Abstract
Background
Hidradenitis suppurativa (HS) predominantly affects women of childbearing age, and sex hormones are thought to play a role in HS pathogenesis. However, there is a paucity of data regarding the pattern of HS perimenstrual flares, as well as patient responses to hormone-based therapies.
Objective
We aimed to characterize the temporal pattern of perimenstrual flares, as well as factors associated with perimenstrual flares. We also sought to investigate responses to hormonal therapies in women with HS.
Methods
An anonymous web-based questionnaire was distributed to various online HS support groups in May 2020.
Results
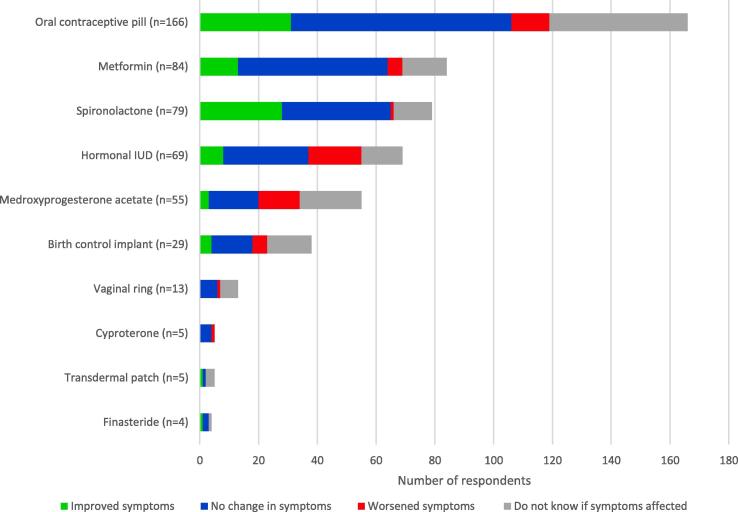
A total of 283 participants met the inclusion criteria as adult women who self-reported having HS and menstrual cycles. The majority (176 of 282 women; 62.4%) reported HS worsening with menses, and 86.9% (153 of 176 women) noted that perimenstrual HS flares occurred always or often. Most women (138 of 175 women; 78.9%) reported that their HS flared in the week preceding menses. Perimenstrual HS flares were more likely in women with a family history of HS compared with those without (49.6% vs. 28.2%; p = .019). More than a third of respondents who had been treated with spironolactone reported improvement of HS symptoms, but more than a quarter of participants who used medroxyprogesterone acetate or used a hormonal intrauterine device reported worsened HS symptoms.
Conclusion
Female patients with HS have high rates of perimenstrual HS flares, specifically during the week preceding the onset of menses. Additional investigations on the role of sex hormones in HS pathogenesis and the efficacy of hormone-based therapies are warranted.
Keywords: Hidradenitis suppurativa, Hormonal therapy, Menses, Perimenstrual flare
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that presents as painful nodules, sinus tracts, and scarring typically within intertriginous regions (Alikhan et al., 2009). HS has been found to disproportionately affect women compared with men in a 2.4:1 ratio (Garg et al., 2017). Many women report a change in HS symptoms in association with menses; therefore, sex hormones are thought to contribute to HS pathogenesis (Von Der Werth and Williams, 2000). There is a paucity of data in the literature on the temporal pattern of perimenstrual flares or how consistently patients experience perimenstrual flares (Riis et al., 2016). This study sought to characterize the pattern of perimenstrual HS flares and factors associated with these flares, as well as investigate responses to hormone-based treatments.
Methods
An anonymous web-based questionnaire was distributed to various Facebook HS support groups in May 2020. The majority of Facebook groups are private and need permission from group administrators to join. Facebook group administrators posted the survey link to their group’s Facebook page. The inclusion criteria for the study were women who self-identified as having HS and who were premenopausal and experiencing menstrual cycles. The study was deemed exempt by the University of Arizona institutional review board. Participants self-reported Hurley staging using a provided description. Respondent characteristics and study variables were summarized for the entire cohort using mean ± standard deviation (SD) and range for continuous measures and frequency (%) for categorical variables. To compare categorical variables between the groups (e.g., worsening of HS with menses and worsening of acne with menses), we used the χ2 test. To assess the association between ordinal ranked or continuous variables (e.g., timing when HS and acne symptoms worsen in relation to menses, which was coded as the week before, during, and after), we used Spearman’s correlation. To compare continuous measures between groups (e.g., body mass index between participants with menstrual HS flares and those who did not have menstrual flares), we used the independent samples t test. The statistical analyses were performed using IBM SPSS, version 25 (Armonk, NY) and p-values <.05 were considered statistically significant.
Results
A total of 283 respondents met the inclusion criteria. Demographic and HS characteristics are presented in Table 1. Mean age at the time of the survey was 35 years (SD: 8.0 years), of menarche was 12.1 years (SD: 1.6 years), and of HS symptom onset was 18.3 years (SD: 8.0 years). The majority of participants (176 of 282 women; 62.4%) reported HS worsening with menses, and 86.9% (153 of 176 women) noted that flares occur always or often with menses. Most respondents (138 of 175 women; 78.9%) reported that their HS flared in the week preceding their menses as opposed to during (33 of 175 women; 18.9%) or after (4 of 175 women; 2.3%) menses. Over a third of respondents (28 of 79 women; 35.4%) who had been treated with spironolactone reported improvement of HS symptoms, which is a higher response rate than all other listed hormonal medications, including finasteride, transdermal patch, oral contraceptive pills (OCPs), metformin, birth control implants, hormonal intrauterine devices (IUDs), medroxyprogesterone acetate, vaginal ring, and cyproterone (Fig. 1). More than a quarter of participants who used medroxyprogesterone acetate (14 of 55 women; 25.5%) or who used a hormonal IUD (18 of 69 women; 26.1%) reported worsened HS symptoms. Respondents who reported menstrual HS flares trended toward reporting improved HS symptoms on OCPs (24.5% vs. 10%; p = .087) and spironolactone (40.4% vs. 23.5%; p = .213) compared with those without menstrual flares, although these findings were not statistically significant.
Table 1.
Participant demographic and HS disease characteristic information.
| Patient characteristics | n (%) |
|---|---|
| Age | |
| At the time of the survey, mean ± SD (range), y (n = 283) | 35.0 ± 8 (18–54) |
| At menarche, mean ± SD (range), y (n = 282) | 12.1 ± 1.6 (8–17) |
| At HS symptom onset, mean ± SD (range), y (n = 280) | 18.3 ± 8.0 (4–48) |
| Body mass index, mean ± SD (range), (n = 282) | 34.6 ± 8.5 (17.4–64.6) |
| Country of residence (n = 279) | |
| United States | 201 (72.0) |
| United Kingdom | 36 (12.9) |
| Canada | 18 (6.4) |
| Australia | 9 (3.2) |
| Puerto Rico | 4 (1.4) |
| Malaysia | 2 (0.7) |
| Other* | 9 (3.2) |
| Race/ethnicity (n = 283) | |
| White/Caucasian | 199 (70.3) |
| Black/African descent | 30 (10.6) |
| Hispanic/Latino | 28 (9.9) |
| Mixed or Multiracial | 15 (5.3) |
| Asian/Pacific Islander | 6 (2.1) |
| Other | 5 (1.8) |
| History of polycystic ovary syndrome (n = 283) | 63 (22.3) |
| History of acne (n = 283) | 128 (45.2) |
| HS severity (n = 282) | |
| Hurley stage 1 | 15 (5.3) |
| Hurley stage 2 | 155 (54.8) |
| Hurley stage 3 | 112 (39.7) |
| HS symptoms in relation to menarche (n = 279) | |
| Prior to menarche | 37 (13.3) |
| At same age as menarche | 22 (7.9) |
| After menarche | 220 (78.9) |
| Family history of HS (n = 283) | |
| Yes | 91 (31.2) |
| No | 112 (39.6) |
| Do not know | 80 (28.3) |
| Body parts affected by HS (n = 283) | |
| Groin/genitals | 258 (91.2) |
| Axillae | 217 (76.7) |
| Buttocks | 191 (67.5) |
| Breast/inframammary | 158 (55.8) |
| Behind ears | 44 (15.5) |
| Posterior neck | 38 (13.4) |
| Scalp | 26 (9.2) |
| Other | 79 (27.9) |
| Duration of menstrual cycle (n = 281) | |
| <21 days | 15 (5.3) |
| 21–35 days | 193 (68.7) |
| >35 days | 6 (2.1) |
| Too irregular to say | 67 (23.8) |
| HS gets worse with period (n = 282) | |
| Yes | 176 (62.4) |
| No | 52 (18.4) |
| Do not know | 54 (19.1) |
| How frequently does your HS get worse with your periods? (n = 176) | |
| Always | 96 (54.5) |
| Often | 57 (32.4) |
| Sometimes | 23 (13.1) |
| HS usually gets worse (n = 175) | |
| In the week before my period | 138 (78.9) |
| During my period | 33 (18.9) |
| In the week after my period ends | 4 (2.3) |
| Does acne get worse with period (n = 127) | |
| Yes | 91 (71.7) |
| No | 21 (16.5) |
| Do not know | 15 (11.8) |
| Acne usually gets worse (n = 91) | |
| In the week before my period | 64 (70.3) |
| During my period | 27 (29.7) |
| In the week after my period ends | 0 (0.0) |
HS, hidradenitis suppurativa.
xxx.
Fig. 1.
Respondent-perceived effects of hormonal treatments on hidradenitis suppurativa symptoms.
The rate of menstrual acne flares is significantly higher in women with menstrual HS flares (64 of 74 women; 86.5%) than in women without menstrual HS flares (13 of 20 women; 65%; p = .027). There is a significant positive correlation between temporal worsening of menstrual flares of HS and acne. Women who report premenstrual HS flares also tended to report premenstrual acne flares (Spearman correlation: 0.60; p < .001). Menstrual HS flares were significantly more likely in participants with a family history of HS than in those without (49.6% vs. 28.2%; p = .019). Race, HS disease severity, body mass index, and the presence of acne or polycystic ovary syndrome did not significantly correlate with worsening HS during menses.
Discussion
Our study found that 62.4% of women of childbearing age reported HS flares with menses, and these flares typically occurred in the premenstrual period. Our study findings are consistent with those of previous studies that reported rates of perimenstrual flares between 44% and 63% (Riis et al., 2016). Participants reported varying perceived efficacy of hormonal treatments, with spironolactone reported to have the most benefit. Meanwhile, medroxyprogesterone acetate and hormonal IUDs, which are progesterone-laden treatments, were reported to worsen symptoms at a higher rate than other hormonal treatments. These findings suggest that providers should inquire about perimenstrual HS flares when managing women of childbearing age. In addition, spironolactone may be considered for women who are not actively planning to become pregnant. Avoidance of medroxyprogesterone acetate and hormonal IUDs may prevent the potential worsening of HS symptoms.
There is limited data in the literature on the pattern of perimenstrual flares or how consistently patients experience perimenstrual flares. This study adds to the previous literature by characterizing the temporal pattern of HS perimenstrual flare as predominantly occurring during the premenstrual phase. In addition, most respondents described consistently experiencing worsening flares with each menstrual cycle. After reaching their peak during the midluteal phase, levels of progesterone and estradiol decline during the week preceding the onset of menses (Reed and Carr, 2000). This decline in sex hormones is hypothesized to cause the premenstrual flares of HS disease, but further investigation is needed to substantiate this hypothesis.
Our study also found that among women who had concomitant acne, 71.7% reported worsening of acne with menses. The higher rate of menstrual acne flares in women with concurrent menstrual HS flares suggests that there may be a common hormonal influence shared by these two diseases, such as the androgenic effect of sexual hormones causing worsening flares. The predominant onset of HS after menarche and the impact of pregnancy on disease course lend further support to a hormonal role in HS. However, at this time, the definitive relationship between progesterone, estrogen, and HS symptoms requires further elucidation. Given that menstrual HS flares were associated with having a positive family history of HS, further investigation into whether hormonal HS may be a unique subtype of HS with strong familial influence is warranted.
The potential benefit of spironolactone for HS shown in this study is consistent with the results of previous studies that demonstrated efficacy with spironolactone treatment (Golbari et al., 2019, Lee and Fischer, 2015). The North American Hidradenitis Suppurativa management guidelines suggest that progestogen-only contraceptive drugs may worsen HS and should be avoided (Alikhan et al., 2019). Our study’s finding that more than a fourth of respondents who were on medroxyprogesterone acetate or a hormonal (progesterone) IUD perceived worsened HS supports this recommendation. Combined OCPs and spironolactone are both used to treat HS in women. Further investigation is needed to determine what patient characteristics (e.g., presence of menstrual flares) may predict an increased likelihood of response to these treatments. Our study also showed a reported efficacy rate of 15.5% for metformin, which is lower than that reported in previous studies on metformin and HS (Jennings et al., 2020, Verdolini et al., 2013), and of 25% in finasteride; however, the cohort of patients endorsing finasteride treatment was small. Metformin and finasteride are two other hormonal medications used in the treatment of HS, although more data are needed to explore efficacy.
Limitations of this study include recall bias, response bias, self-reported HS and Hurley stage, self-reported polycystic ovary syndrome, and generalizability of online social media groups with the general HS population. In addition, other HS comorbidities, such as smoking, were not studied. Given the anonymous nature of the survey and the continuously changing numbers of participants in these Facebook groups, the response rate is unknown. Patients also did not differentiate between the use of progestogen-only versus combined OCPs, which limits the study’s ability to draw conclusions about OCP efficacy given that progestogen-only OCPs may be harmful while combined OCPs are thought to be beneficial. Also, progestogen agents in combined OCPs have varying androgenic or antiandrogenic effects. The duration of hormonal regimens was also not recorded; thus, the lack of an adequate trial may have resulted in perceived treatment failure as opposed to intrinsic treatment inefficacy. Finally, patients did not specify whether they were taking other HS medications concomitantly with their reported hormonal treatment, which may have impacted perceived treatment efficacy.
Conclusion
Female patients with HS have a high rate of perimenstrual flares, and specifically of premenstrual flares, which supports the role of hormonally mediated factors of HS pathogenesis. Further investigation of treatment efficacy of hormonal treatments, including identifying subgroups of patients who are more likely to respond, is needed. Improved understanding of the role of sex hormones in HS pathogenesis and the benefit of hormonal therapies will help optimize care for female patients with HS.
Acknowledgments
Acknowledgments
The authors are thankful for the participation from the following Facebook groups: HS Connect, HS Support Group – Hidradenitis Suppurativa (Pics allowed), Hidradenitis Suppurativa Warrior, Hidradenitis Suppurativa Support Group, HS Unique Fun Facts, Hope for HS – Private Group, HS Hidradenitis Suppurativa NEVER GIVE UP, #HSGLOBAL – Hidradenitis Suppurativa International, International Association of Hidradenitis Suppurativa Network, Inc., and Hidradenitis Suppurativa Warriors for Research.
Financial Disclosures
This research was supported by National Institutes of Health, National Center for Advancing Translational Science, University of California Los Angeles (Clinical and Translational Science Institute Grant Number UL1TR001881).
Conflicts of Interest
Jennifer L. Hsiao, MD has served as an advisor for Novartis. Vivian Y. Shi, MD is a stock shareholder of Learn Health and has served as an advisory board member, investigator, and/or received research funding from Sanofi Genzyme, Regeneron, AbbVie, Eli Lilly, Novartis, SUN Pharma, LEO Pharma, Pfizer, Menlo Therapeutics, Burt’s Bees, GpSkin, the National Eczema Association, Global Parents for Eczema Research, the Foundation for Atopic Dermatitis, and Skin Actives Scientific. There were no incentives or transactions, financial or otherwise, relevant to this manuscript. Haley B. Naik, MD, MHSc has consulted for 23andme and Boehringer Ingelheim, is a board member of the HS Foundation, and has received grant funding from Abbvie. Erin K. Collier, MPH; Kyla N. Price, BS; Tristan R. Grogan, MS have no conflicts of interest.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- Alikhan A., Lynch P.J., Eisen D.B. Hidradenitis suppurativa: A comprehensive review. J Am Acad Dermatol. 2009;60(4):539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- Alikhan A., Sayed C., Alavi A., Alhusayen R., Brassard A., Burkhart C. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81(1):76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A., Kirby J.S., Lavian J., Lin G., Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbari N.M., Porter M.L., Kimball A.B. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80(1):114–119. doi: 10.1016/j.jaad.2018.06.063. [DOI] [PubMed] [Google Scholar]
- Jennings L., Hambly R., Hughes R., Moriarty B., Kirby B. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31(3):261–263. doi: 10.1080/09546634.2019.1592100. [DOI] [PubMed] [Google Scholar]
- Lee A., Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone: Hidradenitis suppurativa, spironolactone. Australas J Dermatol. 2015;56(3):192–196. doi: 10.1111/ajd.12362. [DOI] [PubMed] [Google Scholar]
- Reed B, Carr B. The normal menstrual cycle and the control of ovulation. In: Feingold K, Anawalt B, Boyce A, Al. E, editors. South Dartmouth, MA: MDText.com, Inc.; 2000.
- Riis P.T., Ring H.C., Themstrup L., Jemec G.B. The role of androgens and estrogens in hidradenitis suppurativa - a systematic review. Acta Dermatovenerologica Croat. 2016;24(4):239–249. [PubMed] [Google Scholar]
- Verdolini R., Clayton N., Smith A., Alwash N., Mannello B. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatology Venereol. 2013;27(9):1101–1108. doi: 10.1111/j.1468-3083.2012.04668.x. [DOI] [PubMed] [Google Scholar]
- von der Werth J.M., Williams H.C. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venerol. 2000;14(5):389–392. doi: 10.1046/j.1468-3083.2000.00087.x. [DOI] [PubMed] [Google Scholar]