the most prevalent chronic diseases known to man that begins as a superficial inflammatory response of the gingiva (gingivitis) and later progresses to attachment loss with subsequent destruction of the tooth-supporting structures (periodontitis) [1–4]. Results investigating the distribution of the disease from a national survey conducted in the USA found that over 47% of the adult population was affected with 38.5% of the population having either moderate or severe cases (stage III or stage IV) [5]. This finding is most alarming as the disease is characterized with an exponentially more difficult resolution and regeneration once advanced progression has taken place.
Treatment of periodontal disease is therefore of utmost importance since epidemic studies have linked periodontitis to a number of systemic diseases including cardiovascular diseases (heart attack/stroke), Alzheimer’s, diabetes, obesity, and premature births, among others [6]. It therefore becomes vital to correct the disease as early as possible and halt disease progression and utilize strategies to promote their regeneration [7–9].
True and complete periodontal regeneration is complex since it consists of a complex interaction of epithelium, gingival connective tissue, periodontal ligament, and alveolar bone [1]. True periodontal regeneration should also include Sharpey’s fibers spanning from the cementum through the periodontal ligament (PDL) and into the alveolar bundle bone [1]. To date, many attempts utilizing various strategies including bone grafts, barrier membranes, and biologic agents have been proposed, yet to date complete periodontal regeneration remains very challenging and unpredictable [1].
One strategy that was proposed several years ago for the regeneration of intrabony defects was the use of platelet concentrates [10]. While platelet-rich plasma (PRP) was proposed as a first-generation platelet concentrate, the use of anticoagulants has since been shown to interfere with the angiogenic and regenerative responses mediated by platelets [11]. For these reasons, a second-generation platelet concentrate, termed platelet-rich fibrin (PRF), has more been introduced in regenerative medicine and dentistry [10, 12–15].
Since PRF was first launched more than two decades ago in regenerative medicine, its use has gained widespread acceptance across many fields of medicine including for periodontal regeneration where nearly 40 randomized clinical trials (RCTs) have investigated its regenerative potential. One of the advantages of PRF is that following centrifugation, it forms a fibrin-dense clot with host platelets and leukocytes being entrapped favoring a more extended release of growth factors over time [16, 17]. A number of systematic reviews (SRs) have thoroughly documented the use of PRF in regenerative dentistry, where it has been shown to particularly favor soft tissue healing over hard tissue healing [10, 18, 19]. The aim of this systematic review with meta-analysis was to evaluate the current evidence regarding the use of PRF for the treatment of both intrabony defects in comparison to other treatment options including bone grafts, barrier membranes, enamel matrix derivative (EMD), and a number of other biomolecules commonly utilized for periodontal regeneration.
Materials and methods
Protocol
This SR followed the recommendations of the PRISMA guidelines [20]. The protocol for this SR was based on PRISMA-P [21]. There were no deviations from the initial protocol.
Focused question
What is the effectiveness of PRF for the treatment of periodontal two- and three-walled intrabony defects?
Eligibility criteria and study selection process
The inclusion criteria were based on the PICOS strategy highlighted below [22]. The search-and-screening process was conducted by two independent reviewing authors (R.J.M and V.M.), commencing with the analysis of titles and abstracts. Next, full papers were selected for careful reading and matched with the eligibility criteria for future data extraction. Disagreements between the reviewing authors were resolved through careful discussion. Only studies meeting the following criteria were included:
Population: Systemically healthy humans with periodontal intrabony defects (two or three walls).
Intervention: Surgical treatment of bone defects through the use of PRF alone or in combination with other biomaterials with a follow-up period of at least 6 months.
Comparison: PRF versus open flap debridement (OFD) alone or in combination with other biomaterials.
Outcomes: The outcome variable and data collection included the change in pocket depth (PD), clinical attachment level (CAL), and radiographic bone fill (RBF).
Study design: RCTs with a minimum of 10 patients.
Search strategy
PubMed/MEDLINE, the Cochrane Central Register of Controlled Trials, Scopus, Embase, and Lilacs were used to search for articles that were published before June 2020 without other restrictions regarding date or language. A search of the gray literature using the Literature Report [23] and OpenGrey [24] databases was also conducted. Finally, the study reference lists were evaluated (cross-referenced) to identify other studies for potential inclusion. The search strategy is described in the Supplementary Appendix (S1). Retrospective clinical studies, case reports, or animal studies as well as follow-up of less than 6 months were excluded from the study.
Data synthesis
The study data were extracted by R.J.M. and M.F.K. and systematically reviewed by V.M. The following data, when available, were extracted from the included studies: authors, study design, follow-up, number of treated intrabony defects, type of bone defects, number of subjects, age range, gender, number of smokers, surgical technique, mean difference (mD) in PD, CAL, BF, centrifugation system, volume of blood drawn, and centrifugation parameters.
Assessments of the risk of bias
Two reviewing authors (V.M. and M.D.C.M.) analyzed the risk of bias. The RoB 2 (a revised Cochrane risk-of-bias tool for randomized trials) [25] was used to analyze the risk of bias in RCTs. Each study was analyzed in relation to five domains: risk of bias arising from the randomization process, risk of bias due to deviations from the intended interventions, missing outcome data, risk of bias in the measurement of the outcome, and risk of bias in the selection of the reported research. Studies were classified as having a low risk, some concerns, or high risks of bias for each domain. The overall risk of biased judgment used the following criteria: low risk, when the five areas of the study were judged as low risk; some concerns, when the study is judged as raising some concerns in at least one area; and high risk, when the study is judged to be at high risk in at least one domain or when the study is judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result.
Statistical analysis
The continuous variables (PD, CAL, and BF) of the included studies were categorized in groups and subgroups and analyzed in a meta-analysis through software Review Manager (version 5.2.8, Copenhagen, Denmark, 2014).
The estimates of the intervention effects were expressed as percentages or millimeters with 95% CIs. The inverse variance method was used for the random-effects or fixed-effects models, depending on the heterogeneity between the studies. The heterogeneity was assessed using χ2 tests. Values ≤ 25% were validated as low heterogeneity, while values > 25 < 50% was classified as moderate. Values ≥ 50% were classified as high heterogeneity [26]. The use of the random-effects model was conducted when heterogeneity was found (p < 0.10). In contrast, the fixed-effects model was used in the case of low or medium heterogeneity. The statistical significance level of the effect of meta-analysis was fixed in p < 0.05.
Results
Literature search
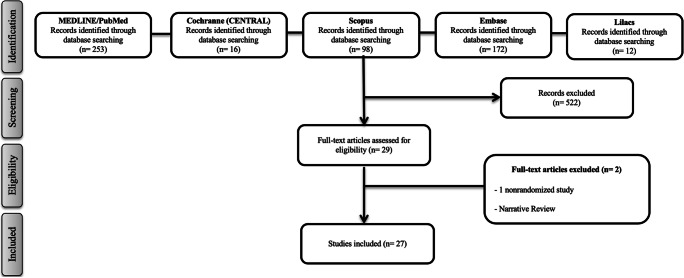
The process of the search and selection and the reasons for excluding potential studies are shown in Fig. 1. Twenty-seven studies on intrabony defects [27–53] published between 2011 and 2019 met the eligibility criteria and were included in this SR. Of the 27 RCTs, the most highly researched centrifugation system utilized in 15 of 27 studies (56% of studies) was the Remi centrifuge whereas the IntraSpin/Hettich PC-02 system was utilized in 1/27 studies (4% of studies). Of the 27 studies, 20/27 utilized 3000 rpm for 10-min protocol (74% of studies) whereas 2/27 studies utilized 2700 rpm for 12-min protocol (7% of studies). Only 2 of 27 studies included smokers into their study.
Fig. 1.
Flow diagram (PRISMA format) of the screening and selection process
Study characteristics
The included studies analyzed 1025 research participants. In addition to OFD alone, the effect of PRF was compared to other groups of biomaterials (autograft, allograft, xenograft, alloplast, barrier membrane, enamel matrix derivative (EMD), metformin, bisphosphonates, and statins). The mean follow-up period of the studies was 8.44 ± 2.04 months. The data extracted from each included study are presented in Table 1.
Table 1.
Main characteristics of the 27 RCTs included in the present study
| Authors (year) |
Study design Follow-up |
Number of participants Gender Mean age |
Groups | Bone defect type | Smokers (no, yes) | Conclusions |
| OFD vs. PRF | ||||||
| Sharma and Pradeep (2011) [48] |
RCT (parallel) 9 months |
42 ♂24/♀18 35.3 |
C: 28, OFD T: 28, OFD + PRF |
3 walls | No |
There was greater PD reduction, CAL gain, and bone fill at sites treated with PRF with OFD compared to OFD alone |
| Thorat et al. (2011) [50] |
RCT (parallel) 9 months |
32 ♂20/♀12 30.7 |
C: 16, OFD T: 16, OFD + PRF |
2 and 3 walls | No | There was greater reduction in PD, more CAL gain, and greater intrabony defect fill at sites treated with PRF than the OFD alone |
| Rosamma et al. (2012) [53] |
CT (split-mouth) 12 months |
15 ♂6/♀9 29.5 |
C: 15, OFD T: 15, OFD + PRF |
2 and 3 walls | No | The use of PRF was more effective than OFD alone in the management of IBDs |
| Ajwani et al. (2015) [28] |
RCT (split-mouth) 9 months |
20 ♂10/♀10 30.5 |
C: 20, OFD T: 20, OFD + PRF |
2 and 3 walls | No | Adjunctive use of PRF with OFD significantly improves defect fill when compared to OFD alone |
| Bajaj et al. (2017) [30] |
RCT (parallel) 9 months |
17 ♂9/♀8 29.7 |
C: 27, OFD T: 27, OFD + PRF |
2 and 3 walls | No | There was greater BF at sites treated with PRF with conventional OFD than conventional OFD alone |
| Patel et al. (2017) [41] |
RCT (split-mouth) 12 months |
13 ♂4/♀9 44 |
C: 13, OFD T: 13, OFD + PRF |
2 and 3 walls | No | The adjunctive use of PRF to conventional OFD may be potentially used in the treatment of IBDs |
| Pradeep et al. (2017) [42] |
RCT (parallel) 9 months |
62 ♂34/♀28 39.7 |
C: 18, OFD T1: 19, OFD + PRF T2: 20, OFD + PRF + HA |
3 walls | No | Treatment of IBD with PRF results in significant improvements of clinical parameters compared to baseline |
| Thorat et al. (2017) [49] |
RCT (split-mouth) 12 months |
15 ♂7/♀8 25 |
C: 15, OFD T: 15, OFD + PRF |
3 walls | NR | Use of PRF significantly enhances the clinical and radiographic outcomes of OFD in the treatment of IBDs |
| BG vs. PRF | ||||||
| Mathur et al. (2015) [38] |
RCT (parallel) 6 months |
25 ♂14/♀11 39.7 |
C: 19, OFD + ABG T: 19, OFD + PRF |
2 and 3 walls | No | The use of either PRF or ABG was effective in the treatment of IBDs |
| Shah et al. (2015) [47] |
RCT (split-mouth) 6 months |
20 NR NR |
C: 20, OFD + DFDBA T: 20, OFD + PRF |
2 and 3 walls | No | PRF has shown significant results after 6 months, which is comparable to DFDBA for periodontal regeneration |
| Chadwick et al. (2016) [32] |
RCT (parallel) 6 months |
36 ♂20/♀16 54.9 |
C: 19, OFD + DFDBA T: 17, OFD + PRF |
2 and 3 walls | Yes | Treatment of IBDs with either DFDBA or PRF resulted in a significant gain in CAL as well as BF after 6 months of healing, with no significant difference |
| Galav et al. (2016) [34] |
RCT (split-mouth) 9 months |
20 NR 45 |
C: 20, OFD + ABG T: 20, OFD + PRF |
2 and 3 walls | No | Both ABG and PRF can be used predictably to reconstruct lost periodontal structures |
| Yajamanya et al. (2017) [51] |
RCT (parallel) 9 months |
32 NR NR |
C: 28, OFD T1: 28, OFD + BioG T2: 28, OFD + PRF |
2 and 3 walls | No | This study shows marked improvements in the clinical parameters and radiographic outcomes with both BioG and PRF to treat periodontal IBDs as compared to OFD alone |
| BG vs. BG + PRF | ||||||
| Bansal and Bharti (2013) [52] |
RCT (split-mouth) 6 months |
10 NR NR |
C: 10, OFD + DFDBA T: 10, OFD + DFDBA + PRF |
NR | NR | There was a significantly greater PD reduction and CAL when PRF was added to DFDBA |
| Elgendy and Abo Shady (2015) [33] |
RCT (split-mouth) 6 months |
20 NR 44 |
C: 20, OFD + HA T: 20, OFD + HA + PRF |
NR | Yes | Both treatment groups showed a significant PD reduction and CAL gain 6 months after surgery. However, there was a significantly greater PD reduction and CAL gain when PRF was added to BG |
| Agarwal et al. (2016) [27] |
RCT (split-mouth) 12 months |
30 ♂15/♀15 52 |
C: 30, OFD + DFDBA T: 30, OFD + DFDBA/PRF |
2 and 3 walls | No | The combination of PRF and DFDBA is more effective than DFDBA alone |
| Naqvi et al. (2017) [39] |
RCT (split-mouth) 9 months |
10 ♂7/♀3 NR |
C: 10, OFD + BioG T: 10, OFD + BioG + PRF |
2 and 3 walls | No | The results of this study showed both groups BioG putty alone and the combination of PRF and BioG putty are effective in the treatment of IBDs |
| Sezgin et al. (2017) [46] |
RCT (split-mouth) 6 months |
15 ♂8/♀7 NR |
C: 15, OFD + ABBM T: 15, OFD + ABBM + PRF |
2 and 3 walls | No | The results of this study indicate that both therapies are effective in the treatment of intrabony defects |
| Bodhare et al. (2019) [31] |
RCT (split-mouth) 6 months |
20 ♂11/♀9 35.9 |
C: 20, OFD + BioGide T: 20, OFD + BioGide + PRF |
2 and 3 walls | No | BioG when used in combination with PRF is found to be more effective in gain in CAL, reduction in PD, and achieving greater bone fill as compared to treatment with BG alone |
| BM vs. PRF | ||||||
| Panda et al. (2016) [40] |
RCT (split-mouth) 9 months |
18 ♂10/♀8 38.1 |
C: 18, OFD + BM T: 18, OFD + BM + PRF |
3 walls | No | The adjunctive use of PRF in combination with BM is more effective in the treatment of IBDs in chronic periodontitis as compared with BM alone |
| PRP vs. PRF | ||||||
| Pradeep et al. (2012) [45] |
RCT (parallel) 9 months |
54 ♂27/♀27 36.8 |
C: 17, OFD T1: 17, OFD + PRP T2: 16, OFD + PRF |
3 walls | No | There was similar PD reduction, CAL gain, and BF at sites treated with PRF or PRP with conventional OFD |
| EMD vs. PRF | ||||||
| Gupta et al. (2014) [35] |
RCT (parallel) 6 months |
30 ♂15/♀15 NR |
C: 22, OFD + EMD T: 22, OFD + PRF |
3 walls | No | Both EMD and PRF were effective in the regeneration of IBDs |
| EMD vs. EMD + PRF | ||||||
| Aydemir Turkal et al. (2016) [29] |
RCT (split-mouth) 6 months |
28 ♂14/♀14 38.5 |
C: 24, OFD + EMD T: 25, OFD + EMD + PRF |
1, 2, and 3 walls | No | Addition of PRF did not improve the clinical and radiographic outcomes |
| PRF vs. PRF + metformin | ||||||
| Pradeep et al. (2015) [44] |
RCT (parallel) 9 months |
120 ♂60/♀60 41 |
C: 30, OFD T1: 30, OFD + 1% MF T2: 30, OFD + PRF T3: 30, OFD + 1% MF + PRF |
3 walls | No | The study showed that the PRF + 1% MF group was more effective than MF, PRF, or OFD alone in the management of IBDs |
| PRF vs. PRF + bisphosphonates | ||||||
| Kanoriya et al. 2016 [36] |
RCT (parallel) 9 months |
90 ♂43/♀47 40.3 |
C: 30, OFD T1: 30, OFD + PRF T2: 30, OFD + PRF/1% ALN |
3 walls | No | Combined approach therapy of PRF + 1% ALN for IBD treatment showed better clinical parameter outcomes compared with PRF and OFD alone |
| PRF vs. PRF + statins | ||||||
| Martande et al. (2016) [37] |
RCT (parallel) 9 months |
96 ♂48/♀48 37.6 |
C: 30, OFD T1: 30, OFD + PRF T2: 30, OFD + PRF + 1.2% ATV |
3 walls | No | PRF + 1.2% ATV showed similar improvements in clinical parameters with a greater percentage radiographic defect depth reduction compared with PRF alone in treatment of IBDs |
| Pradeep et al. (2016) [43] |
RCT (parallel) 9 months |
90 ♂45/♀45 35 |
C: 30, OFD T1: 30, OFD + PRF T2: 30, OFD + PRF + 1.2% RSV |
2 and 3 walls | No | OFD with RSV (1.2%) and PRF results in significantly greater periodontal benefits compared with OFD alone or with PRF |
| Methods for PRF preparation | ||||||
| Authors (year) | Mean difference in PD between baseline and final follow-up (mm) | Mean difference in CAL between baseline and final follow-up (mm) |
Mean difference in BF between baseline and final follow-up (mm) |
Centrifugation system | Volume of blood drawn (ml) |
Centrifugation parameters speed (rpm) × time (min) |
| OFD vs. PRF | ||||||
| Sharma and Pradeep (2011) [48] |
3.21 ± 1.64 (C) 4.55 ± 1.87 (T) |
2.77 ± 1.44 (C) 3.31 ± 1.76 (T) |
0.09 ± 0.11 (C) 2.50 ± 0.78 (T) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Thorat et al. (2011) [50] |
3.56 ± 1.09 (C) 4.69 ± 1.45 (T) |
2.13 ± 1.71 (C) 4.13 ± 1.63 (T) |
1.24 ± 0.69 (C) 2.12 ± 0.69 (T) |
NR | 10 | 2700 × 12 |
| Rosamma et al. (2012) |
2.40 ± 0.63 (C) 4.67 ± 0.90 (T) |
1.40 ± 1.06 (C) 4.73 ± 0.88 (T) |
0.64 ± 0.50 (C) 1.93 ± 1.07 (T) |
KW-70 (Almicro Instruments, Haryana, India) | 10 | 3000 × 10 |
| Ajwani et al. (2015) [28] |
1.60 ± 0.84 (C) 1.90 ± 0.74 (T) |
1.30 ± 0.68 (C) 1.80 ± 0.63 (T) |
0.80 ± 0.35 (C) 1.45 ± 0.50 (T) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Bajaj et al. (2017) [30] |
2.14 ± 1.26 (C) 3.14 ± 1.26 (T) |
1.59 ± 1.01 (C) 2.66 ± 1.07 (T) |
0.84 ± 0.99 (C) 2.24 ± 0.66 (T) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Patel et al. (2017) [41] |
2.40 ± 0.84 (C) 4.20 ± 1.69 (T) |
2.10 ± 0.74 (C) 3.70 ± 0.67 (T) |
NR | REMI-8C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Pradeep et al. (2017) [42] |
2.97 ± 0.93 (C) 3.90 ± 1.09 (T1) 4.27 ± 0.98 (T2) |
2.67 ± 1.09 (C) 3.03 ± 1.16 (T1) 3.67 ± 1.03 (T2 |
0.93 ± 0.83 (C) 3.20 ± 0.89 (T1) 3.87 ± 1.33 (T2) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Thorat et al. (2017) [49] |
1.50 ± 0.34 (C) 4.00 ± 0.63 (T) |
0.33 ± 1.21 (C) 4.00 ± 0.63 (T) |
1.67 ± 0.06 (C) 3.09 ± 0.50 (T) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 12 |
| BG vs. PRF | ||||||
| Mathur et al. (2015) [38] |
2.40 ± 1.06 (C) 2.67 ± 1.29 (T) |
2.67 ± 1.63 (C) 2.53 ± 1.06 (T) |
2.66 ± 1.84 (C) 2.93 ± 1.79 (T) |
R-4C (REMI, Mumbai, India) | NR | 3000 × 10 |
| Shah et al. (2015) [47] |
3.70 ± 0.68 (C) 3.67 ± 0.69 (T) |
2.97 ± 1.68 (C) 2.97 ± 1.56 (T) |
0.32 ± 1.59 (C) 0.42 ± 1.38 (T) |
NR | 10 | 3000 × 10 |
| Chadwick et al. (2016) [32] |
2.00 ± 1.37 (C) 2.12 ± 1.41 (T) |
1.16 ± 1.33 (C) 1.03 ± 0.86 (T) |
1.53 ± 1.64 (C) 1.35 ± 1.60 (T) |
Centrifuge 1310 (Salvin Dental Specialties, Charlotte, NC) | 10 | 3000 × 10 |
| Galav et al. (2016) [34] |
4.80 ± 0.57 (C) 4.10 ± 0.63 (T) |
4.50 ± 0.52 (C) 3.90 ± 0.37 (T) |
4.10 ± 0.47 (C) 4.59 ± 0.70 (T) |
NR | 10 | 3000 × 10 |
| Yajamanya et al. (2017) [51] |
3.68 ± 0.72 (C) 5.57 ± 1.10 (T1) 6.11 ± 0.92 (T2) |
4.14 ± 0.76 (C) 6.57 ± 1.45 (T1) 6.74 ± 1.55 (T2) |
NR | NR | 10 | 3000 × 10 |
| BG vs. BG + PRF | ||||||
| Bansal and Bharti (2013) [52] |
3.10 ± 0.74 (C) 4.00 ± 0.82 (T) |
2.30 ± 0.70 (C) 3.40 ± 0.60 (T) |
1.93 ± 1.21 (C) 2.13 ± 1.28 (T) |
NR | 10 | 3000 × 10 |
| Elgendy and Abo Shady (2015) [33] |
3.33 ± 0.36 (C) 3.30 ± 0.18 (T) |
3.55 ± 0.13 (C) 3.50 ± 0.06 (T) |
NR | NR | 10 | 3000 × 10 |
| Agarwal et al. (2016) [27] |
3.60 ± 0.51 (C) 4.15 ± 0.84 (T) |
2.61 ± 0.68 (C) 3.73 ± 0.74 (T) |
2.49 ± 0.64 (C) 3.50 ± 0.67 (T) |
NR | 10 | 400×g × 12 |
| Naqvi et al. (2017) [39] |
3.15 ± 1.06 (C) 3.20 ± 2.30 (T) |
3.15 ± 1.06 (C) 4.10 ± 1.73 (T) |
5.70 ± 1.37 (C) 7.10 ± 1.37 (T) |
NR | 10 | 3000 × 10 |
| Sezgin et al. (2017) [46] |
4.21 ± 1.21 (C) 4.93 ± 1.22 (T) |
3.27 ± 1.34 (C) 4.47 ± 1.60 (T) |
1.98 ± 0.80 (C) 2.55 ± 1.15 (T) |
PC-02 (Process, Nice, France) | 10 | 2700 × 12 |
| Bodhare et al. (2019) [31] |
5.65 ± 1.66 (C) 5.75 ± 1.16 (T) |
4.20 ± 1.70 (C) 5.05 ± 1.09 (T) |
2.56 ± 0.95 (C) 3.51 ± 1.17 (T) |
NR (REMI, Mumbai, India) | 10 | 3000 × 10 |
| BM vs. PRF | ||||||
| Panda et al. (2016) [40] |
3.19 ± 1.33 (C) 3.88 ± 1.15 (T) |
3.38 ± 1.45 (C) 4.44 ± 1.50 (T) |
0.80 ± 0.28 (C) 2.10 ± 0.64 (T) |
Model C-854/6 (REMI, Mumbai, India) | 5 | 3000 × 10 |
| PRP vs. PRF | ||||||
| Pradeep et al. (2012) [45] |
2.97 ± 0.93 (C) 3.77 ± 1.07 (T1) 3.77 ± 1.19 (T2) |
2.83 ± 0.91 (C) 2.93 ± 1.08 (T1) 3.17 ± 1.29 (T2) |
0.13 ± 1.46 (C) 2.70 ± 0.79 (T1) 2.80 ± 0.89 (T2) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| EMD vs. PRF | ||||||
| Gupta et al. (2014) [35] |
1.80 ± 0.56 (C) 1.80 ± 0.77 (T) |
2.00 ± 0.54 (C) 1.87 ± 0.91 (T) |
2.08 ± 0.78 (C) 1.67 ± 1.17 (T) |
NR (REMI, Mumbai, India) | 10 | 3000 × 12 |
| EMD vs. EMD + PRF | ||||||
| Aydemir Turkal et al. (2016) [29] |
3.88 ± 1.26 (C) 4.00 ± 1.38 (T) |
3.29 ± 1.30 (C) 3.42 ± 1.28 (T) |
1.21 ± 1.24 (C) 1.13 ± 0.83 (T) |
Mikro 22 R (Hettich, Tuttlingen, Germany) | 10 | 400×g × 10 |
| Metformin vs. PRF | ||||||
| Pradeep et al. (2015) [44] |
3.00 ± 0.18 (C) 3.93 ± 0.25 (T1) 4.00 ± 0.18 (T2) 4.90 ± 0.30 (T3) |
2.96 ± 0.18 (C) 3.93 ± 0.25 (T1) 4.03 ± 0.18 (T2) 4.90 ± 0.30 (T3) |
0.49 ± 0.27 (C) 2.56 ± 0.28 (T1) 2.53 ± 0.30 (T2) 2.77 ± 0.30 (T3) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Bisphosphonates vs. PRF | ||||||
| Kanoriya et al. 2016 [36] |
2.86 ± 0.68 (C) 3.70 ± 0.91 (T1) 4.53 ± 0.81 (T2) |
3.03 ± 0.18 (C) 4.20 ± 0.66 (T1) 5.16 ± 0.46 (T2) |
0.38 ± 0.26 (C) 2.42 ± 0.21 (T1) 2.84 ± 0.26 (T2) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
| Statins vs. PRF | ||||||
| Martande et al. (2016) [37] |
2.76 ± 1.43 (C) 3.76 ± 1.12 (T1) 4.06 ± 1.22 (T2) |
2.50 ± 1.33 (C) 3.40 ± 1.13 (T1) 3.66 ± 1.42 (T2) |
0.27 ± 0.19 (C) 2.46 ± 0.33 (T1) 2.58 ± 0.36 (T2) |
R-4C (REMI, Mumbai, India) | 5 | 3000 × 12 to 14 |
| Pradeep et al. (2016) [43] |
3.10 ± 0.30 (C) 4.03 ± 0.18 (T1) 4.90 ± 0.31 (T2) |
2.47 ± 0.77 (C) 3.30 ± 0.65 (T1) 3.93 ± 0.78 (T2) |
1.43 ± 0.50 (C) 3.17 ± 0.65 (T1) 3.63 ± 0.67 (T2) |
R-4C (REMI, Mumbai, India) | 10 | 3000 × 10 |
RCT, randomized clinical trial; OFD, open flap debridement; IBDs, infra-bony defects; NR, not reported; C, control group; T, test group, ♂, male; ♀, female; PRF, platelet-rich fibrin; PRP, platelet-rich plasma; PD, probing depth; CAL, clinical attachment level; BF, bone fill; rpm, rotations per minute; BG, bone graft; HA, hydroxyapatite; DFDBA, demineralized freeze-dried bone allograft; XB, xenogeneic bone; ABBM, anorganic bovine bone mineral; ABG, autogenous bone graft; EMD, Emdogain; BioG, bioactive glass; BM, barrier membrane; MF, metformin; ALN, alendronate; ATV, atorvastatin; RSV, rosuvastatin
Intrabony defects
Probing depth
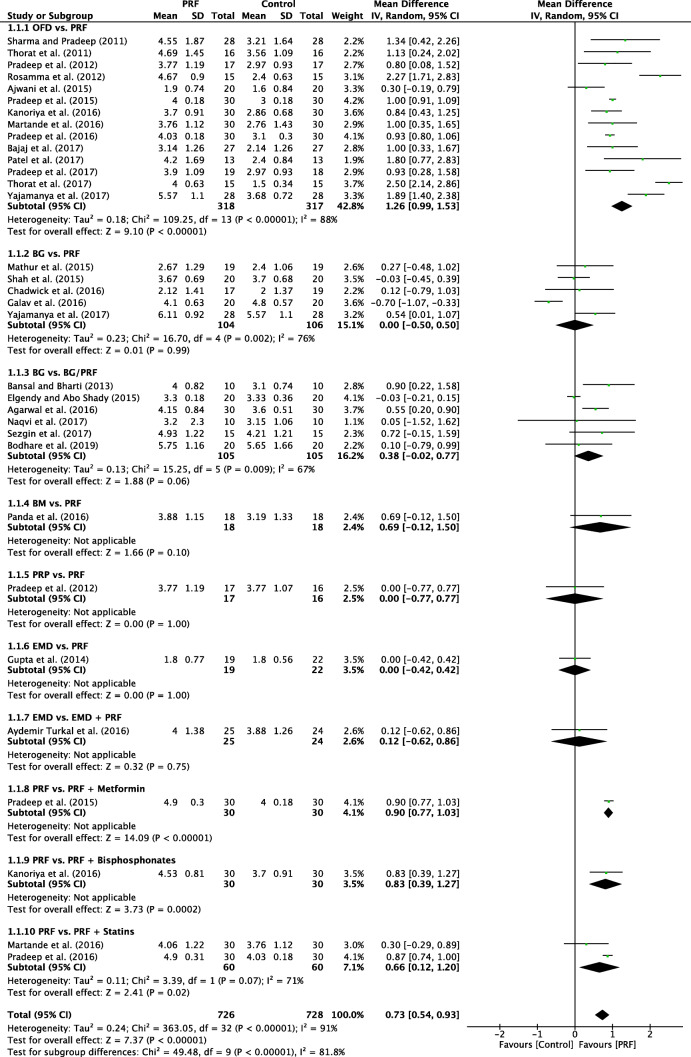
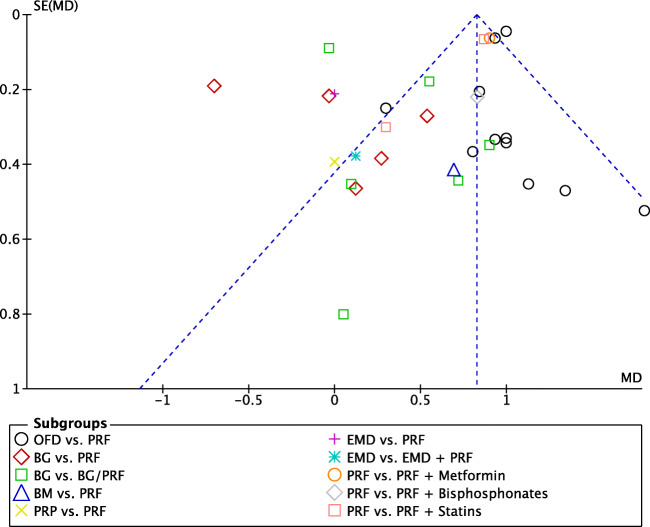
A random-effects model was used to evaluate the PD due to the high heterogeneity that was found between the subgroups (P < 0.00001; I2 = 91%). No subgroup showed a significant result in favor of the control groups when compared to that of PRF. In overall effect, the use of PRF differed significantly (P < 0.00001) in favor of PRF when compared with the control groups, with a mD of 0.82 (95% CI 0.78 to 0.87) (Fig. 2). The funnel plot demonstrated asymmetric distribution indicating high risk of publication bias (Fig. 3). The sensitivity analysis (exclusion of outliners) suggests that the divergence between the size of the sample groups may favor the increase in the possibility of publication bias.
Fig. 2.
Forest plot for the event reduction in “probing depth” (PD) (reported in mm) for intrabony defects
Fig. 3.
Funnel plot for the studies reporting reduction in probing depth (PD) (reported in mm) for intrabony defects
Clinical attachment level
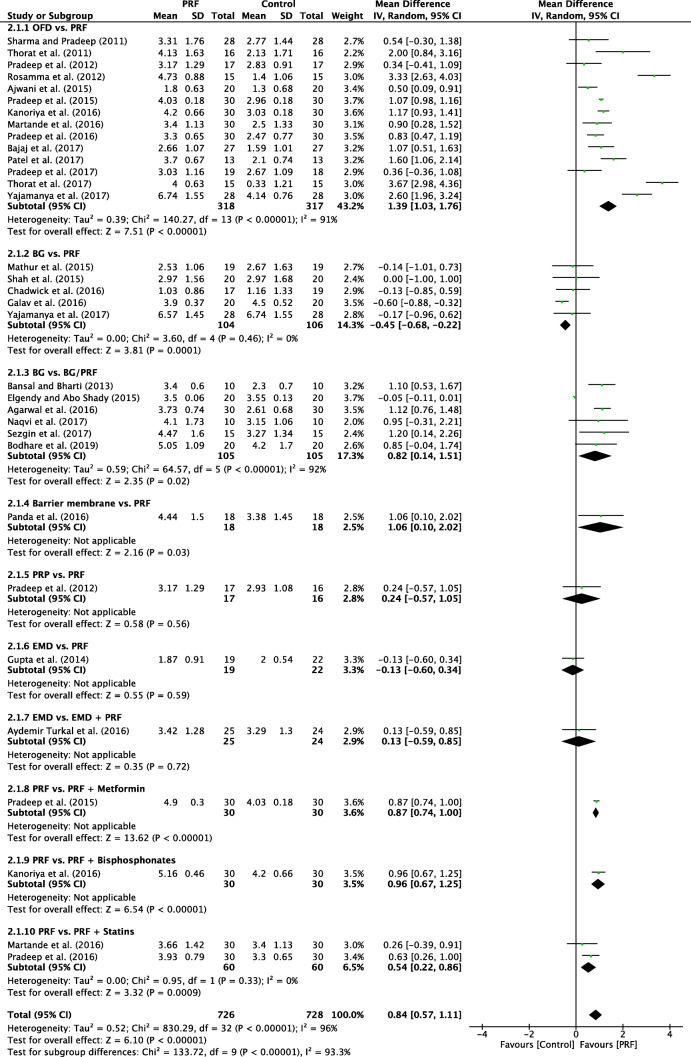
For CAL, the random-effects model was used due to the moderate heterogeneity among the analyzed subgroups (P < 0.00001; I2 = 96%). One subgroup showed a significant difference (P < 0.0001) in favor of autogenous graft when compared to PRF, with a mD of − 0.45 (95% CI − 0.68 to − 0.22). However, in the overall effect there was a significant difference (P < 0.00001) in favor of the PRF group when compared to the control group, with mD of 0.84 (95% CI 0.57 to 1.11) (Fig. 4).
Fig. 4.
Forest plot for the event “clinical attachment level” (reported in mm) for intrabony defects
Radiographic bone fill
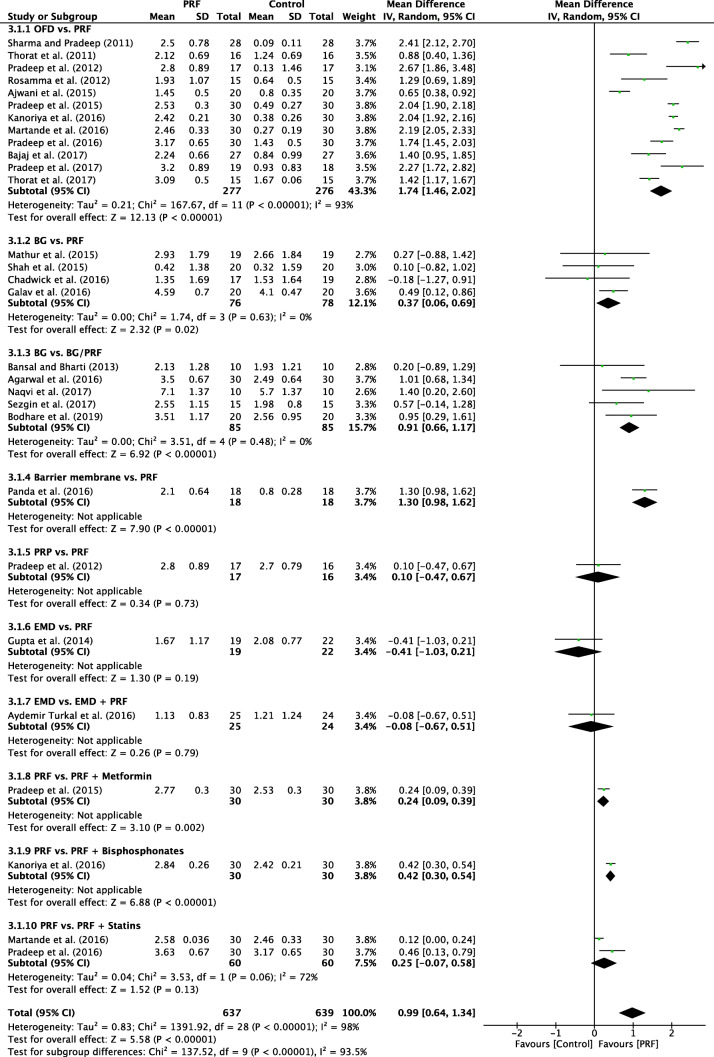
A random-effects model was used to evaluate the RBF due to the high heterogeneity that was found between the subgroups (P < 0.00001; I2 = 98%). No subgroup showed a significant result in favor of the control groups when compared to the PRF group. In overall effect, the use of PRF differed significantly (P = 0.11) in favor of PRF when compared to the control group, with a mD of 0.99 (95% CI 0.64 to 1.34) (Fig. 5).
Fig. 5.
Forest plot for the event “bone fill” (reported in % change) for intrabony defects
Assessment of the risk of bias
The overall risk of biased judgment of all studies was classified as “low risk of bias.” The RoB 2 analysis is shown in the Supplementary Appendix (S2).
Discussion
The present SR and meta-analysis have investigated the use of PRF for reconstructive surgery in intrabony defects as evaluated in RCTs comparing it to all other treatment modalities. The aim was to more specifically address the use and recommendations for PRF for the treatment of periodontal two- and three-walled intrabony defects. Overall, the majority of studies to date compared the use of OFD/PRF versus OFD alone or OFD/BG versus OFD/PRF (Table 1). Furthermore, additional studies were gathered comparing OFD/EMD versus OFD/PRF, OFD/BM versus OFD/PRF, and OFD/PRF versus OFD/PRF/biomolecules. Below, we highlight and discuss the summary of evidence from the current categories and further discuss the strengths and limitations of each comparative analysis (Table 2).
OFD alone versus with PRF
In total, 14 studies evaluated the use of PRF as an adjunct to OFD when compared to OFD alone (Table 1). In summary, 13 of the 14 studies demonstrated statistically significant clinical improvements in mean PD reduction—1.26 mm (Fig. 2), 11 of 14 studies demonstrated statistically significant improvements in mean CAL gain 1.39 mm (Fig. 4), and all studies showed statistically significant improvement in terms of bone fill (Fig. 5). In summary, it was observed that on average, the results from 14 RCTs demonstrated a statistically significant relative PD reduction of ~ 1.3 mm and ~ 1.5 mm CAL gain when PRF was additionally filled into intrabony defects following OFD (Figs. 2, 3, and 4).
Bone graft versus PRF
In a second series of studies, five studies evaluated the use of a BG versus PRF (Table 1). In general, no statistically significant difference was found between the two groups. One study demonstrated statistically significantly better results for PRF [51] and one study demonstrated statistically significantly more favorable outcomes for BG [34]. The three remaining studies demonstrated no statistically significant differences between the groups. The meta-analysis also demonstrated no statistically significant differences in PD reduction, CAL gain, or RBF between the two groups. Therefore, the data indicate that PRF may lead to comparable clinical outcomes than those obtained with BG when used for intrabony defect repair/regeneration.
Bone graft versus bone graft + PRF
In a third series of investigated studies, six studies evaluated the additional use of PRF to BG when compared to BG alone (Table 1). Of the six studies, two demonstrated a statistically significant improvement in PD and CAL gain when compared to BG alone [27, 52] while the other four studies demonstrated no statistically significant difference [31, 33, 39, 46]. A final reported ~ 0.5-mm non-significant reduction in PD was observed. When investigating CAL gain, two studies demonstrated a statistically significant advantage whereas three others have failed to demonstrate statistically significant improvements in CAL level (Fig. 4). Following meta-analysis, it was found that the additional use of PRF to BG led to a statistically significant ~ 1 mm gain in CAL when compared to BG alone and also statistically significant improvements in RBF. Therefore, some clinical benefit was observed when PRF was combined with a BG material. Potential reasons for these findings are likely multi-factorial. Many bone grafting materials, such as xenografts and the majority of synthetic materials, have no incorporation of extracellular matrix components or growth factors. Therefore, one hypothesized reason for the additional benefit of including PRF to a BG could be its new incorporation of regenerative cells and growth factors that contribute to the regenerative process. Previous in vitro research investigating PRF has demonstrated its ability to improve PDL cell migration, proliferation, and wound closure rates [55]. Furthermore, PRF also contains supra-physiological concentrations of defense-fighting leukocytes. Since periodontal pockets harbor a number of periodontal pathogens, it is possible that leukocytes may aid in the defense against potential bacterial contamination/invasion. Lastly, basic science studies have now demonstrated that PRF promotes an anti-inflammatory environment [56, 57]. Recent research has shown that PRF has the ability to favor M2 macrophage polarization and also decreases tissue inflammation [56, 57]. It also possesses some anti-bacterial/antimicrobial activity, thereby favoring potential wound healing of periodontal pockets [58, 59]. Taken together, each of the aforementioned parameters is thought to at least in part contribute toward periodontal regeneration when PRF is utilized in combination with a BG.
Additional randomized clinical studies evaluating PRF
An additional eight studies evaluated the use of PRF in various RCTs as highlighted herein. No meta-analysis could be performed but general trends were reported (Figs. 2, 3, 4, and 5) [29, 35, 37, 40, 43–45]. The comparison investigating PRF versus a collagen barrier membrane yielded no statistically significant difference in terms of PD reduction. However, statistically significant improvements were observed for CAL and RBF favoring the PRF group when compared to collagen membranes [40]. Interestingly, no differences in any of the investigated parameters were found for single RCTs investigating (1) PRP versus PRF [45], (2) EMD versus PRF [35], or (3) EMD versus EMD + PRF [29].
Lastly, four studies have investigated PRF in combination with either (1) metformin [44], (2) bisphosphonates [36] or (3) statins [37, 43]. There was a statistically significant advantage in PD reduction, CAL gain, and RBF for the combined use of PRF with each of the above modalities when compared to utilizing PRF alone. Although few studies have thus far characterized their potential benefit, these relatively novel findings support the more recent trends favoring more “personalized” medicine as regenerative strategies. Thus, future research investigating specific patient populations (e.g., osteoporotic women) may potentially and more specifically target the local use of additional biomolecules (such as bisphosphonates) favoring more specific bioactivity (anti-resorptive properties) favoring a more personalized treatment protocol. Furthermore, the use of antibiotic therapy in certain patients with aggressive periodontitis may benefit from more “personalized” antibiotic therapy. Since PRF may be utilized as a three-dimensional matrix with long-term delivery of small biomolecules, PRF may therefore be utilized as a therapeutic drug delivery system as previously reported [60]. Nevertheless, it remains somewhat unclear the modes of action of certain strategies such as additional combination of metformin to PRF. Future research investigating PRF as a potential drug delivery system for various local therapeutic agents/biomolecules with their better understanding may provide further clinical benefit. At present, however, the above trends are simply reported in single RCTs with much further research needed on the topic.
Implications for clinical practice and future direction
The latest guidelines from the EFP determined in a study titled “Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: a systematic review and meta-analysis” that EMD or GTR in combination with papillary preservation flaps should be considered the treatment of choice for residual pockets with deep (≥ 3 mm) intrabony defects [61]. With the fact that blood clot seems to be a very important aspect for periodontal regeneration, it must first be noted that there remains a great need for human/animal histology with lots of effort to deliver studies that compare standard periodontal regenerative procedures with PRF.
Despite the fact that PRF is only beginning to be more commonly utilized in routine clinical practice for the treatment of intrabony defects, it remains interesting to note that 27 RCTs have thus far evaluated its potential for periodontal regeneration/repair. The formation of a blood clot alone has been shown to be one of the key necessary features in order for periodontal regeneration to take place, as long as bacterial pathogens have been completely eliminated. Evidence from the literature suggests that blood clot formation alone is enough to treat a number of intrabony defects where space maintenance is not an issue (two- or three-walled defects) [62]. PRF therefore acts in a similar fashion whereby the fibrin scaffold can be inserted into the periodontal pocket acting as a stable clot, with significant increases in platelets, leukocytes, and growth factors. While periodontal regeneration remains complex due to the number of tissues needed to be regenerated (new cementum, periodontal ligament, and alveolar bone), as well as the fact that Sharpey’s fibers need to be oriented functionally to support the tooth apparatus, it remains difficult to assess whether PRF actually leads to true periodontal regeneration since no human histological evidence exists to date on the topic (despite the nearly 30 RCTs having been performed). Nevertheless, it is known that periodontal disease is caused by bacterial pathogens and an increase in regenerative growth factors and cells, as well as its incorporation of defense-fighting leukocytes is certainly hypothesized to favor defect resolution and potentially mitigate tissue inflammation. Furthermore, angiogenesis is an important factor for tissue regeneration, and PRF releases a number of pro-angiogenic and pro-fibrotic agents capable of further speeding periodontal tissue repopulation [9, 62].
The biological advantages of PRF have been shown to act locally by quickly stimulating a large number of cell types by influencing their recruitment, proliferation and/or differentiation. These have previously been shown to include endothelial cells, gingival fibroblasts, chondrocytes, and osteoblasts, thereby having the potential effect to act locally and affect various cell types [63, 64]. Thus, PRF may prove beneficial for the regeneration of specific tissues such as the periodontium since several cell types and tissue types are required to regenerate in order for periodontal regeneration to occur. While it is known that beneficial effects of PRF may partially be due to the large number of secreted autologous blood-derived growth factors, it remains interesting to point out the fact that rhPDGF (which is approved by the FDA) has been one of the main recombinant growth factors sold to date in North America for the regeneration of periodontal tissues [65–68]. Although recombinant proteins have a regenerative potential well documented in the literature [69–71], their associated costs and other secondary adverse effects, including biocompatibility, lower stability, and potential swelling, may favor the use of autologous PRF [72, 73]. Future comparative studies including a cost–benefit analysis between both modalities remain necessary.
It is also noted that of a total of 551 papers that were screened and 27 RCTs that met the inclusion criteria, studies were classified and presented into 10 categories, via comparison of clinical outcomes between PRF and other various treatment modalities/approaches. It remains interesting to point out that of the studies, three major groups of studies were gathered as follows: (1) therapeutic modalities with or without PRF (OFD vs. OFD/PRF, OFD/BG vs. OFD/BG/PRF, and OFD/EMD vs. OFD/EMD/PRF); (2) treatment modalities in comparison to PRF (OFD/PRF vs. OFD/BG, OFD/BM, OFD/PRP, and OFD/EMD, respectively); (3) therapeutic modalities in comparison of OFD/PRF with additional small biomolecules (OFD/PRF vs. OFD/PRF/metformin, OFD/PRF/bisphosphonates, and OFD/PRF/statins, respectively).
All therapeutic modalities with addition of PRF to their surgical approach (group 1) demonstrated better outcomes apart from one study comparing OFD/EMD versus OFD/EMD/PRF. Each of the treatment modalities comparing PRF alone to other regenerative strategies (group 2) found similar clinical outcomes between both groups. Each of the therapeutic modalities utilizing PRF with addition of small biomolecules (group 3) found improved clinical outcomes with the addition of either metformin, bisphosphonates, or statins. Most of the studies dealt with the therapeutic modalities with or without PRF (group 1; n = 21, 78%). It thus remains interesting to note that generally speaking, the additional use of PRF tends to favor regenerative outcomes of IBDs, and addition of small biomolecules may further improve such outcomes. Future research investigating more precisely when PRF should be utilized in combination approaches versus as a sole regenerative modality needs further clarification.
Several research topics also remain at the forefront of needed research in this space. As previously mentioned, it remains interesting to point out that no single study has characterized PRF at the histological level in a well-characterized human study. It has already been well established in the literature that PRF favors soft tissue wound healing when compared to hard tissues [74]. Since periodontitis is not only characterized by PDL breakdown but also that of cementum and alveolar bone, the regenerative potential of each of these tissues needs to be further characterized via histological evaluation, ideally in human studies. Furthermore, it remains astonishing that very few included trials addressed with their research these valid questions: (1) collagen membranes without hard substitute support, (2) bone grafting of any kind of material without barriers, (3) PRF as a substitute for collagen membranes, etc. The next wave of research should more specifically address these important topics to better understand the additional role/benefit PRF may serve under such conditions. Furthermore, an array of different surgical procedures utilized to treat IBDs exists. Previously, it was shown that great variability in surgical approaches was discussed when PRF was used for the treatment of gingival recessions [75]. Future research investigating more precisely various surgical difference such as flap design and surgical techniques (e.g., MIST and M-MIST) should be further evaluated in future studies to better determine optimal surgical approaches when using PRF in regenerative therapy of IBDs.
Conclusion
The data from the present SR with meta-analysis demonstrate that OFD/PRF leads to statistically significant clinical improvements in PD reduction, CAL gain, and RBF when compared to OFD alone. Furthermore, the data suggest that comparable results can be obtained when intrabony defects are filled with either PRF or a BG and statistically significant improvements in CAL and RBF were observed when PRF was combined with BG. Future research may be warranted to evaluate the use of PRF in combination with various additional small biomolecules such as metformin, bisphosphonates, statins, and/or antibiotics to additionally improve the clinical outcomes. In addition, animal and human histological evidence is needed to verify if PRF actually leads to true periodontal regeneration.
Supplementary Information
(DOCX 24 kb)
Author contributions
All authors made substantial contribution to the conception and design of the manuscript. R.J.M., M.F.K., and V.M. performed the literature search and interpretation of the data. All authors drafted the work and revised it critically for important intellectual content. All authors agree to be accountable for all aspects of the study design and its content. All authors approved the final submitted version.
Funding
Open Access funding provided by Universität Bern.
Declarations
Conflict of interest
R.J.M. holds intellectual property on PRF. All other authors declare no conflict of interest.
Ethical approval
No ethical approval was required for this study since it was a systematic review.
Informed consent
No informed consent was required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Richard J. Miron and Vittorio Moraschini contributed equally to this work.
References
- 1.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontology. 2009;2000(51):208–219. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Laugisch O, Cosgarea R, Nikou G, Nikolidakis D, Donos N, Salvi GE, Stavropoulos A, Jepsen S, Sculean A. Histologic evidence of periodontal regeneration in furcation defects: a systematic review. Clinical oral investigations. 2019;23:2861–2906. doi: 10.1007/s00784-019-02964-3. [DOI] [PubMed] [Google Scholar]
- 3.Sculean A, Nikolidakis D, Nikou G, Ivanovic A. Chapple IL and Stavropoulos A (2015) Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontology. 2000;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 4.Ivanovic A, Nikou G, Miron RJ, Nikolidakis D, Sculean A (2014) Which biomaterials may promote periodontal regeneration in intrabony periodontal defects? A systematic review of preclinical studies. Quintessence international 45 [DOI] [PubMed]
- 5.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup: James Beck GDRP Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol. 2013;2000(62):271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 7.Grzesik WJ, Narayanan AS. Cementum and periodontal wound healing and regeneration. Crit Rev Oral Biol Med. 2002;13:474–484. doi: 10.1177/154411130201300605. [DOI] [PubMed] [Google Scholar]
- 8.Wikesjo UM, Selvig KA. Periodontal wound healing and regeneration. Periodontol. 1999;2000(19):21–39. doi: 10.1111/j.1600-0757.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, Salkin L, Townsend C, Sheridan P, Genco RJ. Periodontal regeneration. Journal of periodontology. 2005;76:1601–1622. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- 10.Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clinical oral investigations. 2017;21:1913–1927. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 11.Oneto P, Zubiry PR, Schattner M, Etulain J. Anticoagulants interfere with the angiogenic and regenerative responses mediated by platelets. Frontiers in bioengineering and biotechnology. 2020;8:223. doi: 10.3389/fbioe.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar RV, Shubhashini N. Platelet rich fibrin: a new paradigm in periodontal regeneration. Cell and tissue banking. 2013;14:453–463. doi: 10.1007/s10561-012-9349-6. [DOI] [PubMed] [Google Scholar]
- 13.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J (2017) Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clinical oral investigations. 10.1007/s00784-017-2063-9 [DOI] [PubMed]
- 14.Lourenco ES, Mourao C, Leite PEC, Granjeiro JM, Calasans-Maia MD, Alves GG. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. Journal of biomedical materials research Part A. 2018;106:1373–1380. doi: 10.1002/jbm.a.36346. [DOI] [PubMed] [Google Scholar]
- 15.Mourão CFDAB, Calasans-Maia MD, de Mello Machado RC, de Brito Resende RF, Alves GGJO, Surgery M (2018) The use of platelet-rich fibrin as a hemostatic material in oral soft tissues. 22:329–333 [DOI] [PubMed]
- 16.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clinical oral investigations. 2016;20:2353–2360. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. Journal of periodontology. 2017;88:112–121. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 18.Najeeb S, Khurshid Z, Agwan MAS, Ansari SA, Zafar MS, Matinlinna JP. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: a systematic review of clinical studies. Tissue engineering and regenerative medicine. 2017;14:735–742. doi: 10.1007/s13770-017-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue engineering Part B, Reviews. 2017;23:83–99. doi: 10.1089/ten.TEB.2016.0233. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 349 [DOI] [PubMed]
- 22.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC medical informatics and decision making. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grey Literature Report. The New York Academy of Medicine. Available at: http://www.greylit.org. Accessed May 10, 2020. Natl J Maxillofac Surg 7:45-51. doi: 10.4103/0975-5950.196124
- 24.Open Grey. Available at: http://www.opengrey.eu. Accessed May 10, 2020.
- 25.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS and Eldridge SM (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366. [DOI] [PubMed]
- 26.Egger M, Davey-Smith G, Altman D (2008) Systematic reviews in health care: meta-analysis in context. John Wiley & Sons
- 27.Agarwal A, Gupta ND, Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trial. Acta odontologica Scandinavica. 2016;74:36–43. doi: 10.3109/00016357.2015.1035672. [DOI] [PubMed] [Google Scholar]
- 28.Ajwani H, Shetty S, Gopalakrishnan D, Kathariya R, Kulloli A, Dolas RS, Pradeep AR. Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J Int Oral Health. 2015;7:32–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Aydemir Turkal H, Demirer S, Dolgun A, Keceli HG. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. Journal of clinical periodontology. 2016;43:955–964. doi: 10.1111/jcpe.12598. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj P, Agarwal E, Rao NS, Naik SB, Pradeep AR, Kalra N, Priyanka N, Kumari M. Autologous platelet-rich fibrin in the treatment of 3-wall intrabony defects in aggressive periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2017;88:1186–1191. doi: 10.1902/jop.2017.120661. [DOI] [PubMed] [Google Scholar]
- 31.Bodhare GH, Kolte AP, Kolte RA, Shirke PY. Clinical and radiographic evaluation and comparison of bioactive bone alloplast morsels when used alone and in combination with platelet-rich fibrin in the treatment of periodontal intrabony defects—a randomized controlled trial. Journal of periodontology. 2019;90:584–594. doi: 10.1002/jper.18-0416. [DOI] [PubMed] [Google Scholar]
- 32.Chadwick JK, Mills MP, Mealey BL. Clinical and radiographic evaluation of demineralized freeze-dried bone allograft versus platelet-rich fibrin for the treatment of periodontal intrabony defects in humans. Journal of periodontology. 2016;87:1253–1260. doi: 10.1902/jop.2016.160309. [DOI] [PubMed] [Google Scholar]
- 33.Elgendy EA, Abo Shady TE. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. Journal of Indian Society of Periodontology. 2015;19:61–65. doi: 10.4103/0972-124x.148639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galav S, Chandrashekar KT, Mishra R, Tripathi V, Agarwal R, Galav A. Comparative evaluation of platelet-rich fibrin and autogenous bone graft for the treatment of infrabony defects in chronic periodontitis: clinical, radiological, and surgical reentry. Indian journal of dental research : official publication of Indian Society for Dental Research. 2016;27:502–507. doi: 10.4103/0970-9290.195634. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SJ, Jhingran R, Gupta V, Bains VK, Madan R, Rizvi I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. Journal of the International Academy of Periodontology. 2014;16:86–96. [PubMed] [Google Scholar]
- 36.Kanoriya D, Pradeep AR, Singhal S, Garg V, Guruprasad CN. Synergistic approach using platelet-rich fibrin and 1% alendronate for intrabony defect treatment in chronic periodontitis: a randomized clinical trial. Journal of periodontology. 2016;87:1427–1435. doi: 10.1902/jop.2016.150698. [DOI] [PubMed] [Google Scholar]
- 37.Martande SS, Kumari M, Pradeep AR, Singh SP, Suke DK, Guruprasad CN. Platelet-rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2016;87:1039–1046. doi: 10.1902/jop.2016.150306. [DOI] [PubMed] [Google Scholar]
- 38.Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: a comparative analysis. European journal of dentistry. 2015;9:100–108. doi: 10.4103/1305-7456.149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naqvi A, Gopalakrishnan D, Bhasin MT, Sharma N, Haider K, Martande S. Comparative evaluation of bioactive glass putty and platelet rich fibrin in the treatment of human periodontal intrabony defects: a randomized control trial. Journal of clinical and diagnostic research : JCDR. 2017;11:Zc09–Zc13. doi: 10.7860/jcdr/2017/23831.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panda S, Sankari M, Satpathy A, Jayakumar D, Mozzati M, Mortellaro C, Gallesio G, Taschieri S and Del Fabbro M (2016) Adjunctive effect of autologous platelet-rich fibrin to barrier membrane in the treatment of periodontal intrabony defects. The Journal of craniofacial surgery 27:691-696. doi: 10.1097/scs.0000000000002524 [DOI] [PubMed]
- 41.Patel GK, Gaekwad SS, Gujjari SK, CV S. Platelet-rich fibrin in regeneration of intrabony defects: a randomized controlled trial. Journal of periodontology. 2017;88:1192–1199. doi: 10.1902/jop.2017.130710. [DOI] [PubMed] [Google Scholar]
- 42.Pradeep AR, Bajaj P, Rao NS, Agarwal E, Naik SB. Platelet-rich fibrin combined with a porous hydroxyapatite graft for the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2017;88:1288–1296. doi: 10.1902/jop.2012.110722. [DOI] [PubMed] [Google Scholar]
- 43.Pradeep AR, Garg V, Kanoriya D, Singhal S. Platelet-rich fibrin with 1.2% rosuvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2016;87:1468–1473. doi: 10.1902/jop.2016.160015. [DOI] [PubMed] [Google Scholar]
- 44.Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2015;86:729–737. doi: 10.1902/jop.2015.140646. [DOI] [PubMed] [Google Scholar]
- 45.Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of periodontology. 2012;83:1499–1507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 46.Sezgin Y, Uraz A, Taner IL, Çulhaoğlu R. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz Oral Res. 2017;31:e15. doi: 10.1590/1807-3107BOR-2017.vol31.0015. [DOI] [PubMed] [Google Scholar]
- 47.Shah M, Patel J, Dave D, Shah S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. Journal of Indian Society of Periodontology. 2015;19:56–60. doi: 10.4103/0972-124x.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. Journal of periodontology. 2011;82:1705–1712. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 49.Thorat M, Baghele ON, RP S. Adjunctive effect of autologous platelet-rich fibrin in the treatment of intrabony defects in localized aggressive periodontitis patients: a randomized controlled split-mouth clinical trial. The International journal of periodontics & restorative dentistry. 2017;37:e302–e309. doi: 10.11607/prd.2972. [DOI] [PubMed] [Google Scholar]
- 50.Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. Journal of clinical periodontology. 2011;38:925–932. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 51.Yajamanya SR, Chatterjee A, Hussain A, Coutinho A, Das S, Subbaiah S. Bioactive glass versus autologous platelet-rich fibrin for treating periodontal intrabony defects: a comparative clinical study. Journal of Indian Society of Periodontology. 2017;21:32–36. doi: 10.4103/0972-124x.201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bansal C, Bharti V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects. Journal of Indian Society of Periodontology. 2013;17:361–366. doi: 10.4103/0972-124x.115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosamma Joseph V, Sam G, Amol NV. Clinical evaluation of autologous platelet rich fibrin in horizontal alveolar bony defects. Journal of clinical and diagnostic research: JCDR. 2014;8:ZC43. doi: 10.7860/JCDR/2014/9948.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosamma Joseph V, Raghunath A and Sharma N (2012) Clinical effectiveness of autologous platelet rich fibrin in the management of infrabony periodontal defects. Singapore Dental Journal 33:5–12. 10.1016/j.sdj.2012.10.003 [DOI] [PubMed]
- 55.Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. Journal of periodontal research. 2020;55:287–295. doi: 10.1111/jre.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasirzade J, Kargarpour Z, Hasannia S, Strauss FJ, Gruber R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. Journal of periodontology. 2020;91:244–252. doi: 10.1002/JPER.19-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Yin C, Zhao Q, Zhao Z, Wang J, Miron RJ, Zhang Y. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. Journal of Biomedical Materials Research Part A. 2020;108:61–68. doi: 10.1002/jbm.a.36792. [DOI] [PubMed] [Google Scholar]
- 58.Badade PS, Mahale SA, Panjwani AA, Vaidya PD, Warang AD. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian journal of dental research : official publication of Indian Society for Dental Research. 2016;27:300–304. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- 59.Castro AB, Herrero ER, Slomka V, Pinto N, Teughels W, Quirynen M. Antimicrobial capacity of leucocyte- and platelet rich fibrin against periodontal pathogens. Scientific reports. 2019;9:8188. doi: 10.1038/s41598-019-44755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miron RJ, Zhang Y. Autologous liquid platelet rich fibrin: a novel drug delivery system. Acta Biomater. 2018;75:35–51. doi: 10.1016/j.actbio.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Nibali L, Koidou VP, Nieri M, Barbato L, Pagliaro U, Cairo F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: a systematic review and meta-analysis. Journal of clinical periodontology. 2020;47:320–351. doi: 10.1111/jcpe.13237. [DOI] [PubMed] [Google Scholar]
- 62.Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant dentistry. 2006;15:8–17. doi: 10.1097/01.id.0000204762.39826.0f. [DOI] [PubMed] [Google Scholar]
- 63.Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S, Gnyawali U, Bergdall VK, Sen CK. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:753–766. doi: 10.1111/j.1524-475X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189–3209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 65.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183:61–64. [PubMed] [Google Scholar]
- 66.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 67.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. International orthopaedics. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. Journal of dental research. 2012;91:736–744. doi: 10.1177/0022034511435260. [DOI] [PubMed] [Google Scholar]
- 69.Young CS, Ladd PA, Browning CF, Thompson A, Bonomo J, Shockley K, Hart CE. Release, biological potency, and biochemical integrity of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) combined with Augment(TM) bone graft or GEM 21S beta-tricalcium phosphate (beta-TCP) Journal of controlled release : official journal of the Controlled Release Society. 2009;140:250–255. doi: 10.1016/j.jconrel.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 70.Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J Control Release. 2000;67:385–394. doi: 10.1016/S0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 71.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64:103–114. doi: 10.1016/S0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 72.Delgado JJ, Evora C, Sanchez E, Baro M, Delgado A. Validation of a method for non-invasive in vivo measurement of growth factor release from a local delivery system in bone. J Control Release. 2006;114:223–229. doi: 10.1016/j.jconrel.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Oe S, Fukunaka Y, Hirose T, Yamaoka Y, Tabata Y. A trial on regeneration therapy of rat liver cirrhosis by controlled release of hepatocyte growth factor. J Control Release. 2003;88:193–200. doi: 10.1016/S0168-3659(02)00463-7. [DOI] [PubMed] [Google Scholar]
- 74.Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J (2016) Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue engineering Part B, Reviews. 10.1089/ten.TEB.2016.0233 [DOI] [PubMed]
- 75.Miron RJ, Moraschini V, Del Fabbro M, Piattelli A, Fujioka-Kobayashi M, Zhang Y, Saulacic N, Schaller B, Kawase T, Cosgarea R, Jepsen S, Tuttle D, Bishara M, Canullo L, Eliezer M, Stavropoulos A, Shirakata Y, Stähli A, Gruber R, Lucaciu O, Aroca S, Deppe H, Wang HL, Sculean A. Use of platelet-rich fibrin for the treatment of gingival recessions: a systematic review and meta-analysis. Clinical oral investigations. 2020;24:2543–2557. doi: 10.1007/s00784-020-03400-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 24 kb)