Abstract
RNA-binding proteins undergo regulated phase transitions in an array of cell types. The phase separation of RNA-binding proteins, and subsequent formation of RNP condensates or granules, occurs during physiological conditions and can also be induced by stress. Some RNP granules have roles in post-transcriptionally regulating mRNAs, and mutations that prevent the condensation of RNA-binding proteins can reduce an organism’s fitness. The reversible and multivalent interactions among RNP granule components can result in RNP complexes that transition among diffuse and condensed states, the latter of which can be pathological; for example, in neurons solid RNP aggregates contribute to disease states such as amyotrophic lateral sclerosis (ALS), and the dysregulation of RNP granules in human germ cells may be involved in Fragile X-associated primary ovarian insufficiency. Thus, regulating the assembly of mRNAs and RNA-binding proteins into discrete granules appears to provide important functions at both cellular and physiological levels. Here we review our current understanding of the role of post-translational modifications (PTMs) in regulating the condensation of RNA-binding proteins in the germ line. We compare and contrast the in vitro evidence that methylation inhibits phase separation of RNA binding proteins, with the extent to which these results apply to the in vivo germ line environment of several model systems. We also focus on the role of phosphorylation in modulating the dynamics of RNP granules in the germ line. Finally, we consider the gaps that exist in our understanding of the role of PTMs in regulating germ line RNP granules.
Keywords: RNP granules, phase transition, germ line, methylation, phosphorylation, condensate
Introduction
Phase separation is an important principle of cellular organization. Many types of membraneless organelles (MLOs) assemble through the process of liquid-liquid phase separation. Some of the best studied MLOs in the cytoplasm are ribonucleoprotein (RNP) granules composed of RNA and RNA binding proteins, such as stress granules and processing bodies. Much of our understanding of phase separation to date has come from in vitro studies. From such studies, we now understand that phase separation is driven mainly by weak interactions between multivalent protein interaction domains or intrinsically disordered low complexity domains (LCDs) (Kato et al., 2012; Li et al., 2012). Since multivalent interaction motifs and the short linear motifs in intrinsically disordered regions and LCDs are often post-translationally modified (Xie et al., 2007; Bah and Forman-Kay, 2016; Chong and Forman-Kay, 2016), in hindsight it is not surprising that post-translational modifications (PTMs) have been revealed as important regulators of phase separation (Itakura et al., 2018; Rhoads et al., 2018). While a diverse array of PTMs can modulate condensates, in this review we focus on the best-studied paradigms: methylation and phosphorylation.
PTMs can alter the chemical properties of amino acids, such as the steric properties, bulkiness, or charge state. For example, when Arginine (Arg) is methylated, bulkiness is increased, and the distribution of charge and hydrophobicity is altered which affects intermolecular interactions and phase separation. Phosphorylation of Tyr or Ser introduces a negative charge which can either promote or inhibit phase separation (Monahan et al., 2017; Wang et al., 2018); thus, PTMs can weaken or enhance multivalent interactions between phase-separated macromolecules. PTMs can also recruit protein into, or exclude protein from, the condensate (Hofweber and Dormann, 2019). Thus, PTMs can modulate the assembly and disassembly of liquid-like RNP granules, and transitions from the liquid state to gel- or solid-like states.
Condensation of RNA binding proteins and RNA in the germ line of many organisms results in germ granules (Voronina et al., 2011). A variety of terms are used to describe the array of germ granules found across different species which can be confusing but are described in several resources (Table 1 and Schisa, 2012). While some germ granule proteins exhibit liquid-like properties, such as the PGL-1 granules in C. elegans embryos (Brangwynne et al., 2009), other types of germ granules such as the Balbiani body in Xenopus oocytes have solid-like properties (Boke et al., 2016; Woodruff et al., 2018). Careful examination has revealed multiple phases within germ granules; for example, the germ granules of early C. elegans embryos include a liquid-like phase of PGL proteins, and a gel phase of MEG-3 protein that appears to have a scaffolding role in the assembly of germ granules (Putnam et al., 2019). An increasing number of examples of PTMs modulating the assembly of germ granules have been documented over the past two decades. Building upon our understanding from in vitro studies, these in vivo experiments are revealing both conserved and complex roles of PTMs in regulating condensates of RNA binding proteins in the germ line that are associated with critical germ line functions. This review will focus on our understanding of how methylation and phosphorylation regulate germ line RNP condensates across invertebrate and vertebrate model systems.
TABLE 1.
Summary of germ line RNP condensates described in this review.
| Species | Germ granule/RNP condensate | Description |
| All | Germ granule | Refers collectively to the electron-dense, RNP granules in vertebrate and invertebrate germ lines, often given a specific name in a species. |
| Drosophila | ||
| Nuage | Perinuclear, small Vasa-positive granules in nurse cells of ovary, and in several stages of spermatogenesis. | |
| Pole plasm | Posterior cytoplasm of the oocyte that contains polar granules; it is necessary and sufficient for the induction of germ cells. | |
| piNG body (piRNA nuage giant body) | Granules, larger than nuage, that appear late during spermatogenesis; granules contain several components of the piRNA pathway. | |
| C. elegans | ||
| P granule | Germ granules in adult germ cells and germ cell precursors (P lineage) of embryo; perinuclear during most of development. | |
| Mouse | ||
| Chromatoid body | Nuage component of mammalian spermatogenic cells; condensed form detected after completion of meiosis; contains similar proteins as germ granules in female germ cells, e.g., mouse Vasa homolog. | |
| Cajal body | Non-membraneous nuclear organelle; site of spliceosome maturation. | |
| Stress granule | RNP granules induced by heat stress; detected in spermatogonia and preleptotene and early pachytene spermatocytes. | |
| Oocyte aggregate | A subcortical RNP aggregate in germinal vesicle-stage oocytes, contains maternal mRNAs, and P body proteins. | |
| Zebrafish | ||
| Balbiani body | Structure in zebrafish (and other) oocytes analogous to the mitochondrial cloud. | |
| Xenopus | ||
| Oocyte aggregate | Patches of XStau1 in the vegetal subcortical region of Stage VI oocytes and eggs. |
Role of Methylation
Methylation Inhibits Condensate Assembly in in vitro Studies
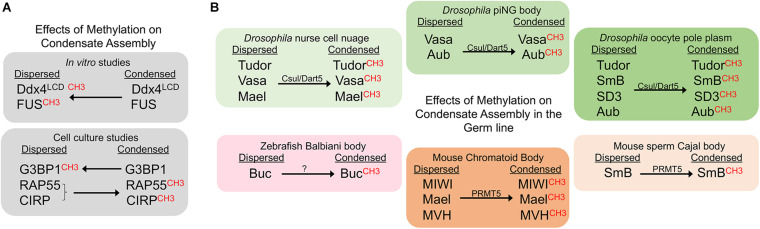
Methylation is a key regulator of phase transitions and RNP granule dynamics. Within many RNP granules are proteins with RGG or RG-rich motifs, and the Arginine residues in these motifs are often methylated by protein arginine methyltransferase (PRMT) enzymes (Bedford and Clarke, 2009). In general, Arg-methylation is considered a less dynamic modification, in how it impacts target proteins, than others such as phosphorylation and acetylation (Fackelmayer, 2005). In in vitro studies methylation of Arg weakens intermolecular interactions and thus inhibits phase separation of RNA binding proteins. For example, droplets of the N-terminal RGG-rich domain of the conserved nuage protein Ddx4/Vasa are destabilized by Arg-methylation (Nott et al., 2015). Methylation of recombinant or purified FUS protein, the protein that phase separates into granules in amyotrophic lateral sclerosis (ALS) mutations, similarly reduces liquid-liquid de-mixing (Qamar et al., 2018; Hofweber and Dormann, 2019). Since no examples have shown Arg-methylation to promote condensation in vitro, it has been suggested that this PTM is a general inhibitor of Arg-aromatic (π) interactions that reduces phase separations (Hofweber and Dormann, 2019). However, ex vivo studies reveal a more complex effect of methylation on phase separation (Figure 1A). Some experiments align with in vitro results showing Arg-methylation suppresses RNP granule formation. Treatments of cultured U2OS cells that increase the methylation of Ras-GAP SH3-binding protein (G3BP1) repress the assembly of stress granules (Tsai et al., 2016). However, there is also evidence for Arg-methylation promoting RNP granule assembly. When methylation of the Lsm4 protein, RAP55A, is decreased, the localization of RAP55A to P bodies is inhibited in cell culture (Matsumoto et al., 2012), and similarly, recruitment of unmethylated CIRP to stress granules is blocked (De Leeuw et al., 2007). Overall, many in vitro and ex vivo examples highlight a role for Arg-methylation in controlling the dynamics of RNP granules; however, these results do not address the extent to which this regulation occurs in vivo or in the germ line. To date, studies in three model systems all demonstrate a role for Arg-methylation in promoting phase separation of RNA binding proteins in the germ line, opposite of the role seen in vitro (Figure 1B).
FIGURE 1.
Methylation promotes condensate assembly in the germ line. CH3 indicates the methylated form of the corresponding protein. Arrows indicate whether methylation promotes or inhibits a condensed state. (A) Methylation suppresses condensates in in vitro studies, but can sometimes promote condensation in cell culture. (B) Methylation promotes condensation of germ line proteins. Effects of methylation are shown for six distinct germ line RNP granules in three model systems.
Methylation Promotes Condensate Assembly in the Germ Line
In Drosophila, components of the methylosome regulate RNA binding proteins in multiple MLOs of the female germ line (Figure 1B). Capsuleen (Csul), also known as Dart5, is the homolog of the methyltransferase PRMT5. In dart5/csul mutant egg chambers the condensation of Tudor, Vasa, and Maelstrom into perinuclear granules of the nurse cell nuage is diminished, suggesting methylation normally promotes condensation of these proteins in the Drosophila female germ line (Gonsalvez et al., 2006; Anne et al., 2007). The Capsuleen-Valois methylosome complex also has a role in assembly of the pole plasm of Drosophila oocytes. The localization of Tudor and the Sm proteins, SmB and SD3, to the posterior pole plasm requires the methylation of Arg residues (Anne et al., 2007; Anne, 2010). The consequence of blocked methylation and disrupted nuage and pole plasm assembly in dart5/csul mutants is a grandchildless phenotype, where embryos of mutant females completely lack pole cells and develop into agametic, sterile adults (Gonsalvez et al., 2006; Anne et al., 2007). Csul also methylates the Piwi protein Aubergine (Aub), which is required for Aub to bind Tudor and to promote the assembly of pole plasm in the developing oocyte (Kirino et al., 2010).
A role for methylation has also been identified in Drosophila primary spermatocytes, where a novel condensate called the piRNA nuage giant body (piNG-body) is enriched for Vasa, Aub, Argonaute 3, and Tudor (Kibanov et al., 2011). In spermatocytes lacking Csul/PRMT5, unmethylated Vasa and Aub fail to condense into the piNG-body; only small Vasa-positive nuage granules, and unlocalized Aub signals are detected. At the same time, the piRNA pathway is disrupted, and male sterility occurs. Thus, methylation by PRMT5 appears to be essential for piNG-body assembly and normal development of the male germ line.
Methylation of Ddx4/Vasa is widely conserved from planar worms to humans (Rouhana et al., 2012); thus, it will be interesting to determine if Arg-methylation also promotes assembly of Vasa granules in systems beyond Drosophila. It is notable that these in vivo germ line studies show an opposite effect of methylation as compared to in vitro studies, where PRMT1-dependent methylation disrupts the phase separation of Ddx4 (Nott et al., 2015). This difference seems likely to be due to the fact in vitro studies generally involve only one or a few purified RBPs while the in vivo environment is much more complex.
Piwi-tudor domain protein interactions promote the assembly of germ granules not only in Drosophila, but also in mouse (Arkov and Ramos, 2010). In vitro studies show that Tudor proteins recognize methylarginine marks on mouse Piwi proteins to drive their localization to cytoplasmic foci (Vagin et al., 2009). In cell culture, treatment with an inhibitor of methyltransferases abolishes interactions between Tdrd1 and the mouse Piwi protein, MILI, suggesting that Arg-methylation and Tudor binding promote assembly of piRNA pathway components into nuage (Vagin et al., 2009). Moreover, immunoprecipitation studies show Tdrd6 interacts with the mouse Piwi proteins Miwi and Mili in vivo, and Miwi is methylated by PRMT5 and binds Tdrd6 in a symmetrical dimethylarginine methylation (sDMA)-dependent manner (Vasileva et al., 2009; Kirino et al., 2010). In tdrd6-/- testes the RNA binding proteins Mael, Miwi, and Mouse Vasa homolog (MVH)/Ddx4 fail to condense into the normal chromatoid bodies (the nuage in mouse spermatogenic cells) (Vasileva et al., 2009). The defects in condensation are accompanied by a lack of elongated spermatids and sperm. Given the proposed role for chromatoid bodies as storage sites during spermatid differentiation, their aberrant architecture and absence of condensed Mael, Miwi, and MVH in chromatoid bodies may directly impact spermatid differentiation (Vasileva et al., 2009). PRMT5 also methylates the SmB splicing protein in mouse spermatocytes. When Arg-methylation is abrogated via mutation of Tdrd6, the assembly of spliceosomes is impaired in primary spermatocytes, resulting in a decreased number of Cajal bodies, the nuclear membraneless condensates where spliceosome maturation occurs (Akpınar et al., 2017). Thus, methylation promotes condensation in both cytoplasmic and nuclear compartments of the mouse male germ line.
In the zebrafish model system methylation appears to promote the condensation of a solid-like germ granule, the Balbiani body. The germ plasm in early embryos originates from the Balbiani body in the oocyte (Kloc et al., 2004). A role for Tudor6 (Tdrd6) has been shown in modulating the aggregation of Buckyball (Buc), the organizer of the Balbiani body (Roovers et al., 2018). Tdrd6a and Tdrd6c interact with Buc via its three symmetrically dimethylated arginines. The three arginines of Buc are required for Buc to condense into a mature Balbiani body in oocytes, and to form germ plasm in embryos. The importance of Arg-methylation in promoting this phase transition to a solid condensate is further underscored by the observation that deleting the three arginines of Buc has a more severe phenotype than a tdrd6a mutant, with defects in germ cell formation and embryonic development (Roovers et al., 2018). It will be interesting to determine if methylation also modulates condensation of germ granule proteins in the adult germ cells or embryonic primordial germ cells where Tdrd7 has a role in maintaining the integrity of the germ granule protein Vasa (Strasser et al., 2008). The recent discovery of a role for PRMT5 in the methylation of Zili and Vasa in the zebrafish gonad should allow researchers to address whether methylation has any role in modulating condensation of Zili, Vasa, or other granule components (Zhu et al., 2019).
No studies to date have identified regulation of RNP condensates by methylation in the C. elegans germ line. However, Arg-methylation of the C. elegans RG proteins PGL-1/-3 by the PRMT1 homolog EPG-11 results in decreased phase separation in vitro (Zhang et al., 2018). In addition, in C. elegans embryos, where the primordial germ cells localize PGL-1/-3 to germ granules, EPG-11 destabilizes PGL-1/-3 aggregates from somatic blastomeres via methylation of the PGL-1/-3 RGG repeats (Li et al., 2013). It remains to be determined if this example of an inhibition of condensation by methylation will be extended to the worm germ line in future studies. In any event, the experiments in the fly, fish, and mouse germ lines clearly indicate differences from how methylation modulates phase separation in vitro and highlight the necessity of additional in vivo studies. Biochemical approaches to further study Ddx4/Vasa may be especially valuable due its broad conservation. Employing a high-resolution mass spectrometry approach to profile PRMT substrates may also be useful, as has been successful in other contexts (Shishkova et al., 2017).
Role of Phosphorylation
Phosphorylation Can Promote or Inhibit Condensate Assembly in vitro
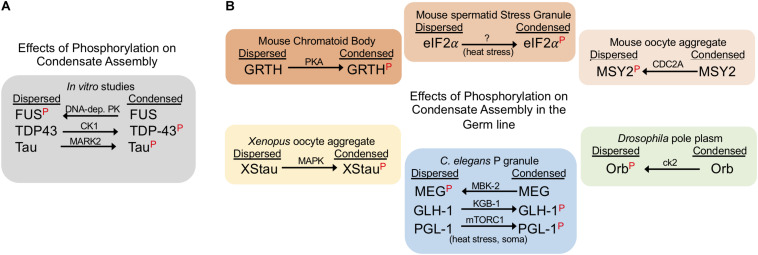
Phosphorylation is a common PTM that is implicated in the regulation of RNP granule dynamics. Phosphorylation is a rapid and reversible process by which proteins acquire negatively charged PO4 groups that alter their intramolecular interactions and consequently impact phase separation (Hofweber and Dormann, 2019). Multivalent interactions among serine and tyrosine residues are especially prominent in the LCDs and intrinsically disordered regions (IDRs) of RNA binding proteins in granules. In in vitro studies phosphorylation sometimes promotes, and other times suppresses, phase separation of RNA binding proteins (Hofweber and Dormann, 2019). For example, phase separation of FUS is blocked when FUS is phosphorylated by the DNA-dependent protein kinase (Monahan et al., 2017; Murray et al., 2017; Luo et al., 2018). In contrast, phosphomimetic S48E substitution in the N-terminal domain of TDP-43 (TAR DNA– binding protein of 43 kDa) blocks the phosphorylation of TDP-43 by Casein Kinase 1 (CK1), and leads to reduced liquid-liquid phase separation of TDP-43 in vitro (Kametani et al., 2009; Wang et al., 2018; Hofweber and Dormann, 2019). Phosphorylation also promotes the condensation of Tau, a neuron-specific microtubule-associated protein (Ambadipudi et al., 2017). Similar to the varied effects of phosphorylation in in vitro studies (Figure 2A), cell culture experiments also reveal both inhibitory and stimulatory roles of phosphorylation on condensation of stress granule proteins (Hofweber and Dormann, 2019). It is not yet clear how well the in vitro and ex vivo studies translate to more complex environments of in vivo tissues and organisms. However, the studies highlighted below elucidate a growing role for phosphorylation in regulating the assembly and disassembly of germ granules in invertebrate and vertebrate systems.
FIGURE 2.
Phosphorylation can suppress or promote condensate assembly. The red P indicates the phosphorylated form of the corresponding protein. Arrows indicate whether phosphorylation drives a dispersed or condensed state; kinase names are above each arrow. (A) Phosphorylation has varying effects on phase separation in in vitro studies. (B) Phosphorylation negatively and positively regulates RNP condensates in the germ line. The effects of phosphorylation are shown for proteins in six distinct germ line RNP granules in three model systems. Note: phosphorylation of the C. elegans P granule protein PGL-1 during heat stress conditions promotes condensates in somatic cells, outside of the germline.
Phosphorylation Can Promote or Inhibit Condensate Assembly in the Germ Line
Studies of C. elegans germ cells reveal insights into the complex roles of phosphorylation in regulating RNP granule assembly (Figure 2B). MBK-2, the C. elegans DYRK3 kinase homolog, and the PP2APPTR–1/2 phosphatase are key players in mediating the dynamics of PGL-1, an RGG protein in germ granules (Seydoux, 2018). Phosphorylation of MEG-1 and MEG-3 (maternal-effect germline defective) proteins by MBK-2 promotes disassembly of PGL-1 granules in zygotes (Wippich et al., 2013). This action is balanced by PPTR-1 and PPTR-2 which have redundant phosphatase functions to dephosphorylate MEG proteins and stabilize PGL-1 granule formation (Wang et al., 2014). In addition to contributing to P granule regulation, the MEG proteins are required for fertility; however, how regulated phosphorylation impacts fertility is not yet clear (Wang et al., 2014). A contrasting example of phosphorylation in C. elegans is the Ddx4/Vasa homolog GLH-1 which is phosphorylated by the KGB-1 MAP kinase (Orsborn et al., 2007). In the kgb-1 knockout the localization of GLH-1 to discrete P granules is partially disrupted, suggesting that KGB-1 normally promotes condensation of GLH-1 via phosphorylation. When GLH-1 is not phosphorylated by KGB-1, elevated levels of GLH-1 protein are detected in the gonad, as well as over-proliferation of germ cells, and a high level of sterility. A second example of phosphorylation promoting condensation of P granules has been described outside of germ cells. mTORC1-mediated phosphorylation of PGL-1/-3 during mild heat-stress promotes the assembly of PGL-1/-3 granules in somatic blastomeres of early embryos (Zhang et al., 2018).
In the Drosophila germ line, phosphorylation has been shown to inhibit protein condensation. Orb, the cytoplasmic polyadenylation element binding protein (CPEB) homolog, regulates the translation of target mRNAs in Drosophila ovaries and is phosphorylated by casein kinase II (ck2) (Wong et al., 2011). When ck2 activity is compromised, Orb transitions from a diffuse state in oocytes into condensed sponge body-like granules, implicating phosphorylation in inhibiting Orb condensation (Wong et al., 2011). Deducing the precise consequence of ectopic Orb condensates in ck2 mutants is not straightforward; however, the phenotype of both orb and ck2 mutants includes dorsal-ventral defects during oogenesis. Interestingly, several examples demonstrate that phosphorylation does not always affect protein condensation. The Drosophila Pan Gu (PNG) kinase directly phosphorylates two de-capping activator proteins Trailer hitch (TRAL)/RAP55 and Me31B/RCK; however, the dispersal of TRAL granules is not affected during in vitro egg activation (Hara et al., 2018). In addition, the phosphorylation of maelstrom (Mael) by polo kinase is not required for its localization to nuage puncta in ovaries (Pek et al., 2012).
Multiple examples in both female and male germ lines of vertebrates demonstrate a role for phosphorylation in regulating protein condensation. In the female mouse germ line, MSY2 is one of several RNA-binding proteins that condense into transient, RNA-containing aggregates in fully grown oocytes but later decondense during oocyte maturation (Flemr et al., 2010). Phosphorylation of MSY2 by CDC2A occurs during oocyte maturation concomitant with its de-condensation, suggesting that phosphorylation may inhibit MSY2 condensation (Medvedev et al., 2008). In contrast, in the male mouse germ line, when Gonadotropin-regulated testicular RNA helicase (GRTH) is not phosphorylated by Protein Kinase A (PKA), GRTH localization to the chromatoid body (CB) is impaired, the CB is reduced in size, testis size is reduced due to germ cell apoptosis, and spermatogenesis arrests at the round spermatid stage (Sheng et al., 2006; Kavarthapu et al., 2019). Phosphorylation of eIF2α by GCN2 also appears to promote its condensation into stress granules in male spermatids in response to temperature stress (Kim et al., 2012; Yoon et al., 2017). In Xenopus oocytes, Staufen proteins (XStau1 and XStau2) are phosphorylated via the MAP Kinase pathway during meiotic maturation (Allison et al., 2004). Phosphorylated XStau1 appears to transition from small aggregates concentrated locally in the vicinity of the ER, to larger aggregates less localized to the ER, suggesting phosphorylation promotes condensation of XStau1 which may impact its association with the ER network (Allison et al., 2004). It is not yet clear how phosphorylation modifies XStau1 function.
Overall, the role of phosphorylation in modulating phase transitions in the germ line has been understudied to date. Dozens of RNA binding proteins have been characterized as components of P granules in C. elegans, and of polar granules and nuage in Drosophila (Updike and Strome, 2010; Kato and Nakamura, 2012). It will be interesting to determine the extent to which phosphorylation regulates condensation of these proteins. For example, the C. elegans MPK-1 ERK (extracellular signal-regulated kinase) controls seven different processes in the adult germ line, including germ cell apoptosis and oocyte maturation (Lee et al., 2007). Genomic approaches have identified 30 ERK substrates including several RNA binding proteins, some of which are P granule proteins (Arur et al., 2009). Future studies should be able to address if ERK modulates condensation of any of its protein substrates.
Perspectives
The germ line has distinct functions from the soma, including the need to accurately transmit genetic information between generations and the requirement of pluripotency. The germ line may also have unique requirements in regulating gene expression, as large pools of maternal mRNAs accumulate in oocytes, many of which are non-translating until after fertilization. Germ line RNP condensates can facilitate post-transcriptional gene regulation of mRNA, and the prevailing theory is that such gene regulation is advantageous for rapid gene activation post-fertilization. PTMs afford some of the fastest changes to protein function that are also reversible and avoid de novo nuclear activities. Since RNP condensates are very dynamic complexes, these advantages may begin to answer why PTMs have evolved as an important regulator of germ line RNP condensates.
In comparison to the many examples of PTMs modulating phase transitions in vitro and in cell culture, there are notably fewer documented cases in the germ line. This discrepancy may simply reflect the challenge of studying PTMs in vivo, with the expectation that additional examples will be identified in the future. Alternatively, the relatively low number of examples may be due to alternative mechanisms regulating condensates in the germ line, with a smaller relative role for PTMs. This review highlights one major difference between in vitro studies that demonstrate a role for methylation in reducing phase separation, and germ line studies that show a role in promoting condensation of RNA binding proteins. In particular, the example of Ddx4/Vasa is striking. The formation of liquid droplets of the disordered N-terminal domain of Ddx4 is suppressed by asymmetric demethylation via PRMT1 expression in bacterial cells (Nott et al., 2015). In contrast, the methylation of Vasa by Dart5/Csul in Drosophila promotes the assembly of nuage granules in nurse cells and of piNG bodies in spermatocytes (Kibanov et al., 2011). The explanation for these opposite effects may simply be a more complex in vivo environment in the germ line, but probing this difference could be helpful in understanding the limitations of applying in vitro findings to the germ line. Another instance of context-specific effects of PTMs can be seen in the C. elegans embryo. MBK-2 phosphorylation of the MEG proteins drives disassembly of P granules, including the PGL-1 protein (Wang et al., 2014); however, phosphorylation of PGL-1 by mTORC1 stimulates the assembly of ectopic P granules in somatic blastomeres during heat stress (Zhang et al., 2018). These differences highlight our incomplete understanding of the role of PTMs in regulating phase separation.
One important consideration not yet discussed is the combinatorial nature of PTMs in modulating the assembly of granules. RNA binding proteins are often both phosphorylated and methylated, suggesting these two types of PTMs can be either synergistic or antagonistic (Bah and Forman-Kay, 2016). In addition, other PTMs such as O-linked GlcNAc modification and lysine acetylation have been shown to interact with phosphorylation in in vitro assays. For example, acetylation of Tau on Lys-321 prevents phosphorylation of a downstream Ser residue, and results in decreased aggregation of Tau filaments (Carlomagno et al., 2017). In regards to the germ line, few combinatorial PTMs of RNA binding proteins have been identified to date. However, the C. elegans PGL-1 protein is methylated by EPG-11 which inhibits PGL-1 aggregation into granules in vitro, and may act similarly in somatic blastomeres of the early embryo (Li et al., 2013; Zhang et al., 2018). The de-condensation of PGL-1 in somatic blastomeres can be balanced during heat stress when phosphorylation of PGL-1 by mTORC1 accelerates phase separations, resulting in ectopic somatic granules (Zhang et al., 2018). It remains to be seen if RNP condensates in the germ line proper are similarly modulated by combinations of PTMs.
Another interesting avenue to pursue is the extent to which stress conditions trigger PTMs that regulate RNP condensates in the germ line. Heat stress, extended meiotic arrest, starvation, hypoxia, and osmotic stress can induce the assembly of large RNP condensates in the germ line; however, PTMs have not been identified as regulators of any of these stress-induced granules to date (Schisa, 2014). In vitro studies, on the other hand, show that certain stresses lead to phosphorylation of FUS in addition to its constitutive Arg-methylation (Rhoads et al., 2018). During environmental stresses, stress granule formation is stimulated by a combination of phosphorylation and O-GlcNAcylation on Ser/Thr residues; however, the precise cause and effect relationship between these combinations of PTMs and phase transitions remains incompletely understood (Hofweber and Dormann, 2019).
Another unresolved question is whether RNP condensates in germ cells are bona fide phase-separated structures. To address this, time-lapse microscopy could be useful to determine the behavior of fusing droplets in vivo. FRAP studies may also be helpful; however, fast FRAP recovery is not sufficient to demonstrate phase separation (Alberti et al., 2019). The use of super-resolution microscopy is a relatively new tool that may be useful in mapping phase diagrams to determine concentration dependent thresholds for assembly (Patel et al., 2015; Rai et al., 2018). Another approach being developed to assess viscosity and porosity of condensates is the use of genetically encoded nanoparticles (GEMS) as microrheology probes (Alberti et al., 2019). One challenge to the field with investigations of the physical properties of in vivo germ line condensates is distinguishing whether genetic perturbations that affect function do so due to altered condensation or independently of condensation alterations.
We have a good appreciation that the dysregulation of protein folding and condensation can result in human disease, as is well-exemplified by multiple neurodegenerative diseases, for example, ectopic aggregates containing TDP-43 and FUS in ALS. Germ cells share several attributes with neurons such as being post-mitotic and differentiated, and relying on regulation of gene expression post-transcriptionally, e.g., the synaptic ends of axons are a distance from nuclei, and maturing oocytes have large stores of maternal mRNAs. Certain stresses and mutations cause increased condensation of RNA binding proteins in C. elegans and Drosophila germ lines. In some cases, the condensates are liquid-like and reversible and have been hypothesized to be protective (Jud et al., 2008; Shimada et al., 2011; Hubstenberger et al., 2013); while in other cases, such as the cgh-1 (tn691) germ line at restrictive temperature, RNA binding proteins condense into sheet-like structures with immobile pools of protein (Hubstenberger et al., 2013; Langerak et al., 2019). The cgh-1 (tn691) phenotype also includes oogenesis defects, increased germ line apoptosis, and embryonic lethality; therefore, the pleiotropic nature of the defects makes it impossible to assess if the condensation of RNA binding proteins into solid structures contributes directly to the infertility (Navarro et al., 2001; Audhya et al., 2005). It will be of interest for future studies to find approaches to address the cause and effect relationships between condensate regulation and gamete development/function in the germ line.
Author Contributions
JS contributed writing and editing of the manuscript and prepared the figures. ME contributed writing of the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Xantha Karp and lab members for helpful feedback on the manuscript.
Footnotes
Funding. JS is funded by the National Institutes of General Medical Sciences 2R15GM109337.
References
- Akpınar M., Lesche M., Fanourgakis G., Fu J., Anasstasiadis K., Dahl A., et al. (2017). TDRD6 mediates early steps of spliceosome maturation in primary spermatocytes. PLoS Genet. 13:e1006660. 10.1371/journal.pgen.1006660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S., Gladfelter A., Mittag T. (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176 419–434. 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R., Czaplinski K., Git A., Adegbenro E., Stennard F., Houliston E., et al. (2004). Two distinct Staufen isoforms in Xenopus are vegetally localized during oogenesis. RNA 10 1751–1763. 10.1261/rna.7450204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambadipudi S., Biernat J., Riedel D., Mandelkow E., Zweckstetter M. (2017). Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat. Commun. 8:275. 10.1038/s41467-017-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne J. (2010). Arginine methylation of SmB is required for Drosophila germ cell development. Development 137 2819–2828. 10.1242/dev.052944 [DOI] [PubMed] [Google Scholar]
- Anne J., Ollo R., Ephrussi A., Mechler B. M. (2007). Arginine methyltransferase Capsuléen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134 137–146. 10.1242/dev.02687 [DOI] [PubMed] [Google Scholar]
- Arkov A. L., Ramos A. (2010). Building RNA-protein granules: insight from the germline. Trends Cell Biol. 20 482–490. 10.1016/j.tcb.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arur S., Ohmachi M., Nayak S., Hayes M., Miranda A., Hay A., et al. (2009). Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc. Natl. Acad. Sci. U.S.A. 106 4776–4781. 10.1073/pnas.0812285106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A., Hyndman F., McLeod I. X., Maddox A. S., Yates J. R., III, Desai A., et al. (2005). A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J. Cell Biol. 171 267–279. 10.1083/jcb.200506124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bah A., Forman-Kay J. D. (2016). Modulation of intrinsically disordered protein function by post-translational modifications. J. Biol. Chem. 291 6696–6705. 10.1074/jbc.R115.695056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M. T., Clarke S. G. (2009). Protein Arginine Methylation in Mammals: who. What, and why. Mol. Cell 33 1–13. 10.1016/j.molcel.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke E., Ruer M., Wühr M., Coughlin M., Lemaitre R., Gygi S. P., et al. (2016). Amyloid-like self-assembly of a cellular compartment. Cell 166 637–650. 10.1016/j.cell.2016.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., et al. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324 1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Carlomagno Y., Chung D. C., Yue M., Castanedes-Casey M., Madden B. J., Dunmore J., et al. (2017). An Acetylation–phosphorylation switch that regulates tau aggregation propensity and function. J. Biol. Chem. 292 15277–15286. 10.1074/jbc.M117.794602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. A., Forman-Kay J. D. (2016). Liquid–liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol. 41 180–186. 10.1016/j.sbi.2016.08.001 [DOI] [PubMed] [Google Scholar]
- De Leeuw F., Zhang T., Wauquier C., Huez G., Kruys V., Gueydan C. (2007). The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp. Cell Res. 313 4130–4144. 10.1016/j.yexcr.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Fackelmayer F. O. (2005). Protein arginine methyltransferases: guardians of the arg? Trends Biochem. Sci. 30 666–671. 10.1016/j.tibs.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Flemr M., Ma J., Schultz R. M., Svoboda P. (2010). P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol. Reprod. 82 1008–1017. 10.1095/biolreprod.109.082057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez G. B., Rajendra T. K., Tian L., Matera A. G. (2006). The Sm-protein methyltransferase, Dart5, is essential for germ-cell specification and maintenance. Curr. Biol. 16 1077–1089. 10.1016/j.cub.2006.04.037 [DOI] [PubMed] [Google Scholar]
- Hara M., Lourido S., Petrova B., Lou H. J., Von Stetina J. R., Kashevsky H., et al. (2018). Identification of PNG kinase substrates uncovers interactions with the translational repressor TRAL in the oocyte-to-embryo transition. ELife 7:e33150. 10.7554/eLife.33150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M., Dormann D. (2019). Friend or foe-post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 294 7137–7150. 10.1074/jbc.TM118.001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A., Noble S. L., Cameron C., Evans T. C. (2013). Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 27 161–173. 10.1016/j.devcel.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura A. K., Futia R. A., Jarosz D. F. (2018). It pays to be in phase. Biochemistry 57 2520–2529. 10.1021/acs.biochem.8b00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud M. C., Czerwinski M. J., Wood M. P., Young R. A., Gallo C. M., Bickel J. S., et al. (2008). Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress and anoxia and are regulated by the major sperm protein pathway. Dev. Biol. 318 38–51. 10.1016/j.ydbio.2008.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani F., Nonaka T., Suzuki T., Arai T., Dohmae N., Akiyama H., et al. (2009). Identification of casein kinase-1 phosphorylation sites on TDP-43. Biochem. Biophys. Res. Commun. 382 405–409. 10.1016/j.bbrc.2009.03.038 [DOI] [PubMed] [Google Scholar]
- Kato M., Han T. W., Xie S., Shi K., Du X., Wu L. C., et al. (2012). Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149 753–767. 10.1016/j.cell.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nakamura A. (2012). Roles of cytoplasmic RNP granules in intracellular RNA localization and translational control in the Drosophila oocyte. Dev. Grow. Differ. 54 19–31. 10.1111/j.1440-169X.2011.01314.x [DOI] [PubMed] [Google Scholar]
- Kavarthapu R., Anbazhagan R., Raju M., Morris C. H. T., Pickel J., Dufau M. L. (2019). Targeted knock-in mice with a human mutation in GRTH/DDX25 reveals the essential role of phosphorylated GRTH in spermatid development during spermatogenesis. Hum. Mol. Genet. 28 2561–2572. 10.1093/hmg/ddz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibanov M. V., Egorova K. S., Ryazansky S. S., Sokolova O. A., Kotov A. A., Olenkina O. M., et al. (2011). A novel organelle, the piNG-body, in the nuage of Drosophila male germ cells is associated with piRNA-mediated gene silencing. Mol. Biol. Cell 22 3410–3419. 10.1091/mbc.E11-02-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Cooke H. J., Rhee K. (2012). DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 139 568–578. 10.1242/dev.075846 [DOI] [PubMed] [Google Scholar]
- Kirino Y., Vourekas A., Sayed N., De Lima Alves F., Thomson T., Lasko P., et al. (2010). Arginine methylation of aubergine mediates tudor binding and germ plasm localization. RNA 16 70–78. 10.1261/rna.1869710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M., Bilinski S., Etkin L. D. (2004). The Balbiani body and germ cell determinants: 150 years later. Curr. Top. Dev. Biol. 59 1–36. 10.1016/S0070-2153(04)59001-4 [DOI] [PubMed] [Google Scholar]
- Langerak S., Trombley A., Patterson J. R., Leroux D., Couch A., Wood M. P., et al. (2019). Remodeling of the endoplasmic reticulum in Caenorhabditis elegans oocytes is regulated by CGH-1. Genesis 57:e23267. 10.1002/dvg.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Ohmachi M., Arur S., Nayak S., Francis R., Church D., et al. (2007). Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177 2039–2062. 10.1534/genetics.107.081356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Banjade S., Cheng H. C., Kim S., Chen B., Guo L., et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483 336–340. 10.1038/nature10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yang P., Tian E., Zhang H. (2013). Arginine methylation modulates autophagic degradation of PGL granules in C.elegans. Mol. Cell 52 421–433. 10.1016/j.molcel.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Luo F., Gui X., Zhou H., Gu J., Li Y., Liu X., et al. (2018). Atomic structures of FUS LC domain segments reveal bases for reversible amyloid fibril formation. Nat. Struct. Mol. Biol. 25 341–346. 10.1038/s41594-018-0050-8 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Nakayama H., Yoshimura M., Masuda A., Dohmae N., Matsumoto S., et al. (2012). PRMT1 is required for RAP55 to localize to processing bodies. RNA Biol. 9 610–623. 10.4161/rna.19527 [DOI] [PubMed] [Google Scholar]
- Medvedev S., Yang J., Hecht N. B., Schultz R. M. (2008). CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers maternal mRNA degradation during mouse oocyte maturation. Dev. Biol. 321 205–215. 10.1016/j.ydbio.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z., Ryan V. H., Janke A. M., Burke K. A., Rhoads S. N., Zerze G. H., et al. (2017). Phosphorylation of the FUS low−complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36 2951–2967. 10.15252/embj.201696394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. T., Kato M., Lin Y., Thurber K. R., Hung I., McKnight S. L., et al. (2017). Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 171 615–627.e16. 10.1016/j.cell.2017.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro R. E., Shim E. Y., Kohara Y., Singson A., Blackwell T. K. (2001). Cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development 128 3221–3232. [DOI] [PubMed] [Google Scholar]
- Nott T. J., Petsalaki E., Farber P., Jervis D., Fussner E., Plochowietz A., et al. (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57 936–947. 10.1016/j.molcel.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsborn A. M., Li W., McEwen T. J., Mizuno T., Kuzmin E., Matsumoto K., et al. (2007). GLH-1, the C. elegans P granule protein, is controlled by the JNK KGB-1 and by the COP9 subunit CSN-5. Development 134 3383–3392. 10.1242/dev.005181 [DOI] [PubMed] [Google Scholar]
- Patel A., Lee H. O., Jawerth L., Maharana S., Jahnel M., Hein M. Y., et al. (2015). A Liquid-to-Solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162 1066–1077. 10.1016/j.cell.2015.07.047 [DOI] [PubMed] [Google Scholar]
- Pek J. W., Ng B. F., Kai T. (2012). Polo-mediated phosphorylation of maelstrom regulates oocyte determination during oogenesis in drosophila. Development (Cambridge) 139 4505–4513. 10.1242/dev.082867 [DOI] [PubMed] [Google Scholar]
- Putnam A., Cassani M., Smith J., Seydoux G. (2019). A gel phase promotes condensation of liquid P granules in C. elegans embryos. Nat. Struct. Mol. Biol. 26 220–226. 10.1038/s41594-019-0193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar S., Wang G. Z., Randle S. J., Ruggeri F. S., Varela J. A., Lin J. Q., et al. (2018). FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell 173 720–734.e15. 10.1016/j.cell.2018.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D., Dey S., Ray K. (2018). A method for estimating relative changes in the synaptic density in Drosophila central nervous system. BMC Neurosci. 19:30. 10.1186/s12868-018-0430-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads S. N., Monahan Z. T., Yee D. S., Shewmaker F. P. (2018). The role of post-translational modifications on prion-like aggregation and liquid-phase separation of FUS. Int. J. Mol. Sci. 19:886. 10.3390/ijms19030886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers E. F., Kaaij L. J. T., Redl S., Bronkhorst A. W., Wiebrands K., de Jesus Domingues A. M., et al. (2018). Tdrd6a regulates the aggregation of Buc into functional subcellular compartments that drive germ cell specification. Dev. Cell 46 285–301.e9. 10.1016/j.devcel.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L., Vieira A. P., Roberts-Galbrait R. H., Newmark P. A. (2012). PRMT5 and the role of symmetrical dimethylarginine in chromatoid bodies of planarian stem cells. Development 139 1083–1094. 10.1242/dev.076182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J. A. (2012). New insights into the regulation of RNP granule assembly in oocytes. Int. Rev. Cell Mol. Biol. 295 233–289. 10.1016/B978-0-12-394306-4.00013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J. A. (2014). Effects of stress and aging on ribonucleoprotein assembly and function in the germ line. WIREs RNA 5 231–246. 10.1002/wrna.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G. (2018). The P granules of C. elegans: a genetic model for the study of RNA–protein condensates. J. Mol. Biol. 430 4702–4710. 10.1016/j.jmb.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y., Tsai-Morris C. H., Gutti R., Maeda Y., Dufau M. L. (2006). Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J. Biol. Chem. 281 35048–35056. 10.1074/jbc.M605086200 [DOI] [PubMed] [Google Scholar]
- Shimada Y., Burn K. M., Niwa R., Cooley L. (2011). Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev. Biol. 355 250–262. 10.1016/j.ydbio.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkova E., Zeng H., Liu F., Kwiecien N. W., Hebert A. S., Coon J. J., et al. (2017). Global mapping of CARM1 substrates defines enzyme specificity and substrate recognition. Nat. Commun. 24:15571. 10.1038/ncomms15571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser M. J., Mackenzie N. C., Dumstrei K., Nakkrasae L. I., Stebler J., Raz E. (2008). Control over the morphology and segregation of Zebrafish germ cell granules during embryonic development. BMC Dev. Biol. 8:58. 10.1186/1471-213X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W. C., Gayatri S., Reineke L. C., Sbardella G., Bedford M. T., Lloyd R. E. (2016). Arginine demethylation of G3BP1 promotes stress granule assembly. J. Biol. Chem. 291 22671–22685. 10.1074/jbc.M116.739573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D., Strome S. (2010). P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31 53–60. 10.2164/jandrol.109.008292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin V. V., Wohlschlegel J., Qu J., Jonsson Z., Huang X., Chuma S., et al. (2009). Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 23 1749–1762. 10.1101/gad.1814809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva A., Tiedau D., Firooznia A., Müller-Reichert T., Jessberger R. (2009). Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr. Biol. 19 630–639. 10.1016/j.cub.2009.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E., Seydoux G., Sassone-Corsi P., Nagamori I. (2011). RNA granules in germ cells. Cold Spring Harb. Perspect. Biol. 3:a002774. 10.1101/cshperspect.a002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Conicella A. E., Schmidt H. B., Martin E. W., Rhoads S. N., Reeb A. N., et al. (2018). A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J. 37 1–18. 10.15252/embj.201797452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Smith J., Chen B. C., Schmidt H., Rasoloson D., Paix A., et al. (2014). Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. ELife 3:e04591. 10.7554/eLife.04591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F., Bodenmiller B., Trajkovska M. G., Wanka S., Aebersold R., Pelkmans L. (2013). Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152 791–805. 10.1016/j.cell.2013.01.033 [DOI] [PubMed] [Google Scholar]
- Wong L. C., Costa A., McLeod I., Sarkeshik A., Yates J., Kyin S., et al. (2011). The functioning of the drosophila CPEB protein orb is regulated by phosphorylation and requires casein kinase 2 activity. PLoS One 6:e24355. 10.1371/journal.pone.0024355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. B., Hyman A. A., Boke E. (2018). Organization and function of non-dynamic biomolecular condensates. Trends Biochem. Sci. 43 81–94. 10.1016/j.tibs.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Xie H., Vucetic S., Iakoucheva L. M., Oldfield C. J., Dunker A. K., Uversky V. N., et al. (2007). Functional anthology of intrinsic disorder. 1. Biological processes and functions of proteins with long disordered regions. J. Proteome Res. 6 1882–1898. 10.1021/pr060392u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Park K., Hwang D. S., Rhee K. (2017). Importance of eIF2α phosphorylation as a protective mechanism against heat stress in mouse male germ cells. Mol. Reprod. Dev. 84 265–274. 10.1002/mrd.22778 [DOI] [PubMed] [Google Scholar]
- Zhang G., Wang Z., Du Z., Zhang H. (2018). mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174 1492–1506.e22. 10.1016/j.cell.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhang D., Liu X., Yu G., Cai X., Xu C., et al. (2019). zebrafish prmt5 arginine methyltransferase is essential for germ cell development (Development (Cambridge)(2019) 146 (dev179572) 10.1242/dev.179572) [DOI] [PubMed] [Google Scholar]