Abstract
Background:
Williams syndrome (WS) is a neurodevelopmental disorder characterized by hypersociability, heightened auditory sensitivities, attention deficits, and strong musical interests despite differences in musical skills. Behavioral studies report individuals with WS exhibit variable beat and rhythm perception skills.
Methods:
We sought to investigate the neural basis of beat tracking in individuals with WS using electroencephalography (EEG). Twenty-seven adults with WS and sixteen age-matched typically developing control subjects passively listened to musical rhythms with accents on either the first or second tone of the repeating pattern, leading to distinct beat percepts.
Results:
Consistent with the role of beta and gamma oscillations in rhythm processing, individuals with WS and typically developing control subjects showed strong evoked neural activity in both beta (13-30 Hz) and gamma (31-55 Hz) frequency bands in response to beat onsets. This neural response was somewhat more distributed across the scalp for individuals with WS. Compared with typically developing control subjects, individuals with WS exhibited significantly greater amplitude of auditory evoked potentials (P1-N1-P2 complex) and modulations in evoked alpha (8-12 Hz) activity, reflective of sensory and attentional processes, compared to typically developing control subjects. Individuals with WS also exhibited markedly stable neural responses over the course of the experiment, and these were significantly more stable than those of controls.
Conclusions:
These results provide neurophysiological evidence for dynamic beat tracking in WS and coincide with the atypical auditory phenotype and attentional difficulties seen in this population.
Keywords: Williams syndrome, rhythm, EEG, beat perception, music, cognitive neuroscience
1. Introduction
Rhythm operates in a complex and dynamic temporal system. Perceived in the context of a musical beat, i.e., a regular pulse, rhythm can be organized hierarchically based on the organization of strong and weak beats. The neurophysiological basis of beat perception may be conceptualized under the framework of dynamic attending theory, which posits that internal neural oscillators, operating at different timescales, can rapidly entrain to the temporal regularities of external rhythmic stimuli over time (1,2). This framework has been applied to explain rhythm and timing impairments in individuals with developmental disorders, including individuals with Attention Deficit Hyperactivity Disorder (ADHD) (3–5) and dyslexia (6), who exhibit disruptions in oscillatory activity both at rest and in response to external stimuli. The present study applies the framework of dynamic attending theory towards examining the neurophysiological correlates of beat perception in individuals with Williams syndrome (WS).
Williams syndrome (WS) is a genetic, neurodevelopmental disorder caused by the deletion of ~28 genes on chromosome 7 (7). Individuals with WS exhibit a specific behavioral phenotype including mild to moderate cognitive impairment, attention problems, hypersociability, and heightened auditory sensitivities (8,9). Notably, individuals with WS exhibit increased emotional responsiveness to music, increased interest in music, and more time spent playing music than their same-aged peers despite variable music perception and production skills in both rhythmic and tonal domains (10–12).
The extant literature on beat and rhythm skills in WS highlights the high degree of individual variability amongst participants within and across studies, with some individuals with WS performing as well as age-matched peers and others performing more poorly. (13). Some studies have found that individuals with WS perform worse than chronological age-matched typically developing (TD) control individuals on same/different judgements of rhythmic patterns (14), reproducing rhythmic patterns by clapping or singing (12), or detecting the beat in music. Other work points to similarities between WS and TD groups on rhythm discrimination or production tasks (8,12,15). Differences in results across studies could relate to experimental paradigms that vary in their social context (12,15). Given that individuals with WS are hypersocial and may perform differently in a social setting, social factors could lead to differences in task performance. Furthermore, differences in results across studies could relate to the type of task (rhythm perception vs. production), assessment method (e.g., subjective judgements of performance), and associated task demands (e.g., working memory confounds in same/different judgments). In the present study, we recorded brain activity during a passive beat perception paradigm, with the advantages of eliminating these potential confounds on performance.
Our experimental design and rationale for the present study in participants with WS have been informed by the substantial literature on the neurophysiological mechanisms underlying beat perception in TD individuals. In a formative magnetoencephalography (MEG) study, listeners heard sequences of tones with a physical accent (via increased amplitude) on either the first or second tone in a sequence, thereby imposing distinct beat percepts (16). In another experimental condition, participants were asked to imagine the physical accents (in these “imagined beat” conditions there were no differences in loudness between the two successive tones). Increases in evoked beta oscillations time-locked to the accented tones (i.e., beats) were evident for both physically accented and imagined musical beats, suggesting evoked beta activity as a neural marker for beat perception of simple rhythms. Subsequent studies linked beta band neural activity to beat processing, particularly for beat anticipation and functional coordination of auditory and motor networks (17,18). Induced beta band power decreases after beat onset and rebounds prior to the subsequent beat in both children and adults (19) and has been linked to both timing and content predictions (20). Additionally, gamma activity may reflect rhythmic expectancy and sensory processing (18,21). Taken together, the patterns of beta and gamma band fluctuations in response to beat structure suggest that such neural activity is linked to sensory and motor processes that optimize attention to auditory rhythmic stimuli, consistent with dynamic attending theory.
The interplay between attention difficulties and atypical auditory processing in WS may contribute to their beat perception skills. Dynamic attending theory, which postulates that attention is facilitated by the temporal saliency of predictable external input, is especially relevant for probing beat perception in WS because of the characteristic problems with attention seen in this population (22,23). Individuals with ADHD, which is often comorbid in WS (23–25), exhibit impaired beat-based timing (4). In combination with their attentional difficulties, the high prevalence of auditory sensitivities in WS suggests difficulties in inhibiting excitatory responses to sound or disengaging from auditory stimuli (10,26). It is also possible that heightened responses to auditory stimuli could lead to enhanced beat perception skills in individuals with WS.
Neuroimaging work further supports an atypical auditory phenotype characteristic of WS. Electroencephalography (EEG) studies identify auditory evoked potentials, consisting of the event-related-potential (ERP) waveform units P1, N1, P2, N2 (27), as canonical neural markers of auditory stimulation. The heightened auditory sensitivities in WS are reflected in increased auditory evoked potentials to tones compared to age-matched controls; the P2 component in particular is more positive compared to controls at any age (28–30) suggesting atypical auditory processing in WS. Functional magnetic resonance imaging (fMRI) studies additionally reveal atypically diffuse processing of both musical and non-musical auditory stimuli in WS (31,32). As the P1-N1-P2 complex is implicated in rhythmic expectancy in perception and production tasks in neurologically healthy adults (33), these components may be greater in amplitude in individuals with WS compared to age-matched controls during beat perception tasks.
In addition to the above ERP correlates of the atypical auditory phenotype in WS, neural oscillatory activity may underlie the sensory and attentional difficulties in this population. Alpha band oscillatory activity is relevant for sensory processing and attentional segregation (34,35). Alpha activity exerts inhibition over processing of task-irrelevant information (34,36) and individuals with attentional difficulties exhibit atypical frontal alpha power (37). One study found greater evoked alpha power to happy vs sad musical stimuli in WS compared to TD, potentially reflecting aberrant sensory and attentional processing (26). In general, inefficient oscillatory responses (i.e.(5)) could potentially lead to downstream difficulties in orienting and preparing for the next stimulus. Atypical sensory processing and attentional difficulties in WS may manifest in atypical modulations of neural activity (such as alpha power) to rhythmically predictable stimuli.
In the current study, we assessed neurophysiological responses known to correlate with beat perception in individuals with Williams syndrome. Specifically, we 1) measured neural responses to beat patterns in both individuals with WS and age-matched TD controls using time-frequency oscillatory representations and auditory evoked potentials and 2) assessed how the strength of neural responses persisted over the course of the experiment. We hypothesized the neural responses to beat patterns in WS would differ from controls and could be attributed to an interaction between the atypical auditory phenotype and attentional difficulties in WS. This is the first study, to the best of our knowledge, to examine the neurophysiological mechanisms of beat perception in Williams syndrome.
2. Methods
Participants
Twenty-seven individuals with WS (13 Female, Age: M=27.90 years, SD=9.18) completed the study. Intellectual function for WS participants was assessed with the Kaufman Brief Intelligence Test, 2nd edition (KBIT-2; 38). The average KBIT-2 Composite IQ for WS participants was 64.0 (SD=13.9). A group of 16 age-matched TD individuals (12 Female, Age: M=33.63 years, SD=11.54) completed the EEG portion of the experiment for comparison purposes to the WS group. Further details about participants are provided in the Supplement.
The study was approved by the Institutional Review Board of the university. All TD controls and parents or guardians of WS participants provided written consent to participate. Participants with WS provided written assent.
Behavioral Measures
Parents of the WS participants completed questionnaires assessing musical and auditory traits in their children (Table 1). These included the Music Interest Scale (MIS; (39)), Sensitivity to Sounds (40), and Beat Alignment Test (BAT, (41), which was completed by the participants with WS. Please see the Supplement for a full description of these measures.
Table 1.
Behavioral measures collected in the WS group.
| Measure | WS mean ± sd (n=27) |
|---|---|
| Composite IQ | 64.0 ± 13.9 |
| Sensitivity to Sounds | 20.48 ± 5.96 |
| MIS Q11 Score (Rhythm) | 4.11 ± 1.15 |
| BAT D-primea | 1.11 ± 0.94 |
Note. IQ scores are from KBIT-2.
n=23 with valid data
EEG dynamic attending paradigm and procedure
The dynamic attending paradigm was adapted from the physical accent conditions of Iversen et al. (2009), which reported similar neural responses between beats with physical accents and “imagined” beats, where there were no amplitude changes present in the stimuli (16). Here, we used the physical accent condition rather than the imagined beat condition to ensure the stimuli contained a clear and consistent beat structure for participants with WS and would not require additional cognitive demands from participants.
Participants were presented with continuous trials of 2 tones (woodblock sounds) and a rest (Figure S1). The stimuli are available at https://osf.io/cxbd7/. Each woodblock sound had an approximate duration of 60 ms and a frequency of 656 Hz. The tones and rests were presented with an interonset interval of 200 ms; thus, each trial was 600 ms in total. Trials ran continuously for 30 seconds (1 block, 50 trials per block), and each block was one of two conditions. In the Accent1 condition, the first tone of each trial was physically accented; in the Accent2 condition, the second tone of each trial was physically accented. The physical accents were achieved by an amplitude increase of approximately 9 dB. The placement of the physical accent was the only aspect that differentiated the two conditions, resulting in distinct beat percepts (perceived beat on tone 1 in the Accent1 condition and perceived beat on tone 2 in the Accent2 condition). Each participant received 9 blocks of each condition, presented in a random order. The first two trials of each block were not analyzed to allow the participant to adjust to the beat percept in the new block.
All participants completed the passive dynamic attending paradigm while watching a silent film on a computer screen. The experiment lasted approximately 10 minutes and EEG data were collected for the full duration. Please see the Supplement for further details on EEG data collection.
EEG preprocessing
EEG data were preprocessed in MATLAB (2018b) with the EEGLAB toolbox version 14.1.2b (42). Please see the Supplement for a description of the EEG preprocessing pipeline.
Time-frequency analyses
Wavelet-based time-frequency decomposition was conducted using the Fieldtrip Toolbox (43) in MATLAB. Evoked (phase-locked) time-frequency representations (TFRs) were computed for each condition using the average event-related potential (ERP) waveform for each participant. The average waveform was convolved with a Morlet wavelet of width=five cycles with convolution from −400 to 800 ms and at 8 to 55 Hz (frequency step of 1 Hz, time step of 2 ms). TFRs were normalized to control for differences in absolute power between participants and to scale power values across frequencies such that baseline power was computed as the average across both conditions for each participant. The relative percent change in power from baseline was then calculated; these values replaced absolute power, resulting in normalized power across conditions.
Event-related potential (ERP) analyses
ERPs were calculated for each participant for each condition. Data for this analysis were bandpass filtered from 1-30 Hz and baseline corrected from −100 to 0ms, with 0ms as the start of each trial. Thus, each epoch was time-locked to the onset of the first tone presented in each condition. Grand-averaged ERPs were computed from −100ms to 500ms for each group and condition and averaged across 10 central electrodes (E6, E7, E13, E30, E55, E79, E80, E106, E112, Cz).
Stability metrics
We conducted a stability analysis to examine if neural responses to the beats persisted over the course of the experiment. Stability of neural responses over time were computed for ERP responses by correlating the ERPs (separately for each participant for the Accent1 and Accent2 conditions) for the first quarter and last quarter of trials for each condition (44). This was done for the same 10 central electrodes in the ERP analysis. Correlations were Fisher z transformed, averaged, and then transformed back to r values to determine the mean stability correlation for each group and condition. To assess differences in stability between the WS and TD groups, we conducted a two-sample t-test on the Fisher’s-z-transformed stability correlations.
Cluster-based permutation testing
We applied nonparametric cluster-based random permutation tests to test for differences in power (evoked time-frequency analyses) and amplitude (ERP analyses) between conditions and groups (45). All statistics were performed on stimuli with nonoverlapping time windows and divided into the following frequency bins: alpha (8–12 Hz), beta (13–30 Hz), and gamma (31–55 Hz). Clustering in the time-frequency analyses identified adjacent power values with a similar direction of effect by conducting t tests at each electrode and time point within the −100 to 500 ms latency, separately for each frequency band. Clustering in the ERP analyses was performed on our 10 central electrodes of interest. Significance was assessed using the Monte Carlo method (p <. 05 was considered significant), and cluster-based correction as implemented in Fieldtrip was used to control for multiple comparisons. The parameters of the cluster randomization tests are provided in the Supplement.
Within-subjects cluster-based permutation testing was performed on Accent1 versus Accent2 conditions, separately in the WS and TD groups in each of the frequency bands and for ERPs, using dependent-samples t tests. Between-group differences (WS vs. TD) were calculated on the condition differences (Accent1-Accent2) in each of the frequency bands and for ERPs using independent-samples t tests. Thus, group differences reported here represent a difference of difference, i.e. the difference in neural response between the Accent1 and Accent2 conditions between the WS and TD groups. Between group analyses accounting for age and sex, as well as analyses exploring WS individual differences in brain-behavior relationships, are presented in the Supplement.
3. Results
Evoked time-frequency analyses: Alpha, beta, and gamma activity between conditions and groups
Both groups exhibited significant evoked beta and gamma activity that was time-locked to beat onset in each condition (i.e., difference in neural activity to the first tone in each condition sequence (Beat1 effect) and difference in neural activity to the second tone in each condition sequence (Beat2 effect); Table 2 and Figure 1). The power of this beta and gamma activity did not differ between groups (WS vs. TD comparisons: p values >0.3).
Table 2.
Significant results of the cluster-based permutation tests for WS and TD control groups.
| Group | Comparison | Frequency band | Time window (ms) | Number of electrodes | p value |
|---|---|---|---|---|---|
| WS | Beat1 effect | Alpha | −74-228 | 125 | p< 0.0001 |
| Beta | −20-196 | 125 | p< 0.0001 | ||
| Gamma | −12-134 | 124 | p< 0.0001 | ||
| Beat2 effect | Alpha | 246-500 | 91 | p=0.004 | |
| Beta | 200-378 | 112 | p< 0.0001 | ||
| Gamma | 204-292 | 95 | p<0.0001 | ||
| TD Controls | Beat1 effect | Alpha | −70-206 | 83 | p<0.0001 |
| Beta | −18-132 | 90 | p=0.004 | ||
| Gamma | −14-92 | 107 | p=0.002 | ||
| Beat2 effect | Alpha | n.s. | n.s. | p=0.45 | |
| Beta | 184-356 | 98 | p=0.002 | ||
| Gamma | 206-288 | 100 | p=0.004 |
Note. Results reflect time windows and topographies where activity was significantly greater for the first tone in the sequence (Beat1 effect in the table) and the second tone in the sequence (Beat2 effect in the table) between conditions.
n.s. = not significant.
Figure 1.
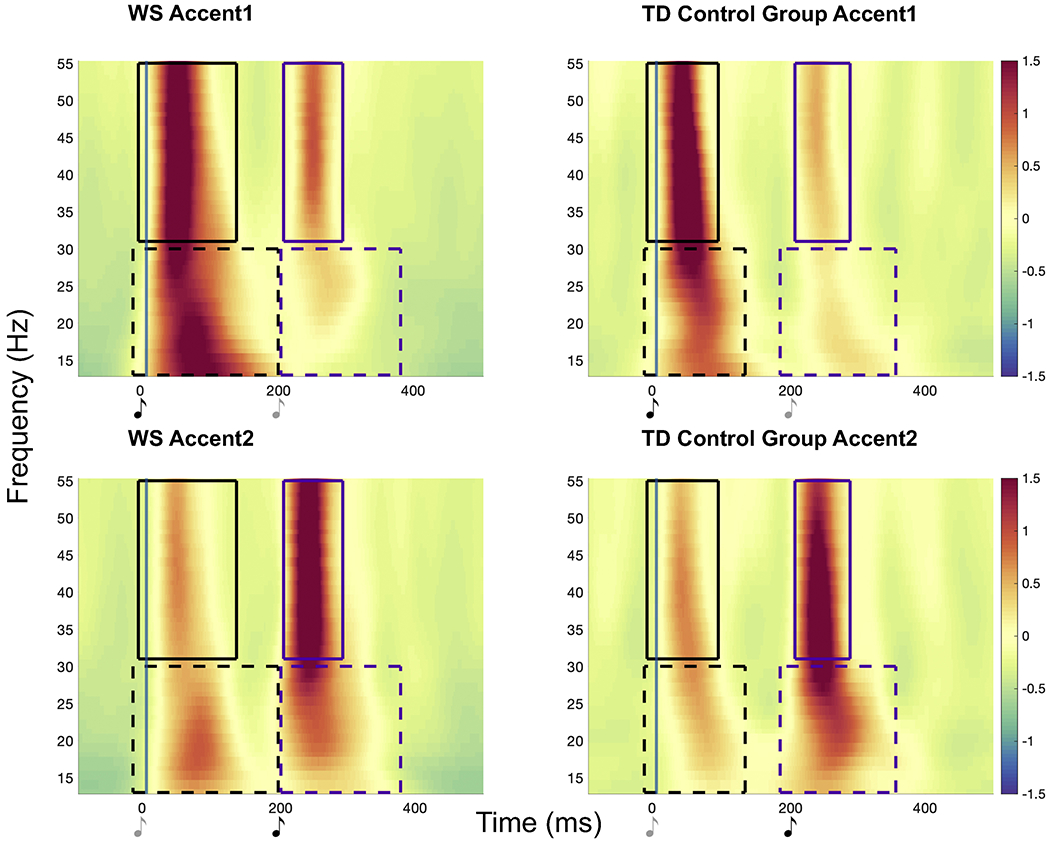
Time-frequency representations of normalized change in power in beta and gamma evoked activity, shown as an average across all electrodes, for both groups in Accent1 and Accent2 conditions. Significant neural activity in each frequency band (Beat1 effect in black and Beat2 effect in purple) is denoted by a box extending through the significant latency of activity, and these boxes represent between condition clustering. Dark music notes indicate accented tones and lighter notes indicate unaccented tones. Increases in evoked activity are time-locked to the physically accented tone (perceived as the beat) in both conditions in both WS and TD groups.
Significant evoked alpha activity time-locked to beat onset was found for both WS and TD groups for the Beat1 effect. The magnitude of this effect was significantly greater in the WS compared to the TD control group over the 74-254ms time window (p=0.021) across 31 electrodes. The WS group also exhibited significant evoked alpha activity for the Beat2 effect that was not present in the TD group. The difference in alpha activity between WS and TD controls for the Beat2 effect did not reach conventional significance levels (p=0.090). Figure 2 depicts alpha power modulations.
Figure 2.
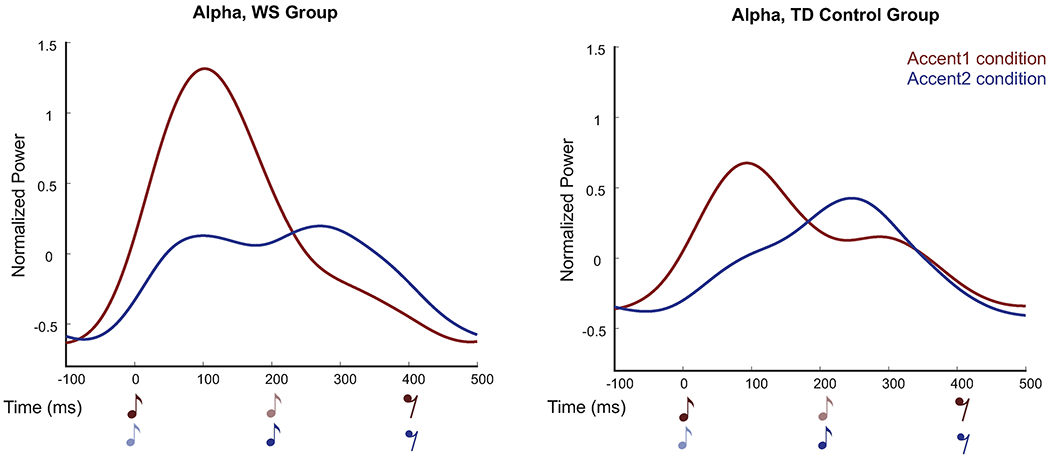
Modulations in alpha power shown as an average across all electrodes for WS group (left panel) and TD control group (right panel). Red=Accent1, Blue=Accent2. Dark music notes indicate accented tones and lighter notes indicate unaccented tones. In both groups, an increase in alpha power coincided with the Beat1 effect (WS: −74-228ms; TD: −70-206ms). Individuals with WS showed significant modulations in alpha power for the Beat 2 effect while TD controls did not (WS: 246-500ms).
Individuals with WS showed an overall more distributed neural response across the scalp compared to TD controls (Table 2; number of electrodes per significant cluster). This was true particularly for the Beat1 effect. See Figure 3 and Figure S2 for a graphical depiction of these results.
Figure 3.
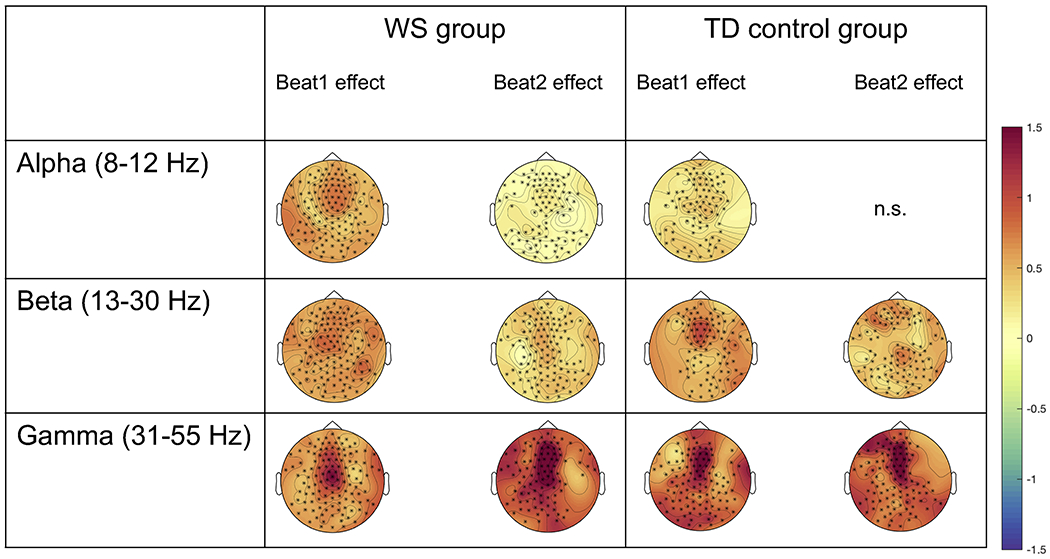
Electrodes contributing to the significant clusters for alpha, beta, and gamma activity for both WS and TD control groups for the Beat1 and Beat2 effects. Asteriks denote electrodes contributing to the significant clusters. Topographies are taken from the entire significant time windows denoted in Table 2. See also Figure S2.
ERP analyses: Auditory evoked potentials
Both the WS and TD control groups showed the canonical P1-N1-P2 auditory response to physically accented tones in both conditions (Figure 4). The WS group exhibited significant amplitude differences between the Accent1 and Accent2 conditions at 3 time windows: 1) 24-72ms, p=0.014 (P1 component, Beat1 effect), 2) 140-216ms, p=0.005 (P2 component, Beat1 effect), 3) 350-418ms, p=0.009 (P2 component, Beat2 effect). There were no significant amplitude differences between conditions for the TD control group.
Figure 4.
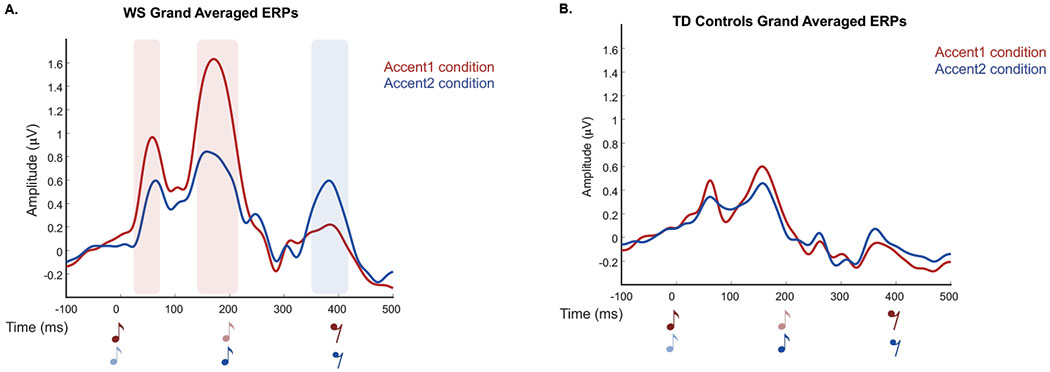
ERP responses for WS group (A) and TD controls (B) in both conditions for ten central electrodes. Red=Accent1, Blue=Accent2. Dark music notes indicate accented tones and lighter notes indicate unaccented tones. Shaded areas indicate significant amplitude differences between the two conditions (color coded), which were only evident in the WS group.
Group differences between WS and TD control groups were calculated for the ERP difference wave (Accent1-Accent2). Between group clustering revealed two time windows in which the WS group displayed a greater neural response (i.e., greater amplitude of the ERP difference wave) compared to the TD control group. The WS group had larger responses from 1) 28-58ms, p=0.044 (P1, Beat1 effect) and 2) 148-202ms, p=0.018 (P2, Beat1 effect) (Figure 5).
Figure 5.
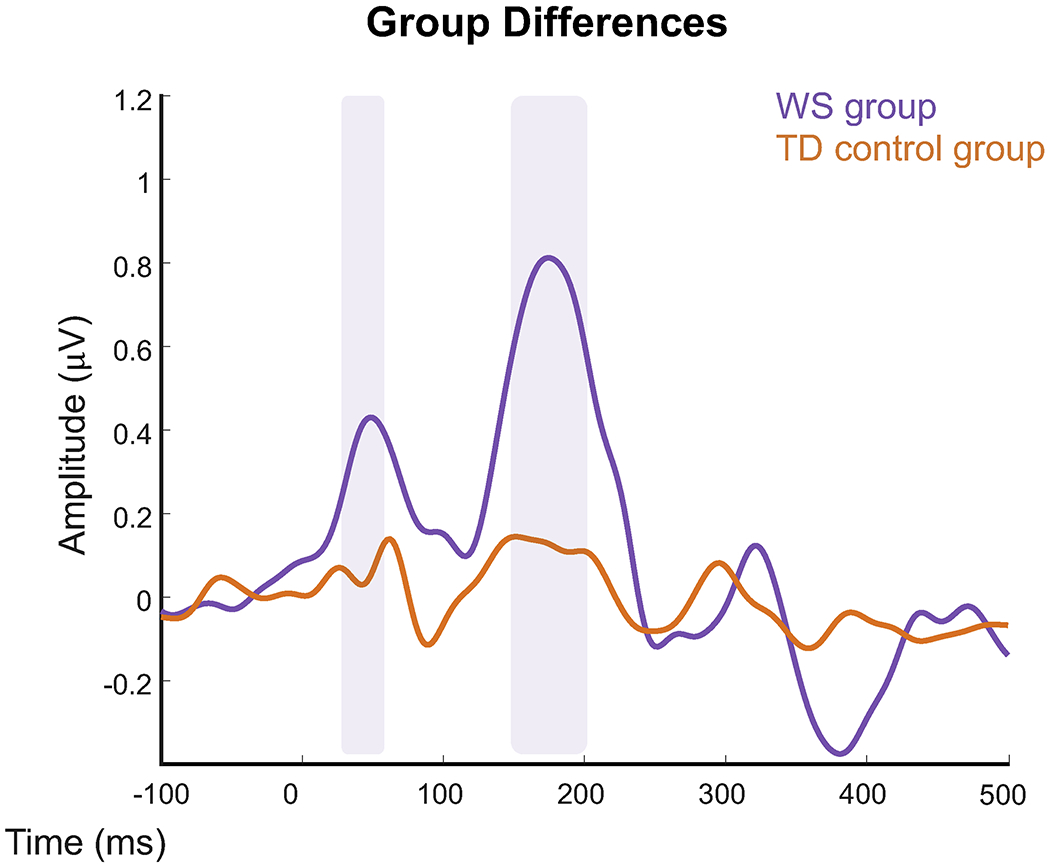
ERP responses for WS group (purple) and TD group (orange). ERPs are a difference between conditions over ten central electrodes. Shaded areas indicate significant amplitude differences between the two groups, with the WS group consistently displaying greater ERP amplitude differences.
Stability
The WS group displayed very stable neural responses over the course of the experiment as indexed by the correlation of the ERP responses between the first and last quarter of trials (44). The mean stability correlation (SD, p) was r = 0.70 (0.16, p< .0001) in the Accent1 condition and r = 0.69 (0.18, p< .0001) in the Accent2 condition. The TD group also showed fairly stable ERP responses, though not to the same degree as the individuals with WS. In the TD group, the mean stability correlation (SD, p) was r = 0.49 (0.16, p = .026) in Accent1 and r = 0.42 (0.22, p = .050) in Accent2. Using a two-sample t test with Fisher’s z-transformed stability correlations, the difference in stability between the WS and TD groups was significant for Accent1 (t(41) = 4.08, p <.001) and Accent2 (t(41) = 4.27, p <.001).
4. Discussion
The present study assessed the neurophysiological mechanisms known to correlate with beat perception using EEG in individuals with Williams syndrome. First, we found that individuals with WS exhibited the canonical evoked beta and gamma activity implicated in beat processing, with activity occurring over a somewhat broader scalp distribution. Second, we found significantly larger auditory evoked potentials (P1-N1-P2 ERP complex) in the WS group compared to typically developing controls. Third, we found greater modulations in evoked alpha power in the WS group. Fourth, ERP stability analyses suggest that neural responses to the beat percepts were remarkably stable in individuals with WS throughout the duration of the experiment. These results provide neurophysiological evidence for dynamic beat tracking in WS with some fundamental similarities to and some marked differences from that of typically developing controls.
Similar evoked beta and gamma activity between groups
Individuals with WS and TD controls showed similar evoked beta and gamma activity time-locked to accented tones perceived as beats, consistent with dynamic attending theory (2) and prior studies in TD individuals(16). The presence of the canonical evoked beta and gamma activity implicated in beat perception (16,21) highlights that individuals with WS are dynamically tracking the beat. The overall similar neural responses in beat perception between the two groups corroborates behavioral studies showing similar abilities in rhythm between WS and TD groups (12,15). Visual inspection of topographies between the two groups revealed a slightly more diffuse neural response in WS than TD. This could suggest that individuals with WS recruit more processing resources, perhaps reflective of heightened auditory sensitivities (simple auditory stimuli highly engaging for the WS group) and/or required greater attentional resources despite the simplicity of the stimuli in our passive listening paradigm. Prior work has also observed more diffuse processing of auditory stimuli in WS (31). Alternatively, electrode scalp distributions may arise from brain morphology and head shape differences in WS (46) or variability within groups; techniques with greater spatial precision than EEG (e.g., fMRI) are needed to further elucidate the spatial processing requirements of beat perception between the two groups.
Increased amplitude of auditory evoked potentials and alpha power modulations in WS
Individuals with WS displayed significantly increased auditory evoked potential amplitudes compared to TD controls. The WS group exhibited differences between the conditions for the P1 (Beat1 effect) and P2 (both Beat1 and Beat2 effects) amplitudes, consistent with literature identifying enhanced auditory evoked potentials for metrically strong positions (47–49). These findings align with Bouwer & Honing (2015) who reported larger P1 responses for sounds on the beat compared to off the beat, but no difference in N1 amplitudes based on metrical position. The P1 and P2 Beat1 effects were significantly greater in the WS than TD groups. Enhanced ERP responses in WS may reflect reduced inhibition of excitatory responses to auditory stimuli and potentially less efficient allocation of attention, even for a repetitive and predictable beat-based task. Our results corroborate and extend the increased auditory evoked potentials in WS in response to non-beat based auditory stimuli (28,30).
Alpha activity was also significantly different for the WS group compared to the TD control group for the Beat1 effect, and trended for Beat2, aligning with previous findings of greater evoked alpha power in WS compared to TD controls in response to auditory stimuli (26). Alpha oscillations are implicated in sensory processing and attention, though the extent to which alpha synchronization or desynchronization relates to inhibitory processes is not straightforward (50). As alpha power is generally indicative of inhibition of processing task-irrelevant information, greater evoked alpha power may map onto inefficient allocation of attentional resources in WS or the inability to disengage attention from a repetitive auditory stimulus. Though the role of attention cannot be disentangled in our study, its role in alpha power modulations in response to rhythmically predictable stimuli may provide insight into the profile of dynamic beat tracking in individuals with WS. In addition, the more robust alpha Beat1 effect than Beat2 effect may reflect increased salience of a strong beat when it follows a rest (silence) compared to a strong beat following another tone. Taken together, the time-frequency oscillatory and ERP analyses suggest that the hyperactive auditory phenotype in WS contributes to simple beat perception.
While TD participants displayed canonical P1-N1-P2 auditory evoked potential components to the tones, they did not show ERP differences to the beat. This is somewhat surprising given findings of modulated ERP amplitudes in prior studies for notes in strong vs. weak metrical positions (49,51). The lack of difference in ERPs in the control group between conditions could in part be due to the small sample size or the simple and repetitive nature of the stimuli. Nevertheless, tracking of the perceived beats was still apparent in the evoked time-frequency activity (beta and gamma activity time locked to beat onset) for TD controls, consistent with(16), which highlights that this paradigm was successful in 1) eliciting neural responses to beat percepts and 2) tapping into meaningful group differences between WS and TD participants.
In neurologically healthy adults, a cortico-subcortical network including the cerebellum, basal ganglia (BG), supplementary motor area, and auditory cortices is activated in beat perception and production tasks (52). Interestingly, individuals with WS exhibit atypical morphology in several of these areas, including increased cerebellar volume (53,54), reduced gray matter in the BG (53), and increased left auditory cortex volume even when controlling for overall brain volume reduction (55). One group found increased left planum temporale volume in WS participants with high musical abilities (12). Additionally, individuals with WS exhibit decreased within-network connectivity in a somatomotor network (56). Differences in brain structure and network connectivity in WS may impact beat perception skills and their neural correlates in this population, perhaps resulting in less efficient allocation of neural resources during beat processing, and a pattern of activation different from controls. Future comparisons between WS and TD controls in these regions would advance understanding of the brain networks important for beat perception in WS.
Highly stable neural responses over time in WS
Atypical attention to auditory stimuli is also corroborated by the striking stability results. While both WS and TD groups showed correlations between the first and last quarter of trials (i.e. they were attending to the stimuli for the duration of the experiment), these correlations were remarkably higher in the WS group. Previous work has investigated the stability of N1-P2 components in neurologically healthy adults during sensorimotor integration (44) and word listening (57), and in children with autism (58). The increased stability in the WS compared to the TD group in the present study suggests stable task engagement in WS and adds to evidence that a passive, repetitive listening paradigm that required minimal cognitive load can be an effective tool for assessing beat perception in this population. The stability finding may also reflect the increased auditory sensitivities in WS and an inability to inhibit attention to auditory information (26), providing further evidence for the increased auditory sensitivities and the characteristic difficulties with inhibiting attention to auditory material in WS.
Limitations and Future Directions
One limitation of the present study is the focus on evoked (phase-locked) and not induced (non-phase-locked) neural activity. Several studies have identified induced beta and gamma activity as markers of beat tracking and predictive timing (18–20). The evoked time-frequency data we present here may be only indicative of stimulus-driven activity and not of underlying neural oscillatory processes, though it is also unclear how phase-locked and non-phase locked activity are biologically dissociable (62). Examining non-phase-locked neural activity to musical beats is an important next step in determining exactly how neural oscillations underlie such cognitive processes.
While the distinct neural responses we observe here to accented tones are very likely due to percepts of beat patterns, we cannot rule out the possibility that amplitude changes in the stimulus are partly driving effects. Future work should explore beat perception in WS with paradigms designed to disentangle neural effects of beat perception from those tied directly to the physical characteristics of the stimulus, such as by priming participants to perceive specific metrical patterns (16). Iversen et al. (2009) found very similar neural responses in TD individuals to physically accented tones and to tones with an imagined accent, aligning with findings on subjective accenting (48,49,59,60). As the present study is the first investigation of neurophysiological correlates of beat perception in WS, we purposefully chose to use stimuli with differences in amplitude to avoid complex task demands, given the cognitive phenotype of the WS population.
Additionally, future studies could 1) explore the neural basis of beat tracking in WS with more complex and ecologically valid stimuli (e.g., perceiving beats in natural music instead of repeating tones), 2) incorporate a larger control comparison group and/or a comparison group of another neurodevelopmental disorder with behavioral measures of rhythm perception and production abilities and cognitive functioning, and 3) explore links between beat perception and social skills in WS. Individuals with WS exhibit social communication difficulties (61), and those who have higher beat and meter perception abilities also have higher adaptive communication and socialization skills (13). Exploring brain-behavior relationships between rhythm and social communication could have potential clinical implications.
Conclusion
Our results suggest individuals with WS exhibit a profile of dynamic beat tracking both similar to and different from neurotypical controls. Individuals with WS exhibit evoked beta and gamma activity similar to that of TD individuals when processing simple beats. Their neural response to beat tracking is additionally characterized by greater amplitude of auditory evoked potentials (P1-N1-P2 ERP complex) and modulation of evoked alpha activity compared to TD controls. Strikingly, the stability of neural responses was quite high in the WS participants throughout the experiment. Our results align with the characteristic heightened auditory sensitivities and attention problems seen in WS and suggest increased attentional resources and a lack of inhibition towards auditory input are implicated in beat processing in Williams syndrome.
Supplementary Material
Acknowledgements
We thank Dr. Alexandra Key and Dr. Elisabeth Dykens for project discussions, Dorita Jones, Chris Daniell, and Carolyn Comes for assistance with data collection, and Dr. Enikő Ladányi, Dr. Dan Gustavson, and Alyssa Scartozzi for manuscript feedback. We thank the participants and their families and the ACM Lifting Lives – Vanderbilt Kennedy Center Music Camp.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development, a National Science Foundation GRFP to AK, the Office of the Director of the National Institutes of Health under Award Number DP2HD098859 to RLG, and R21DC016710 and R61MH123029 awards to MDL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Use of REDCap was made possible by Grant UL1 TR000445 from the National Center for Advancing Translational Sciences (NCATS)/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Jones MR, Boltz M (1989): Dynamic Attending and Responses to Time. Psychol Rev 96: 459–491. [DOI] [PubMed] [Google Scholar]
- 2.Large EW, Jones MR (1999): The dynamics of attending: How people track time-varying events. Psychological Review, vol. 106. pp 119–159. [Google Scholar]
- 3.Arns M, Conners CK, Kraemer HC (2013): A Decade of EEG Theta/Beta Ratio Research in ADHD: A Meta-Analysis. J Atten Disord 17: 374–383. [DOI] [PubMed] [Google Scholar]
- 4.Puyjarinet F, Bégel V, Lopez R, Dellacherie D, Dalla Bella S (2017): Children and adults with Attention-Deficit/Hyperactivity Disorder cannot move to the beat. Sci Rep 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderone DJ, Lakatos P, Butler PD, Castellanos FX (2014): Entrainment of neural oscillators as a modifiable substrate of attention. Trends Cogn Sci 18: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami U (2011): A temporal sampling framework for developmental dyslexia. Trends Cogn Sci 15: 3–10. [DOI] [PubMed] [Google Scholar]
- 7.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, et al. (1993): Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet 5: 11–16. [DOI] [PubMed] [Google Scholar]
- 8.Levitin DJ, Cole K, Lincoln A, Bellugi U (2005): Aversion, awareness, and attraction: Investigating claims of hyperacusis in the Williams syndrome phenotype. J Child Psychol Psychiatry Allied Discip 46: 514–523. [DOI] [PubMed] [Google Scholar]
- 9.Martens MA, Wilson SJ, Reutens DC (2008): Research Review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. J Child Psychol Psychiatry Allied Discip 49: 576–608. [DOI] [PubMed] [Google Scholar]
- 10.Levitin DJ, Cole K, Chiles M, Lai Z, Lincoln A, Bellugi U (2004): Characterizing the musical phenotype in individuals with Williams Syndrome. Child Neuropsychol 10: 223–247. [DOI] [PubMed] [Google Scholar]
- 11.Thakur D, Martens MA, Smith DS, Roth E (2018): Williams syndrome and music: A systematic integrative review. Front Psychol 9. 10.3389/fpsyg.2018.02203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens MA, Reutens DC, Wilson SJ (2010): Auditory cortical volumes and musical ability in Williams syndrome. Neuropsychologia 48: 2602–2609. [DOI] [PubMed] [Google Scholar]
- 13.Lense MD, Dykens EM (2016): Beat perception and sociability: Evidence from Williams syndrome. Front Psychol 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopyan T, Dennis M, Weksberg R, Cytrynbaum C (2001): Music skills and the expressive interpretation of music in children with Williams-Beuren syndrome: Pitch, rhythm, melodic imagery, phrasing, and musical affect. Child Neuropsychol 7: 42–53. [DOI] [PubMed] [Google Scholar]
- 15.Levitin DJ, Bellugi U (1998): Musical abilities in individuals with Williams syndrome. Music Perception, vol. 15. pp 357–389. [Google Scholar]
- 16.Iversen JR, Repp BH, Patel AD (2009): Top-down control of rhythm perception modulates early auditory responses. Ann N Y Acad Sci 1169: 58–73. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka T, Trainor LJ, Large EW, Ross B (2012): Internalized Timing of Isochronous Sounds Is Represented in Neuromagnetic Beta Oscillations. J Neurosci 32: 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujioka T, Trainor LJ, Large EW, Ross B (2009): Beta and gamma rhythms in human auditory cortex during musical beat processing. Ann N Y Acad Sci 1169: 89–92. [DOI] [PubMed] [Google Scholar]
- 19.Cirelli LK, Bosnyak D, Manning FC, Spinelli C, Marie C, Fujioka T, et al. (2014): Beat-induced fluctuations in auditory cortical beta-band activity: Using EEG to measure age-related changes. Front Psychol 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang A, Bosnyak DJ, Trainor LJ (2016): Unpredicted pitch modulates beta oscillatory power during rhythmic entrainment to a tone sequence. Front Psychol 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanto TP, Snyder JS, Large EW (2006): Neural correlates of rhythmic expectancy. Adv Cogn Psychol 2: 221–231. [Google Scholar]
- 22.Lense MD, Key AP, Dykens EM (2011): Attentional disengagement in adults with Williams syndrome. Brain Cogn 77: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes SM, Riby DM, Matthews K, Coghill DR (2011): Attention-deficit/hyperactivity disorder and Williams syndrome: Shared behavioral and neuropsychological profiles. J Clin Exp Neuropsychol 33: 147–156. [DOI] [PubMed] [Google Scholar]
- 24.Leyfer OT, Woodruff-Borden J, Klein-Tasman BP, Fricke JS, Mervis CB (2006): Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am J Med Genet Part B Neuropsychiatr Genet 141: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes SM, Riby DM, Park J, Fraser E, Campbell LE (2010): Executive neuropsychological functioning in individuals with Williams syndrome. Neuropsychologia 48: 1216–1226. [DOI] [PubMed] [Google Scholar]
- 26.Lense MD, Gordon RL, Key APF, Dykens EM (2014): Neural correlates of cross-modal affective priming by music in Williams syndrome. 10.1093/scan/nst017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponton CW, Eggermont JJ, Kwong B, Don M (2000): Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clin Neurophysiol 111: 220–236. [DOI] [PubMed] [Google Scholar]
- 28.Zarchi O, Avni C, Attias J, Frisch A, Carmel M, Michaelovsky E, et al. (2015): Hyperactive auditory processing in Williams syndrome: Evidence from auditory evoked potentials. Psychophysiology 52: 782–789. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs M, Dykens EM, Key AP (2018): Attentional rather than sensory differences characterize auditory processing in Williams syndrome. Brain Cogn 121: 24–37. [DOI] [PubMed] [Google Scholar]
- 30.Neville HJ, Mills DL, Bellugi U (1994): Effects of altered auditory sensitivity and age of language acquisition on the development of language-relevant neural systems: Preliminary studies of Williams syndrome. Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. pp 67–83 ST--Effects of altered auditory sensitivi. [Google Scholar]
- 31.Thornton-Wells TA, Cannistraci CJ, Adam WA, Kim Chai-Youn, Eapen Mariam, John CG, et al. (2010): Auditory attraction: Activation of visual cortex by music and sound in Williams syndrome. Am J Intellect Dev Disabil 115: 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitin DJ, Menon V, Schmitt JE, Eliez S, White CD, Glover GH, et al. (2003): Neural correlates of auditory perception in Williams syndrome: An fMRI study. Neuroimage 18: 74–82. [DOI] [PubMed] [Google Scholar]
- 33.Jones A, Hsu Y, Granjon L, Waszak F (2017): Temporal expectancies driven by self- and externally generated rhythms. Neuroimage 156: 352–362. [DOI] [PubMed] [Google Scholar]
- 34.Van Diepen RM, Foxe JJ, Mazaheri A (2019): The functional role of alpha-band activity in attentional processing: the current zeitgeist and future outlook. Curr Opin Psychol 29: 229–238. [DOI] [PubMed] [Google Scholar]
- 35.Obleser J, Kayser C (2019): Neural Entrainment and Attentional Selection in the Listening Brain. Trends Cogn Sci 23: 1–14. [DOI] [PubMed] [Google Scholar]
- 36.Strauß A, Wöstmann M, Obleser J (2014): Cortical alpha oscillations as a tool for auditory selective inhibition. Front Hum Neurosci 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JS, Oh S, Jeon HJ, Hong KS, Baek JH (2019): Resting-state alpha and gamma activity in affective disorder with ADHD symptoms: Comparison between bipolar disorder and major depressive disorder. Int J Psychophysiol 143: 57–63. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman A, Kaufman N (2004): Kaufman Brief Intelligence Test, 2nd ed. Circle Pines, MN: American Guidance Service. [Google Scholar]
- 39.Blomberg S, Rosander M, Andersson G (2006): Fears, hyperacusis and musicality in Williams syndrome. Res Dev Disabil 27: 668–680. [DOI] [PubMed] [Google Scholar]
- 40.Lense M, Dykens E (2013): Musical learning in children and adults with Williams syndrome. J Intellect Disabil Res 57: 850–860. [DOI] [PubMed] [Google Scholar]
- 41.Iversen JR, Patel AD (2008): The Beat Alignment Test (BAT): Surveying beat processing abilities in the general population. Proc 10th Int Conf Music Percept Cogn 465–468. [Google Scholar]
- 42.Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21. [DOI] [PubMed] [Google Scholar]
- 43.Oostenveld R, Fries P, Maris E, Schoffelen JM (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross B, Barat M, Fujioka T (2017): Sound-Making Actions Lead to Immediate Plastic Changes of Neuromagnetic Evoked Responses and Induced β-Band Oscillations during Perception. J Neurosci 37: 5948–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt JE, Eliez S, Bellugi U, Reiss AL (2001): Analysis of cerebral shape in Williams syndrome. Arch Neurol 58: 283–287. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer RS, Vlek RJ, Desain P (2011): Decomposing rhythm processing: Electroencephalography of perceived and self-imposed rhythmic patterns. Psychol Res 75: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abecasis D, Brochard R, Granot R, Drake C (2005): Differential brain response to metrical accents in isochronous auditory sequences. Music Percept 22: 549–562. [Google Scholar]
- 49.Bouwer FL, Honing H (2015): Temporal attending and prediction influence the perception of metrical rhythm: evidence from reaction times and ERPs. Front Psychol 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klimesch W, Sauseng P, Hanslmayr S (2007): EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- 51.Tierney A, Kraus N (2013): Neural responses to sounds presented on and off the beat of ecologically valid music. Front Syst Neurosci 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grahn JA (2012): Neural Mechanisms of Rhythm Perception: Current Findings and Future Perspectives. Top Cogn Sci 4: 585–606. [DOI] [PubMed] [Google Scholar]
- 53.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Karmiloff-Smith A, et al. (2009): Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res 1258: 96–107. [DOI] [PubMed] [Google Scholar]
- 54.Menghini D, Di Paola M, Murri R, Costanzo F, Caltagirone C, Vicari S, Petrosini L (2013): Cerebellar vermis abnormalities and cognitive functions in individuals with Williams syndrome. Res Dev Disabil 34: 2118–2126. [DOI] [PubMed] [Google Scholar]
- 55.Wengenroth M, Blatow M, Bendszus M, Schneider P (2010): Leftward lateralization of auditory cortex underlies holistic sound perception in Williams syndrome. PLoS One 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vega JN, Hohman TJ, Pryweller JR, Dykens EM, Thornton-Wells TA (2015): Resting-state functional connectivity in individuals with down syndrome and williams syndrome compared with typically developing controls. Brain Connect 5: 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner M, Shafer VL, Haxhari E, Kiprovski K, Behrmann K, Griffiths T (2017): Stability of the Cortical Sensory Waveforms, the P1-N1-P2 Complex and T-Complex, of Auditory Evoked Potentials. 60: 2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunham K, Feldman JI, Liu Y, Cassidy M, Conrad JG, Santapuram P, et al. (2020): Stability of Variables Derived From Measures of Multisensory Function in Children With Autism Spectrum Disorder. Am J Intellect Dev Disabil 125: 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Repp BH (2009): Do metrical accents create illusory phenomenal accents? Atten Percept Psychophys 71: 881–895. [DOI] [PubMed] [Google Scholar]
- 60.Potter DD, Fenwick M, Abecasis D, Brochard R (2009): Perceiving rhythm where none exists: Event-related potential (ERP) correlates of subjective accenting. Cortex 45: 103–109. [DOI] [PubMed] [Google Scholar]
- 61.Laws G, Bishop DVM (2004): Pragmatic language impairment and social deficits in Williams syndrome: A comparison with Down’s syndrome and specific language impairment. Int J Lang Commun Disord 39: 45–64. [DOI] [PubMed] [Google Scholar]
- 62.Cohen MX (2014): Analyzing Neural Time Series Data: Theory and Practice. Cambridge, MA: MIT Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


