Abstract
Breast-conserving surgery with adjuvant radiation therapy has become the standard of care for women with early stage breast cancer, and as a result, a large number of patients are affected by the cutaneous sequelae of radiation therapy. These dermatologic toxicities may present during treatment or years later and can significantly impact patients’ quality of life. In this review, we discuss the clinical presentation, prevention, and management of radiation-induced cutaneous toxicities in women with breast cancer, including radiation dermatitis, radiation recall, radiation-induced morphea, radiation-induced fibrosis, and cutaneous malignancies in irradiated skin.
Keywords: Breast cancer, Radiation therapy, Radiation dermatitis, Oncodermatology, Cutaneous toxicities, Radiation-induced morphea
Introduction
Both in the United States and globally, breast cancer is the most frequently diagnosed malignancy in women (Bray et al., 2018, Siegel et al., 2018). Despite its high incidence, breast cancer mortality rates have steadily decreased over the past few decades due to earlier detection from improved screening as well as therapeutic advancements (Buchholz, 2009). Randomized controlled trials have demonstrated that adjuvant radiation therapy (RT) reduces breast cancer recurrence after breast-conserving surgery (Early Breast Cancer Trialists’ Collaborative Group et al., 2014, Fisher et al., 2002). As a result, RT has become a standard-of-care treatment modality for breast cancer (Whelan et al., 2000).
The delivery of ionizing radiation to a tumor induces double-stranded DNA breaks leading to apoptotic cell death (Leventhal and Young, 2017). Because DNA repair mechanisms are more robust in healthy cells compared with malignant cells, RT preferentially targets tumor cells. However, damage may also occur in the healthy tissues through which radiation beams travel once the radiation dose surpasses their DNA repair threshold. The skin is especially sensitive to the toxic effects of radiation due to its high cellular turnover rate. In fact, an estimated 74% to 100% of patients who receive RT for breast cancer will experience cutaneous toxicities (Schnur et al., 2011, Wengström et al., 2001). A range of dermatologic adverse effects may occur as a result of RT and although most develop shortly after treatment, others may be observed years later.
Breast irradiation is typically administered 5 days per week for 5 to 7 weeks with weekend breaks (Schnur et al., 2011). This intense dosing schedule, coupled with radiation toxicities, can significantly disrupt patients’ work, social, and family roles. RT is associated with higher incidences of depression, anxiety, and fatigue in female patients with breast cancer, and dermatologic toxicities from RT have also been shown to negatively impact patients’ quality of life (QoL; Fuzissaki et al., 2019, Lee et al., 2008, Whelan et al., 2000). As a result, it is imperative that women with breast cancer have timely access to dermatologic care should cutaneous toxicities develop.
In this review, we discuss the clinical features and management of radiation-induced dermatologic toxicities in women with breast cancer. These conditions include radiation dermatitis, radiation recall, radiation-induced morphea, radiation-induced fibrosis, and cutaneous carcinogenesis in irradiated skin.
Radiation dermatitis
Radiation dermatitis is the most common adverse effect of RT, observed in 90% of patients with breast cancer who receive RT, with 30% experiencing moderate to severe presentations (Fisher et al., 2000, Yee et al., 2018). Acute radiation dermatitis typically presents within the first 90 days of RT, whereas chronic radiation dermatitis presents months to years later (Singh et al., 2016).
For patients with breast cancer undergoing postmastectomy RT, risk factors for the development of acute radiation dermatitis include smoking, darker skin, higher radiation dose, larger breast size, and higher body mass index (De Langhe et al., 2014, Kole et al., 2017, Yee et al., 2018). Although no association has been demonstrated between acute radiation dermatitis and the later development of chronic radiation dermatitis, risk factors for chronic radiation dermatitis also include higher cumulative radiation dose, higher total volume of irradiation, older age, concurrent chemotherapy or targeted therapy, connective tissue disease, and inflammatory skin disorders such as psoriasis, eczema, and acne (Collette et al., 2008, Hölscher et al., 2006, Hymes et al., 2006, Porock, 2002, Spalek, 2016, Tejwani et al., 2009, Toledano et al., 2006).
Radiation dermatitis can affect patients’ QoL both during and after treatment, and severe cases may result in dose reduction or RT interruption (Leventhal and Young, 2017). Several studies have demonstrated reduced QoL due to the physical burden of radiation dermatitis and its emotional consequences, such as disturbance of body image (Fuzissaki et al., 2019; Rossi et al., 2018, Schnur et al., 2011, Sundaresan et al., 2015). The physical burden of radiation dermatitis can be significant and includes sleep disturbance, burning, and pruritus (Schnur et al., 2011). Additional out-of-pocket costs are also common for women with radiation dermatitis, including the purchase of new undergarments to replace those that are less comfortable or stained by topical treatments, altered clothing choices, makeup or scarves to conceal radiation dermatitis and telangiectasias, as well as soothing ointments, moisturizers, or pillows to reduce discomfort (Rossi et al., 2018, Schnur et al., 2011, Schnur et al., 2012). One study estimated that the mean skin toxicity cost per patient with breast cancer undergoing RT was $131.64 and that this cost negatively impacted the functional domain in QoL metrics (Schnur et al., 2012).
Clinical presentation
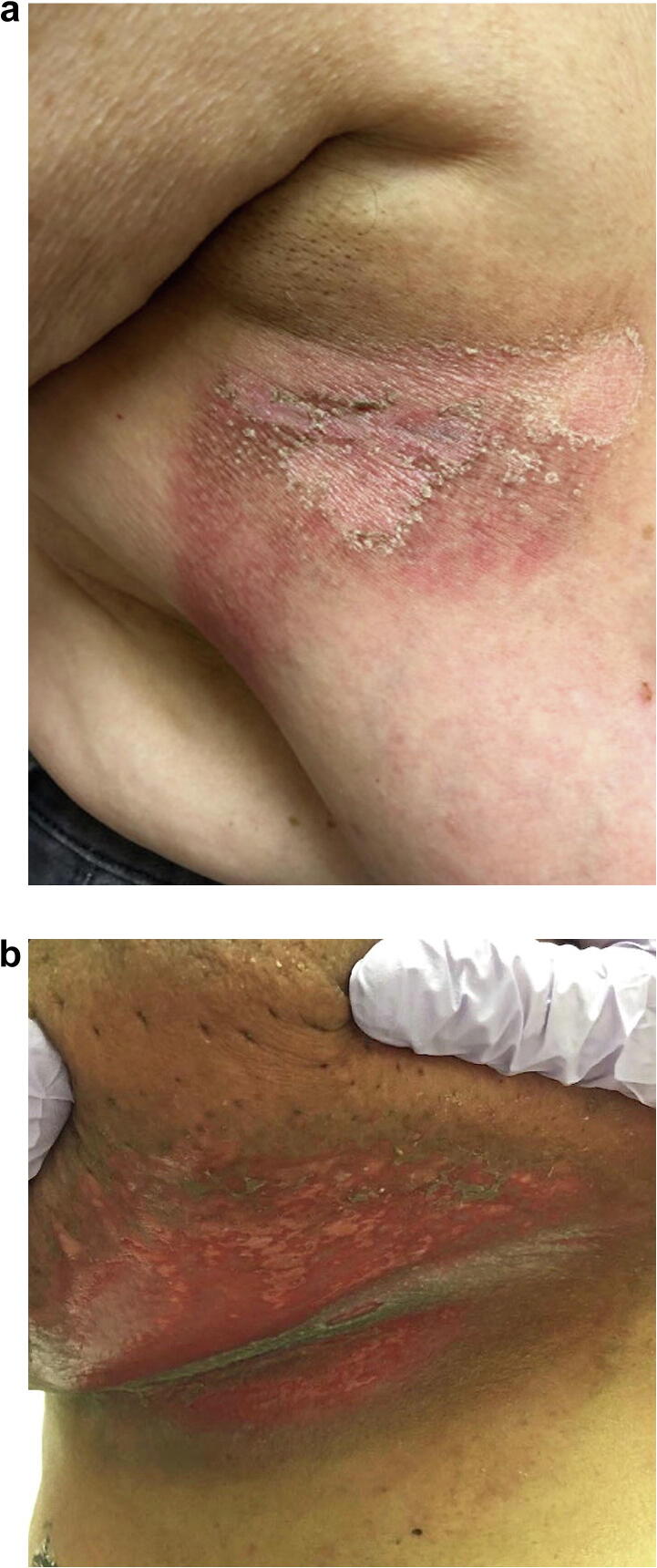
The clinical symptoms of acute radiation dermatitis are partially dependent on the cumulative radiation dose. A transient, faint erythema may occur hours after radiation exposure. Classically, acute radiation dermatitis occurs during the second week of RT and presents as a dry, erythematous patch localized to the field of radiation. After 3 to 4 weeks and high cumulative doses, dry desquamation may occur (Fig. 1A); in severe cases, worsening edema, pruritus, tenderness, moist desquamation (Fig. 1B), and ulceration may ensue (Kole et al., 2017).
Fig. 1.
(A) Acute radiation dermatitis manifesting as erythematous patches with dry desquamation weeks after radiation therapy for breast cancer. (B) Severe presentation of acute radiation dermatitis manifesting as moist desquamation.
Impaired wound healing from irradiation due to inadequate vascularization, tissue hypoxia, and fibrosis can progress to necrosis and skin ulceration, termed radiation necrosis (Bray et al., 2016, Buboltz and Cooper, 2019, Uzun et al., 2013). Radiation necrosis is a late sequela that is estimated to occur in up to 5% of patients (Bray et al., 2016, Buboltz and Cooper, 2019).
Chronic radiation dermatitis encompasses later radiation-induced skin changes, including postinflammatory hyperpigmentation or hypopigmentation, skin atrophy, telangiectasias, and subcutaneous fibrosis. These findings usually present after the completion of RT and are a distinct entity from nonhealing acute radiation dermatitis (Hymes et al., 2006, Singh et al., 2016). Although many manifestations of chronic radiation dermatitis are permanent as a result of significant injury to fibroblasts and the cutaneous microvasculature, transient forms of chronic radiation dermatitis may occur. For example, the peau d’orange appearance of irradiated breast skin that is caused by lymphatic edema often resolves within the first year after therapy, and postinflammatory pigmentary changes may also slowly regress (Hymes et al., 2006).
Grading and management
According to the Common Terminology Criteria for Adverse Events, version 5.0, grade 1 radiation dermatitis consists of faint erythema or dry desquamation, grade 2 consists of moderate erythema with edema and patchy moist desquamation primarily in the skin folds, grade 3 comprises moist desquamation in areas other than skin folds and bleeding by minor trauma, and grade 4 involves full thickness skin necrosis and spontaneous bleeding from the site of radiation (U.S. Department of Health and Human Services, 2017).
Topical corticosteroids have been robustly investigated for the prevention of acute radiation dermatitis (Haruna et al., 2017, Meghrajani et al., 2013). Randomized controlled trials have demonstrated that the prophylactic application of mometasone furoate is superior to topical emollients in reducing the severity of radiation dermatitis and increasing the time to development of grade 3 dermatitis (Hindley et al., 2014, Ho et al., 2018). In one study, using mometasone cream also positively impacted patients’ self-reported QoL scores compared with a topical emollient (Hindley et al., 2014). As a result, treating irradiated skin with mometasone ointment twice daily from the first day of RT to 14 days after completion is often recommended to reduce the severity of acute dermatitis (Ho et al., 2018). Recent studies have also demonstrated that barrier creams and films, as well as melatonin-based creams, may decrease the incidence of radiation dermatitis and moist desquamation and delay the development of radiation dermatitis-associated pruritus (Ben-David et al., 2016, Laffin et al., 2015, Shaw et al., 2015, Zetner et al., 2019). In addition, glutamine supplementation, olive oil/calcium hydroxide emulsions, and many other agents have been shown to reduce the incidence and severity of radiation dermatitis in small studies (Chitapanarux et al., 2019, Eda et al., 2016).
Counseling patients on lifestyle changes to minimize the effects of friction and unnecessary trauma within the radiation field is essential. Examples include wearing loose-fitting clothing; avoiding exposure to sun, extreme heat or cold, and irritating products; using bland emollients; avoiding shaving with straight edge or disposable razors; and using mild soaps. Normal use of deodorants or antiperspirants does not appear to be associated with an increased risk or severity of radiation dermatitis (Leventhal and Young, 2017).
Recent studies have shown that decreasing radiation doses and varying fractionation or delivery methods may reduce the incidence and severity of radiation dermatitis. In particular, the use of hypofractionated RT, intensity modulated RT, accelerated partial breast irradiation, and prone positioning have each resulted in decreased rates of acute radiation dermatitis compared with conventional RT (Buwenge et al., 2017, Freedman et al., 2009, Pignol et al., 2008, Shaitelman et al., 2015, Yee et al., 2018).
Fortunately, most cases of acute radiation dermatitis resolve after treatment is completed. The primary management strategies include keeping the affected area clean and moist with bland emollients or topical corticosteroids, protecting the area from contamination and infection, and managing pain. Application of dressings (hydrogel, hydrocolloid, soft silicone, or silver-based dressings) may reduce mechanical injury to the wound site and promote healing. In the presence of superinfection, antimicrobials such as mupirocin ointment or silver sulfadiazine cream may be indicated. For grade 3 or 4 reactions, brief interruptions of RT along with supportive wound care may be required. Amifostine, zinc, and the combination of oral pentoxifylline and oral vitamin E have been investigated in phase 1 and 2 trials for the treatment of acute radiation dermatitis, with variable success (Jacobson et al., 2013). Other experimental treatments, including autologous fibroblasts, stem cells, and growth factors (e.g., fibroblast growth factors, platelet-derived growth factor, granulocyte and granulocyte–macrophage colony-stimulating factors, and transforming growth factor-beta modulators), are currently being investigated (Haubner et al., 2012, Jacobson et al., 2013). In addition, telangiectasias secondary to chronic radiation dermatitis may be treated with long-pulsed dye lasers (Nymann et al., 2009).
Finally, radiation necrosis may be treated with hyperbaric oxygen therapy, which stimulates angiogenesis (Buboltz and Cooper, 2019, Uzun et al., 2013). Pentoxifylline has also been used with promising results in a small pilot study (Dion et al., 1990). Severe cases of cutaneous radiation necrosis may warrant surgical debridement and/or flap reconstruction of the affected area; grafts have been employed with varying success (Jacobson et al., 2017, Öztürk et al., 2008).
Radiation recall after chemotherapy
Radiation recall dermatitis is a rare and poorly understood phenomenon in which a systemic drug, usually chemotherapy, triggers an inflammatory reaction in previously irradiated skin (Guarneri and Guarneri, 2010). Its incidence is approximately 5% to 10%. In contrast with radiation enhancement, a common phenomenon occurring within 1 week of irradiation and characterized by heightened sensitivity to chemotherapeutic agents, radiation recall reactions occur >1 week after the completion of RT (Bahaj et al., 2019, Burris and Hurtig, 2010, Guarneri and Guarneri, 2010, Melnyk et al., 2012). Importantly, one-third of cases may involve extracutaneous sites, resulting in mucositis, pneumonitis, or esophagitis (Burris and Hurtig, 2010, Camidge and Price, 2001). Interestingly, rechallenge with the offending systemic drug will not always lead to reappearance of radiation recall (Burris and Hurtig, 2010, Haffty et al., 2008, Kodym et al., 2005 Mizumoto et al., 2006, Pardo et al., 2013, Sakaguchi et al., 2018).
The mechanism of radiation recall is not established, and various theories have been proposed, including idiosyncratic hypersensitivity reaction, increased sensitivity of memory stem cells in irradiated skin, and reduced threshold for inflammation in irradiated skin, which is then upregulated by chemotherapy (Camidge and Price, 2001, Melnyk et al., 2012).
Clinical presentation
Radiation recall manifests similarly to radiation dermatitis as an erythematous eruption that may be painful or pruritic, with edema, vesicles, or desquamation (Guarneri and Guarneri, 2010). The reaction ranges from mild erythema to severe skin necrosis (Burris and Hurtig, 2010, Guarneri and Guarneri, 2010). Multiple drugs have been implicated, particularly antimetabolites (gemcitabine, capecitabine, pemetrexed), as well as anthracyclines (doxorubicin) and taxanes (docetaxel, paclitaxel) (Burris and Hurtig, 2010). Gemcitabine, one of the most commonly associated drugs, may result in extracutaneous recall reactions in two-thirds of cases (Burris and Hurtig, 2010). Other oncologic drugs, including antitumor antibiotics (bleomycin, actinomycin, adriamycin), alkylating agents (melphalan), targeted therapies (gefitinib, trastuzumab, bevacizumab, vemurafenib, sorafenib, sunitinib, erlotinib), and endocrine therapy (tamoxifen) have also been associated with radiation recall reactions (Bourgeois et al., 2017, Burris and Hurtig, 2010, Guarneri and Guarneri, 2010, Levy et al., 2013, Mehta et al., 2018, Melnyk et al., 2012, Rhee et al., 2014).
Grading, prevention, and treatment
Radiation recall is graded similarly to radiation dermatitis as described earlier. Given the rare and idiosyncratic nature of this reaction, no preventive interventions currently exist. In mild cases, patients may remain on chemotherapy or try a reduced dose. Symptomatic management with topical steroids, antihistamines, and nonsteroidal antiinflammatory drugs are also commonly used, but these treatments have not been shown to decrease time to resolution (Burris and Hurtig, 2010, Camidge and Price, 2001, Guarneri and Guarneri, 2010).
Severe cases may warrant an interruption of chemotherapy, especially when extracutaneous involvement is present, with reactions subsequently resolving in days to weeks (Burris and Hurtig, 2010). According to one study, systemic steroid prophylaxis upon rechallenge with the causative agent may reduce the inflammatory response, but this strategy has otherwise remained unproven (Camidge and Price, 2001).
Radiation-induced morphea
Radiation-induced morphea (RIM) is an underrecognized sequela of RT that abruptly presents months to years after radiation exposure. The vast majority of cases occur in patients with breast cancer, with an incidence of approximately 1 in 500 patients (Bleasel et al., 1999,Friedman et al., 2018, Fruchter et al., 2017, Leventhal and Young, 2017 Spalek et al., 2015). The disfigurement and pain associated with RIM can substantially affect women’s QoL (Spalek et al., 2015). One recent retrospective study of 25 patients with RIM noted that autoimmune disorders, obesity, smoking history, and breast implantation correlated with more severe presentations (Mittal et al., 2019).
Clinical presentation
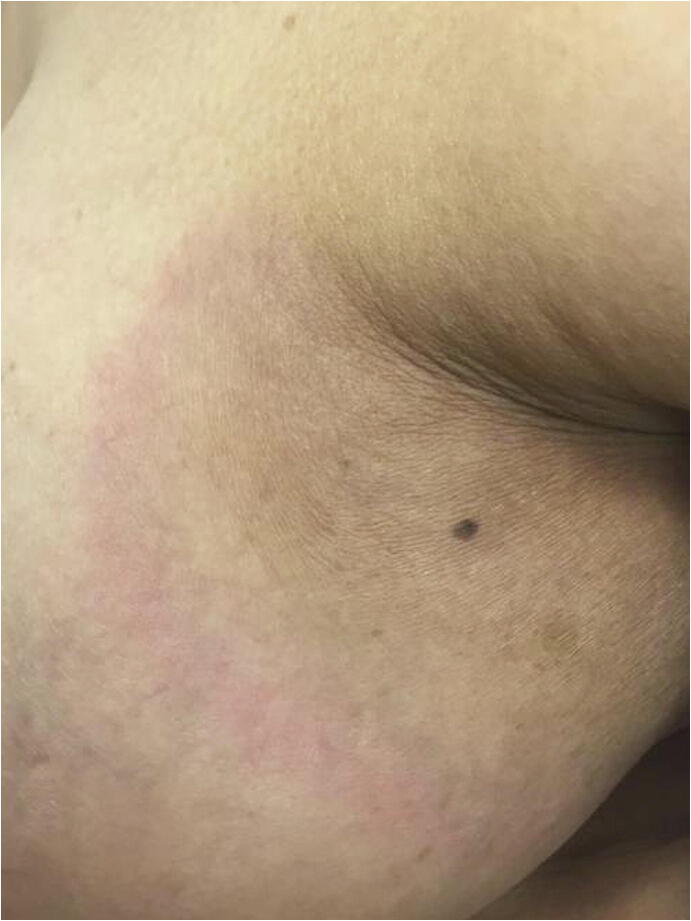
Classically, the presentation of RIM involves two sequential clinical phases. The initial inflammatory phase is characterized by an erythematous and sometimes edematous round plaque at the site of radiation that may mimic cellulitis. Histology demonstrates perivascular and periadnexal lymphocytic infiltrate and slight dermal collagen thickening (Friedman et al., 2018; Spalek et al., 2015). The second phase is the burnout phase, marked by decreased inflammation, the development of fibrosis, and hyperpigmentation (Fig. 2). On histology, the burnout phase shows prominent fibrosis with sclerotized collagen fibers and loss of the initial lymphocytic infiltrate (Spalek et al., 2015).
Fig. 2.
Radiation-induced morphea presenting as a round erythematous plaque with a violaceous border on the lateral breast.
Although RIM tends to manifest as a single round plaque, 50% of lesions may extend beyond the radiation field. Clinically, RIM can be distinguished from radiation dermatitis, which tends to present more diffusely throughout the radiation field and remains localized within its borders. RIM is also a relatively rare phenomenon, occurring in <1% of patients with breast cancer receiving RT, whereas radiation dermatitis occurs in up to 90% of patients with breast cancer (Harper et al., 2004, Leventhal and Young, 2017). In addition, RIM can be distinguished from radiation-induced fibrosis, which develops more gradually and remains localized to the radiation field (Fruchter et al., 2017).
Management
Biopsy is essential to rule out metastatic breast cancer, cellulitis, fat necrosis, and radiation dermatitis. Multiple treatments with varying efficacies have been reported, including topical steroids, calcineurin inhibitors or vitamin D analogs, intralesional steroids, systemic immunomodulatory agents (e.g., prednisone, methotrexate, mycophenolate, tofacitinib, tetracyclines, and acitretin), phototherapy (ultraviolet A and narrowband ultraviolet B), as well as reconstructive surgery for excision of affected skin in refractory cases (Fruchter et al., 2017, Mittal et al., 2019, Spalek et al., 2015). Early intervention in the inflammatory phase correlates with treatment response, whereas the burnout phase often results in irreversible fibrosis. Combination treatment with topicals, systemics, or phototherapy is more likely to induce a clinical response (Cheah et al., 2008,Friedman et al., 2018, Fruchter et al., 2017, Mittal et al., 2019, Newland and Marshman, 2012 Spalek et al., 2015).
Radiation-induced fibrosis
Radiation-induced fibrosis (RIF) is a complication that typically arises within the first 3 months after RT (Schaffer et al., 2000). In contrast to the abrupt onset of RIM, RIF has an earlier yet gradual presentation and often continues to progress over several years (Schaffer et al., 2000, Straub et al., 2015).
Both the incidence and severity of RIF are higher in patients with connective tissue disorders, in particular those with systemic sclerosis or systemic lupus erythematosus (Barnes and Mayes, 2012, Hölscher et al., 2006, Morris and Powell, 1997, Rees et al., 2017, Straub et al., 2015). In a study of patients with breast cancer treated with excisional biopsy and primary RT, breast fibrosis was observed in 23% of patients, and the severity was dependent on daily radiation dose (Clarke et al., 1983). Other risk factors for RIF include high volume of irradiated tissue, an accelerated radiation schedule, and concurrent treatment with chemotherapy (Borger et al., 1994, Geara et al., 1998, Straub et al., 2015).
Clinical presentation
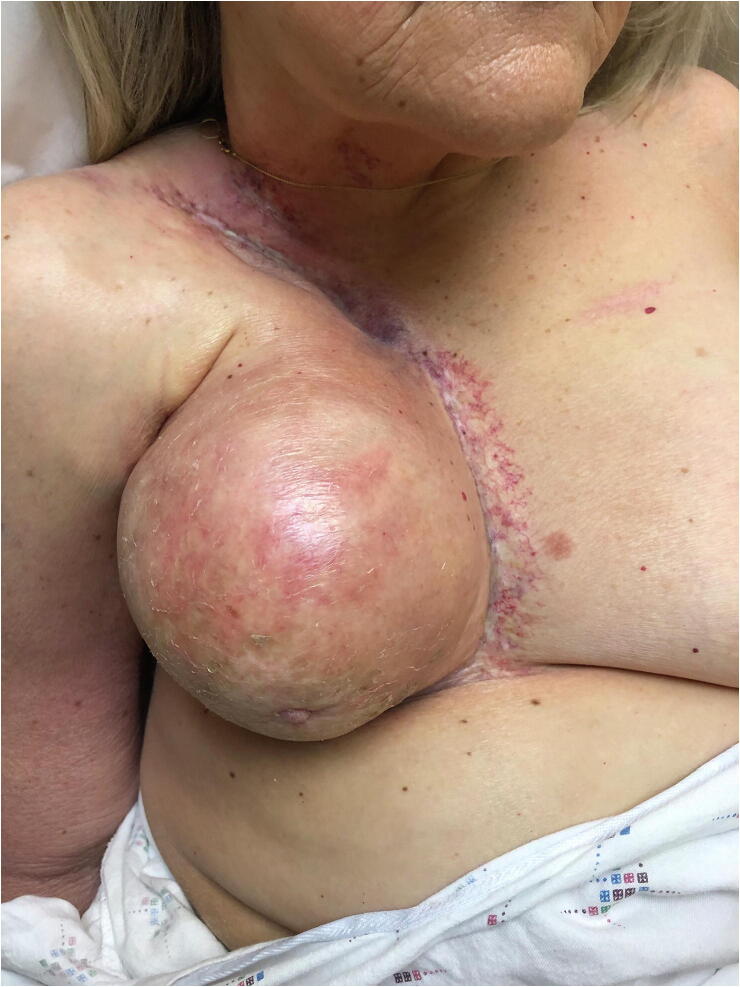
RIF classically presents as fibrosis and scarring of the affected area, and when the breast is involved, contraction and visible deformity may develop (Fig. 3). In addition, RIF is confined to the radiation field and does not extend beyond its borders, unlike RIM (Clarke et al., 1983, Friedman et al., 2018, Schaffer et al., 2000). Importantly, RIF can affect any tissue in the radiation field, including the lungs, gastrointestinal tract, skin, and subcutaneous tissue (Straub et al., 2015). Its consequences range from cosmetic concerns to functionally impairing contractures in the skin and soft tissue (Straub et al., 2015).
Fig. 3.
Radiation-induced fibrosis presenting as marked contracture and breast deformity. Note the surrounding chronic radiation changes, including dyspigmentation and telangiectasias.
Histopathologically, RIF may demonstrate excess collagen deposition, macrophage infiltration in its earlier stages, differentiation of fibroblasts into fibrocytes, and changes in the vascular connective tissue, such as extracellular matrix protein deposition and collagen atrophy (Delanian and Lefaix, 2004, Schaffer et al., 2000).
Prevention and treatment
Prevention of RIF involves adjusting the dose, schedule, and volume of radiation (Straub et al., 2015). With the advent of intensity modulated RT, in which radiation beams are conformed to the shape of the tumor for homogenous dose delivery, decreases in breast induration have been observed (Donovan et al., 2007, Straub et al., 2015). Once RIF occurs, the scarring and contractures are often irreversible, and treatment is aimed at improving functionality. Physical therapy and mechanical massage techniques can improve patients’ range of motion as well as pain and skin induration (Bourgeois et al., 2008, Straub et al., 2015). Pharmacologic interventions, such as the combination of oral vitamin E and oral pentoxifylline, have demonstrated decreased fibrosis in patients with breast cancer and RIF according to some reports (Delanian and Lefaix, 2007, Jacobson et al., 2013). Pirfenidone, an antiproliferative and antifibrotic agent, also improved range of motion in a small pilot study (Simone et al., 2007).
Postradiation cutaneous carcinogenesis
Secondary skin neoplasms are late sequelae of RT and are thought to arise from radiation-induced mutations that drive carcinogenesis (Li and Athar, 2016). Postradiation atypical vascular lesions (AVLs) and cutaneous angiosarcomas are almost exclusively described in the literature as sequelae of chest wall radiation for breast cancer. In addition, nonmelanoma skin cancers (NMSCs), particularly basal cell carcinoma (BCC), followed by squamous cell carcinoma (SCC), make up a substantial proportion of postradiation skin cancers in women with breast cancer (Cuperus et al., 2013, Mattoch et al., 2007).
Vascular proliferations
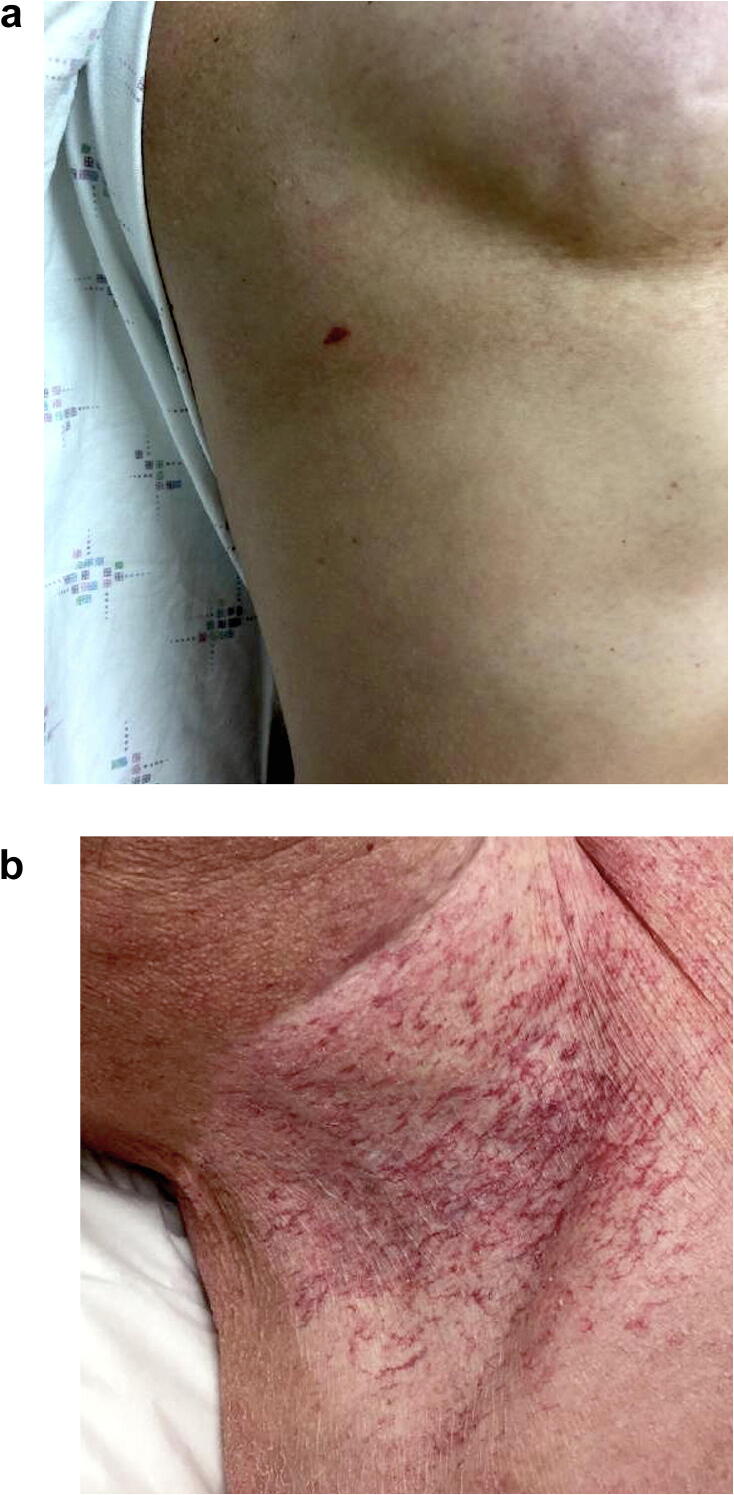
Radiation-induced vascular proliferations include AVLs and angiosarcomas, which are most commonly described in the mammary skin of patients with breast cancer after RT. AVLs are benign vascular proliferations thought to represent the dilation of superficial vascular channels as a result of lymphatic obstruction from radiation or surgery (Ronen et al., 2019). The terms benign lymphangiomatous papules, lymphangiomas, and acquired lymphangiectasias are also used. Classically presenting 3 to 4 years after RT, AVLs can appear as well-circumscribed, red-to-bluish papules or vesicles that are usually <5 mm in diameter (Fig. 4A), which is distinct from the telangiectasias of chronic radiation dermatitis (Fig. 4B; Gengler et al., 2007, Mattoch et al., 2007, Ronen et al., 2019). Histologically, the lesions are generally well circumscribed and located in superficial or mid dermis, where they are composed of dilated or irregular and jagged vascular channels lined by a single layer of bland endothelial cells. The lesions can demonstrate either a predominately lymphangioendothelioma‐like or lymphangioma/lymphangioma circumscriptum‐like growth pattern (Gengler et al., 2007).
Fig. 4.
(A) Atypical vascular lesion presenting as a red papule on an irradiated chest. (B) Radiation-induced telangiectasias localized to the radiation field.
Biopsy is indicated in the management of AVLs to rule out cutaneous metastasis and more aggressive angiosarcomas. Of note, although AVLs are benign, there is clinical and histologic overlap with malignant angiosarcomas (Billings et al., 2004, Mattoch et al., 2007). Most studies demonstrate that AVLs do not progress to angiosarcoma, but rare cases of malignant transformation have been reported (Fraga-Guedes et al., 2014, Patton et al., 2008, Ronen et al., 2019). Resampling is warranted for lesions that recur or rapidly increase in size, and some recommend complete removal with close clinical follow-up to avoid recurrence (Mattoch et al., 2007).
Secondary angiosarcomas from RT are aggressive vascular tumors with a poor prognosis and typically occur 5 to 6 years after RT, although latencies ranging from 1 to 40 years have been reported (Billings et al., 2004, Mattoch et al., 2007, Meattini et al., 2014, Weaver and Billings, 2009). Its incidence in patients with breast cancer has been estimated at 0.1% to 0.3%, but some reports indicate incidences up to 1% in patients who have survived >5 years (Sholl et al., 2017).
Clinically, postirradiation angiosarcoma can present as red–purple plaques or nodules on irradiated skin. In contrast to AVLs, which are typically <5 mm in diameter, angiosarcomas tend to be much larger with an average diameter of 7.5 cm (Ronen et al., 2019, Weaver and Billings, 2009). In addition, angiosarcomas are ecchymotic-appearing, violaceous in color, and more likely to have multiple foci compared with AVLs (Weaver and Billings, 2009).
Diagnosis of angiosarcoma is ultimately made with biopsy and histologic examination, and increased Ki67 and MYC expression can help distinguish angiosarcoma from AVLs (Ronen et al., 2019). Treatment for angiosarcoma is wide local excision, but there is a high rate of local recurrence due its propensity to form satellite lesions (Weaver and Billings, 2009). Several case reports and a single-institution retrospective analysis have demonstrated successful management of primary angiosarcomas with Mohs surgery, but this has not been studied in patients with postirradiation angiosarcoma (Buehler et al., 2014, Bullen et al., 1998). Patients with postirradiation angiosarcoma have a poor overall prognosis with a median survival of 18 months after diagnosis (Weaver and Billings, 2009, Ronen et al., 2019).
Nonmelanoma skin cancer
Patients who have undergone RT are at elevated risk of developing NMSCs within the radiation field, particularly BCCs followed by SCCs, (Karagas et al., 1996, Lichter et al., 2000). Multiple BCCs may present clinically, and the nodular pattern is most frequent histologically, though the fibroepithelioma of Pinkus variant has also been associated with irradiated skin (Cuperus et al, 2013). The average latency period from the time of RT to the development of NMSC was approximated to be at least 20 years (Lichter et al., 2000); however, incubation periods ranging from 2 to >50 years have also been reported (Meibodi et al., 2008). Higher cumulative doses of radiation, exposure of irradiated skin to ultraviolet light, and younger age may be associated with shorter latency periods (Marín-Gutzke et al., 2004, Cuperus et al., 2013).
The management of NMSCs in the setting of RT is complex because these tumors tend to have ill-defined margins and are more aggressive than their ultraviolet-induced counterparts. One study found that SCCs arising in irradiated skin had an associated 5-year survival of 50%, compared with 90% in ultraviolet-associated SCCs (Edwards et al., 1989). Therefore, surgical excision is the recommended treatment, and there is expert consensus that Mohs micrographic surgery is appropriate for ensuring histological clearance in previously irradiated skin, regardless of subtype, size, or depth (Connolly et al., 2012, Karagas et al., 1996). Dermatologists should be cognizant of the increased risk for cutaneous carcinogenesis in patients with a history of RT, and routine total body skin examinations may allow for prompt diagnosis and treatment.
Conclusion
The dermatologic sequelae of RT in women with breast cancer are diverse and can significantly impact patients’ QoL. RT-associated cutaneous toxicities range from acute dermatitis, which develops shortly after treatment initiation, to chronic radiation dermatitis, RIF, RIM, and cutaneous carcinogenesis, which may arise years later. As the number of breast cancer survivors grows each year, dermatologists will likely encounter women who have received RT with dermatologic adverse effects. Familiarity with the range of cutaneous toxicities will allow for prompt diagnosis and management. A collaborative and multidisciplinary approach among dermatologists, radiation oncologists, and medical oncologists is critical for the overall care of women with breast cancer.
Conflicts of Interest
None.
Funding
This investigation was supported by NIH Research Grant P30CA016359 from the National Cancer Institute.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Footnotes
For patient information on skin cancer in women, please click on Supplemental Material to bring you to the Patient Page. Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijwd.2020.07.015.
Appendix A. Supplementary Material – Patient Page
The following are the Supplementary data to this article:
References
- Bahaj W., Ya’qoub L., Toor M., Masood A. Radiation recall in a patient with intrahepatic cholangiocarcinoma: case report and a literature review. Cureus. 2019;11(6):e5020. doi: 10.7759/cureus.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J., Mayes M.D. Epidemiology of systemic sclerosis: Incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol. 2012;24(2):165–170. doi: 10.1097/BOR.0b013e32834ff2e8. [DOI] [PubMed] [Google Scholar]
- Ben-David M.A., Elkayam R., Gelernter I., Pfeffer R.M. Melatonin for prevention of breast radiation dermatitis: a phase II, prospective, double-blind randomized trial. Isr Med Assoc J. 2016;18(3–4):188–192. [PubMed] [Google Scholar]
- Billings S.D., McKenney J.K., Folpe A.L., Hardacre M.C., Weiss S.W. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28(6):781–788. doi: 10.1097/01.pas.0000126055.33916.0b. [DOI] [PubMed] [Google Scholar]
- Bleasel N.R., Stapleton K.M., Commens C., Ahern V.A. Radiation-induced localized scleroderma in breast cancer patients. Australas J Dermatol. 1999;40(2):99–102. doi: 10.1046/j.1440-0960.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- Borger J.H., Kemperman H., Sillevis Smitt H., Hart A., van Dongen J., Lebesque J. Dose and volume effects on fibrosis after breast conservation therapy. Int J Radiat Oncol. 1994;30(5):1073–1081. doi: 10.1016/0360-3016(94)90312-3. [DOI] [PubMed] [Google Scholar]
- Bourgeois A., Grisoli S.B., Soine E.J., Rosen L.B. Tamoxifen-induced radiation recall dermatitis. Dermatol Online J. 2017;23(2) [PubMed] [Google Scholar]
- Bourgeois J.F., Gourgou S., Kramar A., Lagarde J.M., Guillot B. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Ski Res Technol. 2008;14(1):71–76. doi: 10.1111/j.1600-0846.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Bray F.N., Simmons B.J., Wolfson A.H., Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb) 2016;6(2):185–206. doi: 10.1007/s13555-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. http://doi.wiley.com/10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Buboltz J.B., Cooper J.S. StatPearls Publishing; 2019. Hyperbaric soft tissue radionecrosis. [PubMed] [Google Scholar]
- Buchholz T.A. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360(1):63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- Buehler D., Rice S.R., Moody J.S., Rush P., Hafez G.R., Attia S. Angiosarcoma outcomes and prognostic factors. Am J Clin Oncol. 2014;37(5):473–479. doi: 10.1097/COC.0b013e31827e4e7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen R., Larson P.O., Landeck A.E., Nychay S., Snow S.N., Hazen P. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. Dermatologic Surg. 1998;24(10):1105–1110. doi: 10.1111/j.1524-4725.1998.tb04083.x. [DOI] [PubMed] [Google Scholar]
- Burris H.A., Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15(11):1227–1237. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwenge M., Cammelli S., Ammendolia I., Tolento G., Zamagni A., Arcelli A. Intensity modulated radiation therapy for breast cancer: current perspectives. Breast Cancer (Dove Med Press) 2017;9:121–126. doi: 10.2147/BCTT.S113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge R., Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol. 2001;59(3):237–245. doi: 10.1016/s0167-8140(01)00328-0. [DOI] [PubMed] [Google Scholar]
- Cheah N.L., Wong D.W., Chetiyawardana A.D. Radiation-induced morphea of the breast: a case report. J Med Case Rep. 2008;2(1):136. doi: 10.1186/1752-1947-2-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitapanarux I., Tovanabutra N., Chiewchanvit S., Sripan P., Chumachote A., Nobnop W. Emulsion of olive oil and calcium hydroxide for the prevention of radiation dermatitis in hypofractionation post-mastectomy radiotherapy: a randomized controlled trial. Breast Care. 2019;14:394–400. doi: 10.1159/000496062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D., Martinez A., Cox R.S. Analysis of cosmetic results and complications in patients with stage i and ii breast cancer treated by biopsy and irradiation. Int J Radiat Oncol. 1983;9(12):1807–1813. doi: 10.1016/0360-3016(83)90348-6. https://www.sciencedirect.com/science/article/pii/0360301683903486?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Collette S., Collette L., Budiharto T., Horiot J.C., Poortmans P.M., Struikmans H. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer – a study based on the EORTC trial 22881–10882 “boost versus no boost”. Eur J Cancer. 2008;44(17):2587–2599. doi: 10.1016/j.ejca.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Connolly S.M., Baker D.R., Coldiron B.M., Fazio M.J., Storrs P.A., Vidimos A.T. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Cuperus E., Leguit R.J., Albregts M., Toonstra J. Post radiation skin tumors: Basal cell carcinomas, squamous cell carcinomas and angiosarcomas. A review of this late effect of radiotherapy. Eur J Dermatology. 2013;23(6):749–757. doi: 10.1684/ejd.2013.2106. [DOI] [PubMed] [Google Scholar]
- De Langhe S., Mulliez T., Veldeman L., Remouchamps V., van Greveling A., Gilsoul M. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14(1):711. doi: 10.1186/1471-2407-14-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanian S., Lefaix J.L. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiotherapy and Oncology. Amsterdam, the Netherlands; Elsevier. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Delanian S., Lefaix J.L. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17(2):99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Dion M.W., Hussey D.H., Doornbos J.F., Vigliotti A.P., Wen B.C., Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys. 1990;19(2):401–407. doi: 10.1016/0360-3016(90)90549-y. [DOI] [PubMed] [Google Scholar]
- Donovan E., Bleakley N., Denholm E., Evans P., Gothard L., Hanson J. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Eda K., Uzer K., Murat T., Cenk U. The effects of enteral glutamine on radiotherapy induced dermatitis in breast cancer. Clin Nutr. 2016;35(2):436–439. doi: 10.1016/j.clnu.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Edwards M.J., Hirsch R.M., Broadwater J.R., Netscher D.T., Ames F.C. Squamous Cell Carcinoma Arising in Previously Burned or Irradiated Skin. Arch Surg. 1989;124(1):115–117. doi: 10.1001/archsurg.1989.01410010125024. https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- Fisher J., Scott C., Stevens R., Marconi B., Champion L., Freedman G.M. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97–13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307–1310. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- Fraga-Guedes C., Gobbi H., Mastropasqua M.G., Rocha R.M., Botteri E., Toesca A. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146(2):347–354. doi: 10.1007/s10549-014-3020-9. [DOI] [PubMed] [Google Scholar]
- Freedman G.M., Li T., Nicolaou N., Chen Y., Ma C.C.M., Anderson P.R. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol. 2009;74(3):689–694. doi: 10.1016/j.ijrobp.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman O., Barnea Y., Hafner A. Underdiagnosed and disfiguring - Radiation-induced morphea following breast cancer treatment. The Breast. 2018;39:97–100. doi: 10.1016/j.breast.2018.04.006. https://www.sciencedirect.com/science/article/pii/S0960977618300699?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Fruchter R., Kurtzman D.J.B., Mazori D.R., Wright N.A., Patel M., Vleugels R.A. Characteristics and treatment of postirradiation morphea: a retrospective multicenter analysis. J Am Acad Dermatol. 2017;76(1):19–21. doi: 10.1016/j.jaad.2016.08.059. [DOI] [PubMed] [Google Scholar]
- Fuzissaki MA, Paiva C.E., de Oliveira M.A., Lajolo Canto P.P., de Paiva Maia Y.C. The impact of radiodermatitis on breast cancer patients’ quality of life during radiotherapy: a prospective cohort study. J Pain Symptom Manage. 2019;58(1) doi: 10.1016/j.jpainsymman.2019.03.017. 92–9.e1. [DOI] [PubMed] [Google Scholar]
- Geara F.B., Komaki R., Tucker S.L., Travis E.L., Cox J.D. Factors influencing the development of lung fibrosis after chemoradiation for small cell carcinoma of the lung: evidence for inherent interindividual variation. Int J Radiat Oncol. 1998;41(2):279–286. doi: 10.1016/s0360-3016(97)00741-4. [DOI] [PubMed] [Google Scholar]
- Gengler C., Coindre J.M., Leroux A., Trassard M., Ranchère-Vince D., Valo I. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process. Cancer. 2007;109(8):1584–1598. doi: 10.1002/cncr.22586. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383(9935):2127–35. [DOI] [PMC free article] [PubMed]
- Guarneri C., Guarneri B. Radiation recall dermatitis. CMAJ. 2010;182(3):E150. doi: 10.1503/cmaj.090320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffty B.G., Vicini F.A., Beitsch P., Quiet C., Keleher A., Garcia D. Timing of chemotherapy after mammosite radiation therapy system breast brachytherapy: analysis of the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial. Int J Radiat Oncol. 2008;72(5):1441–1448. doi: 10.1016/j.ijrobp.2008.02.070. [DOI] [PubMed] [Google Scholar]
- Harper J.L., Franklin L.E., Jenrette J.M., Aguero E.G. Skin toxicity during breast irradiation: pathophysiology and management. South Med J. 2004;97(10):989–993. doi: 10.1097/01.SMJ.0000140866.97278.87. [DOI] [PubMed] [Google Scholar]
- Haruna F., Lipsett A., Marignol L. Topical management of acute radiation dermatitis in breast cancer patients: a systematic review and meta-analysis. Anticancer Res. 2017;37(10):5343–5353. doi: 10.21873/anticanres.11960. [DOI] [PubMed] [Google Scholar]
- Haubner F., Ohmann E., Pohl F., Strutz J., Gassner H.G. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7(1):162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley A., Zain Z., Wood L., Whitehead A., Sanneh A., Barber D. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol. 2014;90(4):748–755. doi: 10.1016/j.ijrobp.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Ho A.Y., Olm-Shipman M., Zhang Z., Siu C.T., Wilgucki M., Phung A. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol. 2018;101(2):325–333. doi: 10.1016/j.ijrobp.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher T., Bentzen S.M., Baumann M. Influence of connective tissue diseases on the expression of radiation side effects: a systematic review. Radiother Oncol. 2006;78(2):123–130. doi: 10.1016/j.radonc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hymes S.R., Strom E.A., Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Jacobson G., Bhatia S., Smith B.J., Button A.M., Bodeker K., Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol. 2013;85(3):604–608. doi: 10.1016/j.ijrobp.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Jacobson L.K., Johnson M.B., Dedhia R.D., Niknam-Bienia S., Wong A.K. Impaired wound healing after radiation therapy: A systematic review of pathogenesis and treatment. JPRAS Open. 2017;13:92–105. https://www.sciencedirect.com/science/article/pii/S2352587817300256. [Google Scholar]
- Karagas M.R., McDonald J.A., Greendberg E.R., Stukel T.A., Weiss J.E., Baron J.A. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. JNCI J Natl Cancer Inst. 1996;88(24):1848–1853. doi: 10.1093/jnci/88.24.1848. [DOI] [PubMed] [Google Scholar]
- Kodym E., Kalinska R., Ehringfeld C., Sterbik-Lamina A., Kodym R., Hohenberg G. Frequency of radiation recall dermatitis in adult cancer patients. Oncol Res Treat. 2005;28(1):18–21. doi: 10.1159/000082175. [DOI] [PubMed] [Google Scholar]
- Kole A.J., Kole L., Moran M. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer Targets Ther. 2017;9:313–323. doi: 10.2147/BCTT.S109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffin N., Smyth W., Heyer E., Fasugba O., Abernethy G., Gardner A. Effectiveness and acceptability of a moisturizing cream and a barrier cream during radiation therapy for breast cancer in the tropics. Cancer Nurs. 2015;38(3):205–214. doi: 10.1097/NCC.0000000000000161. [DOI] [PubMed] [Google Scholar]
- Lee T.S., Kilbreath S.L., Refshauge K.M., Pendlebury S.C., Beith J.M., Lee M.J. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer. 2008;16(4):399–405. doi: 10.1007/s00520-007-0328-6. [DOI] [PubMed] [Google Scholar]
- Leventhal J., Young M.R. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park) 2017;31(12) 885–7, 894–9. [PubMed] [Google Scholar]
- Levy A., Hollebecque A., Bourgier C., Loriot Y., Guigay J., Robert C. Targeted therapy-induced radiation recall. Eur J Cancer. 2013;49(7):1662–1668. doi: 10.1016/j.ejca.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Li C., Athar M. Ionizing radiation exposure and basal cell carcinoma pathogenesis. Radiat Res. 2016;185(3):217–228. doi: 10.1667/RR4284.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter M.D., Karagas M.R., Mott L.A., Spencer S.K., Stukel T.A., Greenberg E.R. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136(8):1007. doi: 10.1001/archderm.136.8.1007. [DOI] [PubMed] [Google Scholar]
- Marín-Gutzke M., Sánchez-Olaso A., Berenguer B., González B., Rodríguez P., De Salamanca J.E., De Prada I. Basal cell carcinoma in childhood after radiation therapy: case report and review. Ann Plast Surg. 2004;53(6):593–595. doi: 10.1097/01.sap.0000136972.23991.07. [DOI] [PubMed] [Google Scholar]
- Mattoch I.W., Robbins J.B., Kempson R.L., Kohler S. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57(1):126–133. doi: 10.1016/j.jaad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Meattini I., Santi R., Scartoni D., Giacomelli I., De Luca Cardillo C, Scotti V. Multiple cutaneous angiosarcomas after breast conserving surgery and bilateral adjuvant radiotherapy: an unusual case and review of the literature. Case Rep Oncol Med. 2014;2014 doi: 10.1155/2014/413030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibodi N.T., Maleki M., Javidi Z., Nahidi Y. Clinicopathological evaluation of radiation induced basal cell carcinoma. Indian J Dermatol. 2008;53(3):137–139. doi: 10.4103/0019-5154.43222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghrajani C.F., Co H.C.S., Ang-Tiu C.M.U., Roa F.C. Topical corticosteroid therapy for the prevention of acute radiation dermatitis: a systematic review of randomized controlled trials. Expert Rev Clin Pharmacol. 2013;6(6):641–649. doi: 10.1586/17512433.2013.841079. [DOI] [PubMed] [Google Scholar]
- Mehta K., Kaubisch A., Tang J., Pirlamarla A., Kalnicki S. Radiation recall dermatitis in patients treated with sorafenib. Case Rep Oncol Med. 2018;2018:1–3. doi: 10.1155/2018/2171062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk S.M., More K.F., Miles E.F. Idiopathic radiation recall dermatitis developing nine months after cessation of cisplatin therapy in treatment of squamous cell carcinoma of the tonsil. Case Rep Oncol Med. 2012;2012:1–3. doi: 10.1155/2012/271801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A., Mittal V., Panse G., Choi J.N., Kwong B.Y., Leventhal J.S. Radiation-induced morphea: association with autoimmune comorbidities, severity, and response to therapy. J Am Acad Dermatol. 2019;81(1):260–262. doi: 10.1016/j.jaad.2019.02.039. [DOI] [PubMed] [Google Scholar]
- Mizumoto M., Harada H., Asakura H., Zenda S., Fuji H., Murayama S. Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol. 2006;66(4):1187–1191. doi: 10.1016/j.ijrobp.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Morris M., Powell S. Irradiation in the setting of collagen vascular disease: acute and late complications. J Clin Oncol. 1997;15(7):2728–2735. doi: 10.1200/JCO.1997.15.7.2728. [DOI] [PubMed] [Google Scholar]
- Newland K., Marshman G. Success treatment of post-irradiation morphoea with acitretin and narrowband UVB. Australas J Dermatol. 2012;53(2):136–138. doi: 10.1111/j.1440-0960.2011.00864.x. http://doi.wiley.com/10.1111/j.1440-0960.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- Nymann P., Hedelund L., Haedersdal M. Intense pulsed light vs. long-pulsed dye laser treatment of telangiectasia after radiotherapy for breast cancer: a randomized split-lesion trial of two different treatments. Br J Dermatol. 2009;160(6):1237–1241. doi: 10.1111/j.1365-2133.2009.09104.x. [DOI] [PubMed] [Google Scholar]
- Öztürk H., Öztemür Z., Bulut O. Treatment of skin necrosis after radiation synovectomy with yttrium-90: a case report. Rheumatol Int. 2008;28(10):1067–1068. doi: 10.1007/s00296-008-0571-2. [DOI] [PubMed] [Google Scholar]
- Pardo J., Mena A., Prieto I., Hernández M., Soto R., Vara J.C. Radiation recall dermatitis development: an observational study in 350 breast cancer patients. Int J Radiat Oncol. 2013;87(2):S214. [Google Scholar]
- Patton K.T., Deyrup A.T., Weiss S.W. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32(6):943–950. doi: 10.1097/pas.0b013e31815bf8fe. [DOI] [PubMed] [Google Scholar]
- Pignol J.P., Olivotto I., Rakovitch E., Gardner S., Sixel K., Beckham W. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- Porock D. Factors influencing the severity of radiation skin and oral mucosal reactions: development of a conceptual framework. Eur J Cancer Care (Engl) 2002;11(1):33–43. [PubMed] [Google Scholar]
- Rees F., Doherty M., Grainge M.J., Lanyon P., Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- Rhee J., Kim G.E., Lee C.H., Kwon J.M., Han S.H., Kim Y.S. Radiation recall dermatitis induced by tamoxifen during adjuvant breast cancer treatment. Radiat Oncol J. 2014;32(4):262–265. doi: 10.3857/roj.2014.32.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen S., Ivan D., Torres-Cabala C.A., Curry J.L., Tetzlaff M.T., Aung P.P. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46(1):52–58. doi: 10.1111/cup.13363. [DOI] [PubMed] [Google Scholar]
- Rossi A.M., Blank N.R., Nehal K., Dusza S., Lee E.H. Effect of laser therapy on quality of life in patients with radiation-induced breast telangiectasias. Lasers Surg Med. 2018;50(4):284–290. doi: 10.1002/lsm.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Maebayashi T., Aizawa T., Ishibashi N. Docetaxel-induced radiation recall dermatitis with atypical features: a case report. Medicine (Baltimore) 2018;97(36) doi: 10.1097/MD.0000000000012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer J.V., Carroll C., Dvoretsky I., Huether M.J., Girardi M. Postirradiation morphea of the breast presentation of two cases and review of the literature. Dermatology. 2000;200(1):67–71. doi: 10.1159/000018322. [DOI] [PubMed] [Google Scholar]
- Schnur J.B., Graff Zivin J., Mattson D.M.K., Green S., Jandorf L.H., Wernicke A.G. Acute skin toxicity-related, out-of-pocket expenses in patients with breast cancer treated with external beam radiotherapy. Support Care Cancer. 2012;20(12):3105–3113. doi: 10.1007/s00520-012-1435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur J.B., Ouellette S.C., DiLorenzo T.A., Green S., Montgomery G.H. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology. 2011;20(3):260–268. doi: 10.1002/pon.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaitelman S.F., Schlembach P.J., Arzu I., Ballo M., Bloom E.S., Buchholz D. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation. JAMA Oncol. 2015;1(7):931. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.Z., Nien H.H., Wu C.J., Lui L.T., Su J.F., Lang C.H. 3M cavilon no-sting barrier film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: a clinical trial. J Formos Med Assoc. 2015;114(5):407–414. doi: 10.1016/j.jfma.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Sholl L.M., Barletta J.A., Hornick J.L. Radiation-associated neoplasia: clinical, pathological and genomic correlates. Histopathology. 2017;70(1):70–80. doi: 10.1111/his.13069. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Simone N.L., Soule B.P., Gerber L., Augustine E., Smith S., Altemus R.M. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol. 2007;2(1):19. doi: 10.1186/1748-717X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Alavi A., Wong R., Akita S. Radiodermatitis: A review of our current understanding. Am J Clin Dermatol. 2016;17(3):277–292. doi: 10.1007/s40257-016-0186-4. [DOI] [PubMed] [Google Scholar]
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016:473–482. doi: 10.2147/CCID.S94320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalek M., Jonska-Gmyrek J., Gałecki J. Radiation-induced morphea - a literature review. J Eur Acad Dermatology Venereol. 2015;29(2):197–202. doi: 10.1111/jdv.12704. [DOI] [PubMed] [Google Scholar]
- Straub J.M., New J., Hamilton C.D., Lominska C., Shnayder Y., Thomas S.M. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan P., Sullivan L., Pendlebury S., Kirby A., Rodger A., Joseph D. Patients’ perceptions of health-related quality of life during and after adjuvant radiotherapy for T1N0M0 breast cancer. Clin Oncol. 2015;27(1):9–15. doi: 10.1016/j.clon.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Tejwani A., Wu S., Jia Y., Agulnik M., Millender L., Lacouture M.E. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor inhibitor therapy. Cancer. 2009;115(6):1286–1299. doi: 10.1002/cncr.24120. [DOI] [PubMed] [Google Scholar]
- Toledano A., Garaud P., Serin D., Fourquet A., Bosset J.F., Breteau N. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys. 2006;65(2):324–332. doi: 10.1016/j.ijrobp.2005.12.020. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- Uzun G., Candas F., Mutluoglu M., Ay H. Successful treatment of soft tissue radionecrosis injury with hyperbaric oxygen therapy. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009555. bcr2013009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J., Billings S.D. Postradiation cutaneous vascular tumors of the breast: a review. Semin Diagn Pathol. 2009;26(3):141–149. doi: 10.1053/j.semdp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Wengström Y., Häggmark C., Forsberg C. Coping with radiation therapy: strategies used by women with breast cancer. Cancer Nurs. 2001;24(4):264–271. doi: 10.1097/00002820-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Whelan T.J., Levine M., Julian J., Kirkbride P., Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma. Cancer. 2000;88(10):2260–2266. [PubMed] [Google Scholar]
- Yee C., Wang K., Asthana R., Drost L., Lam H., Lee J. Radiation-induced skin toxicity in breast cancer patients: a systematic review of randomized trials. Clin Breast Cancer. 2018;18(5):e825–e840. doi: 10.1016/j.clbc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Zetner D., Kamby C., Mahmood F., Rosenberg J. MELADERM-trial: melatonin cream against acute radiation dermatitis in patients with early breast cancer. Melatonin Res. 2019;2(1):32–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.