Highlights
-
•
PP can develop in patients without atopic background.
-
•
Onset: late 2nd to early 3rd trimester; can persist until delivery.
-
•
Patient should be counseled accordingly.
-
•
These findings should be considered when classifying specific pregnancy dermatoses.
Keywords: Prurigo of pregnancy, Specific dermatoses of pregnancy, Atopy, Atopic predisposition, Atopic dermatitis, Atopic eruption of pregnancy
Abstract
Background
Prurigo of pregnancy (PP), a specific pregnancy dermatosis, has been associated with atopic background in the patient and/or the patient’s family. However, this association was not validated in some studies, and cases without atopic background have been reported.
Objective
This study aimed to evaluate the clinical features of PP not related to atopic background and search for comorbid conditions in medical and obstetric records.
Methods
In this case series, patients with typical PP presentation (i.e., pruritic, discrete papulonodules on the extensor surfaces of the extremities) diagnosed at the dermatology clinics of tertiary referral hospitals were evaluated. The exclusion criteria included missing historical data, inadequate follow-up, laboratory test results suggestive of other pruritic conditions, history of atopic disease, and family history of atopy. Clinical and laboratory data including course, response to treatment, serum total immunoglobulin E level, and comorbidities in the medical and obstetric history were collected.
Results
Twenty patients developed PP predominantly during the late second or early third trimester. Nine patients reported that itching developed first, versus 10 patients who reported that lesions started first (one patient was uncertain about onset). There was no recurrence postpartum (mean postpartum follow-up, 17 months). Serum total immunoglobulin E level was elevated in 3 of 14 patients tested (21.4%). Obstetric history (n = 12) included polymorphic eruption of pregnancy (16.6%), PP (16.6%), and gestational pruritus (8.3%). Two of 20 patients (10%) had a history of pruritic skin condition (prurigo nodularis and pruritus of unclear etiology) while not pregnant. Medical history (n = 20) included psychiatric disease (20%) (obsessive compulsive disorder and anxiety), hypothyroidism (10%), and obesity (10%).
Conclusion
PP can develop in patients without an atopic background. This finding should be considered when classifying specific pregnancy dermatoses. A thorough medical and family history with a focus on atopy should be obtained from every patient with a gestational eruption, and patients should be counseled accordingly.
Introduction
Prurigo of pregnancy (PP) affects approximately 1 in 300 to 1 in 450 pregnancies (Kroumpouzos and Cohen, 2001, Roger et al., 1994, Roth et al., 2016). A higher incidence, often surpassing that of polymorphic eruption of pregnancy (PEP), has been reported in studies performed in India (Chander et al., 2011, Hassan et al., 2015, Kannambal and Tharini, 2017). PP encompasses entities that have been historically reported as Besnier’s prurigo gestationis, early onset prurigo, and papular dermatitis of pregnancy described by Spangler (reviewed by Roth et al., 2016). PP starts in mid-pregnancy, at approximately 25 to 30 weeks’ gestation, although onset in other trimesters has been reported (Roth et al., 2016). The lesions are grouped, extremely pruritic, discrete, erythematous papulonodules distributed over the extensor surfaces of the extremities and dorsa of hands and feet and occasionally the abdomen and other sites. The eruption may become generalized. Histopathologic findings are nonspecific, and there are no diagnostic laboratory abnormalities. PP is not associated with adverse fetal outcomes (Kroumpouzos and Cohen, 2001, Kroumpouzos and Cohen, 2003).
Holmes and Black (1983) suggested that PP may result from pruritus gravidarum (defined by the authors as pruritus during pregnancy) in patients with an atopic diathesis. A prospective study showed an association with personal or family history of atopic dermatitis (AD; Vaughan Jones et al., 1999). A subsequent retrospective study indicated an association with atopy in most patients with PP and classified PP under the new concept of atopic eruption of pregnancy (AEP; Ambros-Rudolph et al., 2006). Nevertheless, the largest prospective study on pregnancy dermatoses to date did not confirm an association with atopy (Roger et al., 1994), and an Indian study found a history of atopy in only 3 of 27 patients with PP (Chander et al., 2011). Furthermore, there has been no history of AD or atopic background (defined as personal or family history of atopy) in some patients with PP (Ambros-Rudolph et al., 2006, Hassan et al., 2015, Kroumpouzos and Cohen, 2013, Roger et al., 1994, Roth et al., 2016).
In this series, we included PP cases without an atopic background, studied their clinical features, and searched for comorbid conditions in medical and obstetric records.
Methods
Patients with a typical clinical presentation of PP (i.e., pruritic, discrete papulonodules on the extensor surfaces of the extremities) and photographic documentation of the lesions were included in this series. Ten cases were collected prospectively from January 2018 to January 2020, and the remaining 10 were collected retrospectively. The course of PP during gestation and the postpartum period and response to treatment were documented. Patients’ perception at the time of the first dermatology visit as to which developed first, itching or lesions, was reviewed. Pathology and serum total immunoglobulin E (IgE) levels were reviewed when available.
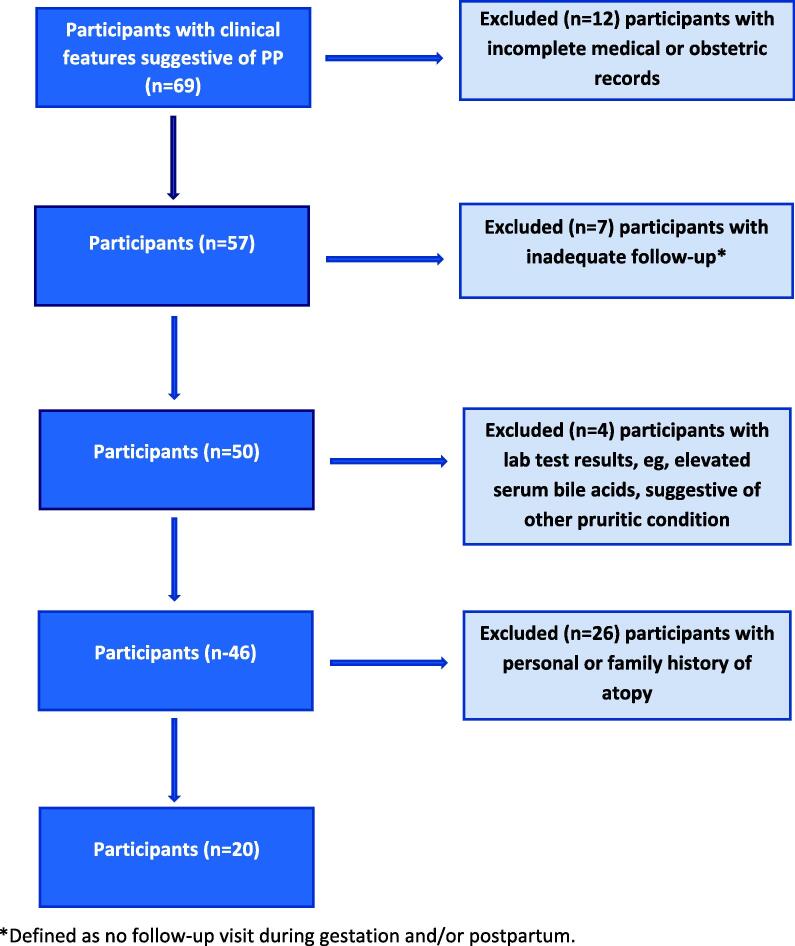
The exclusion criteria included missing data (e.g., incomplete medical or obstetric records), inadequate follow-up (i.e., no follow-up appointment during pregnancy and/or the postpartum period), laboratory test results (e.g., elevated serum bile acid; range, 4.5–19.2 μmol/L) suggestive of other pruritic conditions, a history of atopic disease (i.e., hay fever, allergic rhinitis, AD), and a family history of atopy. Twenty-six patients with a personal and/or family history of atopy were excluded. A flow chart describing participant selection is shown in Fig. 1. Elevated serum total IgE level was not included in the exclusion criteria because IgE is not part of the definition of atopic background and has not been systematically studied in gestational eruptions (Koutroulis et al., 2011). Of note, patients with the features described (nonflexural eruption, absence of personal/family history of atopy) would not satisfy the standard sets of atopy criteria, such as those by Hanifin and Rajka (1980) even if IgE elevation were present (Koutroulis et al., 2011).
Fig. 1.
Flow chart showing participant selection.
Medical history was reviewed also for other skin disease, diabetes, obesity, thyroid and liver disease, neurologic and psychiatric disease, and allergies. Obstetric history was reviewed for parity, multiple gestation pregnancy, adverse fetal outcome, hypertension, cholestasis, and gestational diabetes. Fetal outcome was documented. We also reviewed the serologic work-up that was done to rule out other pruritic skin conditions, including gliadin, transglutaminase, thyroid-stimulating hormone, antinuclear antibody, ferritin, and, when indicated, enzyme-linked immunosorbent assay for bullous pemphigoid antigen 2 (BP180).
Results
Patient characteristics
Twenty patients (age: 21–43 years; median: 30.5 years) are included (clinical and laboratory data are summarized in Table 1). Seventeen patients were Caucasian and three patients were Latin American. Patients lived in the United States, Brazil, and Europe. Skin phototypes I, II, III, and IV were observed in four, six, seven, and three patients, respectively. Clinical features were typical of PP (Fig. 2, Fig. 3). All extremities were affected in 19 (95%), the upper extremities in 1 (5%), and the trunk in 13 (65%) of 20 patients. None of the patients showed associated features (minor criteria) of AD, such as xerosis, keratosis pilaris, ichthyosis vulgaris, palmoplantar hyperlinearity, nipple eczema, and ocular/periocular changes (Hanifin and Rajka, 1980). Serum total IgE level (n = 14) was elevated in three patients (21.4%). There were no adverse fetal outcomes, the pregnancies were full term, and babies were healthy.
Table 1.
Clinical features of PP participants.
| Patient No./Age, y | No. of previous pregnancies | Gestational wk at PP onset | Lesion Distribution | PP duration, wk | Onset with lesions vs. pruritus | Pruritus/ eruption in obstetric history | Medical history (nonpregnant status) | Serum IgE (IU/ml)† | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1/20 s | 0 | 32 | AE | 8 | P | N/A | Obesity | TNP | OAH, prednisone, topical steroids |
| 2/30 s | 1 | 28 | AE | 4 | P | N | Hypothyroidism | TNP | OAH, topical steroids |
| 3/30 s | 1 | 33 | AE, abdomen | 7* | P | PEP | 142 | OAH, prednisone, augmented betamethasone, emollients | |
| 4/30 s | 2 | 28 | AE, abdomen, back, chest | 8 | L | N | Pruritus of unknown etiology; allergies (no hay fever/allergic rhinitis) | 256 | OAH, topical steroid |
| 5/20 s | 1 | 24 | AE, chest | 6 | L | PEP | OCD | 342 | OAH, benzoyl peroxide |
| 6/20 s | 2 | 16 | AE, abdomen, back, chest | 8 | P | PP | Anxiety | TNP | OAH, prednisone, emollients |
| 7/30 s | 1 | 14 | AE, back, chest | 19 | L | N | Diabetes; seasonal allergies (no hay fever/allergic rhinitis) | 53 | OAH, augmented betamethasone, topical antipruritic medication, NBUVB |
| 8/20 s | 0 | 16 | AE, abdomen, back | 21* | L | N/A | 68 | OAH, prednisone, TAC | |
| 9/40 s | 2 | 29 | AE | 11* | L | N | Hypothyroidism | TNP | None |
| 10/20 s | 1 | 27 | AE | 9 | P | P | TNP | OAH, prednisone, topical steroid | |
| 11/30 s | 0 | 28 | AE | 12* | U | N/A | TNP | OAH, topical steroid | |
| 12/20 s | 0 | 19 | AE, abdomen | 10 | L | N/A | 23 | TAC, antipruritic topical medication | |
| 13/20 s | 0 | 31.5 | AE, abdomen, back | 3 | P | N/A | 99 | TAC | |
| 14/20 s | 0 | 17 | AE, abdomen, back, buttocks | 13 | L | N/A | 41 | OAH, TAC, topical antipruritic medications, emollient | |
| 15/20 s | 0 | 29 | AE, abdomen, back | 11* | L | N/A | Obesity; OCD; prurigo nodularis | 95 | OAH, TAC, other topical steroid |
| 16/30 s | 1 | 19 | AE, back | 12 | P | N | 106 | TAC, other topical steroid | |
| 17/30 s | 1 | 26 | AE, abdomen, back | 8 | P | N | 45 | OAH, augmented betamethasone, topical antipruritic medications, emollient | |
| 18/30 s | 1 | 29 | AE | 9 | L | PP | 55 | OAH, prednisone, topical steroids | |
| 19/20 s | 0 | 20 | UE, shoulders | 11 | P | N/A | Anxiety | 1 | OAH, TAC |
| 20/30 s | 1 | 31 | AE, abdomen | 7 | L | N | 20 | OAH, augmented betamethasone, emollient |
AE, all extremities (extensor surfaces); L, onset with lesions; N, no; N/A, not applicable; NBUVB, narrowband ultraviolet B; OAH, oral antihistamine; OCD, obsessive compulsive disorder; P, onset with pruritus; PEP, polymorphic eruption of pregnancy; PP, prurigo of pregnancy; TAC, triamcinolone acetonide 0.1% cream; TNP, test not performed; U, uncertain; UE, upper extremities; Y, yes.
Suboptimal response to treatment; PP resolved at or immediately after delivery.
Reference range, 0–114 IU/ml.
Fig. 2.
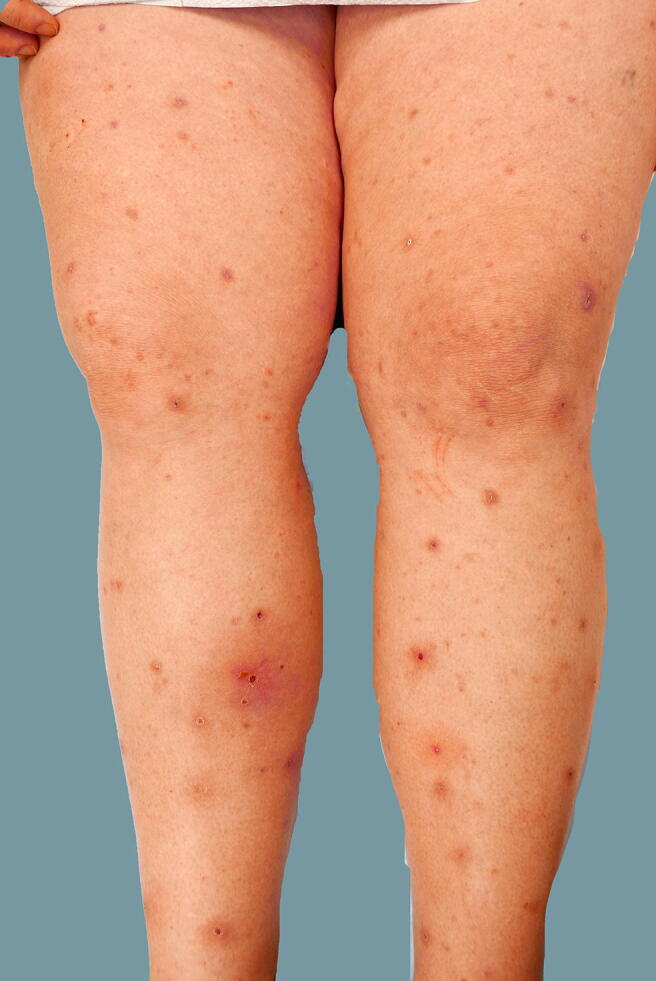
Prurigo of pregnancy: excoriated, erythematous papules on the extensor surfaces of the lower extremities in patient 7. Postinflammatory hyperpigmentation is noted. Patient had a satisfactory response to narrowband ultraviolet light B.
Fig. 3.
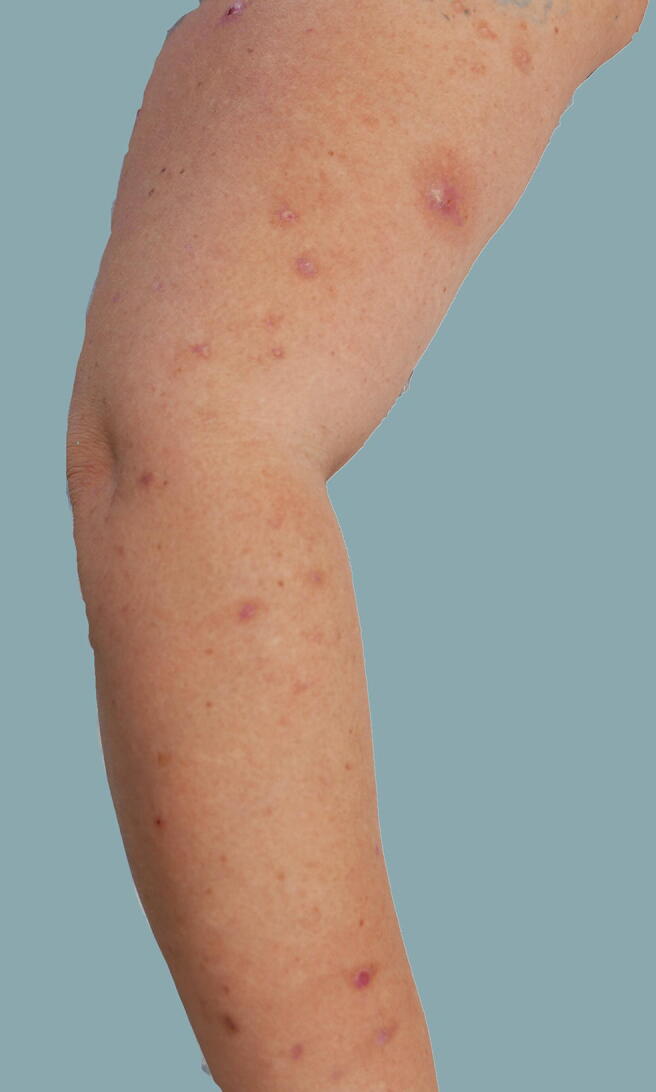
Prurigo of pregnancy: excoriated, scaly, erythematous papules on the extensor surfaces of the upper extremities in patient 7.
Course of eruption
The eruption developed in the late second or early third trimester in most patients (mean gestational age: 24.8 weeks; median gestational age: 27.5 weeks). Nine patients reported that itching developed first versus 10 patients who reported that lesions started first. One patient was uncertain about the onset. In five patients, the eruption responded partially to treatment and resolved at or immediately after delivery (Table 1). In the remaining 15 patients, there was a satisfactory response to treatment, and PP faded prior to delivery. Six patients were treated with oral prednisone because they did not respond satisfactorily to topical steroids and oral antihistamines. Prednisone at a dose of 20 to 30 mg/day for up to 2 weeks was effective. One patient was treated successfully with narrowband ultraviolet light B (Fig. 2, Fig. 3). There was no recurrence of the eruption in the postpartum period (mean postpartum follow-up: 17 months).
Historical data
Obstetric history (n = 12) included PEP (n = 2; 16.6%), PP (n = 2; 16.6%), and gestational pruritus (n = 1; 8.3%). A review of skin history during nonpregnancy (n = 20) revealed pruritic skin conditions in two patients (10%): single cases of prurigo nodularis and pruritus of unclear etiology (Table 1). A review of medical history (n = 20) showed four cases of psychiatric disorder (20%; obsessive compulsive disorder and anxiety), two cases of hypothyroidism (10%), two cases of obesity (10%), and single cases of diabetes, allergies, and seasonal allergies (not associated with hay fever/allergic rhinitis or asthma; negative allergy testing for foods and aeroallergens; Table 1).
Discussion
PP is a clinical diagnosis because it lacks specific histopathologic and laboratory findings (Kroumpouzos and Cohen, 2001, Roth et al., 2016). Its pathophysiology has been elusive, with some authors advocating that PP could develop secondary to scratching in response to pruritus gravidarum (gestational itch; Ingber, 2009, Roger et al., 1994). Associations with obstetric cholestasis or a family history of cholestasis have been reported (Cicek et al., 2007). Other authors have suggested that PP may result from pruritus gravidarum in patients with an atopic diathesis (Holmes and Black, 1983). A prospective study showed an association with a personal or family history of atopy (4 of 12 patients) and elevation of total serum IgE (5 of 12 patients; uncontrolled; Vaughan Jones et al., 1999).
A subsequent retrospective study classified PP under AEP (Ambros-Rudolph et al., 2006), although 4 of 49 patients with PP in the study did not satisfy the eczema-in-pregnancy criterion (mainly flexural eruption and/or personal or family history of atopy and/or serum total IgE elevation) set forth by the group and fulfilled only minor criteria of atopy. The inclusion of uncontrolled serum total IgE elevations as a diagnostic criterion of AEP has been challenged because a patient with eruption and no personal/family history of atopy could be classified under AEP solely on the basis of an elevated total serum IgE level (Koutroulis et al., 2011). These patients would not satisfy standard sets of atopy criteria (Hanifin and Rajka, 1980). Of note, IgE elevations have been noted in other gestational dermatoses, such as PEP (28% of patients; Rudolph et al., 2006). Still, the largest prospective study on pregnancy dermatoses to date did not confirm an association of PP with atopy (Roger et al., 1994). Furthermore, an Indian study found a history of atopy in only 11.1% of patients with PP (Chander et al., 2011).
Herein, we report 20 patients with typical clinical features of PP who did not have an atopic background. Our series did not include patients with darker skin (i.e., phototypes V and VI), which has been associated with papular eczema, a form of AD. This further supports the absence of atopy in our patients. The eruption onset during late pregnancy (median: 27.5 weeks) in this series is in accordance with original PP descriptions (25–30 weeks’ gestation; Roth et al., 2016) and other studies (Chander et al., 2011) and noted at a later point in gestation than the onset of AEP (mean: 18 weeks; Ambros-Rudolph et al., 2006). There were no postpartum recurrences, which is consistent with the absence of an atopic background in the patients. Interestingly, nine patients reported that itching started first, which indicates that itching may trigger the lesions in a percentage of patients with PP. Two of 12 multiparous patients (16.6%) had PEP during previous pregnancies. Two of 12 multiparous patients (16.6%) in this series, compared with 34% of AEP patients (Ambros-Rudolph et al., 2006), had a history of similar eruptions during previous pregnancies.
Patients 4 and 15 (Table 1) had a history of pruritic conditions in while not pregnant, but the features of their gestational (PP) eruption were different, which supports the diagnosis of PP and not a flare of a preexisting condition during gestation. Patient 4 had a history of generalized pruritus of unclear etiology without primary skin lesions while not pregnant that was difficult to manage. She indicated that lesions started before itching during gestation and there was a satisfactory response to topical therapy. Patient 15 had history of difficult-to-control prurigo nodularis that manifested with large nodular lesions while not pregnant. However, her gestational (PP) eruption showed a different morphology and distribution (papular lesions, distributed also in areas not previously affected, such as the abdomen), with a good response to treatment and resolution in the postpartum period.
Serum total IgE level elevation was noted in 3 of 14 tested patients (21.4%) in our series. Serum total IgE elevation has been reported in three patients with PP and a negative history of atopy (Vaughan Jones et al., 1999). As indicated by our group, serum total IgE elevations have not been systematically studied in gestational eruptions (Koutroulis et al., 2011). The regulation of IgE in the distinct type 2 T helper cell (Th2)-skewed immunologic environment of pregnancy has not been adequately studied; one needs to clarify whether stimuli such as itching/scratching may affect IgE levels. A recent observation linked Th2 polarization to IgE elevation in a group of elderly patients with chronic pruritus who had no history of atopy (Xu et al., 2016). In an analogous manner, the Th2 shift that occurs during gestation could be related to itching that may trigger the development of lesions through scratching in the absence of an atopic background.
Further research in the field will help elucidate pathways of itching during gestation. The presence of psychiatric comorbidities among patients with PP (20% in this series) requires further investigation. Although a significant percentage of PP cases show an association with atopic background, the group is likely heterogeneous and multiple etiopathogenetic pathways may be involved (Ingber, 2009, Shornick, 1998). As the present series indicates, the designation PP is preferred over AEP for patients without an atopic background. The AEP label to a PP case in a patient without an atopic history may lead to biased counseling about a patient’s genetic predisposition. We suggest a revision of nomenclature of specific pregnancy dermatoses by a consensus group.
Limitations and strengths
The limitations of our study include its sample size and the retrospective collection of some cases. However, this study went into a higher level of detail regarding comorbidities and historic data than previous PP studies. The strength of this study is its focus on patients with PP without an atopic background, which is a patient population that has not been studied as a group.
Conclusion
This study alerts clinicians that PP may not be related to an atopic background. Such eruption shows typical clinical features, starts in the late second to early third trimester, can persist until delivery, and does not recur postpartum. A thorough medical and family history with a focus on atopy should be obtained from every patient with typical PP presentation, and patients should be counseled accordingly. The absence of an atopic background in a percentage of patients with PP should be considered when classifying specific pregnancy dermatoses.
Financial disclosures
None.
Funding
None.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Acknowledgments
The authors are thankful to Celia Moraes, MD, for contributing a case to this series and being helpful with data collection.
References
- Ambros-Rudolph C.M., Müllegger R.R., Vaughan-Jones S.A., Kerl H., Black M.M. The specific dermatoses of pregnancy revisited and reclassified: Results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395–404. doi: 10.1016/j.jaad.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Chander R., Garg T., Kakkar S., Jain A. Specific pregnancy dermatoses in 1430 females from Northern India. J Dermatol Case Rep. 2011;5:69–73. doi: 10.3315/jdcr.2011.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicek D., Kandi B., Demir B., Turgut D. Intrahepatic cholestasis occurring with prurigo of pregnancy. Skinmed. 2007;6:298–301. doi: 10.1111/j.1540-9740.2007.06387.x. [DOI] [PubMed] [Google Scholar]
- Hanifin J.M., Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92:S44–S47. [Google Scholar]
- Hassan I., Bashir S., Taing S. A clinical study of the skin changes in pregnancy in Kashmir Valley of North India: A hospital based study. Indian J Dermatol. 2015;60:28–32. doi: 10.4103/0019-5154.147782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R.C., Black M. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8:405–412. doi: 10.1016/s0190-9622(83)70046-0. [DOI] [PubMed] [Google Scholar]
- Ingber A. Prurigo of pregnancy. In: Ingber A., editor. Obstetric Dermatology. Springer-Verlag; Berlin Heidelberg: 2009. pp. 151–156. [Google Scholar]
- Kannambal K., Tharini G.K. A screening study on dermatoses in pregnancy. J Clin Diagn Res. 2017;11 doi: 10.7860/JCDR/2017/27207.9907. WC01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutroulis I., Papoutsis J., Kroumpouzos G. Atopic dermatitis in pregnancy: Current status and challenges. Obstet Gynecol Surv. 2011;66:654–663. doi: 10.1097/OGX.0b013e31823a0908. [DOI] [PubMed] [Google Scholar]
- Kroumpouzos G., Cohen L.M. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1–19. doi: 10.1067/mjd.2001.114595. [DOI] [PubMed] [Google Scholar]
- Kroumpouzos G., Cohen L.M. Prurigo, pruritic folliculitis, and atopic eruption of pregnancy. In: Kroumpouzos G., editor. Text Atlas of Obstetric Dermatology. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 205–217. [Google Scholar]
- Kroumpouzos G., Cohen L.M. Specific dermatoses of pregnancy: An evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083–1092. doi: 10.1067/mob.2003.129. [DOI] [PubMed] [Google Scholar]
- Roger D., Vaillant L., Fignon A., Pierre F., Bacq Y., Brechot J.F. Specific pruritic dermatoses of pregnancy. A prospective study of 3192 women. Arch Dermatol. 1994;130:734–739. [PubMed] [Google Scholar]
- Roth M.M., Cristodor P., Kroumpouzos G. Prurigo, pruritic folliculitis, and atopic eruption of pregnancy: Facts and controversies. Clin Dermatol. 2016;34:392–400. doi: 10.1016/j.clindermatol.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Rudolph X.M., Al-Fares S., Vaughan-Jones S.A., Müllegger R.R., Kerl H., Black M.M. Polymorphic eruption of pregnancy: Clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154:54–60. doi: 10.1111/j.1365-2133.2005.06856.x. [DOI] [PubMed] [Google Scholar]
- Shornick J.K. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172–181. doi: 10.1016/s1085-5629(98)80011-4. [DOI] [PubMed] [Google Scholar]
- Vaughan Jones S.A., Hern S., Nelson-Piercy C., Seed P.T., Black M.M. A prospective study of 200 women with dermatoses of pregnancy correlating the clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141:71–81. doi: 10.1046/j.1365-2133.1999.02923.x. [DOI] [PubMed] [Google Scholar]
- Xu A.Z., Tripathi S.V., Kau A.L., Schaffer A., Kim B.S. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J Am Acad Dermatol. 2016;74:1017–1020. doi: 10.1016/j.jaad.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]