Abstract
Background:
Pulmonary embolism (PE) is unexpectedly detected in some donor lungs during organ procurement for lung transplantation. Anecdotally, such lungs are usually implanted, however, the impact of this finding on recipient outcomes remains unclear. We hypothesized that incidentally detected donor PE is associated with adverse short- and long-term outcomes in lung transplant recipients.
Methods:
We analyzed a prospectively maintained database of all lung donors procured by a single surgeon and transplanted at our institution between 2009 and 2018. A standardized approach was used for all procurements and included antegrade and retrograde flush. PE was defined as macroscopic thrombus seen in the pulmonary artery during the donor procurement operation.
Results:
A total of 501 consecutive lung procurements were performed during the study period. The incidence of donor PE was 4.4% (22/501). No organs were discarded due to PE. Donors with PE were similar to those without PE in baseline characteristics and PaO2. Recipients in the two groups were also similar. PE was associated with a higher likelihood of acute cellular rejection grade (ACR) ≧ 2 (10/22, 45.5% vs. 120/479, 25.1%, p=0.03). Multivariable Cox modeling demonstrated an association between PE and the development of chronic lung allograft dysfunction (CLAD) (hazard ratio, 2.02 (1.23–3.30), p=0.005).
Conclusions:
Lungs from donors with incidentally detected PE may be associated with a higher incidence of recipient ACR as well as reduced CLAD-free survival. Surgeons must use caution when transplanting lungs with incidentally discovered PE. These preliminary findings warrant corroboration in larger data sets.
Keywords: embolism, lung, outcomes, pulmonary arteries/ veins, transplantation, lung
Brain dead organ donors are in a prothrombotic state1. Retrospective data indicate that pulmonary embolism (PE) may be a frequent finding in organ donors whose lungs are rejected for transplantation2. During lung procurement for transplantation, PE is unexpectedly detected in some donor lungs. Whether or not to utilize donor lungs with an incidentally discovered PE may create a clinical dilemma for transplant surgeons, as the impact of this finding on recipient outcomes is unclear3. Given that the pulmonary arteries are the sole source of oxygen delivery to lung parenchyma and bronchial tissue after transplantation, PE may result in small airway ischemia that leads to allograft injury and primary graft dysfunction (PGD) 3,4.
Few studies have examined the association between donor PE and recipient outcomes. One report showed that significant donor PE diagnosed with echocardiography or computed tomography (CT) scan prior to brain death was not associated with PGD, chronic lung allograft dysfunction (CLAD) at 1 year, or overall survival (OS). In this study, the pulmonary arteries were examined for the presence of PE during the donor procurement, and again in the recipient operating room, at which time heparinized saline was injected into the arteries prior to implantation. Interestingly, the 5-year CLAD free survival was actually higher in recipients of donor lungs with massive PE5. Similarly, Fisher et al. have reported three cases of successful transplantation of lungs from brain dead donors who developed severe acute PE after cardiac arrest. While one of the recipients unfortunately expired due to sepsis at 6 months, the other two recipients were alive and did not require supplemental oxygen at the time of publication, suggesting that even severe acute PE may be associated with acceptable outcomes6. Another study examined the impact of incidentally discovered donor PE, as detected by retrograde flush in the recipient operating room, which triggered an additional flush until no residual PE was found. Conversely, here the authors found that incidentally discovered PE was an independent risk factor for prolonged recipient intubation and PGD. In this study, only an antegrade flush was carried out at the time of procurement3. Hence, the impact of PE on recipient outcomes after transplant remains debatable and may depend on how the PE is detected and addressed after being discovered. Our institution has developed a standardized donor procurement protocol in which we perform antegrade flush in vivo and immediately retrograde flush the donor lung after cardiac explant7. Anecdotally, we have found that this technique may reveal donor PE that was not detected pre-operatively.
We hypothesized that incidentally detected donor PE is associated with adverse short- and long-term outcomes in lung transplant recipients. To examine this, we conducted a single-center cohort study via a prospectively maintained database to investigate the impact of donor PE on recipient outcomes using consistent procurement and implantation techniques.
Patients and Methods
Data collection and Study Population
We performed a single-center retrospective cohort study of all donor lungs procured by a single, experienced surgeon (MKP) and transplanted at our institution between December 2009 and June 2018. Data for this study were collected in a prospectively maintained repository. The Washington University School of Medicine Institutional Review Board for Human Studies approved the study protocol (ID #201810205).
Lung Procurement and Transplantation
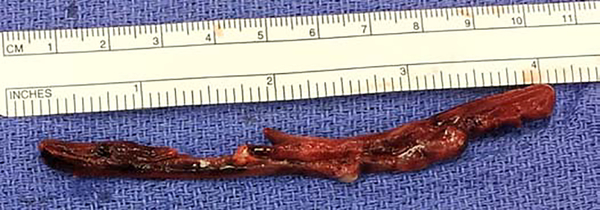
A standardized lung procurement approach was used as previously described7. After bolus administration of prostaglandin E1 (Alprostadil, Pfizer, Kirkland, QC, Canada), antegrade flush perfusion with 5.6 L of cold Perfadex® solution (XVIVO Perfusion AB, Göteborg, Sweden) was performed. After excising the heart, an additional 250 mL of Perfadex® solution was administered as retrograde flush perfusion into each of the four pulmonary veins. Lungs were then stored in cold preservation solution and transferred to the recipient’s site. PE was defined as macroscopic thrombus noted during the lung procurement in the pulmonary artery or coming out of the pulmonary artery during retrograde flush (Figure 1). All lung transplants were performed at a single institution (Barnes-Jewish Hospital, St. Louis, MO) and managed in a standard fashion, as outlined below. Importantly, all lung transplant surgeons at our institution have been trained by the senior most surgeon in this study (GAP) and use the same technique.
Figure 1:
Macroscopic finding of pulmonary embolism that was washed out of the pulmonary artery during retrograde flush.
Postoperative Management
All patients were treated with basiliximab and methylprednisone for induction immunosuppression, followed by a triple drug regimen for maintenance immunosuppression (tacrolimus or cyclosporine, mycophenolate mofetil or azathioprine, prednisone). Surveillance bronchoscopies with transbronchial lung biopsies and bronchoalveolar lavage were performed at 1, 2, 3, 6, and 12 months after transplantation. Acute cellular rejection (ACR) grade, PGD, and CLAD were defined and treated according to accepted standards 8–11.
Outcomes
Short-term outcomes included the incidence of PGD and ACR. ACR was defined according to the International Society for Heart and Lung Transplantation (ISHLT) criteria for lung allograft rejection8,9. PGD grading was based on the ISHLT definition10. The PGD grade was measured at the time of admission to the intensive care unit (0 hours) and then again at 24hrs, 48hrs, and 72hrs post-operatively. The highest grade of PGD at any time point during the first 72 hrs post-operatively was considered the outcome of interest. Long-term outcomes were evaluated by measuring OS and CLAD-free survival.
Statistical Analysis
Recipients were divided into two groups: those receiving a lung transplant from a donor with (PE+) or without PE (PE−) discovered at the time of organ procurement. Donor and recipient characteristics, as well as recipient outcomes, were summarized using descriptive statistics and compared through Chi-square or Fisher’s exact test for categorical variables, and Kruskal-Wallis test for continuous variables. CLAD-free survival was defined as the time from date of transplantation to death or the development of CLAD, whichever occurs first. Otherwise, patients were censored at the last follow-up. OS was defined as the time from the date of transplantation to date of death or was censored at the last follow-up. OS and CLAD-free survival were compared between PE+ and PE− groups using Kaplan-Meier plots, with statistical differences determined by the log-rank test. Cox proportional-hazards models were fitted to evaluate the relationship of key variables (PE, donor age, donor sex, donor cause of death, donor smoking history, donor best PaO2, lung allocation score, recipient age, recipient sex, ischemic time, cytomegalovirus [CMV] mismatch, PGD grade ≧ 2, ACR) and CLAD-free survival. The proportionality assumption was tested by adding a time-dependent covariate for each variable. All variables used in the univariable models were considered in the multivariable model. The final multivariable model was built using the backward stepwise selection approach to identify all significant risk factors. Factors significant at a 10% level were kept in the final model. All statistical tests were two-sided using an α = 0.05 level of significance. SAS Version 9.4 (Cary, NC) was used to perform the statistical analyses.
Results
Donor and Recipient Characteristics
Five hundred and one consecutive lung procurements were performed by one surgeon between December 2009 and June 2018 (Supplemental Figure). The incidence of donor PE was 4.4% (22/501). No organs were discarded due to donor PE. There were no significant differences in the baseline characteristics or PaO2 between donors with PE and those without PE. Recipients in the two groups were also similar in baseline characteristics, underlying cardiopulmonary disease, and lung allocation scores (Table 1). Ninety seven percent of recipients received double lung transplants (489/501), with no significant difference between the PE+ and PE− groups (21/22, 95.5% vs. 468/479, 97.7%, p=0.42).
Table 1:
Baseline characteristics of donors and recipients
| Variable | PE− (N=479) | PE+ (N=22) | p | |
|---|---|---|---|---|
| Donor | ||||
| Age (years) | 36.2±14.9 | 37.5±15.5 | 0.69 | |
| Male | 278 (58.0) | 16 (72.7) | 0.19 | |
| Cause of death | 0.39 | |||
| Cerebrovascular/ stroke | 152 (31.7) | 7 (31.8) | ||
| Head trauma | 196 (40.9) | 12 (54.6) | ||
| Anoxia | 109 (22.8) | 2 (9.1) | ||
| Others | 22 (4.6) | 1 (4.6) | ||
| Smoking history* | 57 (12.0) | 2 (9.1) | 1 | |
| Best PaO2 (mmHg) | 508.4±72.3 | 498.4±63.3 | 0.50 | |
| Recipient | ||||
| Age (years) | 53.9±14.3 | 54.0±13.1 | 0.79 | |
| Male | 276 (57.6) | 15 (68.2) | 0.38 | |
| Indication for transplant | 0.42 | |||
| Obstructive lung disease | 97 (20.3) | 4 (18.2) | ||
| Pulmonary vascular disease | 8 (1.7) | 1 (4.6) | ||
| Cystic fibrosis | 80 (16.7) | 2 (9.1) | ||
| Restrictive lung disease | 227 (47.4) | 10 (45.5) | ||
| Others | 67 (14.0) | 5 (22.7) | ||
| Lung allocation score | 47.3±17.7 | 49.0±20.2 | 0.94 | |
| Preoperative Mechanical Ventilation | 20 (4.2) | 2 (9.1) | 0.25 | |
| Preoperative ECMO | 10 (2.1) | 0 (0) | 1 | |
| Ischemic time (min) | 221.1±63.8 | 216.6±66.9 | 0.62 | |
| CMV mismatch | 207 (43.2) | 14 (63.6) | 0.08 | |
| mPA pressure (n available) (mmHg) | (353) 28.4±10.0 | (20)31.0±15.2 | 0.81 | |
| Intraoperative Cardiopulmonary bypass or ECMO | 193 (40.3) | 9 (40.9) | 0.95 |
Baseline characteristics of donors and recipients of lungs with and without incidentally discovered pulmonary embolism (PE). Values are presented as n (%) or mean ± standard error of the mean. PE+, lungs with incidentally discovered donor PE. PE−, lungs without incidentally discovered donor PE.
Denotes donors with a smoking history ≥ 20 pack years (missing data in 2 donors). The denominator for the percentages is the sum of patients across all categories in the PE+ or PE− group, respectively, excluding missing values. PE, pulmonary embolism; ECMO, Extracorporeal Membrane Oxygenation; CMV, cytomegalovirus; mPA, mean pulmonary artery.
Short-term outcomes
There were no significant differences in the length of hospital stay between the two groups. The incidence of postoperative renal failure was higher in the PE+ group (9.1% vs. 1.0%, p=0.03), while the rate of other postoperative complications was similar between the two groups. Among the 501 lung recipients, 238 (47.5%) developed PGD grade ≧ 2, and 130 (25.9%) developed ACR within the first year after transplantation. The incidence of PGD grade ≧ 2 was similar in the PE+ and PE− groups (7/22, 31.8% vs. 231/479, 48.2%, p=0.13) (Table 2). PE was associated with a higher likelihood of ACR grade ≧ 2 (10/22, 45.5% vs. 120/479, 25.1%, p=0.03) (Table 2).
Table 2:
Clinical outcomes in recipients
| Variable | PE- (N=479) | PE+ (N=22) | p |
|---|---|---|---|
| Double lung transplant | 468 (97.7) | 21 (95.5) | 0.42 |
| Complication | |||
| Pulmonary embolism | 7 (1.5) | 0 (0) | 1 |
| Pulmonary ventilation ≥ 48h | 130 (27.1) | 5 (22.7) | 0.65 |
| Atrial arrhythmia | 177 (37.0) | 10 (45.5) | 0.42 |
| Ventricular arrhythmia | 8 (1.7) | 0 (0) | 1 |
| Myocardial infarction | 2 (0.4) | 0 (0) | 1 |
| Deep vein thrombosis | 48 (10.0) | 1 (4.5) | 0.71 |
| Neurological complication | 5 (1.0) | 0 (0) | 1 |
| Renal failure | 5 (1.0) | 2 (9.1) | 0.03 |
| ACR | 120 (25.1) | 10 (45.5) | 0.03 |
| PGD grade ≧2 | 231 (48.2) | 7 (31.8) | 0.13 |
| Length of hospital stay (days) | 23.8±21.8 | 25.7±26.2 | 0.97 |
| 30-day/hospital mortality | 14 (2.9) | 1 (4.6) | 0.50 |
Clinical outcomes in recipients receiving lungs with and without incidentally discovered pulmonary embolism (PE). Values are presented as n (%) or mean ± standard error of the mean. P values ≤ 0.05 are denoted in bold. The denominator for the percentages is the sum of patients across all categories in the PE+ or PE− group, respectively, excluding missing values. ACR, Acute cellular rejection; PE, pulmonary embolism; PGD, primary graft dysfunction.
Long-term outcomes
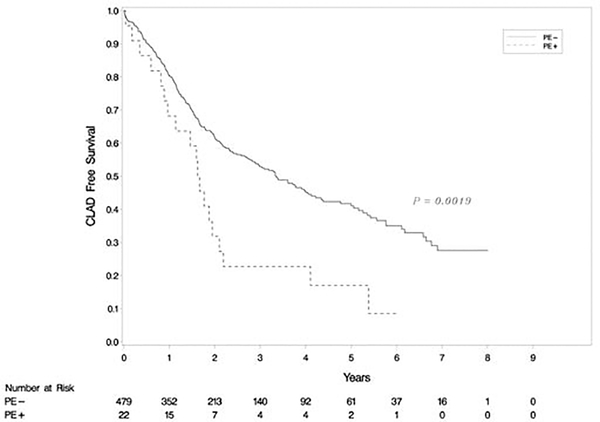
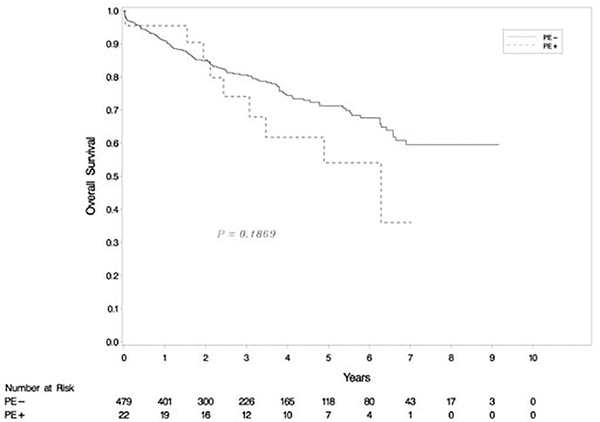
The 501 patients in our cohort were followed up for a mean of 39.1 ±27.4 months (range: 0–110 months). Kaplan-Meier analysis revealed that the CLAD-free survival rate was significantly higher in recipients from donors without PE than with PE (p=0.002) (Figure 2). Cox multivariable modeling showed that donor PE was associated with a higher likelihood of CLAD (hazard ratio [HR], 2.02, 95% confidence interval [CI] 1.23–3.30, p=0.005), while donor smoking status (>20 pack-years) was associated with a reduced incidence of CLAD (HR 0.61, CI 0.39–0.97, p= 0.03) after lung transplantation (Table 3). None of the other donor or recipient characteristics included in our study were associated with the development of CLAD. There was no statistical difference in OS between transplants from donors with and without PE (p=0.19) (Figure 3).
Figure 2:
Kaplan-Meier analysis of chronic lung allograft dysfunction (CLAD)-free survival. The difference in CLAD-free survival rates between two groups was significant (p=0.002).
Table 3:
Univariable and multivariable Cox regression analyses for risk factors for CLAD
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| PE | 2.06 | 1.29–3.29 | 0.003 | 2.02 | 1.23–3.30 | 0.005 |
| Donor age | 1.00 | 0.99–1.01 | 0.84 | 1.005 | 0.99–1.02 | 0.38 |
| Donor male | 1.24 | 0.96–1.60 | 0.10 | 1.24 | 0.93–1.65 | 0.15 |
| Donor cause of death | 1.00 | 0.77–1.30 | >0.99 | 0.95 | 0.69–1.33 | 0.78 |
| Donor smoking history | 0.60 | 0.38–0.93 | 0.02 | 0.61 | 0.39–0.97 | 0.03 |
| Donor best PaO2 | 1.001 | 0.999–1.003 | 0.27 | 1.002 | 1.000–1.003 | 0.09 |
| Lung allocation score | 1.004 | 0.997–1.010 | 0.27 | 1.002 | 0.99–1.01 | 0.66 |
| Recipient age | 0.99 | 0.98–1.00 | 0.07 | 0.995 | 0.99–1.004 | 0.24 |
| Recipient male | 1.05 | 0.81–1.36 | 0.69 | 1.04 | 0.78–1.38 | 0.79 |
| Ischemic time | 1.001 | 0.999–1.002 | 0.54 | 1.000 | 0.998–1.002 | 0.76 |
| CMV mismatch | 1.21 | 0.94–1.55 | 0.13 | 1.08 | 0.83–1.40 | 0.57 |
| PGD grade ≧2 | 1.24 | 0.97–1.59 | 0.09 | 1.30 | 0.99–1.70 | 0.06 |
| ACR | 1.16 | 0.89–1.52 | 0.27 | 1.03 | 0.78–1.35 | 0.86 |
Univariable and multivariable Cox regression model examining association between donor and recipient characteristics and chronic lung allograft dysfunction (CLAD). P values ≤ 0.05 are denoted in bold. ACR, Acute cellular rejection; HR, hazard ration; CI, confidence interval; PE, pulmonary embolism; CMV, cytomegalovirus; PGD, primary graft dysfunction.
Figure 3:
Kaplan-Meier analysis of overall survival (OS). There was no statistical difference in OS rates between two groups (p=0.19).
Comment
We have investigated the impact of incidentally discovered donor PE on recipient outcomes following lung transplantation. We found that recipients receiving lungs from donors with PE detected during lung procurement had a higher incidence of ACR after lung transplantation. Furthermore, recipients receiving lungs from donors with PE had reduced CLAD-free survival rate. We consider our findings preliminary and hypothesis generating, yet they may offer important insights into chronic rejection after lung transplantation.
PE is a relatively common finding in brain dead patients. The incidence of deep-vein thrombosis and PE are higher among patients who sustain a traumatic brain injury than those without head injury12. Ware and colleagues2 noted that the incidence of PE may be as high as 35% in potential donors whose lungs were rejected for transplantation. In this study, lungs were not flushed with cold preservation solution and were examined for both gross and microscopic evidence of PE, explaining the higher incidence compared with our findings. Interestingly, in both this study and ours, donor characteristics and PaO2 were not significantly different between those with and without PE. These findings highlight the importance of the intra-operative evaluation, as donor PE cannot be reliably predicted due to significant variability in evaluation of potential donors via echocardiography and contrast CT scan13.
One prior study has examined the significance of incidentally discovered donor PE prior to lung transplantation3. Here, donor lungs from a cohort of 122 consecutive transplants were flushed antegrade, but not retrograde, in the donor operating room and examined for the presence of PE after cold storage. The authors found that 38% of these lungs contained PE during a delayed retrograde flush in the recipient operating room3; these results mirror those discussed above by Ware and colleagues, who also did not perform an early retrograde cold flush. It is our standard practice to consecutively perform both an antegrade and retrograde flush during organ procurement7. The rationale for this technique is to rapidly cleanse the lungs of static blood products immediately after perfusion is stopped and thereby prevent thrombosis. Here, we report that with this technique the incidence of donor PE is only 4.4%, a figure which is substantially lower than prior reports2, 3.
Lung transplantation remains the only treatment for advanced end-stage lung disease. As lung transplantation is limited by the shortage of donor organs, the use of marginal donors is one strategy to expand the donor pool14. It is unclear whether lungs with unexpected PE impact outcomes after transplantation. However, successful implantation of donor lungs with PE has been performed with good long-term outcomes by utilizing a mechanical thrombectomy and heparin flush protocol5. In the study by Sommer and colleagues5, the lungs from donors with significant PEs were subjected to a heparinized saline flush in the recipient operating room prior to transplantation. Contrary to our results, they found that lungs from donors with PE actually resulted in superior 5-year CLAD free survival. While these findings may in part be explained by the fact that donors with PE were significantly younger than those without (37 vs 46 years of age), they also may be related to the extra heparin flush that was administered. Given that the findings from our study are not congruent, we recommend that surgeons consider utilizing the thrombectomy and heparin flush described by Sommer et al.5 and exercise caution when accepting lungs with PE, weighing the risk of transplantation and the urgency of the procedure. Furthermore, frequent monitoring of recipients from donors with PE after transplantation may contribute to early detection and treatment of ACR or CLAD.
With the changing landscape of donor management and optimization, another dilemma that surgeons may be faced with is whether or not employ ex vivo lung perfusion (EVLP) in marginal lung grafts prior to making the decision for transplantation. EVLP allows for a unique opportunity to treat such lungs with therapies that may improve function. Keshavjee and colleagues have previously described alteplase infusion via EVLP in a donor with PE, which resulted in ex vivo thrombolysis and a favorable recipient outcome15. While future studies are needed to clarify the benefit of EVLP-mediated thrombolysis, this therapy may hold promise for optimizing donor lungs with PE.
It is important to look for the presence of PE in donors as we have noted that incidentally discovered PE may be associated with adverse recipient outcomes. Current donor management does not include venous ultrasound for lower extremity deep venous thrombosis or PE-protocol CT scans13. Chest CT imaging, which has previously been shown to influence the decision to accept or decline lungs for transplantation, is commonly used to evaluate potential lung donors16. However, a standard chest CT has a sensitivity of 66–93% for detecting PE, and PE-protocol CT scan, with appropriate dose and timing of intravenous contrast, may be needed to adequately detect PE17. Furthermore, donors may develop PE between the time of imaging and the procurement operation, undermining the value of these tests.
Anatomically, the lungs are supplied by two separate vascular systems consisting of the pulmonary arteries and bronchial arteries. The bronchial arteries carry oxygenated blood to the lungs at a pressure six times that of the pulmonary arteries18. The bronchial arterial circulation has an important role in maintaining homeostasis in the lungs, however, only the pulmonary artery circulation is restored at the time of lung transplantation and highly oxygenated bronchial artery circulation is sacrificed19,20. This directly damages the microvasculature in lung grafts, results in proinflammatory and oxidative milieu, and leads to the development of bronchiolitis obliterans syndrome (BOS) and CLAD20. In a dog transplant model, Nowak and colleagues showed that bronchial artery revascularization protects pulmonary endothelium and type II pneumocytes21. We hypothesize that chronic small airway ischemia resulting from thromboembolic occlusion of small pulmonary arteries, which are the only source of airway blood supply after lung transplantation, may explain our findings.
We also noted that donor smoking status (>20 pack-years) may be associated with a reduced incidence of CLAD after lung transplantation. Conflicting data exist regarding whether donor smoking is associated with the risk of CLAD. Hennessy and colleagues22 reported that donor tobacco use was an independent risk factor for CLAD, whereas Schultz and colleagues23 reported that donor-smoking status was not associated with CLAD-free survival. In our study, smoking history was obtained from chart review and verification is not possible. Furthermore, our data set does not account for the dose of smoking exposure and smoking other agents such as marijuana, potentially leading to miscategorization or under-reporting of smoking status. Our data set also did not include fat emboli, which may have an adverse impact on recipient outcomes as well. In addition, we have not recorded the burden of PE, which may be associated with the incidence and severity of adverse outcomes. Vedovati and colleagues24 assessed the burden of emboli by multidetector CT scan in patients with acute PE and showed that central emboli are associated with increased risk for all-cause death or clinical deterioration. In future studies we hope to examine the above variables that were not available in our dataset at the time of this analysis.
In our program, heart and lung procurements are performed by a single, highly experienced cardiothoracic surgeon (MKP); if this surgeon is unavailable, then the procurement is performed by a senior level trainee. The findings presented here reflect the observations of our senior procurement surgeon in a prospectively maintained database and represent a standardized and meticulous approach to organ assessment and procurement. Donor level findings for procurements performed by trainees only were not entered into this database, which may limit the generalizability of our results. However, we also feel that this is unlikely to limit the reproducibility of our findings if the presence or absence of a PE is meticulously assessed, regardless of the procuring surgeon.
Since the analysis was performed, we have not seen a donor lung with a PE. However, we have proposed the application of the results from this study at our institution in several ways. Perhaps most notably, in very high-risk recipients we will be reluctant to use donor lungs with significant PE discovered at the time of procurement. While we have not specified a specific lung allocation score cutoff for which these lungs will be declined, we will assess them carefully on a case by case basis and be hesitant to use these lungs in patients who are on a ventilator or ECMO, as well as those for whom we anticipate a technically complex transplant (i.e.: history of severe infections, chest surgery, pleurodesis, etc.). Conversely, some factors will make us more likely to use lungs with PEs, such as recipient sensitization and those with a short stature, rendering them unlikely to get another organ match. Additionally, we plan to use the Sommer protocol5 for meticulous removal of all thrombus in the recipient operating room, followed by an antegrade flush with heparinized saline. The availability of EVLP has also provided another therapeutic avenue, as was shown by Keshavjee and colleagues15.
In conclusion, lung transplants from donors with incidentally discovered PE were associated with a higher incidence of ACR, as well as reduced CLAD-free survival. Additional research is needed to confirm these findings, define their mechanism, and guide the clinical decision making of surgeons faced with this dilemma.
Supplementary Material
Supplemental Figure 1. CONSORT diagram illustrating the selection of patients included in the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lisman T, Leuvenink HG, Porte RJ, Ploeg RJ. Activation of hemostasis in brain dead organ donors: an observational study. J Thromb Haemost. 2011;9:1959–1965. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Fang X, Wang Babcock WD, Jones K, Matthay MA. High prevalence of pulmonary arterial thrombi in donor lungs rejected for transplantation. J Heart Lung Transplant. 2005;24:1650–1656. [DOI] [PubMed] [Google Scholar]
- 3.Oto T, Rabinov M, Griffiths AP, et al. Unexpected donor pulmonary embolism affects early outcomes after lung transplantation: a major mechanism of primary graft failure? J Thorac Cadiovasc Surg. 2005;130:1446. [DOI] [PubMed] [Google Scholar]
- 4.Yun JJ, Unai S, Pattersson G. Lung transplant with bronchial arterial revascularization: review of surgical technique and clinical outcomes. J Thorac Dis. 2019;11:S1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer W, Kirschner H, Ius F, et al. Transplantation of donor lungs with pulmonary embolism – a retrospective study. Transpl Int. 2019;32:658–667. [DOI] [PubMed] [Google Scholar]
- 6.Fisher S, Gohrbandt B, Meyer A, Simon AR, Haverich A, Strüber M. Should lungs from donors with severe acute pulmonary embolism be accepted for transplantation? The Hannover experience. J Thorac Cardiovasc Surg. 2003;126:1641–1643. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Terada Y, Pasque ML, et al. Comparison of outcomes in lung and heart transplant recipients from the same multiorgan donor. Clin Transplant. 2020;34:e13768. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. [DOI] [PubMed] [Google Scholar]
- 10.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT working group on primary graft dysfunction, part I: Definition and grading-A 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier JM, Hachem RR, Kreisel D. Update on Chronic Lung Allograft Dysfunction. Curr Transplant Rep. 2016;3:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echeverria RF, Baitello AL, Pereira de Godoy JM, Espada PC, Morioka RY. Prevalence of death due to pulmonary embolism after trauma. Lung India. 2010;27:72–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaney J, Suzuki Y, Cantu E 3rd, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014;6:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaresan S, Semenkovich J, Ochoa L, et al. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg. 1995;109:1075–1079. [DOI] [PubMed] [Google Scholar]
- 15.Machuca TN, Hsin MK, Ott HC, et al. Injury-specific ex vivo treatment of the donor lung: pulmonary thrombolysis followed by successful lung transplantation. Am J Repir Crit Care Med. 2013;188:878–880. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier JM, Bierhals AJ, Liu J, et at. Chest computed tomography imaging improves potential lung donor assessment. J Thorac Cardiovasc Surg. 2019;157:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng J, Krishnan JA, Segal JB, et al. Accuracy of CT in the Diagnosis of Pulmonary Embolism: A Systematic Literature Review. AJR Am Roentgenol. 2004;183:1819–1827. [DOI] [PubMed] [Google Scholar]
- 18.Walker CM, Rosado-de-Christenson ML, Martinez-Jiménez S, Kunin JR, Wible BC. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics. 2015;35:32–49. [DOI] [PubMed] [Google Scholar]
- 19.Nicolls MR, Zamora MR. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant. 2010;15:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigemura N, Tane S, Noda K. The Bronchial Arterial Circulation in Lung Transplantation: Bedside to Bench to Bedside, and Beyond. Tranplantation. 2018;102:1240–1249. [DOI] [PubMed] [Google Scholar]
- 21.Nowak K, Kamler M, Bock M, et al. Bronchial artery revascularization affect graft recovery after lung transplantation. Am J Repir Crit Care Med. 2002;165:216–220. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy SA, Hranjec T, Swenson BR, et al. Donor factors are associated with bronchiolitis obliterans syndrome after lung transplantation. 2010;89:1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz HH, Møller CH, Zemtsovski M, et al. Donor Smoking and Older Age Increases Morbidity and Mortality After Lung Transplantation. Transplant Proc. 2017;49:2161–2168. [DOI] [PubMed] [Google Scholar]
- 24.Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT Scan for Acute Pulmonary Embolism: Embolic Burden and Clinical Outcome. Chest. 2012;142:1417–1424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CONSORT diagram illustrating the selection of patients included in the data analysis.