Highlights
-
•
Participants with ALS exhibited impaired function between the cortex and cerebellum.
-
•
The cerebellum is associated with complex motor and cognitive processing tasks.
-
•
These findings add to the growing number of ALS reports implicating the cerebellum.
Keywords: Amyotrophic lateral sclerosis, Functional magnetic resonance imaging, Cerebellum, Ultra-high field, 7 Tesla
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of the central nervous system that results in a progressive loss of motor function and ultimately death. It is critical, yet also challenging, to develop non-invasive biomarkers to identify, localize, measure and/or track biological mechanisms implicated in ALS. Such biomarkers may also provide clues to identify potential molecular targets for future therapeutic trials. Herein we report on a pilot study involving twelve participants with ALS and nine age-matched healthy controls who underwent high-resolution resting state functional magnetic resonance imaging at an ultra-high field of 7 Tesla. A group-level whole-brain analysis revealed a disruption in long-range functional connectivity between the superior sensorimotor cortex (in the precentral gyrus) and bilateral cerebellar lobule VI. Post hoc analyses using atlas-derived left and right cerebellar lobule VI revealed decreased functional connectivity in ALS participants that predominantly mapped to bilateral postcentral and precentral gyri. Cerebellar lobule VI is a transition zone between anterior motor networks and posterior non-motor networks in the cerebellum, and is associated with a wide range of key functions including complex motor and cognitive processing tasks. Our observation of the involvement of cerebellar lobule VI adds to the growing number of studies implicating the cerebellum in ALS. Future avenues of scientific investigation should consider how high-resolution imaging at 7T may be leveraged to visualize differences in functional connectivity disturbances in various genotypes and phenotypes of ALS along the ALS-frontotemporal dementia spectrum.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive degeneration of motor neurons and their axonal connections in the brain and spinal cord. The disease presents as a continuous loss of motor functions and ultimately results in death. ALS is known to be associated in many individuals with a spectrum of extra-motor dysfunction including behavioral changes, cognitive impairment, dementia and mood changes (Turner et al., 2016). Although rare, there are growing observations that some individuals with “ALS plus” syndromes may have other associated neurological features including extrapyramidal/parkinsonian features, diminished taste/smell, cerebellar ataxia, parasomnias, or eye gaze abnormalities (Turner et al., 2016, Brooks, 2014, Al-Chalabi et al., 2016, Abrahams et al., 1996, Abrahams et al., 2005, Lulé et al., 2007, Silani et al., 2017, Bede et al., 2018). This suggests that ALS may also be viewed as an overlapping neurodegenerative disorder with widespread involvement of different brain regions.
From a neuropathological standpoint, ALS is recognized as a TDP43 proteinopathy in 98% of all cases (except forms of ALS with SOD1 and FUS gene expressions) (Feneberg et al., 2018). The Braak staging of spreading TDP43 inclusion pathology burden in a large postmortem ALS cohort suggests that the disease pathology extends beyond the well known motor cortical system in later stages of disease to also include precerebellar nuclei, prefrontal cortex, and the striatum (Brettschneider et al., 2013). There is an unmet need to expand ongoing in vivo clinical research to evaluate reliable and sensitive neuroimaging biomarkers (Woo and Wager, 2015) that can identify and map neural networks that degenerate with ALS and more importantly their relevance and predictability to clinical disability progression (Bede and Hardiman, 2014, Chiò et al., 2014, Verstraete et al., 2015, Brooks et al., 2000, Douaud et al., 2011, Turner et al., 2012, Trojsi et al., 2012, Chiò et al., 2014, Christidi et al., 2018). There has also been growth in multimodal neuroimaging techniques in the past decade, which provide unique opportunities to leap forward toward developing novel and quantitative biomarkers of disease localization, tracking disease progression, and improving phenotypic classifications in ALS (Agosta et al., 2010, Agosta et al., 2010, Verstraete and Foerster, 2015).
At 1.5 or 3 Tesla, diffusion magnetic resonance imaging (MRI) studies of ALS have revealed widespread structural damage across motor and non-motor regions (Sach et al., 2004, Thivard et al., 2007, Lulé et al., 2010, Verstraete et al., 2010, Agosta et al., 2011, Verstraete et al., 2011, Canu et al., 2011, Keil et al., 2012, Trojsi et al., 2015, Menke et al., 2016, Schulthess et al., 2016, Müller et al., 2018, Menke et al., 2018, Qiu et al., 2019, Bharti et al., 2020). The role of functional MRI (fMRI) in the study of neurodegenerative disorders continues to expand (Fox and Greicius, 2010) and maps brain networks based upon the temporal coherency of hemodynamic responses in a ‘resting state’ or evoked via one or more tasks. Prior fMRI studies at 1.5 or 3T (and one at 4.7T) have revealed the impact of disease on brain functions (Konrad et al., 2002, Schoenfeld et al., 2005, Konrad et al., 2006, Tessitore et al., 2006, Stanton et al., 2007, Lulé et al., 2007, Lulé et al., 2010, Agosta et al., 2011, Mohammadi et al., 2011, Goldstein et al., 2011, Cosottini et al., 2012, Passamonti et al., 2013, Witiuk et al., 2014, Mohammadi et al., 2015, Li et al., 2015, Jelsone-Swain et al., 2015) and modulation of functional (Mohammadi et al., 2009, Jelsone-Swain et al., 2010, Agosta et al., 2011, Menke et al., 2016, Schulthess et al., 2016, Chenji et al., 2016, Xu et al., 2017, Menke et al., 2018, Qiu et al., 2019, Bharti et al., 2020) or effective (Fang et al., 2016) connectivity. The literature on resting state fMRI (rs-fMRI) in ALS is relatively sparse, and there is minimal convergence of findings across different cross-sectional and longitudinal cohorts (Proudfoot et al., 2019). However, several rs-fMRI studies have noted aberrations (increases or decreases) in functional connectivity in ALS participants involving the sensorimotor network (SMN) (Mohammadi et al., 2009, Agosta et al., 2011, Chenji et al., 2016, Menke et al., 2016, Menke et al., 2018, Bharti et al., 2020) as well as other intrinsic networks (Van Dijk et al., 2010).
To date, only a handful of 7 Tesla (7T) ALS studies have been published in the brain (Verstraete et al., 2010, Kwan et al., 2012, Cosottini et al., 2016, Costagli et al., 2016, Verstraete et al., 2014, Atassi et al., 2017, Cheong et al., 2017) and spinal cord (Cohen-Adad et al., 2013). The benefits of imaging at an ultra-high field of 7T include increases in spatial signal-to-noise and contrast-to-noise ratios, functional contrast-to-noise ratio, and spectral dispersion (Ladd et al., 2018, Obusez et al., 2018, Donatelli et al., 2018, Barry et al., 2018). In the cervical cord, high-resolution *-weighted images demonstrated sharp delineation between gray and white matter, and white matter * hyperintensities were visualized in regions of ALS pathology (Cohen-Adad et al., 2013). In the brain, a previous 7T ALS study reported hyperintensities in * maps in the motor cortex of ALS participants, which correlated with iron accumulation in post mortem pathological studies (Kwan et al., 2012). Anatomical imaging of the deep layers of the primary motor cortex at 7T revealed atrophy and * hypointensities in participants with ALS that correlated with upper motor neuron (UMN) impairment and disease progression rate (Cosottini et al., 2016), and increases in magnetic susceptibility measured via quantitative susceptibility mapping co-localized with * hypointensities in the middle and deep layers (Costagli et al., 2016). Diffusion imaging at 7T did not reveal whole-brain differences between controls and ALS participants, but analyses of the corticospinal tracts (CSTs) revealed decreased fractional anisotrophy and increased magnetization transfer ratio in ALS participants (Verstraete et al., 2014). Single voxel magnetic resonance spectroscopy (MRS) of the left precentral gyrus at 7T revealed decreases in N-acetylaspartate (NAA), glutamate, and total NAA (N-acetylaspartate + N-acetylaspartylglutamate) in ALS participants, which correlated with forced vital capacity; additionally, the ratio of total NAA to total creatine (creatine + phosphocreatine) correlated with overall functional decline as measured by decreasing ALS Functional Rating Scale-Revised (ALSFRS-R) (Atassi et al., 2017) scores. Another 7T MRS study reported a lower ratio of total NAA to myo-inositol in both the motor cortex and pons of ALS participants, and that levels of total NAA, myo-inositol, and glutamate in the motor cortex of ALS participants were influenced by the extent of disease progression (Cheong et al., 2017).
We are not aware of previous reports studying ALS using 7T fMRI. Therefore, the pilot study presented herein contributes to the existing body of 7T work by investigating group-level differences in functional networks between healthy controls and ALS participants using high-resolution rs-fMRI with spatial coverage extending from the cerebrum to the cerebellum. We additionally assessed the association between significant imaging findings and ALS clinical variables.
2. Material and methods
2.1. Study cohort
This cross-sectional pilot study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, Massachusetts, USA. Scans were performed within a 4-year period between 2011 and 2015. Subjects included in the current study () were drawn from a cohort of 25 subjects previously reported by our group (Atassi et al., 2017) who provided a high-resolution rs-fMRI scan upon completion of the main MRS protocol. All subjects provided written, informed consent through a protocol approved by the Partners Human Research Committee.
Twelve participants who met the revised El Escorial criteria for at least possible ALS and nine age-matched healthy controls were enrolled in this study. All ALS participants met the institutional MRI safety criteria and were able to tolerate lying flat for the scan duration. All participants had to have no other neurodegenerative disease diagnosis. Baseline characteristics of the study cohorts are detailed in Table 1. Family history of ALS was not collected for these participants. One ALS participant was confirmed to have C9orf72 repeat expansion. ALS causative genetic mutations remained unknown for the remaining 11 participants. All ALS participants underwent standard clinical outcome assessments collected by certified raters at a matching time point to the scan including ALSFRS-R (Cedarbaum et al., 1999), slow vital capacity (SVC), and a quantitative muscle strength test using hand-held dynamometry.
Table 1.
Baseline characteristics for healthy controls and participants with ALS.
| Baseline characteristic | ALS () | Controls () |
|---|---|---|
| %(n)/ () | %(n)/ () | |
| Age at screening (yrs) | 56.8 (10.3) | 53.2 (11.1) |
| Genetic abnormality | unknown (11) | n/a |
| C9orf72 positive (1) | ||
| Male | 75% (9) | 55% (5) |
| Caucasian | 100% (12) | 100% (9) |
| Symptom onset to diagnosis (months) | 10.3 (7.0) | n/a |
| Symptom onset to scan (months) | 30.3 (23.4) | n/a |
| Limb onset | 67% (8) | n/a |
| Baseline SVC (% predicted) | 93.4% (22.2%) | n/a |
| ALSFRS-R at baseline | 38.1 (4.8) | n/a |
| Estimated ALSFRS-R slope pre-baseline ((48 – baseline ALSFRS-R)/ disease duration) (points/month) | 0.46 (0.35) | n/a |
| Revised El Escorial criteria | definite (5) | n/a |
| probable (3) | ||
| probable lab supported (1) | ||
| possible (3) | ||
| Riluzole use | 75% (9) | n/a |
2.2. Data acquisition
Experiments were performed on a whole-body 7 Tesla system (Siemens Healthineers, Erlangen, Germany) using a single-channel transmit birdcage volume coil and a custom-built 32-channel receive coil (Keil et al., 2010).
All subjects completed a 45-min brain imaging session without contrast, which included a high-resolution T1-weighted anatomical scan, MRS (previously reported (Atassi et al., 2017)), and a high-resolution rs-fMRI scan. High-resolution anatomical images were acquired with the following parameters: field of view (FOV) = 256 mm256 mm176 mm, voxel size = 111 mm3, TE = 1.48 ms, inversion time = 1100 ms, repetition time (TR) = 2530 ms, flip angle = 7°. High-resolution blood oxygenation level dependent (BOLD) functional images were acquired with the following parameters: FOV = 190 mm190 mm108 mm, voxel size = 1.151.151.18 mm3, TE = 22.6 ms, TR = 5000 ms (except one subject where TR = 5560 ms inadvertently), flip angle = 84°, number of volumes = 94 (scan time = 7.8 min). Subjects were instructed to remain still and in a state of wakeful rest prior to the functional scan. Reported fMRI parameters are the mean across subjects due to minor adjustments to the protocol over the course of the 4-year study, and subject-specific adjustments to obviate specific absorption rate limits and peripheral nerve stimulation.
2.3. Data processing and analysis
Data were processed and analyzed using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) release 18.b. Structural scans were translated to (0,0,0) coordinates and normalized to Montreal Neurological Institute (MNI) space (Evans et al., 1993) with simultaneous segmentations of gray matter, white matter, and cerebrospinal fluid (CSF). Functional scans were preprocessed using CONN’s recommended pipeline for volume-based analyses: i) realignment and unwarping (including rigid-body motion estimation and correction), ii) centering to (0,0,0) coordinates, iii) slice-timing correction, iv) ARtifact detection Tools (ART)-based outlier detection for data ‘scrubbing’ (www.nitrc.org/projects/artifact_detect) with thresholds of for deviation from global mean and 1.5 mm for framewise displacement, v) normalization to MNI space with segmentations of tissues and CSF, and vi) spatial smoothing using an 8-mm full-width-at-half-maximum Gaussian kernel (Mikl et al., 2008) to reduce between-subject anatomical variability in preparation for group analyses. Physiological noise regressors included five principal components of anatomically-derived white matter and CSF segments’ time series (Behzadi et al., 2007), respectively, the six estimated rigid-body motion parameters and their first derivatives, and ART-derived outliers. Additional preprocessing steps included linear detrending and band-pass filtering (0.008–0.09 Hz).
An exploratory second-level analysis of functional connectivity was performed using CONN’s seed-to-voxel analysis tool that considers numerous seed regions throughout the brain. Considering the nature of this pilot study, we used a relatively stringent voxel threshold of (uncorrected, two-sided) in tandem with a cluster threshold of with false discovery rate correction. Each seed was a 222 mm3 region centered at a given MNI coordinate. A functional contrast of Controls ALS participants was selected to probe group-level differences between controls and participants with ALS. Results from this contrast (presented hereafter) were saved and projected onto a spatially unbiased atlas of the cerebellum using the spatially unbiased infra-tentorial template (SUIT) version 3.0 (Diedrichsen, 2006, Diedrichsen et al., 2009, Diedrichsen et al., 2011, Diedrichsen and Zotow, 2015). Post hoc seed-to-voxel analyses with the same contrast were then performed using three atlas (AAL)-derived regions: left cerebellar lobule VI, right cerebellar lobule VI, and combined (left and right) cerebellar lobule VI.
3. Results
The cohorts of ALS participants () and controls () did not differ significantly in age or sex ( and , respectively, using Wilcoxon rank sum tests). The aggregate rigid-body motion parameters were also not significantly different between groups (, t-test), which is an important quality assurance observation to ensure that group-level differences in functional connectivity were not erroneously driven by group-dependent motion (Power et al., 2012). The number of data points ‘scrubbed’ between groups was also not significantly different (medianmedian absolute deviation: 53.85 for controls vs. 56.61 for ALS participants; using a Wilcoxon rank sum test). No subjects were excluded due to excessive motion.
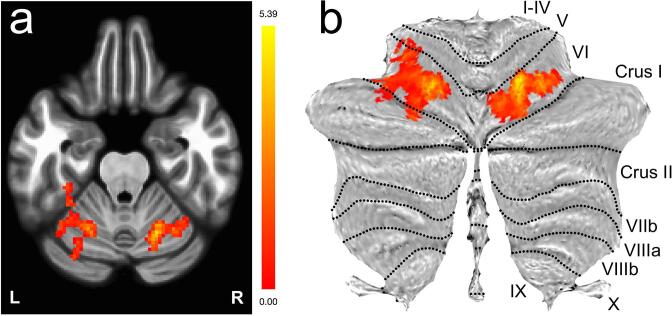
Across all regions considered in this exploratory analysis, we observed a decrease in functional connectivity between the superior sensorimotor cortex (MNI coordinates = (0, −31, 67), which anatomically localizes to the inter-hemispheric fissure and in close proximity to the proximal leg representation area of the motor cortex) and lobule VI of the cerebellum in ALS participants vs. matched controls. Fig. 1a presents the functional contrast Controls ALS participants overlaid onto a single 2D axial slice through the mid-cerebellum. While a bilateral region of the cerebellum exhibited higher connectivity in controls (peak p-values in the left and right clusters were 0.000052 and 0.000033, respectively), no regions exhibited higher connectivity in ALS participants at the same threshold. (Additional post hoc analyses within groups showed that these results were due to an absence of significant correlations in ALS participants.) Fig. 1b projects these results onto a cerebellar flatmap (Diedrichsen and Zotow, 2015). The group-level results are remarkably symmetric and predominantly mapped onto bilateral cerebellar lobule VI (involving both medial and lateral aspects of this cerebellar lobule).
Fig. 1.
Decreased functional connectivity between the sensorimotor cortex and bilateral lobule VI of the cerebellum visualized via the functional contrast Controls ALS participants ( uncorrected, two-sided; cluster threshold: with false discovery rate correction) for a seed region located in the superior sensorimotor cortex (MNI coordinates = (0, –31, 67)). The results are presented as a) an overlay onto a single 2D axial slice through the cerebellum, and b) a projection onto a cerebellar flatmap (Diedrichsen and Zotow, 2015).
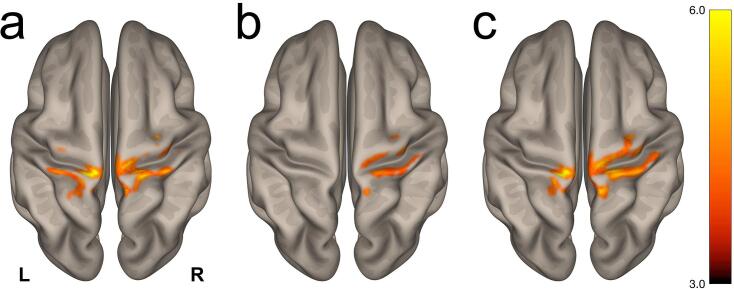
Fig. 2 visualizes results from three post hoc analyses using cerebellar lobule VI seed regions, presented as superior views of the partially-inflated cortical surface (voxel threshold: uncorrected, two-sided; cluster threshold: with false discovery rate correction). Group-level differences between ALS participants and controls predominantly mapped to the postcentral and precentral gyri with secondary differences in the superior parietal lobules and the precuneus cortex. Detailed results are presented in Table S1.
Fig. 2.
Visualization of the post hoc seed-to-voxel contrast Controls ALS participants using atlas-derived a) left, b) right, and c) bilateral cerebellar lobule VI as seed regions. Group-level differences ( uncorrected, two-sided; cluster threshold: with false discovery rate correction) were predominantly observed in the postcentral and precentral gyri. The complete list of clusters is presented in Table S1.
Additional analyses were performed correlating functional connectivity and ALS clinical metrics. These methods and results are presented in Supplementary Information. A meta-analysis of 57 ALS studies performed using NeuroQuery (Dockès et al., 2020), also presented in Supplementary Information, revealed activations in regions we found to be connected (i.e., somatosensory/motor cortex and cerebellum).
4. Discussion
In this data-driven, cross-sectional, ultra-high field rs-fMRI pilot study, we report significantly decreased functional connectivity between the superior sensorimotor cortex and bilateral cerebellar lobule VI in participants with ALS. Post hoc seed-to-voxel analyses of cerebellar lobule VI localize group-level differences in functional connectivity to bilateral precentral and postcentral gyri. While previous rs-fMRI studies have implicated the motor cortex and/or cerebellum in ALS, we are not aware of a previous report highlighting disruption in the specific link between the superior sensorimotor cortex and cerebellar lobule VI.
The upper motor neurons residing in the precentral gyri are well known to be involved in various disease mechanisms and neurodegeneration in ALS (Turner et al., 2012). There is also growing evidence of the involvement of cerebellar structures in imaging and neuropathological studies in sporadic ALS, ALS/neurodegenerative syndromes arising due to C9orf72 or ataxin-2 repeat expansions and TBK1 mutations, and in primary lateral sclerosis (PLS) (Mackenzie et al., 2014, Bede et al., 2015, Wang et al., 2014, Wilke et al., 2018). For example, pathological features associated with the production of C9orf72 transcripts with expanded repeats are the formation of nuclear ribonucleic acid foci in the frontal cortex, hippocampus, and cerebellum (Mackenzie et al., 2014). Functional and diffusion neuroimaging studies support topographical mappings of cerebellar lobule VI to premotor and primary motor cortices reciprocally (Stoodley and Schmahmann, 2018). Task-based fMRI has revealed that ocular and orofacial movements engage the medial aspect of cerebellar lobule VI (Nitschke et al., 2004, Konen et al., 2005, Schraa-Tam et al., 2009, Urban et al., 2003, Dresel et al., 2005). During more complex motor tasks involving motor planning/learning or cognitive processing, activation patterns shift more laterally in lobules VI and VII (Imamizu and Kawato, 2009, Schlerf et al., 2010). Previous rs-fMRI studies have reported on such cortical-cerebellar circuits in healthy volunteers (Habas et al., 2009, Krienen and Buckner, 2009, O’Reilly et al., 2010), further supporting the feasibility that this network is susceptible to disease-related impairment. The multifaceted role of cerebellar lobule VI therefore underscores the significance of its involvement in degeneration in ALS.
Previous studies have reported that cerebellar lobule VI and other regions of the cerebellum play a key role as a hub for diverse and integral motor and non-motor functions in ALS. A recent 3T study using an event-related motor paradigm reported the involvement of lobule VI in ALS participants with UMN predominant dysfunction (Abidi et al., 2020). A 3T diffusion tensor imaging (DTI) analysis of white matter tracts in ALS and PLS participants with pseudobulbar affect found abnormalities in the frontotemporal cortex, transverse pontine, and middle cerebellar peduncular circuits (Floeter et al., 2014). A recent longitudinal 3T voxel-based morphometry study in sporadic ALS (excluding C9orf72 ALS participants) found significant cerebellar gray matter density reductions in multiple regions including bilateral cerebellar lobule VI that evolved on subsequent follow-up scans compared to matched controls (Bede and Hardiman, 2018). Another 3T DTI study showed widespread disruption of cerebellar white matter tracts along the dentato-rubro-thalamo-cortical and spino-cerebellar pathways in PLS that may be greater than ALS, implying the impact of longer duration motor neuron disease (Tu et al., 2019). Such studies highlight the fact that studying the cerebellum and its neural connections is an important endeavor – yet remains challenging due to the relatively small size of many key anatomical structures.
The functional involvement of the cerebellum in ALS (Prell and Grosskreutz, 2013) has been reported in task-based (Schoenfeld et al., 2005, Konrad et al., 2006, Han and Ma, 2006, Abidi et al., 2020) and resting state (Agosta et al., 2011, Zhou et al., 2013, Menke et al., 2016, Bharti et al., 2020) fMRI studies at lower fields, and functional changes have been correlated to disease severity (Zhou et al., 2013), clinical variables (Abidi et al., 2020), and task difficulty (Schoenfeld et al., 2005). However, participants with ALS have a reduced capacity for performing motor tasks associated with task-based fMRI, so rs-fMRI (Mohammadi et al., 2009, Jelsone-Swain et al., 2010, Menke et al., 2016, Schulthess et al., 2016, Fang et al., 2016, Xu et al., 2017, Qiu et al., 2019, Bharti et al., 2020), along with motor imagery paradigms (Lulé et al., 2007), are attractive options in studying diseases with motor implications. There is considerable variability in the literature on functional connectivity network patterns in ALS. Beyond motor task-related disability and heterogeneity in ALS participant characteristics, there are of course other challenges for developing and scaling fMRI as a biomarker for tracking disease progression in ALS including technological (e.g., scanner vendor, field strength) and methodological/statistical (acquisition, preprocessing choices, hypothesis- vs. data-driven approaches) considerations (Cole et al., 2010, Proudfoot et al., 2019).
Earlier studies have reported abnormal connectivity/function involving the cerebellar and SMN areas in ALS (Agosta et al., 2011, Schoenfeld et al., 2005, Bharti et al., 2020). Specifically, 3T studies have highlighted the implications of ALS upon cerebello-cortical pathways. Both ALS participants and ALS mutation carriers have exhibited increased functional connectivity between the cerebellum and the precuneus-cingulate-middle frontal resting state network compared to controls, which may indicate pre-symptomatic ALS cerebral pathology (Menke et al., 2016). ALS participants have also shown decreased functional connectivity between the dentate nucleus in the cerebellum and both the supplementary motor area and left cerebellum lobule IV, which correlated positively with ALSFRS-R (Bharti et al., 2020). These alterations in functional connectivity, especially regarding cerebellar lobule IV and the primary motor cortex, may indicate alterations in the spino-cerebellar tract (Bharti et al., 2020). Furthermore, SMN correlations may increase in the earlier stage of disease (Agosta et al., 2011) and then decrease with more severe and longer duration disease (Chenji et al., 2016, Menke et al., 2018). An early 1.5T study reported decreased functional connectivity between the right SMN and right cerebellar lobule VI in ALS participants vs. healthy controls, but only in a subset of 16 ALS participants with CST damage (as measured via decreased fractional anisotrophy relative to controls and also ALS participants with undetectable CST damage) and only within the right hemisphere (Agosta et al., 2011). A longitudinal rs-fMRI study (with 13 ALS and 3 PLS participants) observed functional connectivity decreases between the SMN and frontal pole and increases between the left fronto-parietal network and left primary motor cortex (Menke et al., 2018). Another longitudinal 3T study (27 ALS participants vs. 56 controls) reported expansions in intrinsic motor, brainstem, ventral attention, and default mode/hippocampal networks that appeared to follow the same neuropathological pattern seen in Braak’s TDP43 staging in ALS (Schulthess et al., 2016). However, a cross-sectional study at 4.7T (20 ALS participants vs. 34 controls) used 10-mm diameter spheres within the pre- and post-central gyri and supplemental motor area to form a combined SMN time course and reported no significant group-level differences in the default mode network or SMN, or between subgroups of high and low UMN burden participants (Chenji et al., 2016).
Due to higher signal-to-noise and functional contrast-to-noise ratios, unique spatial contrasts, and increasing availability of 7T systems, high-resolution structural and functional imaging at 7T is poised to provide new insights into disease manifestation and progression (Beisteiner et al., 2011) in ALS as well as other diseases. The primary advantages of 7T fMRI over lower fields is the greater-than-linear increase in BOLD sensitivity (Menon et al., 1993, Gati and Menon, 1997) that permits smaller voxels without losing statistical power, and weighting toward smaller vessels supplying the superficial cortical structures, such as sensorimotor cortices, and smaller infratentorial tissue structures in close proximity to air-fluid-tissue interfaces such as cerebellum. One must keep in mind, however, that the mechanisms that give rise to an increase in BOLD contrast at higher fields also increase physiological noise (Krüger et al., 2001), and thus care must be taken to mitigate confounding noise sources due to respiration, cardiac pulsatility, and subject motion. In this 7T study, the influence of physiological noise was modeled in CONN using ART-derived outliers, five principal components of anatomically-derived white matter and CSF segments, and the six estimated rigid-body motion parameters and their first derivatives. The appropriate impact of these regressors on each subject’s data was then reviewed using CONN’s suite of tools to visualize first-level fMRI data before and after denoising.
Our investigation has limitations that must, of course, be mentioned. First, this pilot study is a cross-sectional design with relatively small group sizes, so we are therefore not characterizing the trajectory of disease progression for individual participants but rather are performing a group-level comparison that includes inherent heterogeneity within the cohort of ALS participants. Second, while there is one identified C9orf72 positive ALS participant in this cohort, the genetic status and hence contribution of other non-genotyped ALS participants to the abnormal cerebellar connectivity results is unknown. Third, the fMRI data were acquired with a longer TR than what is commonly used nowadays, resulting in fewer degrees of freedom and limitations on how the analyses may be conducted. It is important to note that the high-resolution acquisition strategy was implemented with the methods available when this study commenced in mid-2011 to obtain fMRI data in a regime expected to have minimal physiological noise (Triantafyllou et al., 2005, Triantafyllou et al., 2006). Single-subject analyses were initially pursued in the analyses of these data, but the resultant degrees of freedom per subject after denoising necessitated the second-level analyses presented herein. A longer TR additionally precludes the study of higher frequency resting state signals – up to 0.8 Hz – which has become an important area of investigation in recent years (Hutchison et al., 2013, Chen and Glover, 2015, Gohel and Biswal, 2015, Chen et al., 2019). Thus, future studies should use a TR of 1–1.5 s and also acquire a longer resting state run to increase the fidelity of functional connectivity estimates (Birn et al., 2013). Finally, due to the high-resolution whole-brain acquisition strategy, the imaging FOV did not include the more caudal regions of the cerebellum; therefore, the results presented in this study cannot preclude the possible involvement of these regions of the cerebellum.
Reports of modulation in the cerebellum support the evidence that there are not only adaptive intracortical changes (Kew et al., 1994, Konrad et al., 2002, Schoenfeld et al., 2005, Lulé et al., 2007, Jelsone-Swain et al., 2010, Cosottini et al., 2012, Zhou et al., 2013, Mohammadi et al., 2015, Qiu et al., 2019, Abidi et al., 2020) in response to neurodegeneration in ALS, but also functional reorganization that occurs subcortically (Konrad et al., 2006, Tessitore et al., 2006, Zhou et al., 2013, Mohammadi et al., 2015, Abidi et al., 2020). In sum, these reports highlight the brain’s widespread plasticity and capacity to partially overcome cortical and spinal neuronal degeneration. (Our post hoc analysis of functional modulation restricted to the subcortex (Frazier et al., 2005) may be found in Supplementary Information.) Future studies in ALS may employ recent acquisition advances, most notably simultaneous multi-slice imaging (Moeller et al., 2010), to acquire high-resolution 7T fMRI data from the cerebrum and entire cerebellum with a reduced volume acquisition time. Accurate measurements of cerebellar volume should also be obtained to investigate possible relationships between changes in BOLD signals and gray matter atrophy. In addition to ALSFRS-R, the collection of neurocognitive clinical measures should be considered (Beeldman et al., 2021). Ultimately, larger cohorts and longitudinal studies at ultra-high field are necessary in various genetic subgroups and phenotypes of UMN-predominant motor neuron diseases to better understand the complex functional connectivity architecture from standpoints of both clinical relevance and tracking disease progression. Results from such studies may have a broader impact to inform the design of fMRI studies to better understand the involvement of various neural systems in other neurodegenerative diseases – including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, frontotemporal dementia, and PLS – where the cerebellum is thought to be affected under the shadows of other key motor and cognitive neural pathways (Mormina et al., 2017, Gellersen et al., 2017, Tu et al., 2019).
5. Conclusions
This pilot study acquired 7 Tesla resting state fMRI data in healthy controls and participants with ALS. An exploratory whole-brain analysis revealed a disruption in functional connectivity between the superior sensorimotor cortex (in the precentral gyrus) and bilateral cerebellar lobule VI. Post hoc analyses using atlas-derived left and right cerebellar lobule VI revealed decreased functional connectivity in ALS participants that predominantly mapped to bilateral postcentral and precentral gyri. Cerebellar lobule VI is associated with a wide range of key functions including complex motor and cognitive processing tasks. These findings add to the growing number of ALS reports implicating the cerebellum, and future studies are required with larger cohorts and known genetic status for all ALS participants.
CRediT authorship contribution statement
Robert L. Barry: Formal analysis, Data curation, Visualization, Writing - original draft, Wrifting %E2%80%93 review %26 editing, Funding acquisition. Suma Babu: Formal analysis, Data curation, Visualization, Writing - original draft, Writing - review & editing. Sheeba Arnold Anteraper: Formal analysis, Visualization, Writing - review & editing. Christina Triantafyllou: Methodology, Writing - review & editing. Boris Keil: Resources, Writing - review & editing. Olivia E. Rowe: Writing - original draft, Writing - review & editing. D. Rangaprakash: Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Sabrina Paganoni: Investigation, Writing - review & editing. Robert Lawson: Investigation, Writing - review & editing. Christina Dheel: Investigation, Writing - review & editing. Paul M. Cernasov: Investigation, Writing - review & editing. Bruce R. Rosen: Conceptualization, Writing - review & editing, Funding acquisition. Eva-Maria Ratai: Conceptualization, Investigation, Writing - review & editing, Funding acquisition. Nazem Atassi: Conceptualization, Investigation, Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
Christina Triantafyllou, PhD, is currently employed by Siemens Healthineers. Nazem Atassi, MD, PhD, is currently employed by Sanofi Genzyme. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the generosity of our participants and their families. We also thank Dr. Alfonso Nieto-Castañón for helpful discussions on data preprocessing using the CONN toolbox. Imaging was performed at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital using resources provided by the Center for Functional Neuroimaging Technologies (P41EB015896) and the Center for Mesoscale Mapping (P41EB030006), Biotechnology Resource Grants supported by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (NIH). The NIH also provided support through grants R00EB016689 and R01EB027779 (R.L.B.) and K23NS083715 (N.A). This research was also supported in part by the Harvard NeuroDiscovery Center, the Muscular Dystrophy Association, and the American Academy of Neurology (N.A.), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.nicl.2021.102648.
Contributor Information
Robert L. Barry, Email: Robert.Barry@mgh.Harvard.edu.
Suma Babu, Email: sbabu@mgh.Harvard.edu.
Supplementary data
The following are the Supplementary data to this article:
References
- Abidi M., de Marco G., Couillandre A., Feron M., Mseddi E., Termoz N. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur. J. Neurol. 2020;27:121–128. doi: 10.1111/ene.14042. [DOI] [PubMed] [Google Scholar]
- Abrahams S., Goldstein L.H., Kew J.J., Brooks D.J., Lloyd C.M., Frith C.D. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119(Pt 6):2105–2120. doi: 10.1093/brain/119.6.2105. [DOI] [PubMed] [Google Scholar]
- Abrahams S., Leigh P.N., Goldstein L.H. Cognitive change in ALS: a prospective study. Neurology. 2005;64:1222–1226. doi: 10.1212/01.WNL.0000156519.41681.27. [DOI] [PubMed] [Google Scholar]
- Agosta F., Pagani E., Petrolini M., Sormani M.P., Caputo D., Perini M. MRI predictors of long-term evolution in amyotrophic lateral sclerosis. Eur. J. Neurosci. 2010;32:1490–1496. doi: 10.1111/j.1460-9568.2010.07445.x. [DOI] [PubMed] [Google Scholar]
- Agosta F., Chiò A., Cosottini M., De Stefano N., Falini A., Mascalchi M. The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR Am. J. Neuroradiol. 2010;31:1769–1777. doi: 10.3174/ajnr.A2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Valsasina P., Absinta M., Riva N., Sala S., Prelle A. Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb Cortex. 2011;21:2291–2298. doi: 10.1093/cercor/bhr002. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A., Hardiman O., Kiernan M.C., Chiò A., Rix-Brooks B., van den Berg L.H. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016;15:1182–1194. doi: 10.1016/S1474-4422(16)30199-5. [DOI] [PubMed] [Google Scholar]
- Atassi N., Xu M., Triantafyllou C., Keil B., Lawson R., Cernasov P. Ultra high-field (7tesla) magnetic resonance spectroscopy in amyotrophic lateral sclerosis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R.L., Vannesjo S.J., By S., Gore J.C., Smith S.A. Spinal cord MRI at 7T. Neuroimage. 2018;168:437–451. doi: 10.1016/j.neuroimage.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P., Hardiman O. Lessons of ALS imaging: pitfalls and future directions – a critical review. Neuroimage Clin. 2014;4:436–443. doi: 10.1016/j.nicl.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P., Hardiman O. Longitudinal structural changes in ALS: a three time-point imaging study of white and gray matter degeneration. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:232–241. doi: 10.1080/21678421.2017.1407795. [DOI] [PubMed] [Google Scholar]
- Bede P., Elamin M., Byrne S., McLaughlin R.L., Kenna K., Vajda A. Patterns of cerebral and cerebellar white matter degeneration in ALS. J. Neurol. Neurosurg. Psychiatry. 2015;86:468–470. doi: 10.1136/jnnp-2014-308172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P., Omer T., Finegan E., Chipika R.H., Iyer P.M., Doherty M.A. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imag. Behav. 2018;12:1696–1707. doi: 10.1007/s11682-018-9837-9. [DOI] [PubMed] [Google Scholar]
- Beeldman, E., Govaarts, R., de Visser, M., van Es, M.A., Pijnenburg, Y.A.L., Schmand, B.A., et al., 2021. Screening for cognition in amyotrophic lateral sclerosis: test characteristics of a new screen. J. Neurol. (in press). [DOI] [PMC free article] [PubMed]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisteiner R., Robinson S., Wurnig M., Hilbert M., Merksa K., Rath J. Clinical fMRI: evidence for a 7T benefit over 3T. Neuroimage. 2011;57:1015–1021. doi: 10.1016/j.neuroimage.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K., Khan M., Beaulieu C., Graham S.J., Briemberg H., Frayne R. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: a multi-center and multi-modal neuroimaging study. Neuroimage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J., Del Tredici K., Toledo J.B., Robinson J.L., Irwin D.J., Grossman M. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B.R. ALS-Plus – where does it begin, where does it end? J. Neurol. Sci. 2014;345:1–2. doi: 10.1016/j.jns.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Bushara K., Khan A., Hershberger J., Wheat J.O., Belden D. Functional magnetic resonance imaging (fMRI) clinical studies in ALS – paradigms, problems and promises. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 2):S23–S32. doi: 10.1080/14660820052415790. [DOI] [PubMed] [Google Scholar]
- Canu E., Agosta F., Riva N., Sala S., Prelle A., Caputo D. The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am. J. Neuroradiol. 2011;32:1307–1314. doi: 10.3174/ajnr.A2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen J.E., Glover G.H. BOLD fractional contribution to resting-state functional connectivity above 0.1 Hz. Neuroimage. 2015;107:207–218. doi: 10.1016/j.neuroimage.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.E., Polimeni J.R., Bollmann S., Glover G.H. On the analysis of rapidly sampled fMRI data. Neuroimage. 2019;188:807–820. doi: 10.1016/j.neuroimage.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenji S., Jha S., Lee D., Brown M., Seres P., Mah D. Investigating default mode and sensorimotor network connectivity in amyotrophic lateral sclerosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong I., Marjańska M., Deelchand D.K., Eberly L.E., Walk D., Öz G. Ultra-high field proton MR spectroscopy in early-stage amyotrophic lateral sclerosis. Neurochem. Res. 2017;42:1833–1844. doi: 10.1007/s11064-017-2248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A., Traynor B.J. Motor neuron disease in, Biomarkers for ALS–in search of the Promised Land. Nat. Rev. Neurol. 2014;2015(11):72–74. doi: 10.1038/nrneurol.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A., Pagani M., Agosta F., Calvo A., Cistaro A., Filippi M. Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol. 2014;13:1228–1240. doi: 10.1016/S1474-4422(14)70167-X. [DOI] [PubMed] [Google Scholar]
- Christidi F., Karavasilis E., Rentzos M., Kelekis N., Evdokimidis I., Bede P. Clinical and radiological markers of extra-motor deficits in amyotrophic lateral sclerosis. Front Neurol. 2018;9:1005. doi: 10.3389/fneur.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Adad J., Zhao W., Keil B., Ratai E.M., Triantafyllou C., Lawson R. 7-T MRI of the spinal cord can detect lateral corticospinal tract abnormality in amyotrophic lateral sclerosis. Muscle Nerve. 2013;47:760–762. doi: 10.1002/mus.23720. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosottini M., Pesaresi I., Piazza S., Diciotti S., Cecchi P., Fabbri S. Structural and functional evaluation of cortical motor areas in amyotrophic lateral sclerosis. Exp. Neurol. 2012;234:169–180. doi: 10.1016/j.expneurol.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Cosottini M., Donatelli G., Costagli M., Caldarazzo Ienco E., Frosini D., Pesaresi I. High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am. J. Neuroradiol. 2016;37:455–461. doi: 10.3174/ajnr.A4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagli M., Donatelli G., Biagi L., Caldarazzo Ienco E., Siciliano G., Tosetti M. Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin. 2016;12:965–969. doi: 10.1016/j.nicl.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Maderwald S., Küper M., Thürling M., Rabe K., Gizewski E.R. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Dockès J., Poldrack R.A., Primet R., Gözükan H., Yarkoni T., Suchanek F. NeuroQuery, comprehensive meta-analysis of human brain mapping. Elife. 2020;9 doi: 10.7554/eLife.53385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatelli G., Ceravolo R., Frosini D., Tosetti M., Bonuccelli U., Cosottini M. Present and future of ultra-high field MRI in neurodegenerative disorders. Curr. Neurol. Neurosci. Rep. 2018;18:31. doi: 10.1007/s11910-018-0841-7. [DOI] [PubMed] [Google Scholar]
- Douaud G., Filippini N., Knight S., Talbot K., Turner M.R. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain. 2011;134:3470–3479. doi: 10.1093/brain/awr279. [DOI] [PubMed] [Google Scholar]
- Dresel C., Castrop F., Haslinger B., Wohlschlaeger A.M., Hennenlotter A., Ceballos-Baumann A.O. The functional neuroanatomy of coordinated orofacial movements: sparse sampling fMRI of whistling. Neuroimage. 2005;28:588–597. doi: 10.1016/j.neuroimage.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Evans A.C., Collins D.L., Mills S.R., Brown E.D., Kelly R.L., Peters T.M. 3D statistical neuroanatomical models from 305 MRI volumes. Proc. IEEE Nucl. Sci. Symp. Med. Imaging Conf. 1993:1813–1817. [Google Scholar]
- Fang X., Zhang Y., Wang Y., Zhang Y., Hu J., Wang J. Disrupted effective connectivity of the sensorimotor network in amyotrophic lateral sclerosis. J. Neurol. 2016;263:508–516. doi: 10.1007/s00415-015-8013-z. [DOI] [PubMed] [Google Scholar]
- Feneberg E., Gray E., Ansorge O., Talbot K., Turner M.R. Towards a TDP-43-based biomarker for ALS and FTLD. Mol. Neurobiol. 2018;55:7789–7801. doi: 10.1007/s12035-018-0947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter M.K., Katipally R., Kim M.P., Schanz O., Stephen M., Danielian L. Impaired corticopontocerebellar tracts underlie pseudobulbar affect in motor neuron disorders. Neurology. 2014;83:620–627. doi: 10.1212/WNL.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Greicius M. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Chiu S., Breeze J.L., Makris N., Lange N., Kennedy D.N. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Gati J.S., Menon R.S., Ugˇurbil K., Rutt B.K. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn. Reson. Med. 1997;38:296–302. doi: 10.1002/mrm.1910380220. [DOI] [PubMed] [Google Scholar]
- Gellersen H.M., Guo C.C., O’Callaghan C., Tan R.H., Sami S., Hornberger M. Cerebellar atrophy in neurodegeneration—a meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2017;88:780–788. doi: 10.1136/jnnp-2017-315607. [DOI] [PubMed] [Google Scholar]
- Gohel S.R., Biswal B.B. Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain Connect. 2015;5:23–34. doi: 10.1089/brain.2013.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L.H., Newsom-Davis I.C., Bryant V., Brammer M., Leigh P.N., Simmons A. Altered patterns of cortical activation in ALS patients during attention and cognitive response inhibition tasks. J. Neurol. 2011;258:2186–2198. doi: 10.1007/s00415-011-6088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Ma L. Functional magnetic resonance imaging study of the brain in patients with amyotrophic lateral sclerosis. Chin. Med. Sci. J. 2006;21:228–233. [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H., Kawato M. Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol. Res. 2009;73:527–544. doi: 10.1007/s00426-009-0235-1. [DOI] [PubMed] [Google Scholar]
- Jelsone-Swain L.M., Fling B.W., Seidler R.D., Hovatter R., Gruis K., Welsh R.C. Reduced interhemispheric functional connectivity in the motor cortex during rest in limb-onset amyotrophic lateral sclerosis. Front. Syst. Neurosci. 2010;4:158. doi: 10.3389/fnsys.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsone-Swain L., Persad C., Burkard D., Welsh R.C. Action processing and mirror neuron function in patients with amyotrophic lateral sclerosis: an fMRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil B., Triantafyllou C., Hamm M., Wald L.L. Design optimization of a 32-channel head coil at 7T. Proc. Int. Soc. Magn. Reson. Med. 2010;18:1493. [Google Scholar]
- Keil C., Prell T., Peschel T., Hartung V., Dengler R., Grosskreutz J. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 2012;13:141. doi: 10.1186/1471-2202-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew J.J.M., Brooks D.J., Passingham R.E., Rothwell J.C., Frackowiak R.S.J., Leigh P.N. Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology. 1994;44:1101–1110. doi: 10.1212/wnl.44.6.1101. [DOI] [PubMed] [Google Scholar]
- Konen C.S., Kleiser R., Seitz R.J., Bremmer F. An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans. Exp. Brain Res. 2005;165:203–216. doi: 10.1007/s00221-005-2289-7. [DOI] [PubMed] [Google Scholar]
- Konrad C., Henningsen H., Bremer J., Mock B., Deppe M., Buchinger C. Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp. Brain Res. 2002;143:51–56. doi: 10.1007/s00221-001-0981-9. [DOI] [PubMed] [Google Scholar]
- Konrad C., Jansen A., Henningsen H., Sommer J., Turski P.A., Brooks B.R. Subcortical reorganization in amyotrophic lateral sclerosis. Exp. Brain Res. 2006;172:361–369. doi: 10.1007/s00221-006-0352-7. [DOI] [PubMed] [Google Scholar]
- Krienen F.M., Buckner R.L. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger G., Kastrup A., Glover G.H. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 2001;45:595–604. doi: 10.1002/mrm.1081. [DOI] [PubMed] [Google Scholar]
- Kwan J.Y., Jeong S.Y., Van Gelderen P., Deng H.X., Quezado M.M., Danielian L.E. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 Tesla MRI and pathology. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd M.E., Bachert P., Meyerspeer M., Moser E., Nagel A.M., Norris D.G. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018;109:1–50. doi: 10.1016/j.pnmrs.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Li H., Chen Y., Li Y., Yin B., Tang W., Yu X. Altered cortical activation during action observation in amyotrophic lateral sclerosis patients: a parametric functional MRI study. Eur. Radiol. 2015;25:2584–2592. doi: 10.1007/s00330-015-3671-x. [DOI] [PubMed] [Google Scholar]
- Lulé D., Diekmann V., Kassubek J., Kurt A., Birbaumer N., Ludolph A.C. Cortical plasticity in amyotrophic lateral sclerosis: motor imagery and function. Neurorehabil. Neural. Repair. 2007;21:518–526. doi: 10.1177/1545968307300698. [DOI] [PubMed] [Google Scholar]
- Lulé D., Diekmann V., Anders S., Kassubek J., Kübler A., Ludolph A.C. Brain responses to emotional stimuli in patients with amyotrophic lateral sclerosis (ALS) J. Neurol. 2007;254:519–527. doi: 10.1007/s00415-006-0409-3. [DOI] [PubMed] [Google Scholar]
- Lulé D., Diekmann V., Müller H.P., Kassubek J., Ludolph A.C., Birbaumer N. Neuroimaging of multimodal sensory stimulation in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2010;81:899–906. doi: 10.1136/jnnp.2009.192260. [DOI] [PubMed] [Google Scholar]
- Mackenzie I.R.A., Frick P., Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014;127:347–357. doi: 10.1007/s00401-013-1232-4. [DOI] [PubMed] [Google Scholar]
- Menke R.A.L., Proudfoot M., Wuu J., Andersen P.M., Talbot K., Benatar M. Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk. J. Neurol. Neurosurg. Psychiatry. 2016;87:580–588. doi: 10.1136/jnnp-2015-311945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke R.A.L., Proudfoot M., Talbot K., Turner M.R. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. Neuroimage Clin. 2018;17:953–961. doi: 10.1016/j.nicl.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R.S., Ogawa S., Tank D.W., Ugˇurbil K. 4 Tesla gradient recalled echo characteristics of photic stimulation-induced signal changes in the human primary visual cortex. Magn. Reson. Med. 1993;30:380–386. doi: 10.1002/mrm.1910300317. [DOI] [PubMed] [Google Scholar]
- Mikl M., Mareček R., Hluštík P., Pavlicová M., Drastich A., Chlebus P. Effects of spatial smoothing on fMRI group inferences. Magn. Reson Imaging. 2008;26:490–503. doi: 10.1016/j.mri.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Moeller S., Yacoub E., Olman C.A., Auerbach E., Strupp J., Harel N. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn. Reson. Med. 2010;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B., Kollewe K., Samii A., Krampfl K., Dengler R., Münte T.F. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 2009;217:147–153. doi: 10.1016/j.expneurol.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Mohammadi B., Kollewe K., Samii A., Dengler R., Münte T.F. Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum Brain Mapp. 2011;32:750–758. doi: 10.1002/hbm.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B., Kollewe K., Cole D.M., Fellbrich A., Heldmann M., Samii A. Amyotrophic lateral sclerosis affects cortical and subcortical activity underlying motor inhibition and action monitoring. Hum Brain Mapp. 2015;36:2878–2889. doi: 10.1002/hbm.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormina E., Petracca M., Bommarito G., Piaggio N., Cocozza S., Inglese M. Cerebellum and neurodegenerative diseases: beyond conventional magnetic resonance imaging. World J. Radiol. 2017;9:371–388. doi: 10.4329/wjr.v9.i10.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.P., Agosta F., Riva N., Spinelli E.G., Comi G., Ludolph A.C. Fast progressive lower motor neuron disease is an ALS variant: a two-centre tract of interest-based MRI data analysis. Neuroimage Clin. 2018;17:145–152. doi: 10.1016/j.nicl.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke M.F., Binkofski F., Buccino G., Posse S., Erdmann C., Kömpf D. Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study. Hum. Brain Mapp. 2004;22:155–164. doi: 10.1002/hbm.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obusez E.C., Lowe M., Oh S.H., Wang I., Bullen J., Ruggieri P. 7T MR of intracranial pathology: preliminary observations and comparisons to 3T and 1.5T. Neuroimage. 2018;168:459–476. doi: 10.1016/j.neuroimage.2016.11.030. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L., Fera F., Tessitore A., Russo A., Cerasa A., Gioia C.M. Dysfunctions within limbic–motor networks in amyotrophic lateral sclerosis. Neurobiol. Aging. 2013;34:2499–2509. doi: 10.1016/j.neurobiolaging.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell T., Grosskreutz J. The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:507–515. doi: 10.3109/21678421.2013.812661. [DOI] [PubMed] [Google Scholar]
- Proudfoot M., Bede P., Turner M.R. Imaging cerebral activity in amyotrophic lateral sclerosis. Front. Neurol. 2019;9:1148. doi: 10.3389/fneur.2018.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T., Zhang Y., Tang X., Liu X., Wang Y., Zhou C. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: a multimodal MRI analysis. Hum Brain Mapp. 2019;40:3464–3474. doi: 10.1002/hbm.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sach M., Winkler G., Glauche V., Liepert J., Heimbach B., Koch M.A. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain. 2004;127:340–350. doi: 10.1093/brain/awh041. [DOI] [PubMed] [Google Scholar]
- Schlerf J.E., Verstynen T.D., Ivry R.B., Spencer R.M.C. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J. Neurophysiol. 2010;103:3330–3336. doi: 10.1152/jn.01117.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld M.A., Tempelmann C., Gaul C., Kühnel G.R., Düzel E., Hopf J.M. Functional motor compensation in amyotrophic lateral sclerosis. J. Neurol. 2005;252:944–952. doi: 10.1007/s00415-005-0787-y. [DOI] [PubMed] [Google Scholar]
- Schraa-Tam C.K.L., van Broekhoven P., van der Geest J.N., Frens M.A., Smits M., van der Lugt A. Cortical and cerebellar activation induced by reflexive and voluntary saccades. Exp. Brain Res. 2009;192:175–187. doi: 10.1007/s00221-008-1569-4. [DOI] [PubMed] [Google Scholar]
- Schulthess I., Gorges M., Müller H.P., Lulé D., Del Tredici K., Ludolph A.C. Functional connectivity changes resemble patterns of pTDP-43 pathology in amyotrophic lateral sclerosis. Sci. Rep. 2016;6:38391. doi: 10.1038/srep38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani V., Ludolph A., Fornai F. The emerging picture of ALS: a multisystem, not only a “motor neuron disease”. Arch. Ital. Biol. 2017;155:153–158. doi: 10.12871/00039829201741. [DOI] [PubMed] [Google Scholar]
- Stanton B.R., Williams V.C., Leigh P.N., Williams S.C.R., Blain C.R.V., Jarosz J.M. Altered cortical activation during a motor task in ALS. Evidence for involvement of central pathways. J. Neurol. 2007;254:1260–1267. doi: 10.1007/s00415-006-0513-4. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography of the human cerebellum. Handb. Clin. Neurol. 2018;154:59–70. doi: 10.1016/B978-0-444-63956-1.00004-7. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Monsurrò M.R., Graziano S., Panza D., Russo A. Subcortical motor plasticity in patients with sporadic ALS: an fMRI study. Brain Res. Bull. 2006;69:489–494. doi: 10.1016/j.brainresbull.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Thivard L., Pradat P.F., Lehéricy S., Lacomblez L., Dormont D., Chiras J. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J. Neurol. Neurosurg. Psychiatry. 2007;78:889–892. doi: 10.1136/jnnp.2006.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou C., Hoge R.D., Krueger G., Wiggins C.J., Potthast A., Wiggins G.C. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Triantafyllou C., Hoge R.D., Wald L.L. Effect of spatial smoothing on physiological noise in high-resolution fMRI. Neuroimage. 2006;32:551–557. doi: 10.1016/j.neuroimage.2006.04.182. [DOI] [PubMed] [Google Scholar]
- Trojsi F., Monsurrò M.R., Esposito F., Tedeschi G. Widespread structural and functional connectivity changes in amyotrophic lateral sclerosis: insights from advanced neuroimaging research. Neural Plast. 2012;2012 doi: 10.1155/2012/473538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojsi F., Caiazzo G., Corbo D., Piccirillo G., Cristillo V., Femiano C. Microstructural changes across different clinical milestones of disease in amyotrophic lateral sclerosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., Menke R.A.L., Talbot K., Kiernan M.C., Turner M.R. Cerebellar tract alterations in PLS and ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:281–284. doi: 10.1080/21678421.2018.1562554. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Agosta F., Bede P., Govind V., Lulé D., Verstraete E. Neuroimaging in amyotrophic lateral sclerosis. Biomark. Med. 2012;6:319–337. doi: 10.2217/bmm.12.26. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Goldacre R., Talbot K., Goldacre M.J. Psychiatric disorders prior to amyotrophic lateral sclerosis. Ann. Neurol. 2016;80:935–938. doi: 10.1002/ana.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P.P., Marx J., Hunsche S., Gawehn J., Vucurevic G., Wicht S. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch. Neurol. 2003;60:965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E., Foerster B.R. Neuroimaging as a new diagnostic modality in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:403–416. doi: 10.1007/s13311-015-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E., van den Heuvel M.P., Veldink J.H., Blanken N., Mandl R.C., Hulshoff Pol H.E. Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E., Biessels G.J., van Den Heuvel M.P., Visser F., Luijten P.R., van Den Berg L.H. No evidence of microbleeds in ALS patients at 7 Tesla MRI. Amyotroph. Lateral Scler. 2010;11:555–557. doi: 10.3109/17482968.2010.513053. [DOI] [PubMed] [Google Scholar]
- Verstraete E., Veldink J.H., Mandl R.C.W., van den Berg L.H., van den Heuvel M.P. Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E., Polders D.L., Mandl R.C.W., Van Den Heuvel M.P., Veldink J.H., Luijten P. Multimodal tract-based analysis in ALS patients at 7T: a specific white matter profile? Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:84–92. doi: 10.3109/21678421.2013.844168. [DOI] [PubMed] [Google Scholar]
- Verstraete E., Turner M.R., Grosskreutz J., Filippi M., Benatar M. attendees of the 4th NiSALS meeting. Mind the gap: the mismatch between clinical and imaging metrics in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:524–529. doi: 10.3109/21678421.2015.1051989. [DOI] [PubMed] [Google Scholar]
- Wang M.D., Gomes J., Cashman N.R., Little J., Krewski D. Intermediate CAG repeat expansion in the ATXN2 gene is a unique genetic risk factor for ALS–a systematic review and meta-analysis of observational studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilke C., Baets J., De Bleecker J.L., Deconinck T., Biskup S., Hayer S.N. Beyond ALS and FTD: the phenotypic spectrum of TBK1 mutations includes PSP-like and cerebellar phenotypes. Neurobiol. Aging. 2018;62:244.e9–244.e13. doi: 10.1016/j.neurobiolaging.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Witiuk K., Fernandez-Ruiz J., McKee R., Alahyane N., Coe B.C., Melanson M. Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J. Neurosci. 2014;34:14260–14271. doi: 10.1523/JNEUROSCI.1111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Wager T.D. Neuroimaging-based biomarker discovery and validation. Pain. 2015;156:1379–1381. doi: 10.1097/j.pain.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Li H., Li C., Yao J.C., Hu J., Wang J. Abnormal cortical-basal ganglia network in amyotrophic lateral sclerosis: a voxel-wise network efficiency analysis. Behav. Brain Res. 2017;333:123–128. doi: 10.1016/j.bbr.2017.06.050. [DOI] [PubMed] [Google Scholar]
- Zhou F., Gong H., Li F., Zhuang Y., Zang Y., Xu R. Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. Neuroreport. 2013;24:657–662. doi: 10.1097/WNR.0b013e328363148c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.