Abstract
Intracerebral hemorrhage (ICH) is a stroke subtype with high mortality and severe morbidity. Hemorrhages frequently develop within white matter, but whether white matter fibers within the hematoma survive after ICH has not been well studied. The current study examines whether white matter fibers persist in the hematoma after ICH, fibers that might be impacted by evacuation, and their relationship to macrophage infiltration in a porcine model. Male piglets had 2.5 ml blood with or without CD47 blocking antibody injected into the right frontal lobe. Brains were harvested at from 3 days to 2 months after ICH for brain histology. White matter fibers were detected within the hematoma 3 and 7 days after hemorrhage by brain histology and myelin basic protein immunohistochemistry. White matter still remained in the hematoma cavity at 2 months after ICH. Macrophage scavenger receptor-1 positive macrophages/microglia and heme oxygenase-1 positive cells infiltrated into the hematoma along the intra-hematomal white matter fibers at 3 and 7 days after ICH. Treatment with CD47 blocking antibody enhanced the infiltration of these cells. In conclusion, white matter fibers exist within the hematoma after ICH and macrophages/microglia may use such fibers as a scaffold to infiltrate into the hematoma and aid in hematoma clearance.
Keywords: intracerebral hemorrhage, microglia/macrophages, phagocytosis, white matter
Introduction
Spontaneous intracerebral hemorrhage (ICH) affects four million patients worldwide each year.1 It is the second most common cause of stroke and is associated with high mortality and severe morbidity.2 Mass effect associated with the hematoma and clot components released into surrounding cerebral tissues contribute to brain injury following ICH3–6. The initial injury occurs within minutes to hours of the ICH and is primarily induced by rapidly progressive mass effect leading to decline in cerebral perfusion pressure as well as mechanical injury to surrounding cerebral tissue.7, 8 Following the initial injury, cellular debris and clot components initiate secondary injury that takes place over days to weeks.7–9 There are currently two major forms of hematoma removal, surgical evacuation and endogenous clot reabsorption. With respect to surgical evacuation, the STICH and STICH II trails failed to prove that operative intervention was superior to conservative management of ICH.1, 10 The MISTIE trial strived to liquefy the hematoma with recombinant tissue plasminogen activator (rt-PA) and then perform subsequent aspiration. Though this approach reduced hematoma size and mortality, it did not improve functional outcome, the primary endpoint of the study.11
An alternate approach may be to enhance endogenous clot resorption. Resident microglia or infiltrating macrophages can phagocytize the clot and thus a number of approaches have been used to accelerate such a process in animal models4, 12. Our own lab has utilized CD47 blocking antibody13, 14 which neutralizes the CD47 on erythrocytes, a “don’t eat me” signal to prevent phagocytosis, to enhance phagocytosis of erythrocytes in animal models.
White matter fibers have long been considered to be extremely fragile and a key predictor of prognosis in various neurological disorders. Clinical neurology has shown that white matter serves a critical role in the organization and distribution of neural networks.15, 16 With evidence that more than 77% of ICH patients have white matter injury17, attempts are underway to develop therapeutic agents to reduce white matter injury post ICH, though without significant success.18 In part, this is due to the fact that white matter accounts for 10–20% of the rodent brain volume, whereas, in humans, it accounts for up to 50%19 stressing the need for large animal models.
Previous studies have focused on peri-hematomal white matter damage after ICH20–22; however, the presence and changes to white matter fibers within the ICH has not been well studied. In fact, intraclot white matter fibers have the potential of significant injury during surgical evacuation. We aim to utilize a swine ICH model to study the presence and survival of intraclot white matter tracts. In addition, we will evaluate the role of such white matter tracts as a route of microglia/macrophage infiltration into the hematoma core and the effect of a CD47 blocking antibody on such infiltration.
Methods
Animal Preparation
Approval of all animal procedure protocols was obtained from the University Committee on Use and Care of Animals, University of Michigan, and all experiments were conducted in accordance with United State Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. A total of 25 male piglets (8–10 kg; Michigan State University, East Lansing) were used in this study. One piglet was excluded because of high fever after surgery. The ICH models were performed as previously described23. Pigs were sedated with ketamine (25 mg/kg, IM) and anesthetized with 2% isoflurane via nose cone. The isoflurane concentration was maintained at 1.0 to 1.5% during the surgical procedures. Core body temperature was maintained at 37.5 ± 0.5 °C by a feedback-controlled heating pad. A polyethylene catheter (PE-160) was inserted into the right femoral artery to obtain blood for injection and to monitor arterial blood pressure, blood gases, and glucose concentrations. A cranial burr hole (1.5 mm) was drilled at a point 11 mm lateral to the sagittal suture and 11 mm anterior to the coronal suture. An 18-mm-long 20-gauge sterile plastic catheter was placed stereotactically into the right frontal cerebral white matter. Two consecutive injections were performed with an infusion pump: first 1.0 mL of autologous arterial blood was infused over 10 min and then, after a 5 min break, 1.5 mL blood was injected over 10 min. The needle was removed and the skin incision was closed with sutures.
Experimental groups
There were two parts of experiments. In the first part, piglets had 2.5 ml autologous blood injected into the right frontal lobe. Brains were harvested at 3 days, 7 days and 2 months after ICH for brain histology (n=4 for each time point). In the second part, piglets had 2.5 ml autologous blood with CD47 blocking antibody (Invitrogen, 16-0479-85, 10 μg/ml, final concentration in blood). Brains were then harvested at days 3 and 7 for brain histology (n=6 for each time point).
Brain histology
Piglets were re-anesthetized 2% isoflurane via nose cone and transcardially perfused in situ with 10% formalin at 3 days, 7 days or 2 months after ICH onset. Paraffin embedded brain was cut into 10 μm thick coronal sections.
Hematoxylin and eosin (H&E) staining
Regular H&E staining of brain sections was performed to detect the morphology of the white matter fibers in the hematoma. The paraffin brains sections were de-paraffinized, stained with hematoxylin and eosin sequentially and washed with tap water.
Immunohistochemistry
Immunohistochemistry staining was performed as described previously23. In brief, the sections were deparaffinized in xylene and rehydrated in a graded series of alcohol dilutions. Antigen retrieval was performed by the microwave method with citrate buffer (10 mmol/L, pH 6.0). Immunohistochemistry studies were performed with avidin-biotin complex techniques. The primary antibodies were polyclonal rabbit anti-heme oxygenase-1 (HO-1, Enzo, ADI-SPA-895-F, 1:400 dilution), rabbit anti-macrophage scavenger receptor 1 (MSR1) (NOVUS, NBP1–00092, 1:200 dilution), and mouse anti-myelin basic protein (MBP) (Abcam, ab62631, 1:1000 dilution). Secondary antibodies were horse anti-mouse (1:500 dilution, Vector Laboratories Inc.) and goat anti-rabbit (1:500 dilution, Invitrogen). Eliminating primary antibodies were used as negative controls.
Cell count
HO-1 positive cells were counted on high-power images (40x magnification) taken using a digital camera. Counts were performed on three randomly selected regions in the center of the hematoma without white matter fibers and three regions in the hematoma with white matter fibers (1 mm2 frame). Measurements were performed by personnel blinded to treatment groups.
Statistical analysis
Quantitative data are presented as the mean ± SD. Comparisons were performed by Student t-test. A p-value of <0.05 was considered to be statistically significant.
Results
There was no mortality in this study. The process of hematoma absorption after ICH in the porcine model is depicted in Figures 1 and 2. White matter fibers (white arrows) were noted within the blood clot at 3 and 7 days after ICH in brain sections (Figure 1A). H&E staining also supported the existence of white matter fibers within hematomas 3 and 7 days after ICH (Figure 1B).
Figure 1.
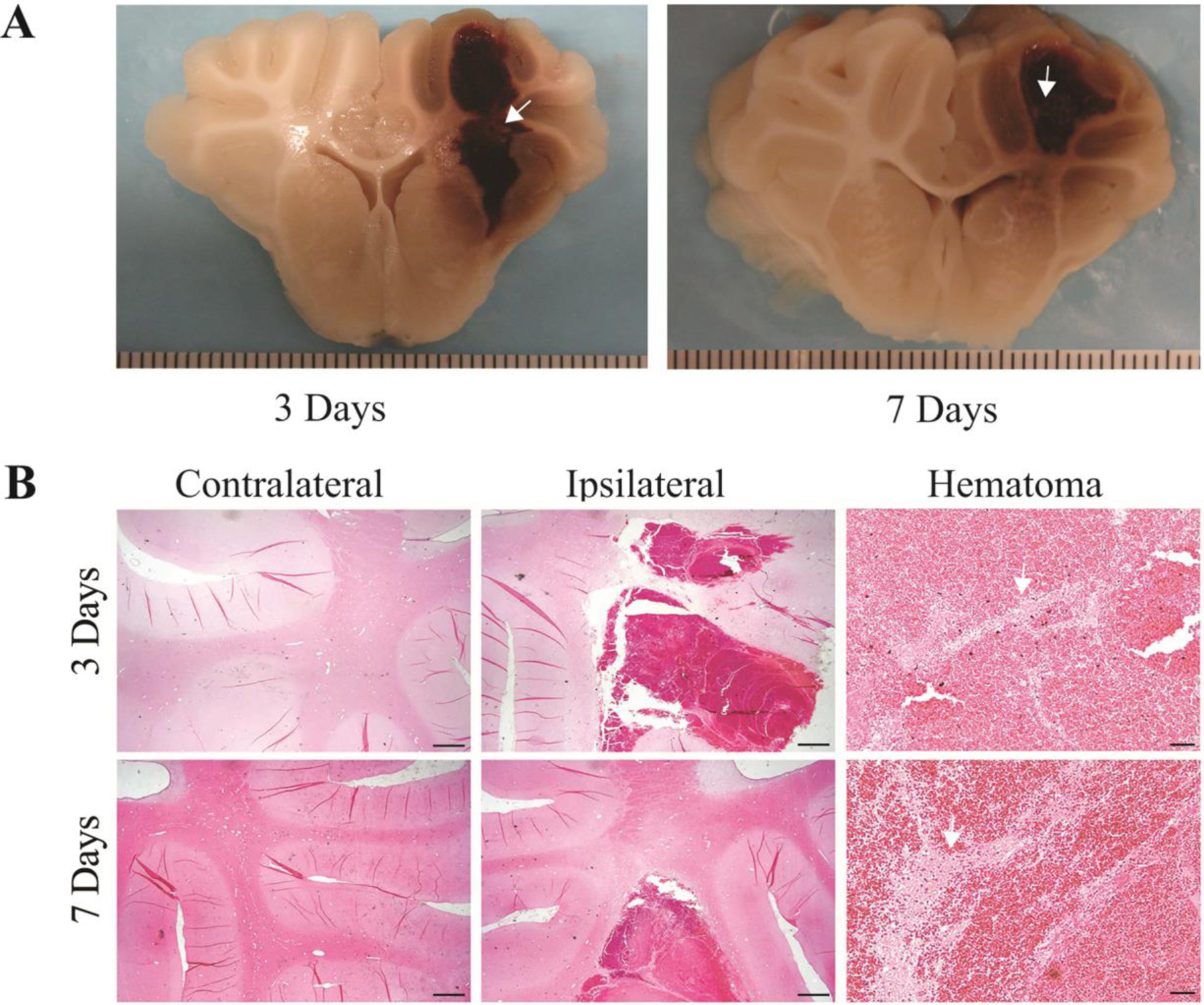
White matter survival in the hematoma. A) Coronal sections of perfused piglet brain showing hematomas with white matter inside (arrows) at 3 and 7 days. Scale intervals in (A) = 1 mm. B) H&E staining of white matter fibers (arrows) inside the hematoma at 3 and 7 days after ICH. Scale bar in the contralateral and the ipsilateral hemisphere = 1 mm; Scale bar in the hematoma = 50 μm.
Figure 2.
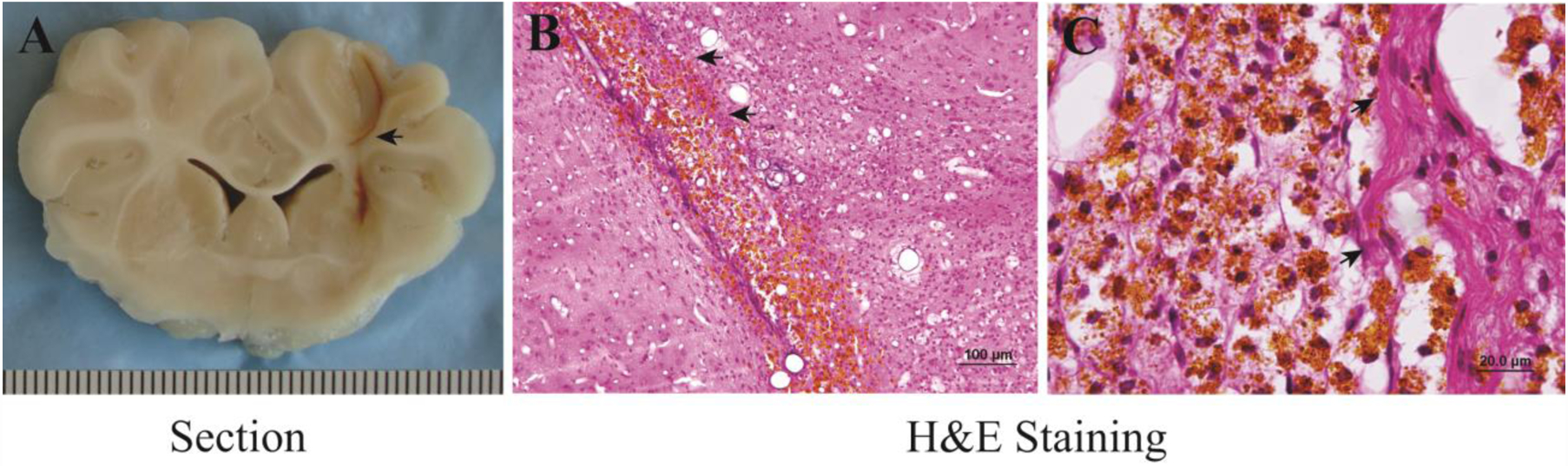
White matter survival in the hematoma cavity 2 months after ICH. A) A brain section showing hematoma cavity formation in the brain. B, C) H&E staining showing hemosiderin deposition and white matter in the cavity. Arrows indicate survived white matter. Scale intervals in (A) = 1mm, scale bar (B) = 100 μm, scale bar in (C) = 20 μm.
At 2 months, hematomas resolved leaving a small hematoma cavity (Figure 2A). In the cavity, there were many hemosiderin positive cells and white matter fibers (Figures 2B & 2C). The white matter fibers that had survived through the hematoma absorption process showed evidence of significant hemosiderin deposition.
To further confirm the presence of white matter in the hematoma, MBP (one of the major constituents of myelin) immunohistochemistry was performed. Survived MBP positive fibers were found within the hematoma at days 3 and 7 (Figure 3).
Figure 3.
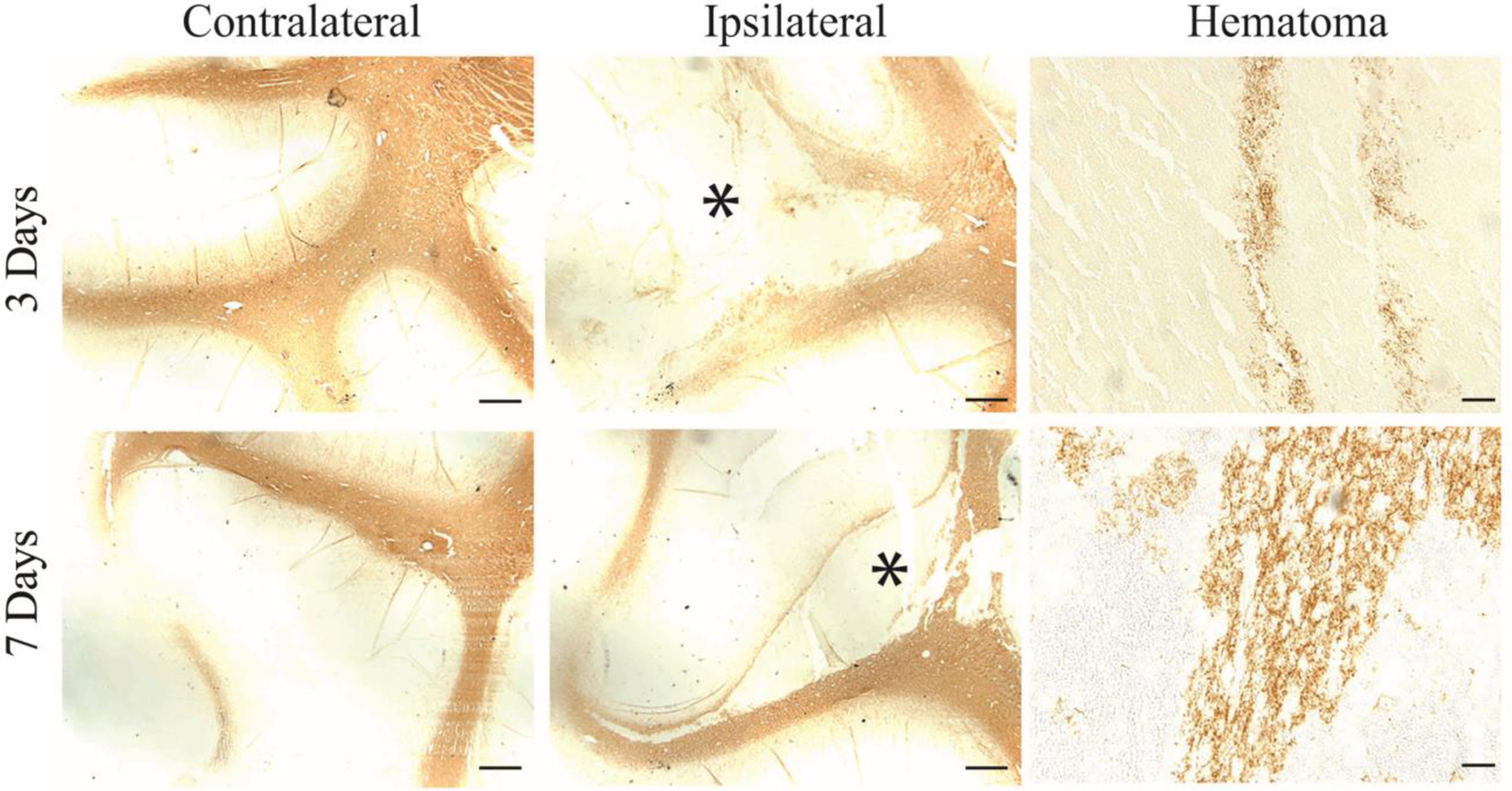
MBP staining of white matter fibers inside the hematoma at days 3 and 7 after ICH. The asterisk indicates the hematoma. Scale bar in the contralateral and the ipsilateral hemisphere = 1 mm; Scale bar in the hematoma = 50 μm.
To examine the role of intra-hematomal white matter fibers in macrophages/microglia infiltration, HO-1 and MSR1 positive cells in regions of the hematoma either with or without white matter fibers were quantified (Figure 4). The number of HO-1 positive cells in hematoma regions with white matter fibers was significantly higher at days 3 (340 ± 43/mm2) and 7 (333 ± 60/mm2) compared to those without white matter fibers (69 ± 6/mm2 at day 3, p<0.01; 63 ± 20/mm2 at day 7, p<0.01; Figure 4A). The morphological characteristics of the HO-1 positive cells were similar to microglia/macrophages. The number of MSR1 positive cells in the hematoma with white matter fibers was also significantly higher at day 3 (501 ±27/mm2) and day 7 (195 ± 101/mm2) compared to that without white matter fibers (75 ± 34/mm2 at day 3, p<0.05; 41 ± 43/mm2 at day 7, p<0.05; Figure 4B). The majority of the microglia/macrophages were centered around the white matter fibers.
Figure 4.
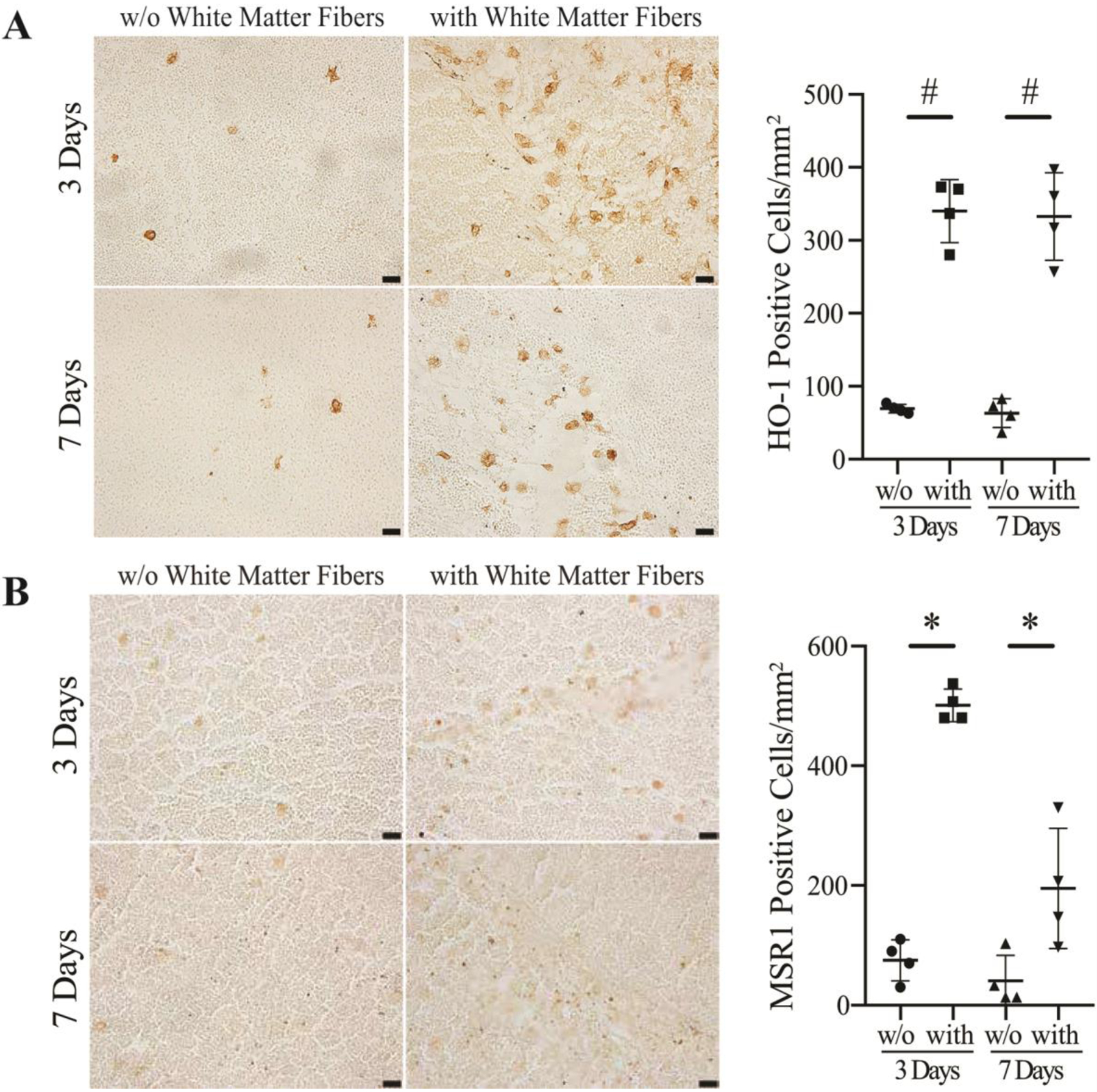
HO-1 (A) and MSR1 (B) immunoreactivity and numbers of positive cells in the hematoma in areas without (w/o) white matter fibers vs. with white matter fibers (with) at 3 and 7 days after ICH. Values are mean ± SD, n=4, *p<0.05, #p<0.01 vs. w/o white matter fibers. Scale bar=20 μm.
To further examine the relationship between hematoma white matter fibers and infiltration of macrophages/microglia into the hematoma, therapeutic grade CD47 blocking antibody was co-injected with autologous blood at the time of ICH. In the presence of the CD47 blocking antibody, the number of microglia/macrophage-like HO-1 positive cells that infiltrated into the hematoma in regions with white matter fibers was significantly higher at day 3 (544 ± 95/mm2) and day 7 (611 ± 220/mm2) after ICH compared to areas without white matter fibers (67 ± 17/mm2 at day 3, p<0.01; 56 ± 27/mm2 at day 7, p<0.01; Figure 5). The infiltration of HO-1 positive cells into the hematoma areas with white matter fibers was enhanced by using CD47 blocking antibody at days 3 and 7 after ICH compared to control ICH pigs (day 3, p<0.01; day 7, p<0.05; Figure 6). Similar results were found in MSR1 positive macrophages/microglia. The MSR1 positive cells infiltrated into the hematoma with white matter fibers was significantly higher at day 3 (a 5.4-fold increase, p<0.01) and day 7 (a 14-fold increase, p<0.05) compared with that without white matter fibers existing.
Figure 5.
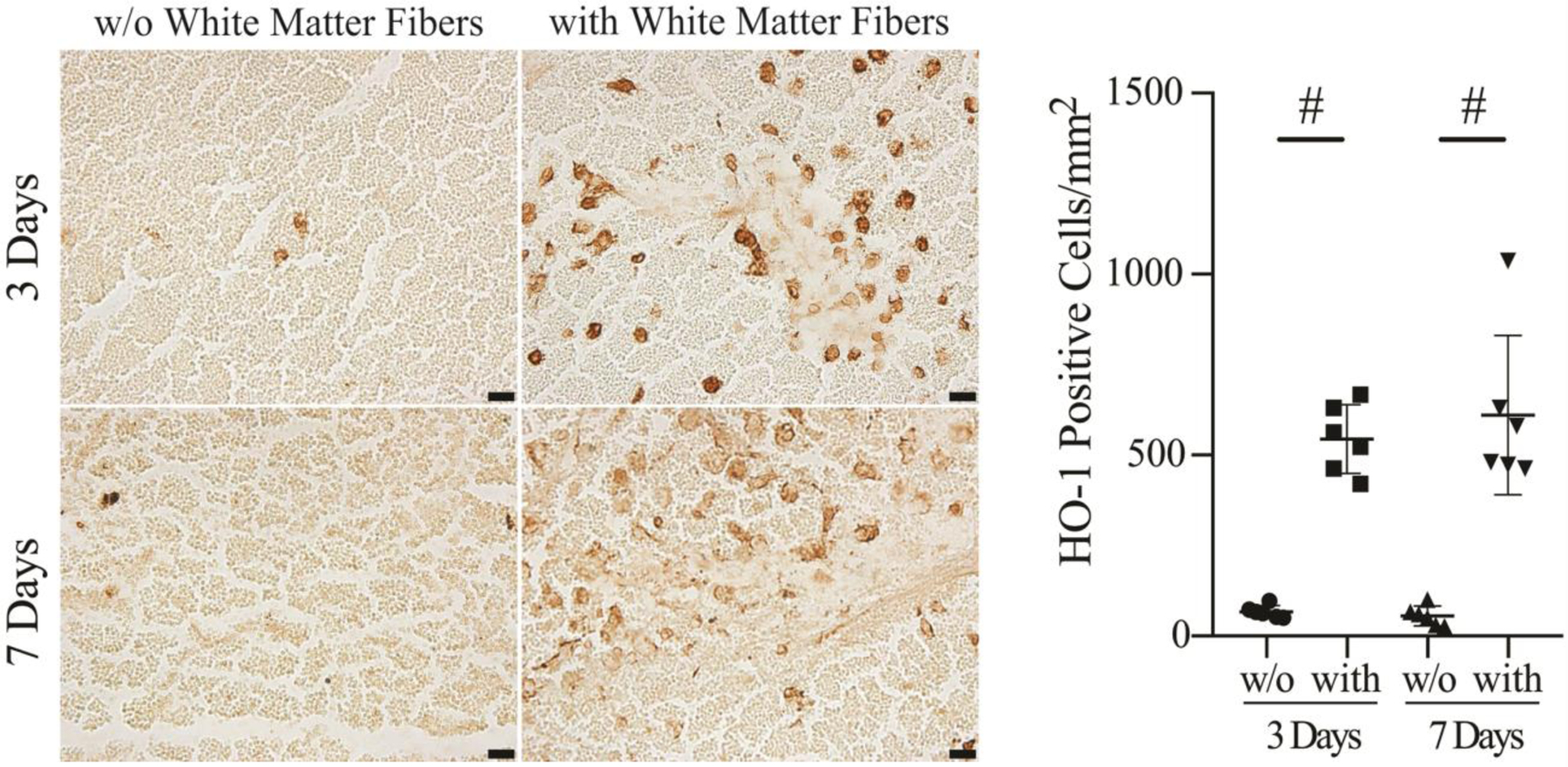
HO-1 immunoreactivity and numbers of positive cells in the hematoma in areas without (w/o) white matter fibers vs. with white matter fibers (with) at 3 and 7 days after ICH in animals treated with CD47 blocking antibody. Values are mean ± SD, n=6, #p<0.01 vs. w/o white matter fibers. Scale bar=20 μm.
Figure 6.
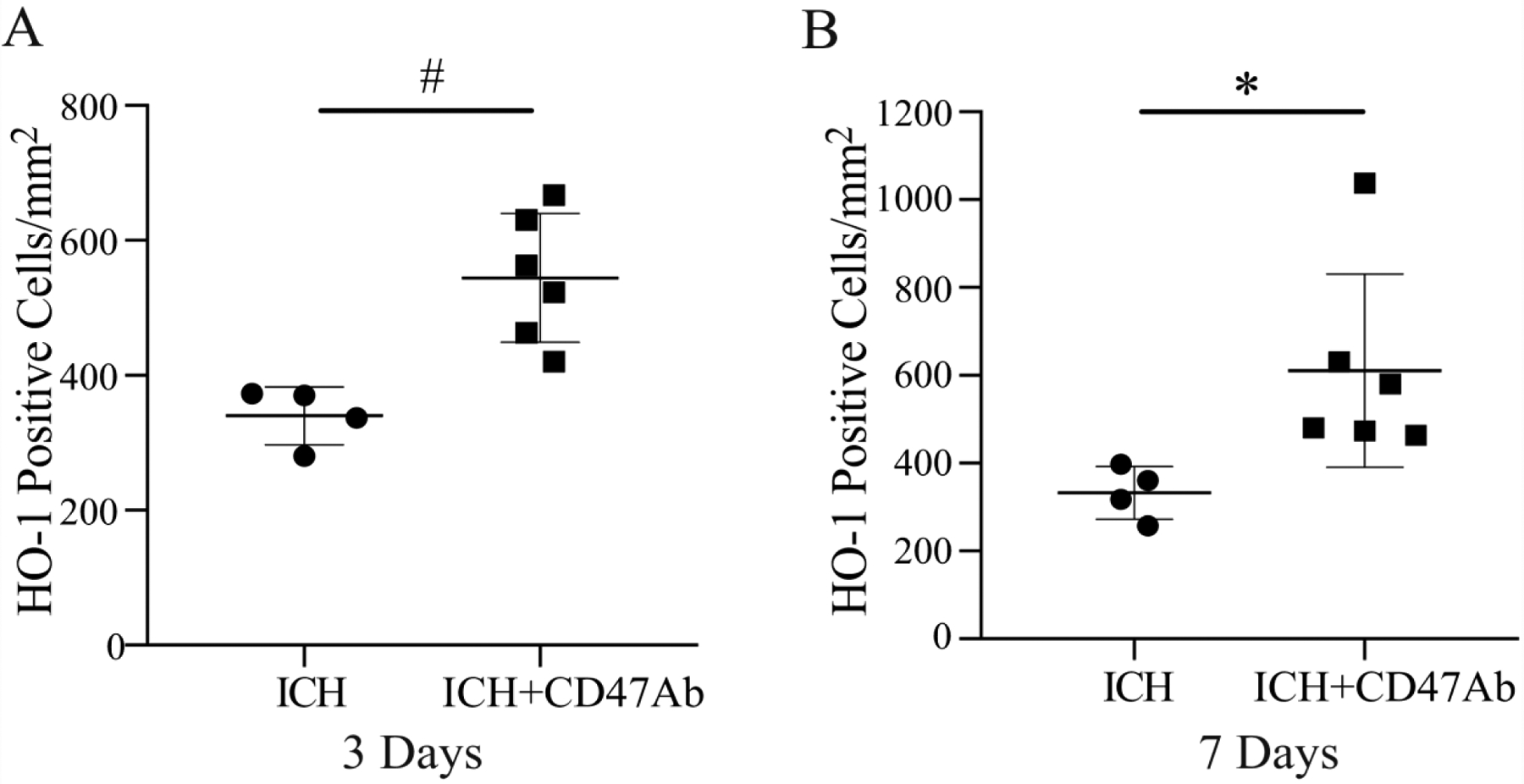
Numbers of HO-1 positive cells in the hematoma in areas with white matter fibers at 3 days (A) and 7 days (B) in pigs with ICH or ICH + CD47 blocking antibody (ICH+CD47Ab). Values are mean ± SD, n=4 in ICH and n=6 in ICH+CD47 blocking antibody, *p<0.05, #p<0.01 vs. ICH.
Discussion
The major findings of this study include: (1) survived white matter fibers exist in the hematoma core during the 2-month period after ICH examined; (2) HO-1 positive cells (microglia/macrophages) infiltrating into the hematoma core are associated with surviving intraclot white matter fibers; (3) a CD47 blocking antibody increased the infiltration of HO-1 and MSR1 positive cells into the hematoma via the white matter fibers, which may enhance the process of erythrophagocytosis post ICH.
Over the past few decades, the majority of ICH preclinical studies have focused on grey matter and neurons,20 with very little research on white matter and its injury progression.21 In general, white matter functions in the transfer of information within distributed neural networks, while gray matter performs information processing.24 Previous research in rodent ICH animal models showed absence white matter fibers within the hematoma and mainly focused on injury in the peri-hematomal region. However, this may be secondary to the fact that rodent brains have a lower percentage of white matter compared with humans (10–20% vs. 50%).19 To date, there is no literature discussing the white matter change inside the hematoma after ICH. This study, via the porcine model, reveals that morphologically normal white matter fibers are present within the hematoma core post ICH and that these fibers survive even after the hematoma resolves at 2 months after ICH.
Neuroinflammation is an important component of many forms of injury including stroke, traumatic brain injury, and neurodegenerative diseases. Microglia/macrophages constitute 5% to 20% of the total glial population in the central nervous system. In ICH, microglia/macrophages are activated as the primary inflammatory cells of the brain. After activation, these cells can release a variety of neurotoxic products such as cytokines, nitric oxide and other potentially toxic factors, which lead to secondary brain injury.25 However, therapeutic strategies aiming at dampening the inflammatory process have not always resulted in protective effects. In fact, several studies have shown the potentially beneficial role of an inflammatory response, including physically confining the damage, producing trophic factors, and removing cellular debris.26–28 Microglia/macrophages are phagocytes that have an important role in preserving tissue integrity and function by engulfing old and damaged cells including erythrocytes as a contributor to hematoma clearance.29, 30,4, 31, 32 The transformation of inactive microglia/macrophage into phagocytes is associated with alterations in cell surface receptors.33 These activated cells infiltrate white matter fibers following axonal damage, suggesting that they are responding to, rather than initiating, damage after ICH.20,34 In the current study, many microglia/macrophage-like HO-1 and MSR1 positive cells infiltrated into the hematoma along the white matter fibers. HO-1 is an enzyme for heme degradation associated with blood clearance by enhancing erythrophagocytosis after stroke.4, 35 MSR1 is a key phagocytic receptor for the uptake of lipids and cell debris in macrophages.36 Based on these results, intra-hematomal white matter fibers may act as a scaffold for the infiltration of microglia/macrophages throughout the hematoma and enhance the process of erythrophagocytosis. These white matter fibers may be playing an important role in creating a conducive microenvironment for microglia/macrophages migration to the damaged zones for hematoma clearance.
This study also indicates that CD47 blocking antibody enhances the infiltration of microglia/macrophage-like HO-1 and MSR1 positive cells into the hematoma along white matter fibers. The dose of CD47 blocking antibody was adopted from our previous mouse study13. CD47, as a “don’t eat me” signal, is expressed on red blood cells, and can inhibit phagocytosis via interaction with an inhibitory receptor, SIRP-α, on microglia/macrophages.37 Recently, it was noted that intra-hematomal CD47 levels decrease with time in pigs.23 Depleting CD47 in blood had an effect of increasing hematoma clearance in mice,38 and CD47 blocking antibody can increase hematoma clearance and reduce brain injury in mice by increasing microglia/macrophages activation13. In this study, the CD47 blocking antibody increased the infiltration of microglia/macrophage-like HO-1 and MSR1 positive cells into the blood clot along the intra-hematomal white matter fibers. It further demonstrates the important role of white matter fibers inside the hematoma in erythrophagocytosis after ICH.
At present, surgical intervention for spontaneous ICH remains controversial although MISTIE III reveals a trend to positive outcome in those who had the greatest volume of hematoma evacuated4. Although some studies have reported that early hematoma evacuation improved functional recovery in a certain subgroup of patients with spontaneous ICH, most randomized trials comparing surgery to conservative management have not demonstrated improved outcome with surgical treatment. The STICH trial, which represents the largest international, pragmatic, multicenter randomized trial to date, failed to prove a statistically significant difference in functional outcome between operative treatment and conservative management. MISTIE III was recently completed with some evidence of reduced mortality with hematoma removal but no evidence of improved functional outcomes.11 On the contrary, a case-control study on fibrinolytic therapy with rt-PA demonstrated that fibrinolytic therapy had beneficial short-term outcomes with reduced 30-day mortality. The mortality rate ranges from 15% to 25% with fibrinolytic therapy whereas it is 40% to 60% with craniotomy for hematoma evacuation in ICH.39 It could be hypothesized that during the surgical approach, in order to reach the hematoma, healthy cerebral tissue needs to be dissected and furthermore, any intra-hematomal white matter fibers may be disrupted.40 Even after surgical evacuation, there is always a residual hematoma, which can be a source of injurious clot derived factors like hemoglobin and iron41. In fact, even with minimally invasive catheter evacuation approach, the intra-hematomal white matter fibers observed in this study are vulnerable and easily damaged. This could lead to a negative impact on cellular trafficking, regeneration, and clot clearance, whereas undisrupted brain tissue and white matter fibers overlying and within the blood clot could contribute to axon regeneration and hematoma clearance.
There are several limitations to this study. These include that the overall sample size for each time point is relatively small. Piglets were all male, reducing the generalizability of these results to the female population. Finally, the CD47 experiments were carried out as either autologous blood with CD47 blocking antibody or without the antibody. A more appropriate control group would have involved autologous blood with a non-blocking antibody. Although our previous study found that CD47 blocking antibody speeded hematoma clearance and reduced neurological deficits in a mouse model of ICH13, the effects of CD47 blocking antibody on hematoma clearance and functional outcomes were not determined in the current study. Despite the limitations of this study, it serves as an excellent proof of concept that white matter fibers exist within the hematoma and serve an important purpose in macrophages/microglia infiltration, which may be negatively impact if disrupted with surgical evacuation.
Conclusion
This study indicates that white matter fibers exist within the hematoma post ICH and that microglia/macrophages may utilize these intraclot fibers as a scaffold for infiltrating the hematoma core for erythrocyte clearance. Once present, these macrophages likely play a significant role in erythrophagocytosis and hematoma clearance. Further experiments are needed to evaluate the molecular mechanisms involved in the interaction between the hematomal white matter fibers and macrophages/microglia as well as clinical studies to develop more targeted methods for hematoma evacuation avoiding damaging such fibers when treating ICH patients.
Sources of Funding:
NC, YH, RFK, ASP and GX were supported by grants NS-096917, NS104663, NS106746, NS108042, NS112394 and NS116786 from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: Jingyin Chen, Sravanthi Koduri, Shuhui Dai, Yasunori Toyota, Ya Hua, Neeraj Chaudhary, Aditya S. Pandey, Richard F. Keep, and Guohua Xi declare that they have no conflict of interest.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (stich ii): A randomised trial. Lancet. 2013;382:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giurgiutiu DV, Yoo AJ, Fitzpatrick K, Chaudhry Z, Leslie-Mazwi T, Schwamm LH, et al. Severity of leukoaraiosis, leptomeningeal collaterals, and clinical outcomes after intra-arterial therapy in patients with acute ischemic stroke. J Neurointerv Surg. 2015;7:326–330 [DOI] [PubMed] [Google Scholar]
- 3.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurology. 2012;11:720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson DA, Keep RF, Hua Y, Xi G. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. Journal of cerebral blood flow and metabolism. 2018;38:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R, Li H, Hua Y, Keep RF, Xiao J, Xi G, et al. Early hemolysis within human intracerebral hematomas: An mri study. Transl Stroke Res. 2019;10:52–56 [DOI] [PubMed] [Google Scholar]
- 6.Pandey AS, Daou BJ, Chaudhary N, Xi G. A combination of deferoxamine mesylate and minimally invasive surgery with hematoma lysis for evacuation of intracerebral hemorrhage. Journal of cerebral blood flow and metabolism. 2020;40:456–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413 [DOI] [PubMed] [Google Scholar]
- 8.Nam KW, Lim JS, Kang DW, Lee YS, Han MK, Kwon HM. Severe white matter hyperintensity is associated with early neurological deterioration in patients with isolated pontine infarction. Eur Neurol. 2016;76:117–122 [DOI] [PubMed] [Google Scholar]
- 9.Bian L, Zhang J, Wang M, Keep RF, Xi G, Hua Y. Intracerebral hemorrhage-induced brain injury in rats: The role of extracellular peroxiredoxin 2. Transl Stroke Res. 2020;11:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. Lancet. 2005;365:387–397 [DOI] [PubMed] [Google Scholar]
- 11.Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (mistie iii): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J, Wang M, Jing C, Keep RF, Hua Y, Xi G. Multinucleated giant cells in experimental intracerebral hemorrhage. Transl Stroke Res. 2020;11:1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing C, Bian L, Wang M, Keep RF, Xi G, Hua Y. Enhancement of hematoma clearance with cd47 blocking antibody in experimental intracerebral hemorrhage. Stroke. 2019;50:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao C, Keep RF, Xi G, Hua Y. Cd47 blocking antibody accelerates hematoma clearance after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2020;11:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catani M, Dell’acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012;48:1262–1287 [DOI] [PubMed] [Google Scholar]
- 16.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613 [DOI] [PubMed] [Google Scholar]
- 17.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612 [DOI] [PubMed] [Google Scholar]
- 18.Zuo S, Pan P, Li Q, Chen Y, Feng H. White matter injury and recovery after hypertensive intracerebral hemorrhage. Biomed Res Int. 2017;2017:6138424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang YB, Wei KY, Zhang XY, Feng H, Hu R. White matter repair and treatment strategy after intracerebral hemorrhage. CNS Neurosci Ther. 2019;25:1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman JK, Schlichter LC. White matter injury in young and aged rats after intracerebral hemorrhage. Exp Neurol. 2008;214:266–275 [DOI] [PubMed] [Google Scholar]
- 21.Joseph MJ, Caliaperumal J, Schlichter LC. After intracerebral hemorrhage, oligodendrocyte precursors proliferate and differentiate inside white-matter tracts in the rat striatum. Transl Stroke Res. 2016;7:192–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45:290–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao S, Zheng M, Hua Y, Chen G, Keep RF, Xi G. Hematoma changes during clot resolution after experimental intracerebral hemorrhage. Stroke. 2016;47:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filley CM, Fields RD. White matter and cognition: Making the connection. J Neurophysiol. 2016;116:2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moxon-Emre I, Schlichter LC. Evolution of inflammation and white matter injury in a model of transient focal ischemia. J Neuropathol Exp Neurol. 2010;69:1–15 [DOI] [PubMed] [Google Scholar]
- 27.Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol Neurobiol. 2006;34:221–242 [DOI] [PubMed] [Google Scholar]
- 28.Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: Networking for survival? Neurochem Res. 2003;28:293–305 [DOI] [PubMed] [Google Scholar]
- 29.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788 [DOI] [PubMed] [Google Scholar]
- 30.Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–145 [DOI] [PubMed] [Google Scholar]
- 31.Chang CF, Goods BA, Askenase MH, Hammond MD, Renfroe SC, Steinschneider AF, et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest. 2018;128:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, et al. Tgf-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest. 2017;127:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86 [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Wang L, Sun XG, Tang J. Haematoma scavenging in intracerebral haemorrhage: From mechanisms to the clinic. J Cell Mol Med. 2018;22:768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schallner N, Pandit R, LeBlanc R 3rd, Thomas AJ, Ogilvy CS, Zuckerbraun BS, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H, et al. Mafb prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through msr1. Nat Med. 2017;23:723–732 [DOI] [PubMed] [Google Scholar]
- 37.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. Cd47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119:5512–5521 [DOI] [PubMed] [Google Scholar]
- 38.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of erythrocyte cd47 in intracerebral hematoma clearance. Stroke. 2016;47:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiex R, Rohde V, Rohde I, Mayfrank L, Zeki Z, Thron A, et al. Frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. J Neurol. 2004;251:1443–1450 [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Crit Care. 2020;24:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhary N, Pandey AS, Griauzde J, Gemmete JJ, Chenevert TL, Keep RF, et al. Brain tissue iron quantification by mri in intracerebral hemorrhage: Current translational evidence and pitfalls. Journal of cerebral blood flow and metabolism. 2019;39:562–564 [DOI] [PMC free article] [PubMed] [Google Scholar]


