Abstract
In 1982, oral isotretinoin was first licensed as a treatment option for severe recalcitrant cystic acne in the United States. Since its introduction into the pharmaceutical market, several instances of amenorrhea in women of child-bearing age taking isotretinoin have been reported. In each documented instance, amenorrhea spontaneously resolved once the medication was discontinued. The Patient Introductory Brochure for iPLEDGE, the risk management distribution program mandated by the U.S. Food and Drug Administration for isotretinoin, does not currently include menstrual irregularities as a side effect of treatment; thus, patients who experience this side effect may also experience the unnecessary stress of a possible pregnancy, or, if a minor, explaining a lack of menses to their parent/guardian. This review synthesizes the limited literature available on this subject to advocate for more widespread acknowledgment of menstrual irregularities as a side effect of isotretinoin therapy. To perform the literature review, the search was conducted on June 13, 2020 on PubMed using the following search terms: (((isotretinoin) AND (oligomenorrhea))) OR ((isotretinoin) AND (amenorrhea)) OR ((isotretinoin) AND (PCOS)) OR ((isotretinoin) AND (menstrual irregularities)) OR ((isotretinoin) AND (polycystic ovary syndrome)). All years available were included. Articles were excluded if they were not published in English or did not address the topic of menstrual irregularities in the setting of isotretinoin, with or without the presence of polycystic ovary syndrome. A total of six articles met our criteria and are described.
Keywords: Amenorrhea, Oligomenorrhea, Isotretinoin, Women's health, OCPs
Introduction
In 1982, oral isotretinoin was first licensed as a treatment option for severe recalcitrant cystic acne in the United States (McElwee et al., 1991). Since its introduction into the pharmaceutical market, several instances of amenorrhea in women of childbearing age taking isotretinoin have been reported (Cox, 1988, McElwee et al., 1991; Saljoughi et al., 2017). In each documented instance, the amenorrhea spontaneously resolved once the medication was discontinued. The patient introductory brochure for iPLEDGE, the risk management distribution program mandated by the U.S. Food and Drug Administration for isotretinoin, does not currently include menstrual irregularities as a side effect of treatment; thus, patients who experience this side effect may also experience the unnecessary stress of a possible pregnancy, or, if a minor, explaining a lack of menses to their parent/guardian. This review synthesizes the limited literature available on this subject to advocate for more widespread acknowledgment of menstrual irregularities as a side effect of isotretinoin therapy.
Methods
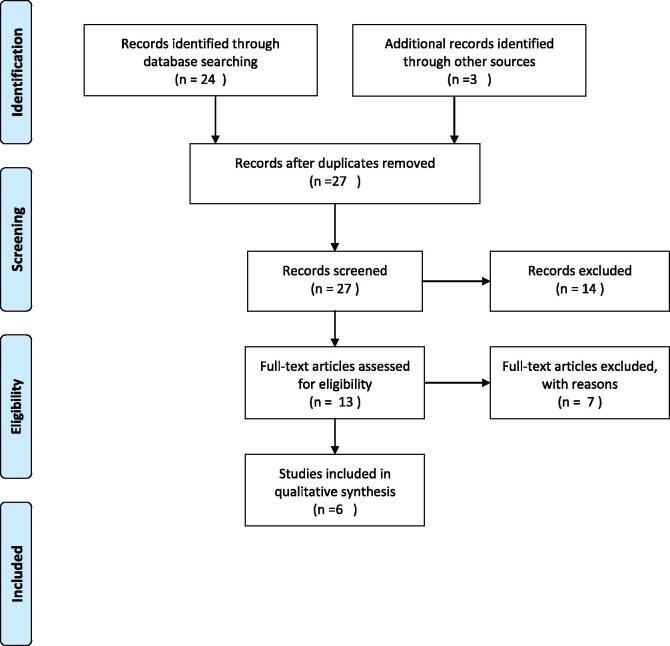
To perform the literature review, a search was conducted on June 13, 2020 on PubMed using the following search terms: (((isotretinoin) AND (oligomenorrhea))) OR ((isotretinoin) AND (amenorrhea)) OR ((isotretinoin) AND (PCOS)) OR ((isotretinoin) AND (menstrual irregularities)) OR ((isotretinoin) AND (polycystic ovary syndrome)). All years available were included. Articles were excluded if they were not published in English or did not address the topic of menstrual irregularities in the setting of isotretinoin, with or without the presence of polycystic ovarian syndrome (PCOS). A total of six articles met our criteria (Fig. 1; Table 1).
Fig. 1.
PRISMA flow diagram.
Table 1.
Characteristics of included journal articles.
| No. of participants in study | No. with menstrual irregularities | PCOS evaluated? | First author, year |
|---|---|---|---|
| 466 | 2 | Not assessed | McElwee et al., 1991 |
| 1 | 1 | Not assessed | Cox, 1988 |
| 5 | 5 | Not assessed | Christmas, 1988 |
| 40 | 8 | Not assessed | Kwon et al., 2007 |
| 50 | 17 | Confirmed; 10% had PCOS | Saljoughi et al., 2017 |
| N/A | N/A | Confirmed; best practice guidelines | Goodman et al., 2015 |
N/A, not applicable; PCOS, polycystic ovarian syndrome.
Results
There are several case reports that document menstrual abnormalities associated with female patients who are not taking oral contraceptive pills (OCPs) to regulate their menstrual cycles. For instance, a 1988 case report by Cox (1988) described a 14-year-old girl who was 10 weeks into a course of isotretinoin when she reported that she had not menstruated during that time frame despite having regular menstruation for the 3 years prior. Ten days after stopping the medication, her menstruation resumed (Cox, 1988). Furthermore, in a 2016 trial of 50 patients started on isotretinoin for acne vulgaris, Saljoughi et al. (2017) reported that 34% experienced a menstruation-related side effect (oligomenorrhea, amenorrhea, or dysmenorrhea) that corresponded with an increase in serum luteinizing hormone (Saljoughi et al., 2017). Additionally, evidence suggests that such cases of amenorrhea are underreported due to the encouraged use of concurrent OCPs while on isotretinoin (Cox, 1988). In a 1991 retrospective study of 466 male and female patients (53.9% male and 46.1% female) on isotretinoin therapy, McElwee et al. (1991) reported that three patients (two male, one female) experienced increased liver enzymes and two female patients experienced amenorrhea. Another case study reported in the Journal of the American Academy of Dermatology demonstrated that a 17-year-old female patient who previously had menstrual cycles lasting 28 days with 5 days of bleeding experienced prolonged menstrual bleeding after initiating isotretinoin. She was taking an OCP. Her bleeding stopped 2 days after isotretinoin was discontinued, but she had another episode of prolonged bleeding after resuming isotretinoin at a later time (Christmas, 1988).
A retrospective chart review of 40 female patients taking isotretinoin found that 8 patients reported new menstrual irregularities. None of the patients were on OCPs, and they presented with normal levels of follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, and prolactin. All eight female patients resumed normal menstrual cycles after discontinuation of the drug. The authors concluded that in younger patients, normal menstrual cycles before and after administration of the drug was suspicious for involvement of isotretinoin in altering the menstrual cycles of the patients (Kwon et al., 2007). Despite the prevalence of amenorrhea in women on isotretinoin that has been demonstrated to stop and start with the use of the medication, it was not listed as a potential side effect of the medication during patient education prior to enrollment in iPLEDGE, Thus, menstrual side effects of the medication continue to cause anxiety and confusion within this population due to a lack of patient education.
The best practice guidelines for PCOS that state “girls with severe acne refractory to treatments including isotretinoin may have a 40% likelihood of developing PCOS” (Goodman et al., 2015). This has led to concern over the differentiation between PCOS and menstrual irregularities secondary to isotretinoin treatment. Although PCOS is clearly a confounding factor in evaluating menstrual irregularities, many of the papers evaluated demonstrated that irregularities began and stopped with isotretinoin treatment, leading authors to the conclusion that the effects may be directly related isotretinoin (Kwon et al., 2007).
Conclusions
The iPLEDGE program is essential in avoiding major maternal-fetal complications associated with isotretinoin use while pregnant, but menstrual irregularities can cause unnecessary anxiety and stress for women taking isotretinoin who are not on OCPs. These women are simply unaware of or uneducated about this as a potential medication side effect. Despite the screening and reporting of other adverse effects with similar incidence rates, amenorrhea continues to be excluded from the list of potential side effects. As of June 2020, the Absorica, 2019, Amnesteem, 2019, and Myorisan package insert (updated 2018) include abnormal menses, but the iPLEGE booklet does not (iPLEDGE, 2016). Thus, we argue that patient care can be improved by including amenorrhea in the list of potential side effects for women of childbearing potential who are planning to take isotretinoin.
Financial disclosures
None.
Funding
None.
Study Approval
N/A.
References
- Absorica (isotretinoin). Highlights of prescribing. Humcao, P. G. P. R., Inc.; 2019.
- Amnesteem (isotretinoin). Package insert. Morgantown, W. M. P. I.; 2019.
- Christmas T. Roaccutane and menorrhagia. J Am Acad Dermatol. 1988;18(3):576–577. doi: 10.1016/s0190-9622(88)80289-5. [DOI] [PubMed] [Google Scholar]
- Cox N.H. Amenorrhoea during treatment with isotretinoin. Br J Dermatol. 1988;118(6):857–858. doi: 10.1111/j.1365-2133.1988.tb02609.x. [DOI] [PubMed] [Google Scholar]
- Goodman N.F., Cobin R.H., Futterweit W., Glueck J.S., Legro R.S., Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–Part 1. Endocr Pract. 2015;21(11):1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- iPLEDGE. Patient introductory brochure. iPLEDGE; 2016.
- Kwon H.J., Lee J.Y., Cho B.K., Park H.J. Menstrual irregularity during isotretinoin treatment. J Eur Acad Dermatol Venereol. 2007;21(4):562–563. doi: 10.1111/j.1468-3083.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- McElwee N.E., Schumacher M.C., Johnson S.C., Weir T.W., Greene S.L., Scotvold M.J. An observational study of isotretinoin recipients treated for acne in a health maintenance organization. Arch Dermatol. 1991;127(3):341–346. [PubMed] [Google Scholar]
- Myorisan (isotretinoin). Package insert. Lake Forest, I. A. P. I.; 2018.
- Saljoughi N., Jebraili R., Yarjanli M., Tehrani S., Ghaedi F. The effects of oral isotretinoin on sex hormones and menstrual cycle in women with severe acne. J Dermatol Cosmet. 2017;7(4):200–205. [Google Scholar]