Abstract
OBJECTIVE
The effectiveness of household contact investigations is limited by low referral uptake for clinic-based TB testing by symptomatic household contacts. We qualitatively investigated the acceptability and perceived benefits of home-based TB testing using a portable GeneXpert-I instrument (GX-I) in an urban South African township.
METHODS
In-depth interviews were conducted with household contacts tested and those that observed testing. Semi-structured interviews explored household contact’s understanding of TB, perceptions of the GX-I device and testing procedures, confidentiality, willingness to refer others, and views on home- vs. clinic-based testing. Focus group discussions with home-based TB testing implementing staff assessed operational considerations for scale-up. Data were analysed using a constant comparison approach to qualitatively evaluate the acceptability and feasibility of home-based TB testing.
RESULTS
Thirty in-depth interviews and two focus group discussions were conducted. Observing one’s own sputum being tested resulted in an emergent trust in home-based TB testing, the GX-I device and one’s test results. Home-based TB testing was considered convenient, helped to overcome apathy towards testing and mitigated barriers to clinic-based testing. Perceptions that home-based TB testing contributes to improved household and community health resulted in an emergent theme of alleviation of health insecurities. Operational concerns regarding inadvertent disclosure of one’s diagnosis to household members and time spent in people’s homes were identified.
CONCLUSIONS
Home-based TB testing was acceptable and feasible. Individuals expressed belief in the machine by being able to witness the testing process. Though most themes mirrored qualitative studies of home-based HIV testing, the alleviation of health insecurities theme is unique to home-based TB testing. Future research must evaluate the impact of home-based TB testing on case finding yield, time-to-treatment initiation and household outcomes.
Keywords: tuberculosis, household contacts, contact tracing, active case finding, home-based testing, GeneXpert, feasibility, acceptability, qualitative, South Africa
Introduction
In 2018, WHO reported that 10 million people developed TB disease; of whom, 4.3 million were undiagnosed [1]. Missed opportunities to screen for TB in primary and community health facilities [2–4], as well as limited resource capacity, use of screening and diagnostic tools with less than optimal sensitivity [5–8], healthcare access barriers and individual health-seeking behaviours [9–14] all contribute to the missing TB cases. Efforts have been made to diminish these barriers [15,16]; however, benefits have mainly flowed to TB cases passively presenting to the health system, suggesting that a renewed effort to implement and optimised community-based active case finding is greatly needed, as such efforts can curtail early transmission [17,18].
Targeted and community-wide household screening interventions are forms of active case finding fundamental to TB control programmes [1] that are cost-effective and improve case detection [19–24]. Strategies to optimise the impact of targeted and community-wide household screening include the slip method (i.e. providing TB index patients with referral letters to give to their contacts), home-based screening with referral for clinic-based testing or home-based sputum collection for laboratory-based testing [24–27]. However, design, implementation factors and low uptake of referrals for clinical-based testing by symptomatic contacts have limited the effectiveness and impact of household contact screening interventions to TB case detection [13,14,27–29].
Home-based TB testing would follow the tradition of home-based HIV counselling and testing, which is an acceptable and effective intervention for identifying individuals living with HIV [30–34]. As part of home-based HIV counselling and testing implementation, several qualitative studies described its high acceptability through the convenience, confidentiality and credibility that home testing offered compared to clinic-based testing [35–38]. Moreover, healthcare workers felt that home-based HIV counselling and testing and early diagnosis could encourage healthcare seeking behaviour and help surmount a number of financial and structural barriers (e.g. transport and slow service delivery) [35–38]. Unfortunately, applying the home-based HIV counselling and testing model to TB has been extremely difficult, as there has never been a highly sensitive and mobile diagnostic test that could rapidly diagnose TB as part of household contact investigations.
The introduction of GeneXpert MTB/RIF has greatly improved TB case finding; however, this technology has been mainly relegated to laboratories and healthcare facilities [39,40]. Recent intensified case finding studies have explored the feasibility and effectiveness of integrating GeneXpert platforms into community-based mobile HIV testing vans [41,42]. Given the need to improve active case finding and linkage to care amongst household contacts of TB patients, we adapted a GeneXpert single module (GX-I) instrument for portability and use during household contact tracing investigations [43]. Here, we present a qualitative investigation of the acceptability and feasibility of home-based TB testing using this adapted technology in households in Buffalo City Metro Health District, Eastern Cape Province, South Africa.
Methods
This qualitative study was nested within a larger randomised study, conducted between July 2018 and June 2019. This larger exploratory study aimed at investigating the acceptability and feasibility of home-based TB testing using a GX-I instrument adapted for portability and determining the potential impact of home-based TB testing on time along the TB case finding cascade. Three attempts were made to schedule testing visits with household contacts. Households that were randomised in the intervention arm and received home-based TB testing using the GX-I were invited to participate in interviews. We used a constant comparison approach to understand household contacts experiences of home-based TB testing. This was further complemented by interviews conducted with field-based staff.
Study setting
This study was conducted in Duncan Village, an urban township with a large informal settlement area located in Buffalo City Metro Health District, Eastern Cape Province, South Africa. Duncan Village, including its surrounding communities, has a population of ~64 523, an extremely high population density in excess of 2500 people per hectare in some areas, and an estimated TB incidence in excess of 831 per 100 000 population [44,45].
Participant recruitment
Participants were household contacts of TB index patients engaged in TB care and treatment at one of six government health clinics in the Duncan Village area. All household contacts were screened for TB using the WHO simplified four-symptom screener [46]. In the larger study, households with symptomatic contacts were randomised to either receive home-based TB testing or referred to the clinic. A total of 23 households underwent successful home-based TB testing (1 invalid). An eligibility criterion for testing was ≥18 years. In-depth interviews eligible participants were those who underwent home-based TB testing, were successfully contacted by telephone and agreed to participate in an in-depth interview. In-depth interviews were conducted with a total of 17 households. Other members that observed the testing process were also approached. This sampling method led to a sample size of 30 in-depth interviews with a larger representation of household contacts tested vs. those who observed. In-depth interviews were performed >30-day post-home-based TB testing so as to not influence the primary quantitative outcome of time-to-clinic presentation. Study staff implementing home-based TB testing were invited and consented to participate in a focus group discussion. Participants were not compensated for their participation in in-depth interviews or focus group discussions.
Home-based diagnostic testing
Contact investigation teams, composed of lay community health workers, were dispatched to homes of TB index patients with a home-based TB testing platforms consisting of a GeneXpert® I (Cepheid, Sunnyvale, CA, USA), GeneXpert® MTB/RIF testing cartridges, a standard laptop and a China Delong Smart Uninterrupted Power Supply (UPS) 500W portable power supply; testing platforms and materials were carried into the communities using a backpack. All testing procedures were conducted in front of household members (Box 1). Immediately upon test completion, individuals were shown their results using the laptop. Participants were counselled and/or referred for treatment in the case of a positive Xpert result.
Data collection
Semi-structured in-depth interviews were conducted with symptomatic household contacts tested in their home and household members who observed the testing process. In-depth interview protocol domains included understanding of TB, TB attitudes and practice; perceptions of the GeneXpert machine; TB testing process; confidentiality; willingness to refer others; and views on home- vs. clinic-based testing. Additionally, two focus group discussions were conducted amongst field-based study staff to capture their experiences in the field. Focus group discussion protocol domains included: GX-I technical considerations, testing experience, participant responses to the testing process and community engagement during the testing process. Focus group discussions were conducted after the in-depth interviews were completed and within a month of last household tested.
Research assistants (male and female) who conducted home-based TB testing received a 2- to 3-day training in qualitative research. In addition to the interview guide that was developed by the co-principal investigators, staff were trained in interviewing, observation and probing techniques. Interviewer qualifications included social work, development studies or previous work experience in HIV/TB/public clinics. They were also trained in the study protocol and protection of human subjects. In-depth interviews were conducted in a participants’ preferred language (e.g. English or isiXhosa), within the privacy of their households and were ~60 min in length. Focus group discussions, conducted in English, were moderated by a female qualitative research team member familiar with the study protocol and trained in qualitative interviewing but whom did not have previous contact with the field staff. Participants were informed that the researcher wanted to capture their experiences, including any implementation challenges as a home-based TB tester focus group discussions lasted ~90 min in length. Field note guides were used during both in-depth interviews and focus group discussions. Interviews were audio-recorded, translated and transcribed into English, as needed, for analysis; to ensure quality control, all transcripts were reviewed. Transcripts were not returned to participants for comment, and they were not informed of the in-depth interview results. However, during the translation and transcription process, a second researcher assessed transcripts for accuracy by reviewing a random selection of transcripts with the respective audio recording. Weekly research team meetings were held to discuss and refine interview and data collection processes.
Data analysis
A constant comparison method was used for data analysis [47]. First, two separate codebooks were developed for the in-depth interviews and focus group discussions. To do so, a subset of the transcripts were preliminary coded using an inductive approach. Codes were defined and organised by domains through discussions amongst three members of the research team. Developed codebooks were reviewed and finalised by the lead qualitative researcher. Final codebooks were applied to all transcripts, and emerging codes were added using an iterative approach. Afterwards, researchers analysed data iteratively by participant groups over a series of meetings, for example household contacts and staff, household contacts tested and household contacts observed, household contacts who tested positive or negative for TB [48]. Then, using matrices, data were triangulated to identify and determine the consistency or divergence of feasibility themes across participant groups. This was supported by quantification of related codes [48–50]. Memo writing and causal diagrams were completed and discussed amongst the research team to refine results. A formal presentation of the preliminary results was delivered to the full research team, which further directed analysis; applying the same processes as outlined here. Final results were classified as aspects of feasibility (feasibility, acceptability, willingness and safety) as per the objectives of this study [51,52]. All coding was conducted inATLAS.ti 8 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany).
Data representation
In-text quotes are denoted as ‘household contact, test outcome, sex, age’ for those tested in their home; ‘household contact, observer, sex, age’ for those who observed home-based TB testing processes; and ‘Field worker #, FGD #, sex, age’ for implementation staff that participated in a focus group discussion.
Ethics
Ethics approval was obtained from the Faculty of Health Sciences Research Ethics Committee, University of Pretoria, South Africa (Ref no.: 06/2016). The study was explained to participants using an informed consent information leaflet. Participation and recording of interviews were voluntary. Permission was obtained through a consent form. An additional consent form was used to obtain permission for taking photographs within households.
Results
Thirty household contacts were invited to participate in an in-depth interview (individuals tested = 23, of whom five tested positive for TB; household observers = 7). The mean age of participants was 38.2 years (standard deviation = 16.7), and 63% were male. Of participants approached, all 30 (100%) agreed and consented to participate. Nine study implementation staff were invited to partake in a focus group discussion, and all (100%) consented.
Through the experiences of household contacts, we aimed to understand the acceptability and feasibility of home-based testing. These are the emerging themes that developed from the findings: (i) emerging trust in the machine through observation, and the perceived benefits of testing at home, (ii) decreasing health insecurity for households and community through home-based TB testing, (iii) previous lack of urgency and overcoming testing apathy and (iv) how technology gathers attention.
Emergent trust and testing convenience
Household contacts perceived the machine as trustworthy by being able to observe the testing process. As expressed in the following quotes, household members observing the machine in action and the generation of immediate results contributed to the credibility in the GX-I machine, and contributed to the trust and belief in their test results:
Yes sister, I believed after seeing my results because I believe. Because the work was done and here are the results that said it was done and the results say this. So, I thought I cannot say this is not me or deny, so I believed the result. Everything was done in front of me.
(Household contact, tested negative, Male, Age 30)
Yes, I did trust because I was watching. It’s because I saw when they took my sputum and put it on the machine and then there was something that was running, proving that they are testing the sputum and I also saw in the screen [laptop] that really they are testing my sputum because I can see clearly.
(Household contact, tested negative, Female, Age 35)
Because I saw and witnessed the testing process and I believe it.
(Household contact, observer, Male, Age 23)
I believed, I believed because immediately after they have setup the machine the way I told you before, the machine runs and TB was exposed, I believed that I do have TB… The disease that I have is out easily with the machine.
(Household contact, tested positive, Male, Age 40)
These individuals mention watching their sputum being processed and run on the machine, and seeing their test run on the laptop screen as contributing to their trust and belief in their test results; this is especially poignant coming from the individual that tested positive for TB. Together, household contacts’ direct observations of all testing activities allowed them to trust and believe in the produced test results. Time-to-test results also contributed to a trust in home-based testing activities, as described in this following quote:
Because most of the time, when you go to the facility for testing TB you get your results after some few days, because there are so many people testing for TB and since there are many people getting tested, [that] is the reason why they have to wait for their results. It happened that after I got tested at home and get the results at the same day, that made me see the fact that it [the machine] is working with us and our health.
(Household contact, tested negative, Male, Age 18)
This household contact refers to the overburdened patient demand for clinic-based TB testing services, and how this demand results in long wait times for testing and receiving one’s results. He perceived the machine to be working with and for ‘us’, by being tested at home and receiving his test results the same day.
Household contacts seemed particularly receptive to home-based testing due to its perceived convenience. Specifically, many felt that home-based TB testing helped surmount certain access barriers, including the numerous trips that were required for TB testing services, as expressed in the following quotes:
yho[!], it was easier [testing at home] than having to go to the clinic and taking that sputum bottle and going home with it [the sputum bottle] and sometimes the clinic had a problem with labs. So, it was easier, rather this way, because you get results the same day.
(Household contact, tested negative, Female, Age 18)
No clinic, ha-ah [negative]…They give you a bottle and I take it [home] with me and cough in the morning and then bring it back [to the clinic]. But when I cough here at home, I cough so that I can be alright… and not being seen by other people.
(Household contact, tested positive, Male, Age 21)
These individuals described a standard procedure in some clinics to send patients home with a bottle for sputum production and then having to return to the clinic to submit it. One contact alluded to challenges and delays in laboratory turnaround time and delivery of test results, which contributed to her suggestion that home-based TB testing was easier, faster and more convenient. The contact that tested positive for TB further expressed preference for coughing and testing at home, as he does not want to be seen by other people in the clinic. Household contacts often recommended home-based testing as a result of the perceived benefits and testing transparency:
I would recommend [testing at home]… Because he or she will know everything. Here are the machines, you can see them, they come to them [the households]. Just to make example, if someone is bed ridden and can’t go to the clinic it is easy for them to come help you at home using their machines. Everything is fast and the results come out immediately.
(Household contact, tested negative, Female, Age 27)
This contact expresses that the value of home-based TB testing is the transparency of the testing procedures being performed in front of an individual, and them being able to see exactly what is happening to their specimen (‘because he or she will know everything’). Additionally, this household contact supports the notion that home-based TB testing is faster and more convenient, especially if someone is unable to get the clinic.
While most household contacts were not fazed when the machine was set up in their homes, some did express surprise and initial uncertainty:
I don’t want to lie [giggles], I’ve never seen it before. I was so surprised, I thought that they were going to… to just test maybe blood or something. It was new. I was so surprised that very valuable machines could come to our homes. It was a good thing.
(Household contact, tested negative, Female, Age 30)
In this case, the machine was perceived as novel and valuable (e.g. it was a ‘good thing’). Of note, this individual expected to be tested using blood, revealing her familiarity with other community-based testing programmes such as home-based HIV testing, but learned that fluid (sputum) other than blood can be used for testing. This demonstrates that although a portable GeneXpert device is perceived as new, the concept of home-based testing is not. Observing the machine and testing process engendered trust in the results amongst participants.
Decreasing household and community health insecurities
Household contacts discussed the importance of TB testing for themselves, their household, and their community. Specifically, while household contacts expressed disquiet about TB transmission in their home, many also felt that home-based TB testing could alleviate this concern and restore a sense of security to their home. Furthermore, individuals also expressed that increased testing via home-based testing services could reduce TB in their communities. Together, the expression that testing could improve individual, household and community-level safety speaks to the health insecurities that TB introduces at multiple levels.
Many household contacts discussed TB as a major cause of illness and death in their communities and South Africa in general. As expressed in the following quote, this individual stated that additional testing is needed to stop TB from killing South Africans:
Yes, I am saying people should get tested because this TB kills, and it helps when someone has been tested and see if you have TB or not. So, it is right when people come to our homes to test us [for] TB because it kills the nation now-a-days.
(Household contact, tested negative, Female, Age 46)
This individual supported home-based TB testing to address TB’s impact on her community and nation. She directly expressed an awareness of TB mortality resulting from undetected TB. Other household contacts expressed a sense of household-level insecurity from living with someone with TB, and the re-introduction of household health security when TB testing was brought into their home, as expressed in the following quote:
It helped [testing at home] because we didn’t know if we were infected with TB or not, now that we are living with someone who had TB, they [the field-based staff] helped to come and check us.
(Household contact, tested negative, Female, Age 51)
This individual further indicates an awareness of household transmission, and the uncertainty and insecurity of not knowing if they too were infected. Moreover, the constant use of ‘we’ and ‘us’ spoke to the recognised household-level risk when one of its members has TB. The introduction of home-based TB testing helps to alleviate this household-level health insecurity.
The acknowledgement that some household members may not seek clinic-based care adds to this health insecurity. Especially when there are TB status uncertainties amongst household members:
I was not going to be able to return to the clinic because he doesn’t want anything to do with the clinic so I was happy to see that he didn’t have TB because I thought he must have had it a long time ago.
(Household contacted, observer, Female, Age 60)
This household contact expressed barriers to confirming her housemate’s TB status, as he was reluctant to go to the clinic. Yet, knowing that her housemate was TB negative eliminated her concerns, and reintroduces a sense of health security to the household.
Household contacts also acknowledged an association between coughing and TB, and accusations that if another household member coughed, that they must have TB:
They [other household members] were very excited [about the test] because we always accuse each other for TB when one of us is coughing. They were very happy to find that I do not have TB, I only had flu’, ….’I was very excited [about testing], I wanted them [other household members] to know that I do not have TB so that they must be safe.
(Household contact, tested negative, Male, Age 65)
This household contact expressed relief when his test result was negative, showing how household members can experience blame and stigma (‘accuse each other’) when TB is suspected. He expressed that other household members were excited about his test result, and no longer felt insecure or threatened by his cough, as he likely ‘only had flu’. Home-based testing alleviated TB concerns on multiple levels, that is individual, household and community levels, re-enforcing household security.
Lack of urgency and overcoming apathy towards testing
Many individuals expressed concerns about potential household transmission and the importance of TB testing as presented above. However, this did not spur many participants to go to a local clinic for clinic-based TB testing. Though individuals may be dissuaded by several barriers from seeking or accessing care, more conspicuous were behaviours associated with a lack of urgency to test given the common understanding that TB can lead to severe illness and death in their communities.
Home-based testing offered household contacts an opportunity to overcome their apathy towards testing, as mentioned in the following quotes:
It was right to be tested in the home because I might be lazy to go and get tested in the clinic.
(Household contact, tested negative, Female, 51)
Ey, my sister, it is good that I got tested at home, the help came to me. But there is [a] thing of having to go [to] the clinic, I keep on saying I am going tomorrow but I never get to go. But now that I have confirmation that I have TB and I am starting not to feel well. I will definitely go.
(Household contact, tested positive, Male, Age 59)
Both individuals confessed that they lacked a sense of urgency to go to the clinic, and were glad that the testing came to them. The contact that tested positive specifically expressed an intent to seek care, but had not gone to date. He expressed appreciation that ‘the help came to me’, and when confronted with his TB status, decided to finally seek care, as confirmed by field staff in the following focus group discussion quote:
Yes, it assisted on the other case, where we got one guy, the first person that was [tested] positive, cause he was reluctant to go to the clinic. He was always sending his mother, and when we gave him his results he immediately went to the clinic and got his medication, and he’s looking better now.
(Field worker 1, focus group discussion 2, Male, Age 38)
In this situation, home-based testing provided this contact with reliable information (i.e. a positive TB test result), which informed his decision-making processes and changes in health-seeking behaviours. This contact’s immediate action to seek care and initiate treatment was further corroborated by the field staff focus group discussions, suggesting that home-based testing may help individuals overcome their apathy towards seeking care. Although some individuals expressed challenges with seeking care in the face of TB-related concerns, home-based testing was perceived as a way to overcome these barriers.
Technology gathers attention
Though household contacts were receptive to home-based testing, there were some concerns that the testing process took too long, disrupting a person’s day, and the machine garnered attention from neighbours and generated pressure to test within households, therefore compromising confidentiality.
Generally, household contacts were unperturbed by the amount of time staff spent in their home – approximately two hours for TB screening and testing. However, ensuring the availability of sufficient GX-I machines and batteries proved to be essential to household contacts comfort levels. Although rarely occurring, some household tests took longer when equipment was not functioning optimally. The lack of available equipment almost resulted in staff overstaying their welcome:
But, what I did not like was that I stayed here for 6 hours [in the house] while the test was running. So what happened is that the equipment that was helping the machine to run [the UPS battery] was being borrowed by the other person. So, they were carrying one equipment, yet they were testing two houses. […] So, my time was being wasted [and] I wanted to do my house chores….I thought they were going to take two hours or three hours not more. So, I was bothered in my soul.
(Household contact, tested negative, Female, Age 35)
In this case, the external battery to run the machine was borrowed by another nearby team resulting in a significant delay in testing this contact. This contact was comfortable with a 2- to 3-h test. However, staff were in her house for nearly 6 h, which impacted her chores and a negative perspective of the testing process.
Additionally, given the duration and the community nature of home-based testing, this may also pose challenges to participant confidentiality. Though not entirely unexpected, there were several instances of inquisitive neighbours entering a home during the testing process. Staff described such events in the following quote:
Yes, the neighbours, whenever you visit the household contacts the neighbours would say, we would also like to know what’s going on’. The neighbours were inquisitive and then sometimes in their household, they would come in unannounced and then they would ask ‘what’s this about’ and we would tell them that, ‘it’s about TB’. ‘Then they would tell you about the other people that have either dropped from taking their medication or that are sickly that they would like for us to go and test them, they would recommend the other people there, in their households.
(Field worker 1, focus group discussion 2, Male, Age 38).
Staff often had to deal with unannounced visitors while engaged in testing activities, leaving them to manage and mitigate potential breaches in participant confidentiality. Of similar concern, although most participants stated a comfort with being tested in front of other household members, not all wanted their test results ‘exposed’:
Yes like I said [recommend] not being tested in front of family members. Privacy and confidentiality should come first because I don’t want my results being exposed in front of everyone.
I: So you would prefer being tested separately?
P: Separately
(Household contact, tested negative, Female, Age 26)
Field staff also described examples of family members pressuring other household contacts who were reluctant to get tested; ‘They [household members] were always supportive to the one who was taking the test and [the household members] always wanted to know, like: “Ai, you must know your status. You must know where you stand. You have been coughing here for two weeks”…’ (Field worker 1, focus group discussion 2, Male, Age 38). Family members prodding other family members to get tested occurred on occasion, but most of this nudging was interpreted as being supportive, not coercion. Household members were excited and welcomed testing, but on occasion, the amount of time spent in the home with the machine was longer than planned due to technical challenges, which generated unwelcomed interest from neighbours, and at times testing compromised participants’ ability to manage TB disclosure within households.
Discussion
This study is the first to qualitatively assess the acceptability and feasibility of home-based TB testing using a GX-I device adapted for portability. The high level of acceptability and willingness to home-based TB testing by household contacts was driven by its convenience and its value of helping to overcome clinic-based access barriers and a general apathy towards TB testing. Furthermore, home-based TB testing was perceived as contributing to a decrease in TB-related health insecurities. Regarding feasibility, field staff were able to introduce and properly execute the testing process within households. Household contacts were not deterred by the duration and privacy limitations when testing in one’s home. However, field-based staff reported examples of operational challenges and confidentiality concerns.
Health insecurity has been defined as the inability to secure or access adequate health care, or the increasing of one’s perceived risk and vulnerability [53]. In this study, we found TB-related health insecurities to be emergent at two levels: community and household. Community-level health insecurity was exemplified by household contacts expressing concern about the burden of TB in their communities, the threat of undetected TB, and an awareness that TB is a major cause of morbidity and mortality in their communities and throughout South Africa. Household contacts exemplified household-level health insecurity by expressing concerns of household transmission, and the stigma of accusations associated with having a cough. The introduction of home-based testing was seen as a way to decrease apathy towards TB testing, provide TB testing services to those who were unwilling or unable to access clinic-based TB testing, secure better health within the community by decreasing the levels of undetected TB, and therefore providing a means to de-stigmatise coughing.
In our study, acceptability presented itself through household contacts’ perceived benefits and convenience of home-based TB testing; these attributes have also been reported by qualitative studies of HIV home-based testing [35,37,38]. Household contacts felt that home-based TB testing helped circumvent existing barriers to clinic-based TB testing, including distance and transport to clinics, waiting times and staff attitudes, and need for multiple clinic visits to submit sputum for testing [10,54,55]. Convenience and circumventing existing barriers were similarly found amongst home sputum collection experiences for GeneXpert laboratory TB testing [56]. Furthermore, household contacts perceived home-based testing to be more private than clinic-based testing and helped to overcome their apathy towards testing. Although home-based testing may not remove certain existing clinic presentation barriers, home-based TB testing has the potential to encourage healthcare seeking behaviour and linkage to care [38].
Home-based TB testing was not perceived as profoundly new. This could be explained by household contacts exposure to other community-based testing services, including home-based HIV counselling and testing, which have been widely implemented in South Africa [57–60]. Similar to qualitative assessments of home-based HIV testing, we found high levels of acceptability and comfort amongst households. Qualitative work done on household contact tracing showed similar results. Participants were not concerned about the community and felt that home-based TB testing did not inconvenience them [61]. Moreover, the transparency resulting from TB testing being performed in front of household members, and their receiving of same-day test results, contributed to an emergent trust in the machine and credibility of home-based TB testing services [35–38]. This emergent trust in home-based TB testing by household contact tracing may have a knock-on effect of engendering positive health-seeking behaviours and treatment initiation amongst those with a positive test result.
Although home-based TB testing was acceptable and feasible, with perceived and real benefits, there are some limitations. The theme related to the alleviation of health insecurities emerged from households in which contacts did not test positive for TB. Given that this theme was not an a priori concept that in-depth interview guides explored, we did not probe household contacts of the impact of a positive test result on the perception of household-level health insecurities. Recall bias may further impact our findings given that households were interviewed more than 30 days following home-based TB testing activities. Staff who conducted home-based TB testing also received qualitative interview training to conduct in-depth interviews. This may have led to some degree of social desirability bias if households were already familiar with the staff. However, some households were visited by different staff members to those who conducted the testing. The differences in responses between the two groups were not obvious. Also, the experiences, perspectives and perceptions of those that received a positive test result (n = 5) may be underrepresented compared to those with a negative test result (n = 18). This may have limited our ability to identify key themes associated with the impact of home-based TB testing on stigma. Our sample limits the generalisability of the study. However, the findings presented here inform our understanding of GeneXpert acceptability, may be relevant and further explored in similar settings. Findings complement previous qualitative household contact tracing and home-based HIV counselling and testing studies. Furthermore, certain technological challenges may have influenced participant responses, but these challenges were not common.
Overall, our findings suggest that home-based TB testing is acceptable to household contacts of TB index patients, and feasibly integrated into household contact investigations and performed by lay health workers. Home-based TB testing mitigated the apathy towards TB testing and help to overcome the inertia and barriers associated with presenting to a clinic for TB testing services. Furthermore, the perception that home-based TB testing decreased health insecurities associated with TB was a unique theme not previously identified with community-based testing services. While this study revealed important feasibility considerations for future implementation, testing time and confidentiality concerns for some household members did not influence the overall acceptability and feasibility of home-based TB testing. This said, such limitations will need to be addressed to further optimise the implementation of home-based TB testing. While home-based TB testing can deliver TB testing services to those at risk and/or unable or unwilling to access clinic-based services, the cost effectiveness of deploying this strategy requires further investigation and evaluation.
Box 1.
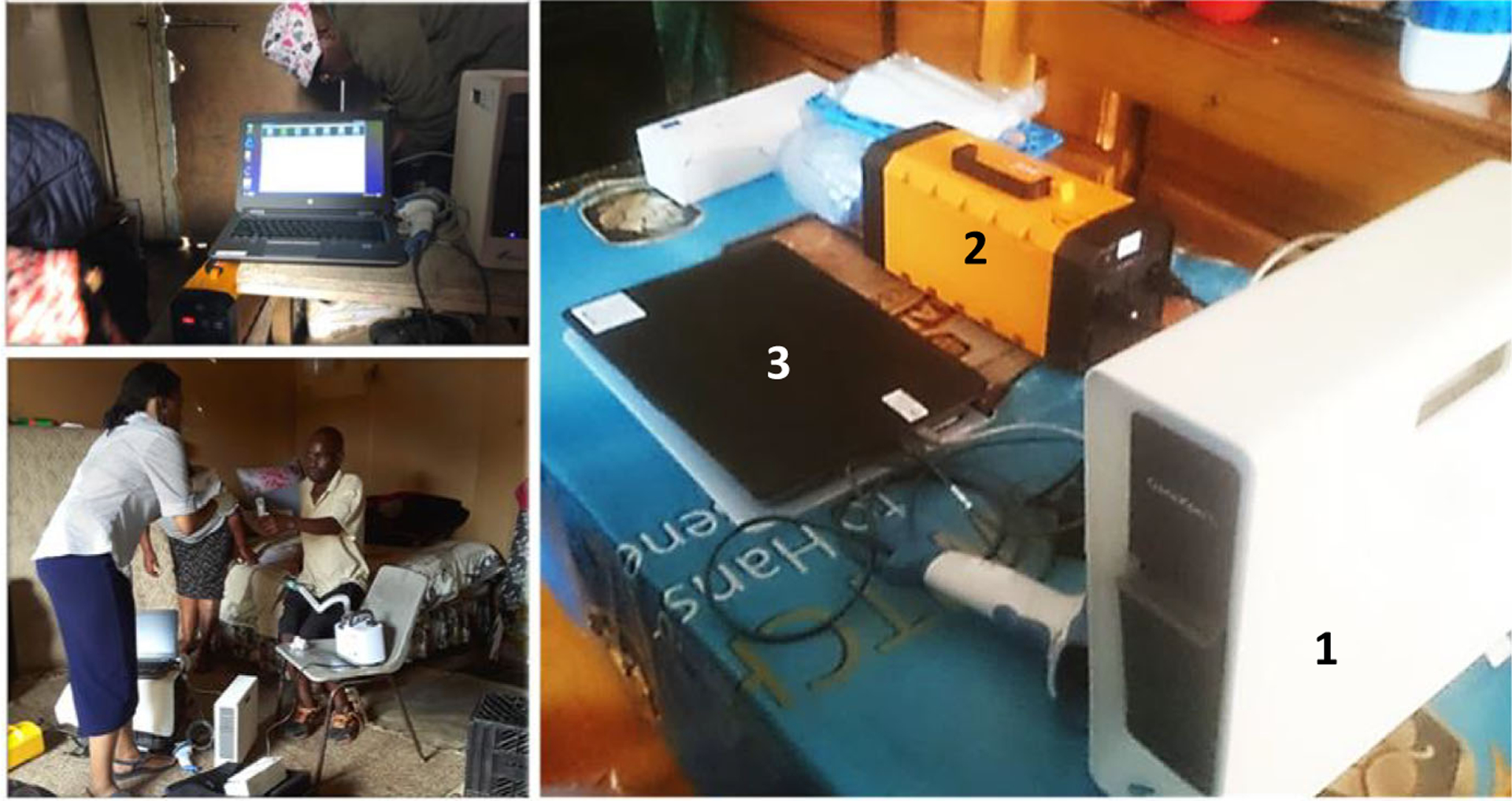
Typical home-based testing set-up (floor vs countertops) of the GeneXpert-I single module device. 1 = GX-I device; 2 = UPS portable battery; 3 = standard laptop
Acknowledgements
The study was conducted with the permission of the Eastern Cape Provincial and Buffalo City Metro District Departments. We owe special thanks to Mr. Ralph Mawarire for his key support in project implementation and management of field-based staff, and to the field staff and participants for sharing their insights and for their valuable contributions to the study. Finally, we thank Cepheid for provision of GX-I devices and donation of MTB/Rif test cartridges. The work was funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the U.S. National Institutes of Health under award number R21EB023679 to AMM. Access to portable GX-I devices and MTB/Rif test cartridges was sponsored under a November 2015 RFA agreement with the Foundation for Innovative New Diagnostics (FIND). Neither NIH nor Cepheid had a role in data collection and analysis nor in writing the initial manuscript draft.
References
- 1.World Health Organization. Global Tuberculosis Report 2019. 2019.
- 2.Kweza PF, Schalkwyk CV, Abraham N, Uys M, Claassens MM, Medina-Marino A. Estimating the magnitude of pulmonary tuberculosis patients missed by primary health care clinics in South Africa. Int J Tuberc Lung Dis 2018: 22: 264–272. [DOI] [PubMed] [Google Scholar]
- 3.Claassens MM, Jacobs E, Cyster E et al. Tuberculosis cases missed in primary health care facilities: should we redefine case finding? Int J Tuberc Lung Dis 2013: 17: 8. [DOI] [PubMed] [Google Scholar]
- 4.Chihota VN, Ginindza S, McCarthy K, Grant AD, Church-yard G, Fielding K. Missed opportunities for TB investigation in primary care clinics in south africa: experience from the XTEND Trial. PLoS One 2015: 10: e0138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. World Health Organization: Geneva, Switzerland, 2013. [PubMed] [Google Scholar]
- 6.Chadha V, Anjinappa S, Rade K et al. Sensitivity and specificity of screening tools and smear microscopy in active tuberculosis case finding. Indian J Tuberc 2019: 66: 99–104. [DOI] [PubMed] [Google Scholar]
- 7.Niveditha S, Jagmohan S, Abhishek KV, Meka M. Diagnostic accuracy of Xpert MTB compared to smear microscopy in pulmonary vs extrapulmonary tuberculosis. IJCMR 2019: 6. https://www.ijcmr.com/uploads/7/7/4/6/77464738/ijcmr_2589_v1.pdf [Google Scholar]
- 8.Assefa Y, Woldeyohannes S, Gelaw YA, Hamada Y, Getahun H. Screening tools to exclude active pulmonary TB in high TB burden countries: systematic review and meta-analysis. Int J Tuberc Lung Dis 2019: 23: 728–734. [DOI] [PubMed] [Google Scholar]
- 9.Christian C, Burger C, Claassens M, Bond V, Burger R. Patient predictors of health-seeking behaviour for persons coughing for more than two weeks in high-burden tuberculosis communities: the case of the Western Cape, South Africa. BMC Health Serv Res 2019: 19: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008: 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pronyk PM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. Int J Tuberc Lung Dis 2001: 5: 619–627. [PubMed] [Google Scholar]
- 12.Kigozi G, Engelbrecht M, Heunis C, Janse van Rensburg A. Household contact non-attendance of clinical evaluation for tuberculosis: a pilot study in a high burden district in South Africa. BMC Infect Dis 2018: 18: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kigozi NG, Heunis JC, Engelbrecht MC. Yield of systematic household contact investigation for tuberculosis in a high-burden metropolitan district of South Africa. BMC Public Health 2019: 19: 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro AE, Variava E, Rakgokong MH et al. Community-based targeted case finding for tuberculosis and hiv in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med 2012: 185: 1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010: 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stop TB Partnership. Stop TB Field Guide 4 Intensified TB Case Finding at Facility Level. 2018.
- 17.Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol 2016: 5: 374–378. [DOI] [PubMed] [Google Scholar]
- 18.Abebe M, Doherty M, Wassie L et al. TB case detection: can we remain passive while the process is active? Pan Afr Med J 2012: 5. [PMC free article] [PubMed] [Google Scholar]
- 19.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013: 41: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison J, Pai M, Hopewell P. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008: 8: 359–368. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Recommendations for Investigating Contacts of Persons With Infectious Tuberculosis in Low- and Middle-income Countries. World Health Organization: Geneva, 2012. [PubMed] [Google Scholar]
- 22.Blok L, Sahu S, Creswell J, Alba S, Stevens R, Bakker MI. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLoS One 2015: 10: e0119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekandi JN, Dobbin K, Oloya J, Okwera A, Whalen CC, Corso PS. Cost-effectiveness analysis of community active case finding and household contact investigation for tuberculosis case detection in urban Africa. PLoS One 2015: 10: e0117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks GB, Nguyen NV, Nguyen PTB et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019: 381: 1347–1357. [DOI] [PubMed] [Google Scholar]
- 25.Stop TB Partnership. Stop TB Field Guide – Using Contact Investigation to Find the Missing People with TB. Stop TB Partnership; 2018. [Google Scholar]
- 26.Mwansa-Kambafwile J, McCarthy K, Gharbaharan V, Venter FWD, Maitshotlo B, Black A. Tuberculosis case finding: evaluation of a paper slip method to trace contacts. PLoS One 2013: 8: e75757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayles H, Muyoyeta M, Du Toit E et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 2013: 382: 1183–1194. [DOI] [PubMed] [Google Scholar]
- 28.Hanrahan CF, Nonyane BAS, Mmolawa L et al. Contact tracing versus facility-based screening for active TB case finding in rural South Africa: a pragmatic cluster-randomized trial (Kharitode TB). PLoS Medicine 2019: 16: e1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deery CB, Hanrahan CF, Selibas K, Bassett J, Sanne I, Rie AV. A home tracing program for contacts of people with tuberculosis or HIV and patients lost to care. Int J Tuberc Lung Dis 2014: 18: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health 2009: 14: 849–855. [DOI] [PubMed] [Google Scholar]
- 31.Naik R, Tabana H, Doherty T, Zembe W, Jackson D. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC Public Health 2012: 12: 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in Sub-Saharan Africa: a systematic review and meta-analysis. PLoS Medicine 2012: 9: e1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS 2010: 24: 735–741. [DOI] [PubMed] [Google Scholar]
- 34.Suthar AB, Ford N, Bachanas PJ et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Medicine 2013; 10: e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jürgensen M, Sandøy IF, Michelo C, Fylkesnes K, Mwangala S, Blystad A. The seven Cs of the high acceptability of home-based VCT: Results from a mixed methods approach in Zambia. Soc Sci Med 2013: 97: 210–219. [DOI] [PubMed] [Google Scholar]
- 36.Kyaddondo D, Wanyenze RK, Kinsman J, Hardon A. Home-based HIV counseling and testing: client experiences and perceptions in Eastern Uganda. BMC Public Health, 2012: 12: 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight LC, Van Rooyen H, Humphries H, Barnabas RV, Celum C. Empowering patients to link to care and treatment: qualitative findings about the role of a home-based HIV counselling, testing and linkage intervention in South Africa. AIDS Care 2016: 27: 1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perriat D, Plazy M, Gumede D et al. “If you are here at the clinic, you do not know how many people need help in the community”: Perspectives of home-based HIV services from health care workers in rural KwaZulu-Natal, South Africa in the era of universal test-and-treat. PLoS One 2018: 13: e0202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Rath G, Schnippel K, Long L et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One 2012: 7: e36966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erasmus L, Coetzee G, Stevens WS. Scale up of Xpert MTB/RIF from the national laboratory perspective: issues and challenges. Int J Tuberc Lung Dis 2011: 15(Suppl 3): S61. [Google Scholar]
- 41.Bassett IV, Forman LS, Govere S et al. Test and Treat TB: a pilot trial of GeneXpert MTB/RIF screening on a mobile HIV testing unit in South Africa. BMC Infect Dis 2019: 19: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calligaro GL, Zijenah LS, Peter JG et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect Dis 2017: 17: 441–450. [DOI] [PubMed] [Google Scholar]
- 43.Medina-Marino A, Bresenham D, Mawarire R et al. Integrating a portable, battery-powered GeneXpert device into contact investigations: Implications for early case finding and time-to-treatment initiation – Buffalo City Metro Health District, South Africa. 2019; 50th Union World Conference on Lung Health. [Google Scholar]
- 44.Census Frith A. 2011. Statistics South Africa [Internet]. (Available from: https://census2011.adrianfrith.com/) [15 Jun 2020].
- 45.National Institute for Communicable Diseases. TB Surveillance Dashboard and Other Resources: Microbiologically Confirmed Pulmonary TB - Centre for Tuberculosis [Internet]. 2018. (Available from: https://www.nicd.ac.za/tb-surveillance-dashboard/). Accessed on 25 May 2020. [Google Scholar]
- 46.World Health Organization. Guidelines for Intensified Tuberculosis Case-finding and Isoniazid Preventative Therapy for People Living with HIV in Resource-constrained Settings [Internet] World Health Organization, Department of HIV/AIDS, Stop TB Department, 2011. (Available from: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf) [30 Jun 2020]. [Google Scholar]
- 47.Mitchell JW, Torres MB, Joe J, Danh T, Gass B, Horvath K. Formative work to develop a tailored HIV testing smartphone app for diverse, at-risk, HIV-negative men who have sex with men: a focus group study. JMIR mHealth uHealth 2016: 4: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Researcher 2011: 18: 52–62. [DOI] [PubMed] [Google Scholar]
- 49.van Loggerenberg F, Gray D, Gengiah S et al. A Qualitative study of patient motivation to adhere to combination antiretroviral therapy in South Africa. AIDS Patient Care STDs 2015: 29: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniels J, Struthers H, Maleke K, Lane T, McIntyre J, Coates T. ‘My tablets are on top of the fridge’: the roles of relationship desire and medical mistrust in art adherence for HIV-positive MSM and transgender women living in rural South Africa. AIDS Behav 2019: 23: 2849–2858. [DOI] [PubMed] [Google Scholar]
- 51.Harichund C, Moshabela M, Kunene P, Abdool Karim Q. Acceptability of HIV self-testing among men and women in KwaZulu-Natal, South Africa. AIDS Care 2019: 31: 186–192. [DOI] [PubMed] [Google Scholar]
- 52.Ngure K, Heffron R, Mugo N et al. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc 2017: 20: 21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gama E Health insecurity and social protection: pathways, gaps, and their implications on health outcomes and poverty. Int J Health Policy Manag 2016: 5: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Schacht C, Mutaquiha C, Faria F et al. Barriers to access and adherence to tuberculosis services, as perceived by patients: A qualitative study in Mozambique. PLoS One 2019: 14: e0219470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skordis J, Hanson K, Mills A. Confusion, caring and tuberculosis diagnostic delay in Cape Town, South Africa. Int J Tuberc Lung Dis 2010: 14: 171–180. [PubMed] [Google Scholar]
- 56.Armstrong-Hough M, Ggita J, Turimumahoro P et al. “Something So Hard”: a mixed-methods study of home sputum collection for TB contact investigation in Uganda. Int J Tuberc Lung Dis 2018: 22: 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shamu S, Farirai T, Kuwanda L et al. Comparison of community-based HIV counselling and testing (CBCT) through index client tracing and other modalities: outcomes in 13 South African high HIV prevalence districts by gender and age. Torpey K, editor. PLoS One 2019: 14: e0221215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.High uptake of community-based HIV testing by adolescent girls and young women aged 15–24: Implications and Synergies for PrEP Roll Out? Abstract presented at: TUAC0201; 2017; Paris, France. [Google Scholar]
- 59.Lewis L, Maughan-Brown B, Grobler A et al. Impact of home-Based HIV testing services on progress toward the UNAIDS 90–90–90 targets in a hyperendemic area of South Africa. J Acquir Immune Defic Syndr 2019: 80: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Department of Health. National HIV Testing Services Policy. Pretoria, South Africa: South African National Department of Health, 2016. [Google Scholar]
- 61.Sathar F, Velen K, Peterson M, Charalambous S, Chetty-Makkan CM. “Knock Knock”: a qualitative study exploring the experience of household contacts on home visits and their attitude towards people living with TB in South Africa. BMC Public Health 2020: 20: 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


