Abstract
Background
Plant cell walls are the main physical barrier encountered by pathogens colonizing plant tissues. Alteration of cell wall integrity (CWI) can activate specific defenses by impairing proteins involved in cell wall biosynthesis, degradation and remodeling, or cell wall damage due to biotic or abiotic stress. Polygalacturonase (PG) depolymerize pectin by hydrolysis, thereby altering pectin composition and structures and activating cell wall defense. Although many studies of CWI have been reported, the mechanism of how PGs regulate cell wall immune response is not well understood.
Results
Necrosis appeared in leaf tips at the tillering stage, finally resulting in 3–5 cm of dark brown necrotic tissue. ltn-212 showed obvious cell death and accumulation of H2O2 in leaf tips. The defense responses were activated in ltn-212 to resist bacterial blight pathogen of rice. Map based cloning revealed that a single base substitution (G-A) in the first intron caused incorrect splicing of OsPG1, resulting in a necrotic phenotype. OsPG1 is constitutively expressed in all organs, and the wild-type phenotype was restored in complementation individuals and knockout of wild-type lines resulted in necrosis as in ltn-212. Transmission electron microscopy showed that thicknesses of cell walls were significantly reduced and cell size and shape were significantly diminished in ltn-212.
Conclusion
These results demonstrate that OsPG1 encodes a PG in response to the leaf tip necrosis phenotype of ltn-212. Loss-of-function mutation of ltn-212 destroyed CWI, resulting in spontaneous cell death and an auto-activated defense response including reactive oxygen species (ROS) burst and pathogenesis-related (PR) gene expression, as well as enhanced resistance to Xanthomonas oryzae pv. oryzae (Xoo). These findings promote our understanding of the CWI mediated defense response.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12284-021-00478-9.
Keywords: Rice, Leaf tip necrosis, Polygalacturonase, Bacterial blight, Cell wall
Introduction
Rice (Oryza sativa L.) is not only the world’s most important food crop for more than half of the world’s population, but also a model species for genetic, cytological, and agricultural studies (Itoh et al. 2005). As the main photosynthetic organs, the development status of rice leaves is closely related to the final yield. Premature withering of rice leaves has a significant impact on plant growth and yield. Thus, understanding the molecular mechanisms of leaf development and identifying genes causing premature withering are of great interest to both plant biologists and plant breeders.
Leaf necrosis starts to appear mainly at the apical meristem. The necrotic tips may be a specific regulation of the plant response to changes in the external environment or gene expression in vivo. The factors that induce leaf tip necrosis have been described at a molecular level in recent studies. The ectopic content of elements may result in leaf tip necrosis. The rice leaf tip necrosis 1 (ltn 1) mutant results in excessive accumulation of phosphate in shoots and thus causes plants to develop a leaf tip necrotic phenotype (Hu et al. 2011). OsPT8 is involved in phosphate homeostasis in rice, and overexpressing OsPT8 resulted in excessive phosphate in shoots and chlorosis and necrosis in leaf tips under high phosphate (0.3 mM) supply (Jia et al. 2011).
Leaf tip necrosis or withering usually occur at the beginning of leaf senescence. A rice mutant Leaf Tip Senescence 1 (LTS1) resulted in dwarfism and withered leaf tips, ultimately causing early leaf senescence (Wu et al. 2016). The loss of function of DEL1 resulted in the leaf apex and leaf margin exhibiting a faint yellow color after germinating for 5 days, while leaf tips exhibited withering and cracking at the maturity stage (Leng et al. 2017). The phenotype of necrotic leaf tips correlates with plant resistance. Lr34 confers durable and partial field resistance to wheat against an obligate biotroph associated with leaf tip necrosis (Singh. 1992). Transforming resistant Lr34 into Nipponbare allele showed increased resistance against multiple isolates of Magnaporthe oryzae and plants also developed a typical, senescence-based leaf tip necrosis phenotype (Krattinger et al. 2016). The same phenotype also occurred in transgenic barley with Lr34 (Risk et al. 2013). Necrotic leaves caused by programmed cell death (PCD) also alter cell wall structure. Mutation of a PECTATE LYASE-LIKE gene. DEL1, led to PCD and changes in cell wall composition and structure in rice (Leng et al. 2017). In other cases, the cell wall is degraded in abscission zones and is a key event in lace plant PCD (Gunawardena et al. 2007).
The typical plant cell wall consists of a primary cell wall, secondary cell wall and middle lamella, comprised of various carbohydrate-based polymers (cellulose, pectins and hemicelluloses) (Sarkar et al. 2009). PGs belong to one of the largest hydrolase families (GH28) and are expressed in a wide range of tissues and developmental stages in plants and fungus (Markovic and Janecek. 2001). PGs are one of the primary cell wall hydrolases affecting cell wall architecture by degrading pectin (Micheli. 2001). PGs have been identified to have multiple functions in a diverse range of processes including fruit ripening, organ abscission, pollen maturation, pathogen infection and plant resistance to pathogens (Atkinson et al. 2002; Wang et al. 2005; Villarreal et al.; 2009). For example, MdPG1 and MdPG2 regulate apple fruit softening and are induced by ethylene (Wakasa et al. 2006; Li et al. 2010). FaPG1 was up-regulated during strawberry fruit ripening and silencing FaPG1 by antisense transformation significantly reduced strawberry fruit softening (Quesada et al. 2009). Down-regulation of PpBGAL10 and PpBGAL16 expression inhibited cell wall degradation and ethylene production and delayed peach fruit softening by decreasing PG and pectin methyl esterases (PME) activity (Liu et al. 2018).
In Arabidopsis, PGs mainly function in growth and developmental processes including cell separation and expansion. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) and ADPG2 are essential for silique dehiscence; ADPG2 and QUARTET2 (QRT2) contribute to floral organ abscission; and all three genes contribute to anther dehiscence (Ogawa et al. 2009). Mutation of QRT3 resulted in failure of microspore separation during pollen development (Rhee et al. 2003). POLYGALACTURONASE INVOLVED IN EXPANSION1 (PGX1) and PGX2 function in cell expansion in seedling growth and rosette expansion in adult plants; overexpressing them resulted in enhanced hypocotyl elongation (Xiao et al. 2014; Xiao et al., 2017). Different from the two previously characterized PGX genes, PGX3 functions in seed germination, which contributes to the determination of etiolated hypocotyl length and root length in young seedlings (Rui et al. 2017). Microbial PGs stimulate plant cell walls to release oligogalacturonides (OGs) to activate defense responses during infections. Phytopathogenic fungi secrete PGs to degrade the pectic homogalacturonan (HG) backbone and aid host colonization by degrading CWI (Annis and Goodwin. 1997). Transgenic expression of a fungal PG in tobacco and Arabidopsis results in higher resistance to microbial pathogens and constitutively activated defense responses (Ferrari et al. 2007).
Although many PGs have been genetically and biochemically characterized in plants, few studies investigating PGs in rice have been reported. OsBURP16 belongs to the PG1β-like subfamily of BURP-family genes and encodes one putative PG1β subunit precursor, which showed enhanced PG activity and reduced pectin content and increased abiotic stress sensitivity in rice when it was overexpressed (Liu et al. 2014). In this study, we report the isolation of a leaf tip necrosis mutant named ltn-212 that exhibits necrotic leaf tips. The mutation in ltn-212 led to H2O2 accumulation and cell death in the necrotic part, but also caused enhanced resistance to bacterial blight pathogen Xoo. Using a map-based cloning strategy, we isolated the gene that encodes OsPG1. Our data suggested that OsPG1 is responsible for leaf tip necrosis in ltn-212 and functions directly in defense responses by mediating cell wall structure.
Results
Phenotypic Characteristics of ltn-212
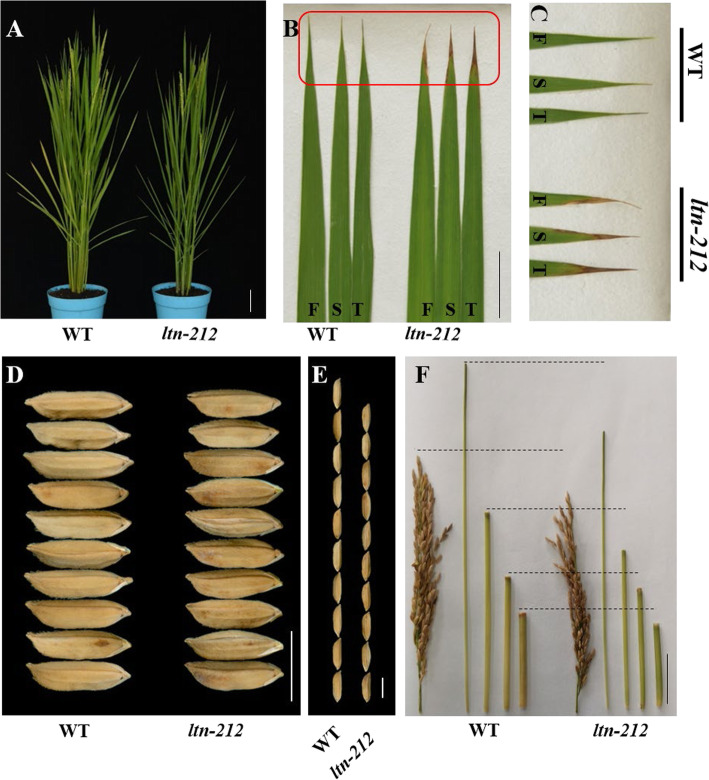
We isolated a leaf tip necrosis mutant from an ethyl methane sulfonate (EMS)-derived mutant population in the JiaHe212 background, which we named ltn-212 (leaf tip necrosis). Under natural summer field conditions, the leaf tip of ltn-212 had a small necrosis region and dark brown necrotic spots were formed at the tillering stage (Additional file 1: Figure S1A-B). Necrosis or withering at the leaf tip is typically the beginning of plant senescence and extends to the bottom of the leaf as the plant grows. Unlike other plant materials, initial necrosis of ltn-212 only occurred at all leaf tips at the tillering stage and developed into 3–5 cm of dark brown necrotic tissue until ripening stage (Fig. 1a-c). In general, the necrosis changed during plant growth but was limited to the leaf tip in ltn-212.
Fig. 1.
Leaf tip necrosis and agronomic traits of ltn-212. a Phenotype of WT and ltn-212 at heading stage (scale bar = 10 cm). b Leaf tip necrosis identification of WT and ltn-212 at heading stage (scale bar = 5 cm). c Enlarge of red part of Fig. b. d-e The phenotype of grain width and grain length (scale bar = 10 mm). f Comparison of panicles and culm length of WT and ltn-212 (scale bar = 5 cm).** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test). F: The first top leaf. S: The second top leaf. T: The third top leaf
The leaf is the mainly photosynthetic organ for energy assimilation of rice. As a result, ltn-212 significantly reduced plant height, 1000-grain weight, and grain length, compared to wild-type (WT, JiaHe212), but there were no changes in grain width or tiller number (Fig. 1d-e, Additional file 1: Figure S2A-D). The reduced plant height for ltn-212 was due to shortened panicles along with shortened first three internodes compared with WT (Fig. 1f, Additional file 1: Figure S2E). The data suggested that the leaf-necrosis mutation also significantly affected agronomic performance.
H2O2 Accumulation and Cell Death Occur in ltn-212
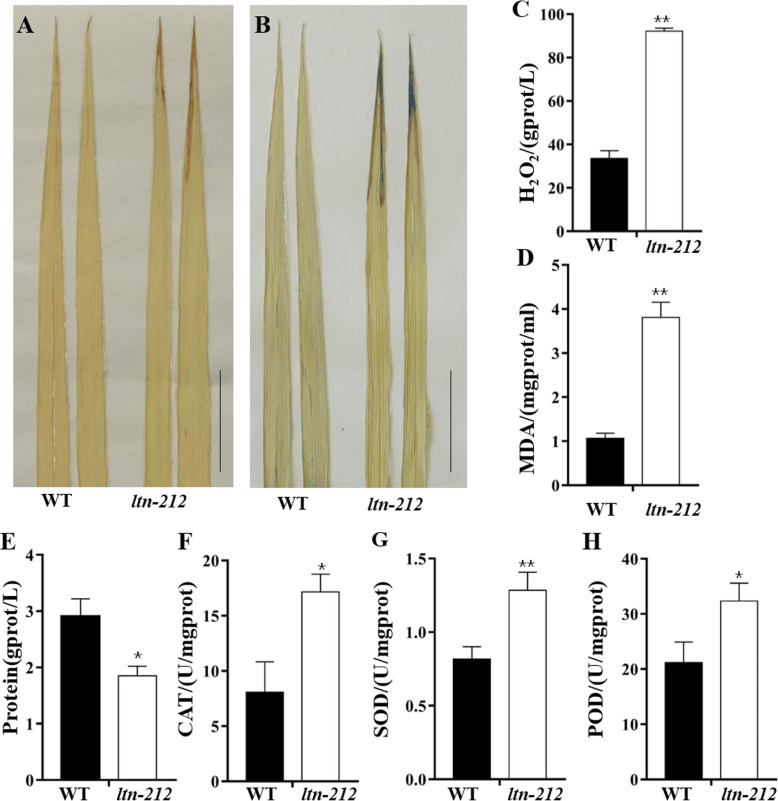
Necrosis has been defined as a type of cell death, mainly caused by ROS burst and H2O2 accumulation (Van and Dat, 2006). To determine whether the development of necrosis in ltn-212 involves altered H2O2 accumulation and cell death, we performed 3,3-Diaminobenzidine (DAB) and Evans blue staining. DAB staining results in the formation of reddish-brown formazan precipitates indicative of H2O2 accumulation. The red brown precipitate was observed on the ltn-212 leaf tip, whereas no precipitate was observed in WT (Fig. 2a). In addition, the H2O2 contents in the ltn-212 leaf tips was approximately 3-fold higher than in WT leaf tips (Fig. 2c). Evans blue staining is an indicator of irreversible membrane damage or cell death (Liu et al. 2008). The ltn-212 leaf tip with necrosis had blue stained cells, whereas no staining was observed in ltn-212 leaves without necrosis or WT (Fig. 2b). To confirm membrane damage and cell death, we further measured the levels of malonaldehyde (MDA). The ltn-212 mutant showed significantly higher levels of MDA content compared to WT (Fig. 2d).
Fig. 2.
Histochemical staining of WT and ltn-212. a-b DAB staining and Evans blue staining at heading stage (scale bar = 5 cm). c-d H2O2 content and MDA content at heading stage (n = 3). e-h Total protein content and the activity of reactive oxygen species scavenging enzymes (CAT, SOD, POD, n = 3). Data are means ± SD. ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test)
Plants can remove ROS by synthesizing anti-oxidative enzymes when injured and suffering from stress. We quantitatively determined the activity of these enzymes in ltn-212 and WT leaves. The results indicated that content of total protein significantly decreased in ltn-212 compared to WT. The activities of catalase (CAT) and superoxide dismutase (SOD) and peroxidase (POD) increased in ltn-212 plants, by 17.15 U/mgprot, 1.28 U/mgprot and 32.35 U/mgprot in ltn-212 leaf tips, respectively, which were approximately 2.1-, 1.6- and 1.5-fold higher than the levels of 8.08 U/mgprot, 0.82 U/mgprot and 21.19 U/mgprot in the WT, respectively (Fig. 2e-h). Taken together, these results demonstrate that the formation of leaf necrosis was associated with ROS accumulation and irreversible membrane damage in the ltn-212 cells.
Activation of Defense Responses in ltn-212
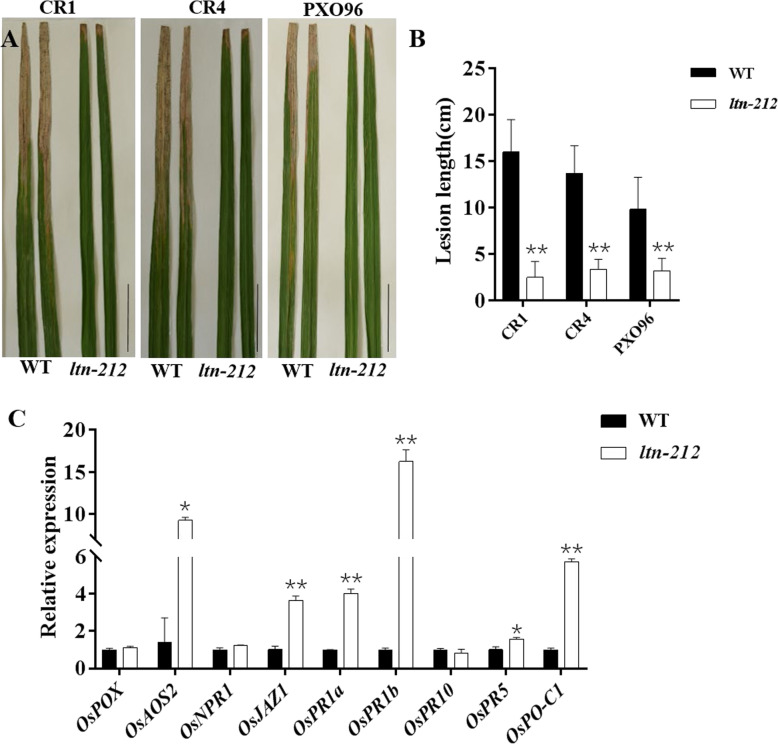
Accumulation of ROS commonly activates defense responses that result in enhanced resistance to one or more pathogens. To examine whether the ltn-212 mutant also gains disease resistance, we inoculated the mutant and the WT plants with isolates of three Xoo pathotypes virulent against the WT using the leaf clipping method at the tillering stage. The results showed that ltn-212 exhibited significantly enhanced resistance to races CR1, CR4, and PXO96 compared to WT (Fig. 3a-b). To check whether the enhanced disease resistance response stemmed from elevated expression of defense related genes, we measured the expression of nine PR genes by real-time quantitative PCR analysis. These genes were mainly involved in SA and JA signaling pathways. The results showed the mRNA levels of OsJAZ1, OsPR1a, OsPR1b, OsPR5 and OsPO-C1 significantly increased compared to WT; in particular, the expression of OsPR1b was upregulated 15-fold in ltn-212 compared to WT (Fig. 3c). Thus, we speculated that the leaf tip necrosis also triggered a defense response, resulting in enhanced resistance to Xoo in ltn-212.
Fig. 3.
Enhanced resistance in ltn-212 to Xoo. a Disease symptoms of WT and ltn-212 leaves after Xoo inoculation at 15 days (scale bar = 5 cm). b Lesion lengths measured at 15 days after inoculation with 3 different Xoo. Data are means ±SD of at least 15 leaves. c Expression analysis of defense-related genes. Data are means ± SD. ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test)
Map-Based Cloning of OsPG1
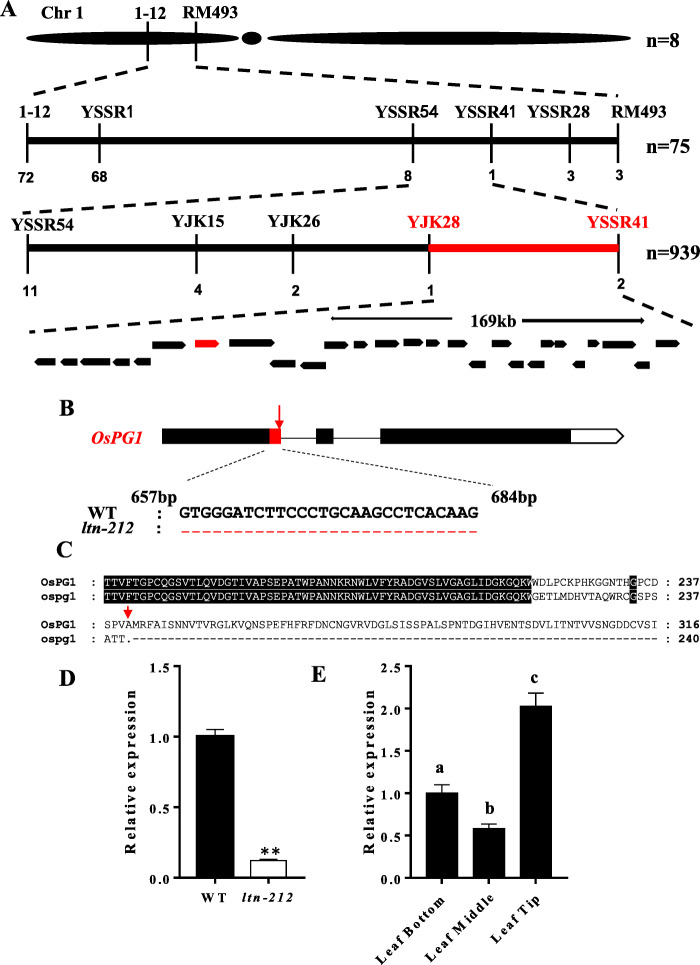
To determine the genetic control of the mutation, we crossed ltn-212 and the WT JiaHe212. F1 progenies had a normal phenotype matching that of WT and in the F2 segregating populations, normal and mutant phenotypes showed a typical segregation ratio of 3:1 (158:42, χ2 = 1.56), indicating that the leaf tip necrotic phenotype of ltn-212 was controlled by a single recessive nuclear gene. To map the gene responsible for the ltn-212 mutant phenotypes, an F2 mapping population was constructed by crossing ltn-212 with the indica variety ZH8015. Eight F2 plants with the ltn-212 phenotype were used for primary mapping, and the mutation was located on the short arm of chromosome 1 between markers 1–12 and RM493 (Fig. 4a). By using 75 homozygous mutant plants, the mutation was further located to markers YSSR54 and YSSR41 (Fig. 4a). Subsequently, using an additional 939 F2 mutant individuals and three newly developed polymorphic markers, the mutation was finally narrowed down to an approximately 169 Kb region between markers YJK28 and YSSR41 (Fig. 4a). Twenty-seven open reading frames (ORFs) were predicted in this region (Fig. 4a).
Fig. 4.
Cloning analysis of the OsPG1. a Fine mapping of OsPG1. b Gene structure and the mutation of genome and cDNA in OsPG1. The red arrow indicates the mutant site and the red dotted line indicates the incorrected splicing sequences. c Protein sequence alignment of OsPG1 and ospg1. d Expression analysis of OsPG1 in WT and ltn-212 leaves (n = 3). e Expression analysis of OsPG1 in WT leaf bottom, middle and tip (n = 3). Data are means ± SD. ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05. a, b, c indicates significance at P ≤ 0.01 (Student’s t test)
Sequencing and comparison of those ORFs cloned from ltn-212 and WT revealed that the seventh ORF (LOC_Os01g19170) had a single base substitution (G-A) in the first intron (Fig. 4b, Additional file 1 Figure S3). We then sequenced cDNA of LOC_Os01g19170 from both genotypes. The sequence alignment revealed that the mutation disrupted the recognition site for an exon, causing incorrect splicing 28 bp at the first exon in ltn-212 (Fig. 4b, Additional file 1: Figure S3). This splicing error was predicted to introduce a premature stop codon at the 240th amino acid (Fig. 4c). We performed qRT-PCR to understand if the mutation caused changes in expression of OsPG1 and the expression level in leaves at different positions. The expression of OsPG1 was significantly down-regulated in ltn-212 leaves compared to WT leaves and the mRNA level was highest in leaf tips (Fig. 4d-e). On the basis of these data, we speculated that OsPG1 was responsible for the necrotic leaf tips.
Functional Analysis and Verification of OsPG1
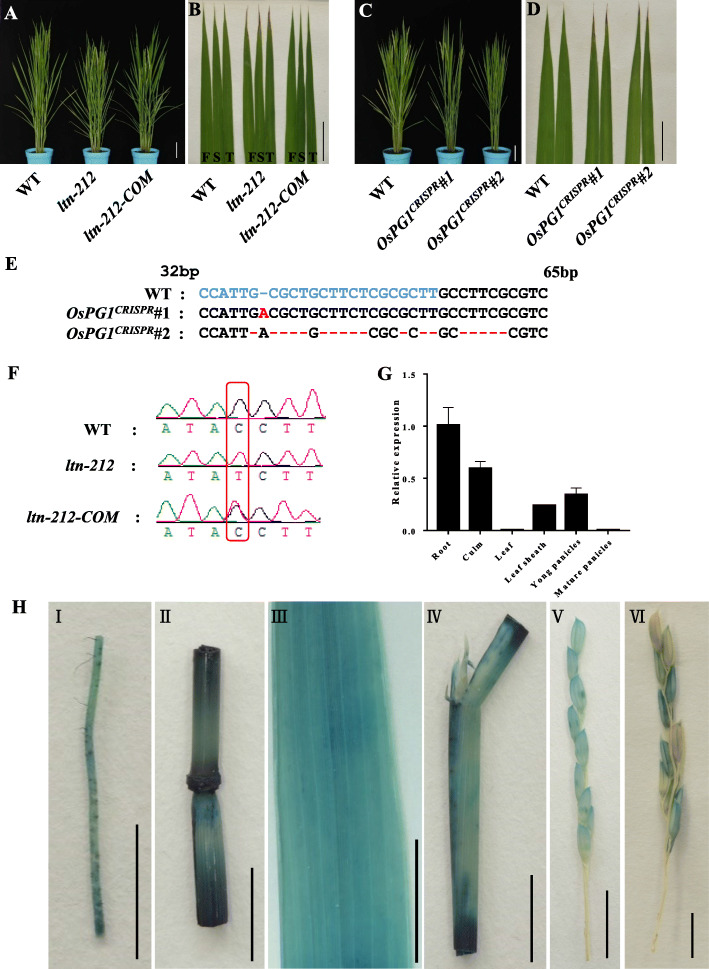
To verify whether the incorrect splicing of OsPG1 was responsible for the ltn-212 phenotype, we constructed a complementation vector containing a WT-derived 5790 bp genomic DNA fragment consisting of the entire OsPG1 coding region, 2826 bp upstream and 1098 bp downstream sequences, and introduced it into ltn-212 using Agrobacterium-mediated transformation. The resulting complemented plants are referred to as ltn-212-COM. No necrosis appeared on the leaf tip of any ltn-212-COM plants at any point in the life cycle (Fig. 5a-b). We carried out genotyping of T1 plants with no necrosis to check for the transgene by sequencing the region of OsPG1 that contains the mutation. We found that all progenies were heterozygous at the mutation site (Fig. 5e). Furthermore, we transformed a CRISPR-Cas9 construct targeting the first exon of OsPG1 into the WT, and obtained OsPG1 knock-out individuals (Fig. 5c-d). All the knockout plants exhibited the leaf tip necrotic phenotype (Fig. 5c-d, e). Together, our results indicate the mutation of OsPG1 was responsible for the formation of the necrotic leaf tip.
Fig. 5.
Genetic complementation, knock-out and expression analysis of OsPG1. a Phenotype of WT, ltn-212 and T1 complementation plant (scale bar = 10 cm). b Leaf tip necrosis disappear on the leaves of T1 complementation plant (scale bar = 5 cm). c Phenotype of WT, and OsPG1 knock-out individuals (OsPG1CRISPR#1, OsPG1CRISPR#2) at JiaHe212 background (scale bar = 10 cm). d Leaf tip necrosis on the leaves of knock-out individuals (OsPG1CRISPR#1, OsPG1CRISPR#2) (scale bar = 5 cm). e Mutational sites of knock-out individuals (OsPG1CRISPR#1, OsPG1CRISPR#2). The blue color words indicate target sequence and the red color words indicate insertion and deletion mutations. f The T1 transgenic plants were verified by sequencing of the mutation site. g Expression levels of OsPG1 in various tissues (n = 3). h Gus staining of OsPG1 promoter–GUS reporter transgenic plants (scale bar = 1 cm). I: Roots. II:Colum. III: leaf. IV: Sheath. V: Young spikelet. VI: Mature spikelet. Data are means ± SD. ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test)
Sequence comparison between genomic DNA and cDNA showed that OsPG1 is composed of three exons separated by two introns. The coding sequence (CDS) of OsPG1 consists of 1512 nucleotides, and encodes a putative PG with a 504 amino acid protein. PG is one of the hydrolases of pectin and is involved in controlling the stability of cell wall structure. The typical PG genes in plants and fungi have four conserved domains: Domain I (SPNTDG), Domain II (GDDC), Domain III (CGPGHGISIGSLG), and Domain IV (RIK). Sequence alignment of PGs homologous among rice, Arabidopsis thaliana, Brassica rapa L. ssp. pekinensis, Ciboria shiraiana, Rhizoctonia solani, and Glycine max revealed that OsPG1 was highly conserved (Additional file 1: Figure. S4). The rice genome contains 45 PG-like genes. Phylogenetic analysis of the PG in rice showed that OsPG1 was classified into Clade A. Genes in Clade A are mainly expressed at fruiting and abscission (Additional file 1 Figure S5). The results indicate that OsPG1 is a typical PG gene.
Expression Pattern of OsPG1
To elucidate the spatial and developmental expression pattern of OsPG1 in rice, qRT-PCR assays were performed in roots, culm, leaves, sheath, young panicles, mature panicles in the WT. OsPG1 was highly expressed in the roots, culm, sheath and young panicles while expression was barely detectable in leaves and mature panicles (Fig. 5g). We generated transgenic plants of OsPG1pro::GUS expressing the β-glucuronidase (GUS) reporter gene driven by the native promoter of OsPG1 to more precisely examine the spatial and temporal expression patterns of OsPG1. GUS staining showed activity of the OsPG1 promoter in all tissues examined (Fig. 5h). In summary, the results showed that OsPG1 was broadly expressed in all tissues.
Mutation of OsPG1 Altered Cell Wall Structure and Composition and Enhanced Resistance to Bacterial Blight Pathogen
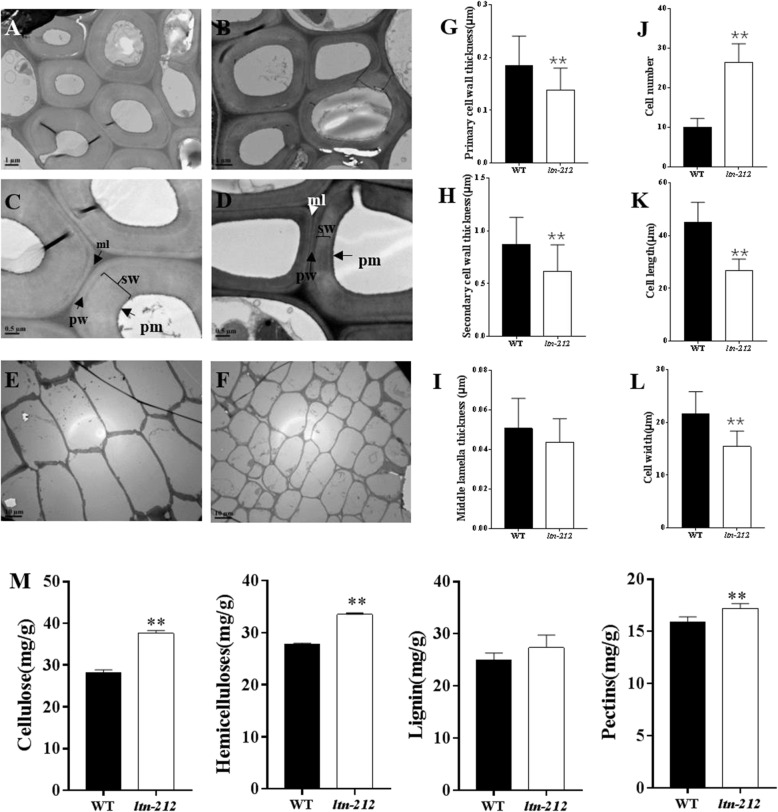
Previous studies have demonstrated that pectin is fundamental and the most complex polysaccharide in plant cell walls (Bonnin et al., 2014). Functional analysis of OsPG1 suggested that the cell wall structure in mutant plants may be altered. We therefore analyzed the structure of ltn-212 and WT plants using Transmission Electron Microscope (TEM). The results revealed that the cell wall thicknesses of bundle sheath fiber cells in ltn-212 were altered (Fig. 6a-d.). The thicknesses of the primary and secondary cell walls were reduced by 25.2% and 29.2% compared to WT, respectively, but there were no significant differences of middle lamella in ltn-212 and WT (Fig. 6g-i). The cell wall thickness in different leaf sites of ltn-212 were reduced compared to WT (Additional file 1: Figure. S6). Moreover, the results of TEM in internodes showed that the cell numbers in ltn-212 increased by approximately 2.6-fold compared to the levels in WT; the cell length and width were reduced by 40.6% and 39.6%, respectively (Fig. 6e-f, j-l). To examine whether ospg1 affected cell wall composition, we analyzed the cellulose, hemicellulose, lignin and pectin contents of WT and ltn-212. The cellulose, hemicellulose and pectin contents of ltn-212 were increased by 33.6%, 20.8%, and 8.1%, respectively, while the lignin was no significantly changed (Fig. 6m). The results indicate OsPG1 mutation casues complex cell wall structure and composition altertion.
Fig. 6.
Cell wall structure in WT and ltn-212. a-b TEM micrographs of the bundle sheath fiber cells in leaves of WT (a) and ltn-212 (b) (scale bar =1 μm). c Magnification in (a), and D. magnification in (b) (scale bar = 0.5 μm). E-F. TEM micrographs of the internode cell of WT (e) and ltn-212 (f) (scale bar = 10 μm). g-i Statistical analysis of the primary cell wall, secondary cell wall, and middle lamella thicknesses of bundle sheath fiber cells in leaves of WT and ltn-212. Data are means ± SD of 20 cells. j-l Statistical analysis of the cell number, cell length and cell width of WT and ltn-212. Data are means ±SD of 30 cells. m The cell wall composition of WT and ltn-212 (n = 5). ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test). ml: middle lamella, pw: primary cell wall, sw: secondary cell wall, pm: plasma membrane
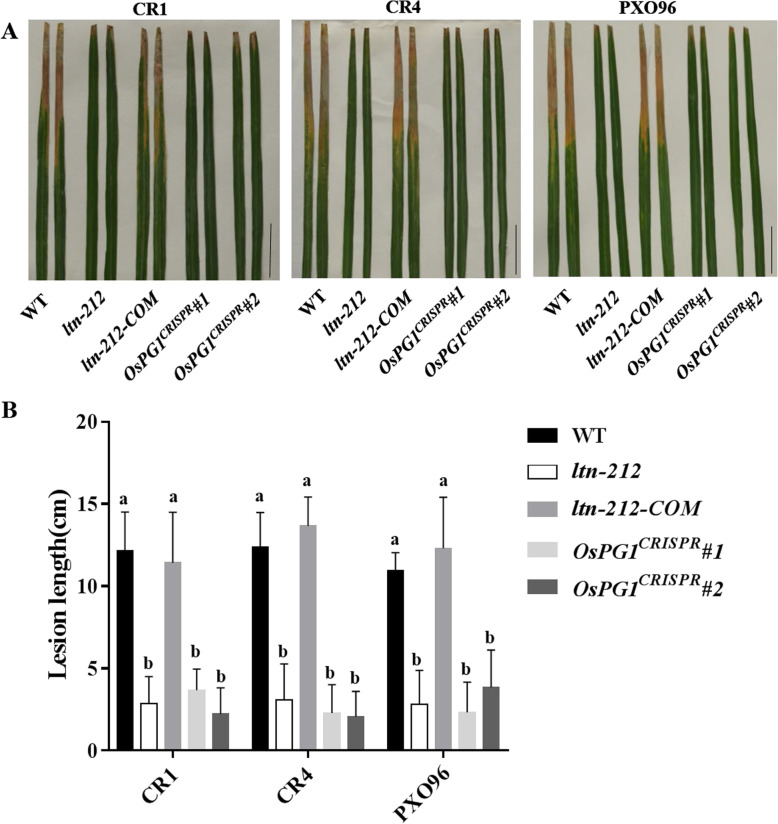
The cell wall is the first barrier against pathogen infection. To determine whether changes in cell wall structure enhanced resistance to the bacterial blight pathogen, we performed a disease resistance assessment with CR1, CR4, and PX096 of ltn-212-COM and OsPG1CRISPR plants. We observed that, similar to WT, the ltn-212-COM plants were more susceptible than ltn-212 plants to the compatible Xoo, while OsPG1CRISPR exhibited shorter lesions similar to those in ltn-212, compared to WT (Fig. 7a-b). These results suggest that the OsPG1 mutation causes structural alterations in cell walls, and OsPG1 negatively regulates disease resistance in rice.
Fig. 7.
Disease reactions of the WT, ltn-212 and transgene plants to Xoo. A. Disease symptoms of WT, ltn-212, ltn-212-COM, OsPG1CRISPR#1, and OsPG1CRISPR#2 leaves after Xoo inoculation at 15 days (scale bar = 5 cm). B. Lesion lengths measured at 15 days after inoculation with 3 different Xoo strains. Data are means ±SD of at least 15 leaves. a, b indicates significance at P ≤ 0.01 (Student’s t test)
Discussion
It has been more than 50 years since the PG gene was first identified, but few PG genes have been reported in crops. In the present study, we identified a novel leaf tip necrotic mutant ltn-212 in an EMS mutagenesis mutant population of rice cultivar JiaHe212. OsPG1 was identified as LOC_Os01g19170, which encodes a PG. Functional complementation and a knock out assay confirmed that the necrotic leaf tips were caused by incorrect splicing of OsPG1, indicating that OsPG1 functions directly in regulating normal leaf growth and development.
The necrosis or withering of leaves commonly accompanies PCD (Hong et al., 2018; Ruan et al., 2019). ZmPGH1, a PG gene homolog, functions as a suppressor of PCD in maize (He et al. 2019). ltn-212 showed dark brown necrotic spots in leaf tips at the tillering stage, which developed into 3–5 cm of dark brown necrosis. Evans blue staining and increased MDA content of ltn-212 showed obvious cell death in leaf tips. TEM observation of the ltn-212 cells showed that areas without necrosis did not have cellular structure destruction or chloroplast degradation (Data not provide). Thus, we speculated that the necrotic leaf tips of ltn-212 were due to cell death and OsPG1 is a general suppressor of PCD in rice. The production of intracellular ROS is closely associated with cell death (Zurbriggen et al. 2010). As expected, DAB staining and H2O2 content in leaf tips occurred at higher levels in the ltn-212 mutants than in WT plants. To eliminate the accumulation of oxidative damage caused by ROS, plants have evolved ROS scavenging enzymes, like SOD, POD, and CAT. In the present study, the activities of three scavenging enzymes were significantly increased, which indicated that the balance between ROS production and scavenging was uncontrolled. ROS burst usually causes serious damage to plants, including brown spots, wilting and necrosis on leaves (Ma et al. 2019; Sathe et al. 2019; Rani et al., 2020). In ltn-212, the necrosis caused by uncontrolled ROS was confined to leaf tips. Interestingly, the qPCR results of OsPG1 in leaf showed that tips had the highest expression levels. This indicates that leaf tips have the highest mRNA levels, which might be responsible for tip necrosis.
PGs are essential in plant growth and development. In Arabidopsis, PGX1, PGX2 and PGX3 are all widely expressed in expanding tissues, including seedlings, roots, leaves, and flower, and all of the loss-of-function mutant plants have growth defects (Rui et al. 2017; Xiao et al. 2017; Xiao et al. 2014). In ltn-212, mutation of OsPG1 caused dark brown necrosis at leaf tips accompanied by decreased plant height, reduced spikelet length, and lower grain size and thousand seed weight relative to WT. This finding suggests that OsPG1 is distributed in multiple tissues at different developmental stages. Given the necrotic phenotype of leaf tips, and changes of cell morphology in internodes and agronomic traits, we speculate that OsPG1 affects apical development in rice. PGs comprise a large family, with 45 annotated PG-encoding genes in rice (Kim et al. 2006; Mccarthy et al. 2014). Phylogenetic analyses of PGs in rice revealed that OsPG1 is classified in Clade A, genes in this clade are expressed in the main fruit or abscission zone (Hadfield and Bennett. 1998). This result is consistent with the GUS staining of grains. Therefore, we conclude that the Clade A genes contributed multiple functions and might thus play a unique role in cell wall remodeling in leaves and other tissues.
Plant cell walls are complex and composed of polysaccharides and protein polymers (Somerville et al. 2004), which are essential in monitoring plant-pathogen interactions (Cantu et al. 2008; Malinovsky et al. 2014; Bacete et al. 2018). Plant cell walls are the first obstacle for pathogens attempting to infect plant tissues and thus function as a passive defensive barrier. Recent studies have shown that plant cell walls have mechanisms for maintaining CWI, including diverse sensors and pattern recognition receptors (PRRs) in the plasma membrane. Alteration of CWI by impairment of proteins involved in biosynthesis or remodeling cell walls significantly affect disease resistance or abiotic stresses (Bellincampi et al. 2014; Miedes et al. 2014; Kesten et al. 2017). PGs, a type of endogenous pectinases, catalyze the degradation of pectin via cleaving HG backbones in plant cell walls (Peaucelle et al. 2012). PGs are normally defined as pathogenicity factors, which are secreted by pathogens to facilitate degradation of the cell wall barrier and promote pathogenesis. However, plants also produce PG. In Arabidopsis, knockdown and overexpression of ADPG2 impacts resistance to P. syringae (Wang et al. 2017). In ltn-212, we found that incorrect splicing of OsPG1 enhances the resistance to bacterial blight pathogen, meaning that the CWI could be altered in ltn-212 plants. The cell wall remodeling also results in PR gene expression. Ectopic activation of endogenous pectin degrading enzyme ADPG1 induces release of pectic oligosaccharide elicitors of PR gene expression (Gallegogiraldo et al. 2020). Similarly, we also detected that some PR genes were upregulated or down-regulated, which may be associated with CWI alteration. Our results highlight the importance of CWI in plant-pathogen interactions.
Pectins are a complex family of polysaccharides that make up a significant fraction of the plant primary cell wall and middle lamella (Bonnin et al. 2014; Atmodjo et al. 2013). Previous studies have shown that pectin has important functions involved in pollen tube growth, fruit softening, providing structural support, and promoting cell-to-cell adhesion and defense responses (Marinrodriguez et al. 2002; Wolf et al. 2009; Hongo et al. 2012). Many examples of disease susceptibility/resistance phenotypes have been reported. Mutations in pectate lyase-like gene PMR6 alter the composition of the plant cell wall and enhance resistance to powdery mildew in Arabidopsis (Vogel et al. 2002). Screening of mutants with alterations in cell wall monosaccharides indicated an important function of pectic polymers for penetration resistance and hyphal growth of Colletotrichum higginsianum at the biotrophic phase (Engelsdorf et al. 2016). Three pectin methylesterase genes AtPMEI10, AtPMEI11 and AtPMEI12 function as mediators maintaining CWI in Arabidopsis immunity (Lionetti et al. 2017). In this study, we observed that the thicknesses of the primary and secondary cell walls were significantly reduced in ltn-212 compared with WT. The changes in cell walls were probably caused by the loss of OsPG1 function, which leads to the degradation of pectin. Consistent with this, the cell numbers increased almost threefold, while the cell size and shape significantly decreased. These results indicate that the function of OsPG1 affects not only the cell wall, but also cell morphology.
Taking these findings together, it is clear that OsPG1 regulated cell death and plant immunity systems through cell wall remodeling. But given the complexity of cell walls, the details in CWI-mediated immunity are still incompletely understood.
Conclusion
OsPG1 functions as a suppressor of programmed cell death and affects CWI, which regulates plant immunity. This finding facilitates efforts to understand the biological functions of PGs, and the role of the cell wall in mediating defense responses.
Methods
Plant Materials and Growth Conditions
The wild-type used in this study was ‘JiaHe212’(Oryza sativa L. ssp. japonica cv). The ltn-212 mutant was obtained from the mutant population generated by EMS treatment of JiaHe212. All plants used in this study were grown in paddy under natural conditions in Hangzhou, Zhejiang province and Lingshui, Hainan province, China. For disease characterizations, the plants were grown in a greenhouse with a photoperiod of 12h white light at 30 °C and 12 h dark at 25 °C.
Construct for Complementation and CRISPR Vectors and Rice Transformation
For the genetic complementation test, a 5790-bp genomic fragment covering the entire coding sequence and 2826-bp native promoter and 1098-bp terminator regions was amplified from the Japonica rice variety Jiahe212 genomic DNA by using KOD FX (Toyobo). The full length product was inserted to the pCAMBIA1300 vector using an In-Fusion Advantage Cloning kit (catalog no. PT4065; Clontech). For OsPG1 knock-out vector construct and rice transformation entrusted BioRun company. The primer sequences used in this experiment listed in Additional file 1: Table S1.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA was extracted from rice leaves, roots, stems at different developmental stages using the RNAprep Pure kit for plants (Tiangen, China). Frist-strand cDNA was synthesized with a ReverTra Ace qPCR RT kit (Toyobo). The qRT-PCR assays were performed with Lightcycler 480 SYBR green (Roche, Sweden) using a LightCycler 480 II real-time PCR instrument (Roche, Sweden). The expression level of the target genes was normalized to that of the UBQ gene. The sequences of all primers used for qRT-PCR are given in Additional file 1: Table S1.
GUS Assay
A 2.8-Kb native promoter region of OsPG1 was amplified and inserted to the pCAMBIA 1305 vector. The transgene rice tissues were stained with GUS staining solution (50 mM PBS buffer; 10 mM EDTA, pH 8.0; 0.1% Triton X-100; 1 mg/mL X-gluc; 1 mM potassium ferricyanide; 1 mM potassium ferrocyanide). After incubating at 37 °C in the dark for 12 h, the chlorophyll was removed by boiling in 95% ethanol until removed completely.
DAB and Evans Blue Staining and Measurement of Enzymatic Activity Related Parameters
Top leaves were collected at heading stage and immediately submerged in 10% SDS. After 10 min, the leaves were subsequently submerged in a 1 mg/mL solution of DAB or 0.5 mg/mL solution of Evans Blue incubated for 8 h in the dark at room temperature, respectively. Samples were then decolored in 95% boiling ethanol for 30 min and soaked for 48 h in 95% ethanol until all of the chlorophyll had been removed. The cleared leaves were then photographed.
Malondialdehyde (MDA) content, Peroxidase (POD), Super Oxide Dismutase (SOD), Catalase (CAT) activity, Soluble Protein (SP) and H202 content of wild type and ltn-212 top leaves were measured from heading stage plants and using kits from Nanjing Jiancheng Bioengineering Research Institute.
Transmission Electron Microscopy (TEM) and Cell Wall Composition Analysis
The materials were cut into small pieces and fixed in 2.5% glutaraldehyde and 0.1 M phosphate buffer at 4 °C overnight and washed three times in the phosphate buffer (0.1 M, pH 7.0) for 15 min at each step; postfixed with 1% OsO4 in phosphate buffer for 1-2 h and washed three times in the phosphate buffer (0.1 M, pH 7.0) for 15 min at each step. For TEM, the samples were subsequently dehydrated through a graded series of ethanol and then embedded in acrylic resin. Ultrathin sections (70-90 nm) were double stained with uranyl acetate and lead citrate aqueous solutions and observed with a Hitachi H-7650 transmission electron microscope (JEOL) at 80 kV. The content assay of cell wall components was entrusted to Convinced-Test company (Nanjing, China).
Sequences Alignment and Phylogenetic Analyses
The gDNA and cDNA sequences of OsPG1 and ospg1 were amplified from WT and ltn-212 using KOD FX, respectively. All the sequence alignments were used MegAlign and Gendoc software. The amino acid sequences of the OsPG1 and Homologous proteins were downloaded from NCBI BLAST server (http://www.ncbi.nlm.nih.gov/). Sequence alignment was performed with ClustalW (Additional file 1: Table S1). A neighbor-joining method implemented in MEGA5 was used to generate the phylogenetic tree; the bootstrap values indicated at the nodes in the phylogenetic tree are based on 1000 replications.
Pathogen Infection
Rice plants were inoculated with Xoo by the leaf-clipping method. CR1, CR4, and PXO96 strains were separately suspended in distilled water and adjusted to 109 viable cells/mL (OD 600 = 1). The Pathogen infection were performed as described previously (Liu et al. 2017).
Supplementary Information
Additional file 1: Figure S1. Leaf tip necrosis of ltn-212 at tillering stage. A. Phenotype of WT and ltn-212 at tillering stage (scale bar = 10 cm). B. Leaf tip necrosis identification of WT and ltn-212 at tillering stage (scale bar = 5 cm). Figure S2. Data statistics of agronomic traits. A-B. Comparison of tiller numbers and plant height of WT and ltn-212 (n = 10). C-D. Comparison of grain length and thousand grain weight of WT and ltn-212 (n = 10). E. Comparison of panicles and culm length of WT and ltn-212 (n = 10). ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test). Figure S3. Genome DNA and cDNA alignments between WT and ltn-212. Red star indicates start codon and stop codon, respectively. The red box indicates the incorrected splicing sequences. The red arrow indicates the mutant site. Figure S4. Amino acid sequence alignment of PG homologous. ADPG2: Arabidopsis thaliana, BCMF9: Brassicarapa L. ssp. Pekinensis, CsPG1: Ciboria shiraiana, RSPG1: Rhizoctonia solani, SDPG: Glycinemax. Figure S5. Phylogenetic analysis of OsPG1 with other homologues in rice. Figure S6. Cell wall structure of leaf bottom, middle, and tip Statistical analysis of the primary cell wall, secondary cell wall, and middle lamella thicknesses of bundle sheath fiber cells in leave bottom(A), middle(B), and tip(C) of WT and ltn-212. Data are means ± SD of 20 cells. ** indicates significance at P ≤ 0.01. Table S1. Primers used in this study.
Acknowledgements
We thank Quanzhi Zhao, Junzhou Li (Henan Agricultural University) for providing opportunity to study at CNRRI. We also thank Bio-Ultrastructure Analysis Lab. of Analysis Center of Agrobiology and Environmental Sciences, Zhejiang Univ. for TEM observation.
Abbreviations
- PG
Polygalacturonase
- CWI
Cell wall integrity
- ROS
Reactive oxygen species
- PR
Pathogenesis-related
- PCD
Programmed cell death
- OGs
Oligogalacturonides
- PME
Pectin methyl esterases
- DAB
3,3-Diaminobenzidine
- MDA
Malonaldehyde
- CAT
Catalase
- POD
Peroxidase
- SOD
Superoxide dismutase
- Xoo
Xanthomonas oryzae pv. oryzae
- WT
Wild type
- ORF
Open reading frame
- CDS
Coding sequence
- GUS
β-glucuronidase
- TEM
Transmission electron microscope
- PRR
Pattern recognition receptor
Authors’ Contributions
CL, WX, CS, CY, LQ, ZY designed the research. CY, LQ, ZY, CY, YN, ZY, WW, CD performed the research and analyzed the data. CY, LQ, CL, WX, CS wrote the manuscript and revised the manuscript. All authors have read the manuscript and approved to submit it to your journal.
Funding
National Natural Science Foundation of China (31801726, 31871236, and 31521064), the National Key Transgenic Program (2016ZX08001002), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of the Agricultural Sciences (CAAS-ASTIP-2013-CNRRI). the Fundamental Research Funds of Central Public Welfare Research Institutions (2017RG001–1).
Availability of Data and Materials
All relevant data are provided within the article and its supplementary information files.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yongrun Cao and Yue Zhang contributed equally to this work.
Contributor Information
Yingxin Zhang, Email: zhangyingxin@caas.cn.
Qunen Liu, Email: liuqunen202@163.com.
References
- Annis SL, Goodwin PH. Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur J Plant Pathol. 1997;103(1):1–14. doi: 10.1023/A:1008656013255. [DOI] [Google Scholar]
- Atkinson RG, Schroder R, Hallett IC, Cohen D, MacRae EA. Overexpression of Polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002;129(1):122–133. doi: 10.1104/pp.010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmodjo MA, Hao ZY, Mohnen D. Evolving views of pectin biosynthesis. Annu Rev Plant Biol. 2013;64(1):747–779. doi: 10.1146/annurev-arplant-042811-105534. [DOI] [PubMed] [Google Scholar]
- Bacete L, Melida H, Miedes E, Molina A. Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J. 2018;93(4):614–636. doi: 10.1111/tpj.13807. [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant–pathogen interactions. Front Plant Sci. 2014;5:228–228. doi: 10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin E, Garnier C, Ralet M. Pectin-modifying enzymes and pectin-derived materials: applications and impacts. Applied microbiology. Biotechnology. 2014;98:519–532. doi: 10.1007/s00253-013-5388-6. [DOI] [PubMed] [Google Scholar]
- Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13(11):610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Engelsdorf T, Will C, Hofmann J, Schmitt C, Merritt BB, Rieger L, Frenger MS, Marschall A, Franke R, Pattathil S. Cell wall composition and penetration resistance against the fungal pathogen Colletotrichum higginsianum are affected by impaired starch turnover in Arabidopsis mutants. J Exp Bot. 2016;68:701–713. doi: 10.1093/jxb/erw434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Pontiggia D, Manfredini C, Lionetti V, Bellincampi D, Cervone F, De LG. Transgenic expression of a fungal endo-Polygalacturonase increases plant resistance to pathogens and reduces Auxin sensitivity. Plant Physiol. 2007;146:669–681. doi: 10.1104/pp.107.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegogiraldo L, Liu C, Posealbacete S, Pattathil S, Peralta AG, Young J, Westpheling J, Hahn MG, Rao XL, Knox JP, Knox JP, Meester BD, Boerjan W, Dixon RA. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proc Natl Acad Sci U S A. 2020;117(6):3281–3290. doi: 10.1073/pnas.1914422117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena AH, Greenwood JS, Dengler NG. Cell wall degradation and modification during programmed cell death in lace plant, APONOGETON MADAGASCARIENSIS (APONOGETONACEAE) Am J Bot. 2007;94(7):1116–1128. doi: 10.3732/ajb.94.7.1116. [DOI] [PubMed] [Google Scholar]
- Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. Plant Physiol. 1998;117(2):337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YJ, Karre S, Johal GS, Christensen SA, Balint-Kurti P. A maize polygalacturonase functions as a suppressor of programmed cell death in plants. BMC Plant Biol. 2019;19(1):310. doi: 10.1186/s12870-019-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YB, Zhang YX, Sinumporn S, Yu N, Xd Z, Shen XH, Chen DB, Yu P, Wu WX, Liu QE, Cao ZY, Zhao CD, Cheng SH, Cao LY. Premature leaf senescence 3, encoding a methyltransferase, is required for melatonin biosynthesis in rice. Plant J. 2018;95(5):877–891. doi: 10.1111/tpj.13995. [DOI] [PubMed] [Google Scholar]
- Hongo S, Sato K, Yokoyama R, Nishitani K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell. 2012;24(6):2624–2634. doi: 10.1105/tpc.112.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Zhu CG, Li F, Tang JY, Wang YQ, Lin AH, Liu LC, Che RH, Chu CC. LEAF TIP NECROSIS1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice. Plant Physiol. 2011;156(3):1101–1115. doi: 10.1104/pp.110.170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh J, Nonomura K, Ikeda K, So Y, Inukai Y, Yamagishi H, Kitano H, Nagato Y. Rice plant development from zygote to spikelet. Plant Physiology Cell. 2005;46(1):23–47. doi: 10.1093/pcp/pci501. [DOI] [PubMed] [Google Scholar]
- Jia HF, Ren HY, Gu M, Zhao JN, Sun SB, Zhang X, Chen JY, Wu P, Xu GH. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in Rice. Plant Physiol. 2011;156(3):1164–1175. doi: 10.1104/pp.111.175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesten C, Menna A, Sanchezrodriguez C. Regulation of cellulose synthesis in response to stress. Curr Opin Plant Biol. 2017;40:106–113. doi: 10.1016/j.pbi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Kim J, Shiu S, Thoma S, Li W, Patterson SE. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 2006;7:1–14. doi: 10.1186/gb-2006-7-9-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger SG, Sucher J, Selter LL, Chauhan H, Zhou B, Tang MZ, Upadhyaya NM, Mieulet D, Guiderdoni E, Weidenbach D, Schaffrath U, Lagudah ES, Keller B. The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnol J. 2016;14(5):1261–1268. doi: 10.1111/pbi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng YJ, Yang YL, Ren DY, Huang LC, Dai LP, Wang YQ, Chen L, Tu ZJ, Gao YH, Li XY, Zhu L, Hu J, Zhang GH, Gao ZY, Guo LB, Kong ZS, Lin YJ, Qian Q, Zeng DL. A Rice PECTATE LYASE-LIKE gene is required for plant growth and leaf senescence. Plant Physiol. 2017;174(2):1151–1166. doi: 10.1104/pp.16.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JG, Zhu H, Yuan RC. Profiling the expression of genes related to ethylene biosynthesis, ethylene perception, and cell wall degradation during fruit abscission and fruit ripening in apple. J Am Soc Hortic Sci. 2010;135(5):391–401. doi: 10.21273/JASHS.135.5.391. [DOI] [Google Scholar]
- Lionetti V, Fabri E, De CM, Hansen AR, Willats WGT, Piro G, Bellincampi D. Three pectin Methylesterase inhibitors protect Cell Wall integrity for Arabidopsis immunity to Botrytis. Plant Physiol. 2017;173(3):1844–1863. doi: 10.1104/pp.16.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ma Y, Chen NA, Guo SY, Liu HL, Guo XY, Chong K, Xu YY. Overexpression of stress-inducible OsBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ. 2014;37(5):1144–1158. doi: 10.1111/pce.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang Y, Xu J, Su T, Liu G, Ren D. Ethylene Signaling is required for the acceleration of cell death induced by the activation of AtMEK5 in Arabidopsis. Cell Res. 2008;18:422–432. doi: 10.1038/cr.2008.29. [DOI] [PubMed] [Google Scholar]
- Liu Hk, Qian M, Song CH, Li JJ, Zhao CP, Li GF, Wang AZ, Han MY (2018) Down-Regulation of PpBGAL10 and PpBGAL16 Delays Fruit Softening in Peach by Reducing Polygalacturonase and Pectin Methylesterase Activity. Frontiers in Plant Science 9:1015. [DOI] [PMC free article] [PubMed]
- Liu QE, Ning YS, Zhang YX, Yu N, Zhao CD, Zhan XD, Wu WX, Chen DB, Wei XJ, Wang GL, Cheng SH, Cao LY. OsCUL3a Negatively Regulates Cell Death and Immunity by Degrading OsNPR1 in Rice. Plant Cell. 2017;29:345–359. doi: 10.1105/tpc.16.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang YF, Ma XD, Meng LZ, Jing RN, Wang F, Wang S, Cheng ZJ, Zhang X, Jiang L, Zhao ZC, Guo XP, Lin QB, Wu FQ, Zhu SS, Wu CY, Ren YL, Lei CL, Zhai HQ, Wan JM. Disruption of gene SPL35, encoding a novel CUE domain-containing protein, leads to cell death and enhanced disease response in rice. Plant Biotechnol J. 2019;17(8):1679–1693. doi: 10.1111/pbi.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovsky FG, Fangel JU, Willats WGT. The role of the cell wall in plant immunity. Front Plant Sci. 2014;5:178–178. doi: 10.3389/fpls.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinrodriguez MC, Orchard J, Seymour GB. Pectate lyases, cell wall degradation and fruit softening. J Exp Bot. 2002;53(377):2115–2119. doi: 10.1093/jxb/erf089. [DOI] [PubMed] [Google Scholar]
- Markovic O, Janecek S. Pectin degrading glycoside hydrolases of family 28: sequence-structural features, specificities and evolution. Protein Eng. 2001;14(9):615–631. doi: 10.1093/protein/14.9.615. [DOI] [PubMed] [Google Scholar]
- Mccarthy TW, Der JP, Honaas LA, Depamphilis CW, Anderson CT. Phylogenetic analysis of pectin-related gene families in Physcomitrella patens and nine other plant species yields evolutionary insights into cell walls. BMC Plant Biol. 2014;14(1):79–79. doi: 10.1186/1471-2229-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6(9):414–419. doi: 10.1016/S1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Miedes E, Vanholme R, Boerjan W, Molina A. The role of the secondary Cell Wall in plant resistance to pathogens. Front Plant Sci. 2014;5:358–358. doi: 10.3389/fpls.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S, Swain SM. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell. 2009;21(1):216–233. doi: 10.1105/tpc.108.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Hofte H. Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci. 2012;3:121–121. doi: 10.3389/fpls.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada MA, Blancoportales R, Pose S, Garciagago JA, Jimenezbermudez S, Munozserrano A, Caballero JL, Pliegoalfaro F, Mercado JA, Munozblanco J. Antisense Down-regulation of the FaPG1 gene reveals an unexpected central role for Polygalacturonase in strawberry fruit softening. Plant Physiol. 2009;150(2):1022–1032. doi: 10.1104/pp.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani MH, Liu QE, Yu N, Zhang YX, Wang BF, Cao YR, Zhang Y, Islam A, Anley ZW, Cao Liyong Cheng SH (2020) ES5 is involved in the regulation of phosphatidylserine synthesis and impacts on early senescence in rice (Oryza sativa L). Plant Mol Biol 102(4-5):501–515. 10.1007/s11103-019-00961-4 [DOI] [PMC free article] [PubMed]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 2003;133(3):1170–1180. doi: 10.1104/pp.103.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars L, Richardson T, Buesing G, Troller A, Lagudah ES, Beat K. The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol J. 2013;11(7):847–854. doi: 10.1111/pbi.12077. [DOI] [PubMed] [Google Scholar]
- Ruan BP, Hua ZH, Zhao J, Zhang B, Ren DY, Liu CL, Yang SL, Zhang AP, Jiang HZ, Yu HP, Hu J, Zhu L, Chen G, Shen L, Dong GJ, Zhang GH, Zeng DL, Guo LB, Qian Q, Gao ZY. OsACL-A2 negatively regulates cell death and disease resistance in rice. Plant Biotechnol J. 2019;17(7):1344–1356. doi: 10.1111/pbi.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Xiao CW, Yi H, Kandemir B, Wang JZ, Puri VM, Anderson CT. POLYGALACTURONASE INVOLVED IN EXPANSION3 functions in seedling development, rosette growth, and Stomatal dynamics in Arabidopsis thaliana. Plant Cell. 2017;29(10):2413–2432. doi: 10.1105/tpc.17.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bosneaga E, Auer M. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J Exp Bot. 2009;60(13):3615–3635. doi: 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- Sathe AP, Su XN, Chen Z, Chen T, Wei XJ, Tang SQ, Zhang XB, Wu JL. Identification and characterization of a spotted-leaf mutant spl40 with enhanced bacterial blight resistance in rice. Rice. 2019;12:1–15. doi: 10.1186/s12284-019-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP. Association between gene Lr34 for leaf rust resistance and leaf tip necrosis in wheat. Crop Sci. 1992;32(4):874–878. doi: 10.2135/cropsci1992.0011183X003200040008x. [DOI] [Google Scholar]
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H. Toward a systems approach to understanding plant-cell walls. Science. 2004;306(5705):2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- Van BF, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal NM, Martínez GA, Civello PM. Influence of plant growth regulators on polygalacturonase expression in strawberry fruit. Chin Academy Agric Sci. 2009;176:0–757. [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC. PMR6, a Pectate Lyase–like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell. 2002;14(9):2095–2106. doi: 10.1105/tpc.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasa Y, Kudo H, Ishikawa R, Akada S, Senda M, Niizeki M, Harada T. Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Technol Postharvest Biol. 2006;39(2):193–198. doi: 10.1016/j.postharvbio.2005.10.005. [DOI] [Google Scholar]
- Wang X, Hou SG, Wu QQ, Lin MY, Acharya BR, Wu DJ, Zhang W. IDL6-HAE/HSL2 impacts pectin degradation and resistance to Pseudomonas syringae pv tomato DC3000 in Arabidopsis leaves. Plant J. 2017;89(2):250–263. doi: 10.1111/tpj.13380. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Wan ZY, CAO JS, YU Xiao-Lin, XIANG X, LU G (2005) Cloning and characterization of the microspore development-related gene BcMF2 in Chinese cabbage Pak-Choi (Brassica campestris L. ssp. chinensis Makino). J Integr Plant Biol, 47: 863–872, 7, DOI: 10.1111/j.1744-7909.2005.00101.x
- Wolf S, Gregory M, Jerome P. Homogalacturonan methyl-esterification and plant development. Mol Plant. 2009;2(5):851–860. doi: 10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- Wu LW, Ren DY, Hu SK, Li GG, Dong GJ, Jiang L, Hu XM, Ye WJ, Cui YT, Zhu L, Hu J, Zhang GH, Gao ZY, Zeng DL, Qian Q, Guo LB. Down-regulation of a Nicotinate Phosphoribosyltransferase gene, OsNaPRT1, leads to withered leaf tips. Plant Physiol. 2016;171(2):1085–1098. doi: 10.1104/pp.15.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CW, Barnes WJ, Zamil MS, Yi H, Puri VM, Anderson CT. Activation tagging of Arabidopsis POLYGALACTURONASE INVOLVED IN EXPANSION2 promotes hypocotyl elongation, leaf EXPANSION, stem lignification, mechanical stiffening, and lodging. Plant J. 2017;89(6):1159–1173. doi: 10.1111/tpj.13453. [DOI] [PubMed] [Google Scholar]
- Xiao CW, Somerville C, Anderson CT. POLYGALACTURONASE INVOLVED IN EXPANSION1 functions in cell elongation and flower development in Arabidopsis. Plant Cell. 2014;26(3):1018–1035. doi: 10.1105/tpc.114.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen MD, Carrillo N, Hajirezaei M. ROS signaling in the hypersensitive response: when, where and what for? Plant Signal Behav. 2010;5(4):393–396. doi: 10.4161/psb.5.4.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Leaf tip necrosis of ltn-212 at tillering stage. A. Phenotype of WT and ltn-212 at tillering stage (scale bar = 10 cm). B. Leaf tip necrosis identification of WT and ltn-212 at tillering stage (scale bar = 5 cm). Figure S2. Data statistics of agronomic traits. A-B. Comparison of tiller numbers and plant height of WT and ltn-212 (n = 10). C-D. Comparison of grain length and thousand grain weight of WT and ltn-212 (n = 10). E. Comparison of panicles and culm length of WT and ltn-212 (n = 10). ** indicates significance at P ≤ 0.01 and * indicates significance at P ≤ 0.05 (Student’s t test). Figure S3. Genome DNA and cDNA alignments between WT and ltn-212. Red star indicates start codon and stop codon, respectively. The red box indicates the incorrected splicing sequences. The red arrow indicates the mutant site. Figure S4. Amino acid sequence alignment of PG homologous. ADPG2: Arabidopsis thaliana, BCMF9: Brassicarapa L. ssp. Pekinensis, CsPG1: Ciboria shiraiana, RSPG1: Rhizoctonia solani, SDPG: Glycinemax. Figure S5. Phylogenetic analysis of OsPG1 with other homologues in rice. Figure S6. Cell wall structure of leaf bottom, middle, and tip Statistical analysis of the primary cell wall, secondary cell wall, and middle lamella thicknesses of bundle sheath fiber cells in leave bottom(A), middle(B), and tip(C) of WT and ltn-212. Data are means ± SD of 20 cells. ** indicates significance at P ≤ 0.01. Table S1. Primers used in this study.
Data Availability Statement
All relevant data are provided within the article and its supplementary information files.