Abstract
Inhibition of vascular endothelial growth factor is the mode of action for several approved therapies, including aflibercept, for the treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Lack of compliance due to the frequent intravitreal dosing requirements may result in inadequately treated disease, leading to irreversible vision impairment. To date, the majority of gene therapy clinical trials providing sustained anti-VEGF levels in the retina have been limited to subretinal injections requiring a vitrectomy. A single intravitreal injection of a gene therapy product could drastically reduce the treatment burden and improve visual outcomes. ADVM-022, an adeno-associated virus vector encoding aflibercept, has been optimized for intravitreal delivery and strong protein expression. Long-term expression and efficacy of ADVM-022-derived aflibercept were evaluated in a laser-induced choroidal neovascularization (CNV) model in non-human primates. Ocular safety was evaluated following long-term suppression of VEGF by clinical scoring (inflammatory parameters) as well as optical coherence tomography (OCT) and electroretinography (ERG). Intravitreal administration of ADVM-022 was well tolerated and resulted in sustained aflibercept levels in ocular tissues. In addition, ADVM-022 administration 13 months before laser-induced CNV prevented the occurrence of clinically relevant CNV lesions, to the same degree as a bolus of aflibercept delivered at the time of laser. These results demonstrate that a single intravitreal administration of ADVM-022 may provide a safe and effective long-term treatment option for nAMD and DME, and may ultimately improve patients' visual outcomes. Clinical trials are currently underway, evaluating safety and efficacy following a single intravitreal injection of ADVM-022.
Keywords: anti-VEGF, adeno-associated virus, gene therapy, neovascular AMD, intravitreal
Introduction
Neovascular age-related macular degeneration (nAMD) is the leading cause of severe visual loss in adults older than 60 years of age. Vision loss results from abnormal blood vessel proliferation and leakage due to vascular endothelial growth factor (VEGFA); inhibition of VEGFA is a validated therapeutic approach for both nAMD and diabetic macular edema (DME). DME is a complication of diabetic retinopathy (DR), a leading cause of visual loss in the working-age population.1 Three recombinant anti-VEGFA proteins (ranibizumab, bevacizumab, and aflibercept), all of which are administered through IVT injection every 4 to 8 weeks, are typically the first line of treatment for both indications.2–4 Although studies have shown that patients with nAMD and DME, who receive regular and frequent injections of anti-VEGF protein therapy, show significantly improved vision,5 other long-term follow-up studies have shown that mean dosing frequencies in the real-world setting are less than half of what is recommended, leading to disease progression and vision loss.6–8 Discomfort from repeated IVT injections, treatment cost, frequent office visits and the burden these visits place on patients and their caregivers are key factors that lead to lack of compliance with recommended treatment schedules.9 In addition, monthly bolus injections of anti-VEGF protein therapies result in peaks and troughs of protein concentration throughout the dosing cycle, potentially leading to suboptimal consequences. Indeed, significant systemic exposure until ≥7 days postinjection has been described,10 while in the period preceding the next monthly/bimonthly injection, potential lack of sufficient anti-VEGF levels in the choroid/retina can lead to an increase in disease activity.11
As a therapeutic alternative, gene therapy offers the potential of a unique drug delivery platform to chronically express anti-VEGF proteins for the treatment of neovascular disease, thus eliminating the treatment burden of frequent injections and solving the compliance issues that can lead to vision loss. In 2018, Luxturna was approved as the first ocular adeno-associated virus (AAV)-based gene therapy approach to deliver a functional copy of the RPE65 gene by subretinal injection to patients with Lebers congenital amaurosis, an inherited retinal disease (IRD). The success of Luxturna set the stage for ocular gene therapy not only as a viable approach for IRDs but also opened the door for the continuous delivery of protein therapeutics, for chronic diseases that require repeated treatments, to provide sustained clinical benefit to patients. The continuous expression of anti-VEGF proteins by way of gene therapy for the treatment of neovascular diseases could alleviate the need for repeated injection and reduce the burden on patients, their caregivers, and the health care system at large. The chronic and steady expression of anti-VEGF proteins from a single gene therapy treatment would also be preferable to the peaks and troughs in drug concentrations from bolus injections of recombinant protein therapies as mentioned above.
ADVM-022 is an AAV-based gene therapy that utilizes a strong ubiquitous promoter, which drives continuous and durable expression of aflibercept, a standard of care anti-VEGF protein therapy for the treatment of nAMD and DME. Its goal is to provide a stable and long-term source of ocular aflibercept following a single IVT injection. This gene therapy approach would address both the compliance issues related to dosing frequency and discomfort, as well as the variable pharmacokinetic profile that currently leads to suboptimal outcomes in patients with nAMD and DME undergoing standard of care (i.e., anti-VEGF) therapy. Two ongoing clinical studies currently in progress are evaluating ADVM-022: a Phase 1 trial for the treatment of “wet” nAMD (OPTIC; NCT NCT03748784) and a Phase 2 trial for DME (INIFINITY; NCT NCT04418427).
Herein, we will review the preclinical studies that supported the initiation of the OPTIC and INFINITY clinical trials. All these studies were performed in non-human primates (NHPs), an ideal species based on similarities in retinal anatomy and physiology to the human eye, allowing us to generate relevant data to plan first-in-human studies. Another important advantage of NHPs for the development of ocular therapeutics is the applicability of noninvasive methods and instrumentation of ocular imaging and electrophysiology used in humans.12 The NHP studies reviewed in this study evaluated the pharmacokinetics, pharmacology, and safety of a single IVT injection of ADVM-022. IVT administration of ADVM-022 to African green monkeys (AGMs) (Chlorocebus sabaeus) resulted in sustained expression of aflibercept in the eye out to 30 months,13 the longest time point evaluated thus far. Ocular levels of ADVM-022-derived aflibercept were comparable to those resulting from a bolus administration of recombinant aflibercept protein.14 In addition, IVT administration of ADVM-022 protected against Grade IV lesions as well as reduced the size of fibrovascular complexes in the laser-induced choroidal neovascularization (CNV) model in these animals.15 Importantly, IVT injection of ADVM-022 in AGMs has demonstrated a good safety profile within the eye out to 30 months with negligible systemic exposure to aflibercept.13–14 Taken together, these preclinical data suggest that ADVM-022 could provide a durable and stable source of aflibercept for the treatment of wAMD and DME, and supports evaluation in the ongoing OPTIC and INFINITY trials.
The initial efficacy, expression, and 12.5-month safety data, which were published in January 2019,15 were presented in March 2019 at the AOPT meeting in the session entitled “Disruptive Technologies: Ophthalmic Tools and Methods that Have Changed the Ways We See the Eye.” Additional data describing the pharmacokinetics14 and long-term safety13 of ADVM-022 administered to AGMs by IVT injection have since been published. All 3 data sets describing the preclinical path forward for ADVM-022 are reviewed in this article.
Overview of ADVM-022
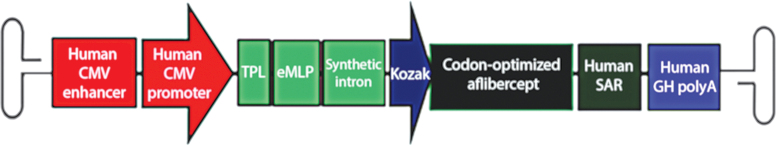
ADVM-022 is a novel, recombinant AAV-based gene therapy vector for the treatment of ocular neovascular diseases, including nAMD, DR, and DME, which has been optimized for IVT administration and robust expression of aflibercept, an approved anti-VEGF therapy for people living with nAMD and other VEGF-driven retinal diseases (Fig. 1).15 The AAV2.7m8 capsid of ADVM-022 was engineered from AAV2 and selected using directed evolution for its ability to transduce retinal cells with high efficiency following IVT administration.16 The cassette has also been engineered for strong and ubiquitous expression of a codon-optimized cDNA encoding aflibercept.
FIG. 1.
Design of aflibercept expression cassette (C11). The aflibercept transgene expression cassette is flanked by AAV2 ITRs. The C11.CO.aflibercept cassette includes regulatory elements, including the human CMV immediate-early enhancer and promoter, an adenovirus TPL followed by an eMLP, a synthetic intron, and a Kozak sequence driving expression of aflibercept. The cDNA of aflibercept is followed by a human SAR and the human GH polyadenylation site. Aflibercept is a recombinant chimeric protein consisting of the VEGFA binding portion of human VEGFR-1 (domain 2) and VEGFR-2 (domain 3 or KDR) fused to the Fc portion of human IgG1 immunoglobin. Printed with permission from Grishanin et al.15 AAV, adeno-associated virus; CMV, cytomegalovirus; eMLP, enhancer element from the major late promoter; GH, growth hormone; ITRs, inverted terminal repeats; KDR, kinase domain receptor; SAR, scaffold attachment region; TPL, tripartite leader sequence; VEGFA, vascular endothelial growth factor.
Expression of aflibercept following IVT administration of ADVM-022
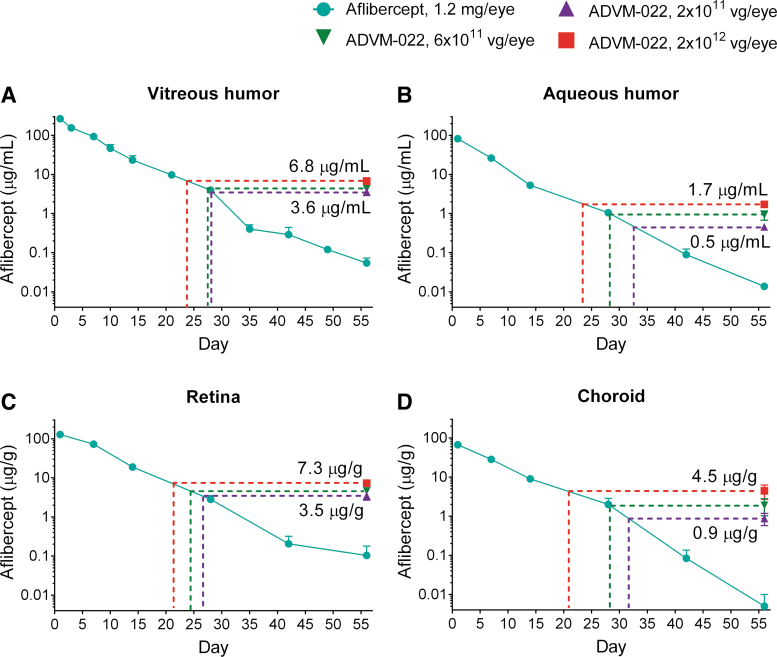
A key objective in the development of ADVM-022 as a treatment for nAMD was to provide expression of aflibercept at levels affording protection from neovascularization for the lifetime of the patient following a single IVT dosing. Toward this objective, we first evaluated aflibercept levels from ADVM-022 and compared them to levels achieved following IVT delivery of a bolus of aflibercept recombinant protein. In the NHP pharmacokinetics study described in Kiss et al.,14 levels of aflibercept from 3 single IVT doses of ADVM-022 (2 × 1011, 6 × 1011, or 2 × 1012 vg/eye; n = 2 animals per dose) were compared to levels following a bolus of a clinically relevant IVT-delivered dose of aflibercept recombinant protein (1.2 mg/eye of Eylea, Regeneron). At all 3 doses of ADVM-022, aflibercept concentrations by day 56 postadministration were similar to those observed in the aflibercept recombinant protein-injected eyes between days 21 and 32 postdose (Fig. 2). It should be noted that aflibercept levels in vitreous humor, aqueous humor, retina, and choroid 56 days post-ADVM-022 (Fig. 2A–D, respectively) were higher than those in the animals that received bolus aflibercept protein, which ranged from below the level of quantitation to 3% of aflibercept in the ADVM-022-injected eyes.
FIG. 2.
IVT administration of ADVM-022 provides levels of intraocular aflibercept expression comparable with those measured 21–32 days following IVT injection of aflibercept recombinant protein (n = 4 eyes, 2 animals for each data point) (A) Vitreous humor. (B) Aqueous humor. (C) Retina. (D) Choroid. The dotted lines indicate the times on the decay curves when recombinant aflibercept levels are equivalent to those seen in the ADVM-022-treated groups' low (2 × 1011 vg/eye, purple), middle (6 × 1011 vg/eye, green), and high (2 × 1012 vg/eye, red) doses tested on day 56. Error bars represent standard deviations. Printed with permission from Kiss et al.14 IVT, intravitreal.
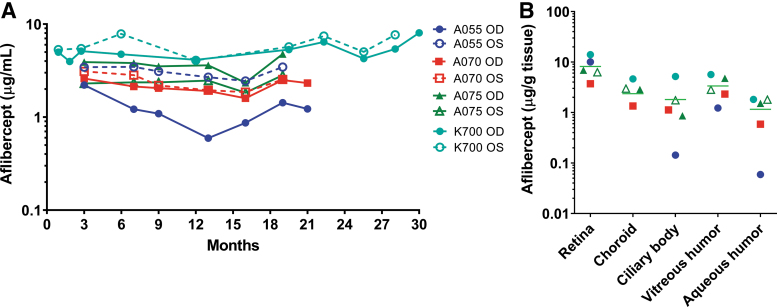
Durability of aflibercept expression over time following a single injection of ADVM-022 has been assessed in studies comprising the preclinical development of ADVM-022. The ability of ADVM-022 to achieve this objective was assessed by longitudinal sampling of the vitreous humor in 3 AGMs that were included in the original efficacy and short-term safety assessments,15 but followed out to 21 months, plus an additional animal from a separate study that was followed for 30 months.13 As shown in Fig. 3A, a single IVT injection of ADVM-022 (2 × 1012 vg/eye) resulted in robust levels of aflibercept ranging from 1.2 μg/mL (Animal A055 OD, at month 21 terminal measurement) to 8.1 μg/mL (Animal K700, at month 30 terminal measurement), which were sustained over the 21- and 30-month observation periods. Note that these levels are consistent with the levels achieved between 21 and 30 days following bolus administration of aflibercept protein in Fig. 2 (turquoise line). Most eyes also had comparable levels of aflibercept in retina, choroid, ciliary body, vitreous humor, and aqueous humor (Fig. 3B), although levels were somewhat lower in ciliary body and aqueous humor in the right eye of animal A055; expression in this eye also dipped midway through the study, concomitant with the vitreous haze seen in only this eye at this time point.
FIG. 3.
A single intravitreal injection of ADVM-022 resulted in sustained levels of aflibercept in the vitreous humor throughout the 21- and 30-month observation periods (A), and at terminal collection (B). Levels of aflibercept were also quantified from the terminally collected ocular tissues (retina, choroid, ciliary body, and aqueous and vitreous humor) (B). Printed with permission from Kiss et al.13
ADVM-022 demonstrated long-term efficacy in the laser-induced CNV model
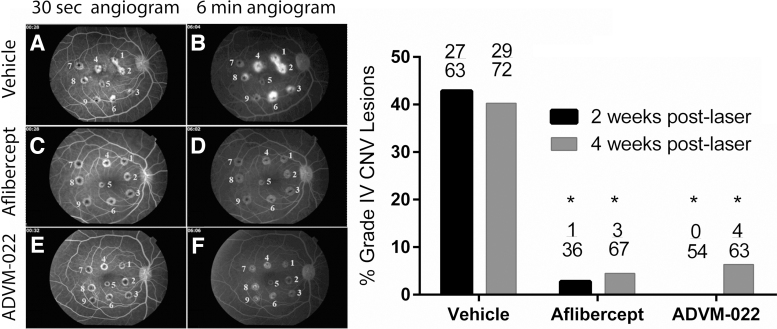
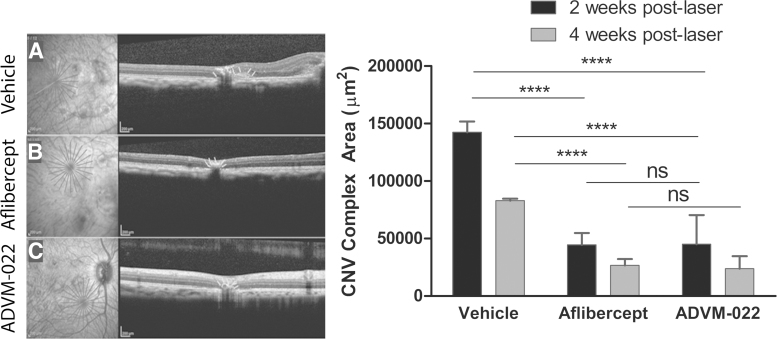
Long-term efficacy following a single IVT injection of ADVM-022 was assessed in the NHP laser-induced CNV model,12,17 in which laser photocoagulation is performed in the perimacular region of the retina to induce CNV lesions.15 Eight animals received a single 50 μL IVT injection of ADVM-022 (2 × 1012 vg/eye; n = 4) or vehicle (n = 4) 13 months before the laser coagulation, and 4 additional animals received an IVT injection of aflibercept recombinant protein (1.2 mg/30 μL) at the time of photocoagulation.15 Efficacy was assessed 2 and 4 weeks postlaser by fluorescein angiography and was determined based on the reduction in the frequency of clinically relevant grade IV exudative lesions, which have previously been shown to be representative of the response following IVT injection of approved anti-VEGF protein therapies.17,19 These lesions appear as bright hyperfluorescence early or midtransit, with late fluorescein leakage extending beyond the borders of the laser spot. Cross-sectional measures of CNV complexes generated by optical coherence tomography (OCT) imaging were also obtained to support the angiography findings.
Representative early and late fluorescein angiograms are shown in Fig. 4A–F. The right panel of Fig. 4 shows the percentage of assessable lesions that were grade IV at 2 and 4 weeks postlaser. The incidence of grade IV lesions at 2 and 4 weeks postlaser was 43% and 40% in the vehicle group, 3% and 5% in the aflibercept group, and 0% and 6% in the ADVM-022 group. The difference between each of the treatment groups and the vehicle group was statistically significant (P < 0.0001 for aflibercept compared with vehicle and P < 0.0001 for ADVM-022 compared with vehicle, Fisher's exact test), while there was no statistical difference in the grade IV lesion incidence between the ADVM-022 and aflibercept recombinant protein groups. A statistically significant difference between each of the treatment groups and the vehicle group was also observed when groups were evaluated based on the number of grade IV lesions per eye (data not shown; P < 0.05 for ADVM-022 and aflibercept compared with vehicle; no significant difference between ADVM-022 and aflibercept).
FIG. 4.
Left, representative early (30-s; A, C, E)- and late-phase (6-min; B, D, F) fluorescence angiograms from vehicle-injected (A, B), aflibercept-injected (C, D), and ADVM-022-injected eyes (E, F). Right, incidence of grade IV lesions in groups treated 13 months before the CNV induction with vehicle or ADVM-022 or treated with aflibercept immediately after laser photocoagulation. Numbers on the top of bars show the absolute number of grade IV lesions scored over the total number of assessable lesions. P < 0.0001 versus vehicle (Fisher's exact probability test). There was no statistical difference between the ADVM-022 and aflibercept groups.15 Printed with permission from Grishanin et al.15 CNV, choroidal neovascularization.
The anatomic appearance of CNV lesions was assessed using spectral domain (SD)-OCT to explore the potential impact of grade IV lesion suppression on the size of CNV fibrovascular complexes.15 This approach has been shown to provide a reliable metric for evaluating the size of these complexes and has been used to assess the efficacy of other novel antiangiogenic agents.20–23 As shown in Fig. 5, a single IVT injection of ADVM-022 administered 13 months before laser significantly reduced the size of fibrovascular CNV complexes. Representative OCT images at 4 weeks postlaser are shown on the left of Fig. 5. The mean CNV complex area measured from principal axis images at 2 and 4 weeks postlaser was 142,369 and 82,923 mm2 in the vehicle group, 44,503 and 26,622 mm2 in the aflibercept group, and 45,078 and 23,792 mm2 in the ADVM-022 group (Fig. 5, right). Consistent with the angiography results, there was a statistically significant difference between each of the treatment groups and the vehicle group (P < 0.0001 at both time points for ADVM-022 or aflibercept vs. vehicle) and no significant difference was observed between the ADVM-022 and aflibercept groups. In summary, ADVM-022 administration 13 months before laser-induced CNV prevented the occurrence of clinically relevant CNV lesions and fibrovascular complexes, to the same degree as a bolus of aflibercept delivered at the time of laser.
FIG. 5.
Representative OCT images at 4 weeks postlaser from eyes receiving vehicle (A), aflibercept (B), and ADVM-022 (C). Right, size of CNV complex evaluated at 2 and 4 weeks postlaser photocoagulation. CNV complex size is presented as the area of the CNV in the optical cross-section. Mean CNV complex areas were significantly smaller in the ADVM-022 and aflibercept groups compared with vehicle. Data show mean maximum cross-sectional CNV area with bars indicating SEM (7–8 eyes analyzed per group). Number of lesions assessed (n) were 71, 68, and 62 for vehicle-, aflibercept-, and ADVM-022-treated groups, respectively, at 2 weeks post laser, and 70, 67, and 63 at 4 weeks post laser. There was no statistical difference between the ADVM-022 and aflibercept groups. (****P < 0.0001; ns, nonsignificant; Mann–Whitney U test).15 Printed with permission from Grishanin et al.15 OCT; SEM, standard error of the mean.
Ocular safety following a single IVT injection of ADVM-022
Ocular safety parameters were evaluated in the eyes of all NHPs in the short-term (56 days) study, as well as in the longer term (21 and 30 months post-ADVM-022 injection) studies. Inflammation scoring by slit lamp examination was performed on all the animals; retinal morphology and function were evaluated by OCT and electroretinography (ERG), respectively, on the long-term study animals.
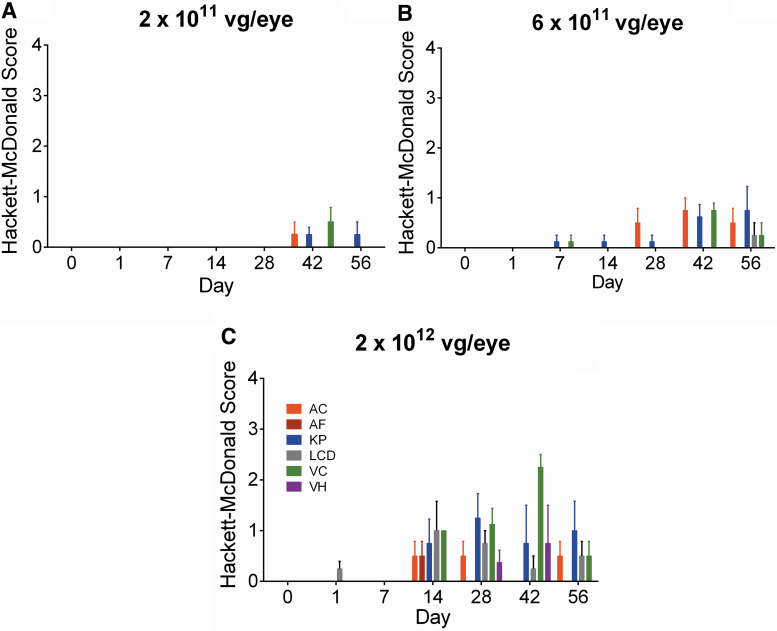
Ocular inflammatory responses were initially assessed for 56 days following a single injection of ADVM-022 at doses of 2 × 1011, 6 × 1011, or 2 × 1012 vg/eye, and showed a dose-related mild to moderate inflammatory response as assessed by Hackett-McDonald Scoring (Fig. 6).14 A longer term ocular safety study evaluated the inflammatory responses following a single IVT injection of ADVM-022 at the highest dose evaluated from the previous study (2 × 1012 vg/eye), out to 21 and 30 months postinjection using slit-lamp biomicroscopy and fundoscopy. In addition, retinal morphology and functional integrity were assessed by OCT and ERG, respectively.13
FIG. 6.
(A–C) AC, AF, KP, LCD, VC, and VH were parameters observed in the eyes treated with ADVM-022 injected at 2 × 1011, 6 × 1011, or 2 × 1012 vg/eye, out of 21 parameters scored by slit-lamp examination.18 Scores are shown as mean ± SEM; n = 4 eyes. Printed with permission from Kiss et al.14 AC, aqueous cell; AF, aqueous flare; LCD, lens capsule deposit; KP, keratic precipitate; VCs, vitreous cells; VH, vitreous haze.
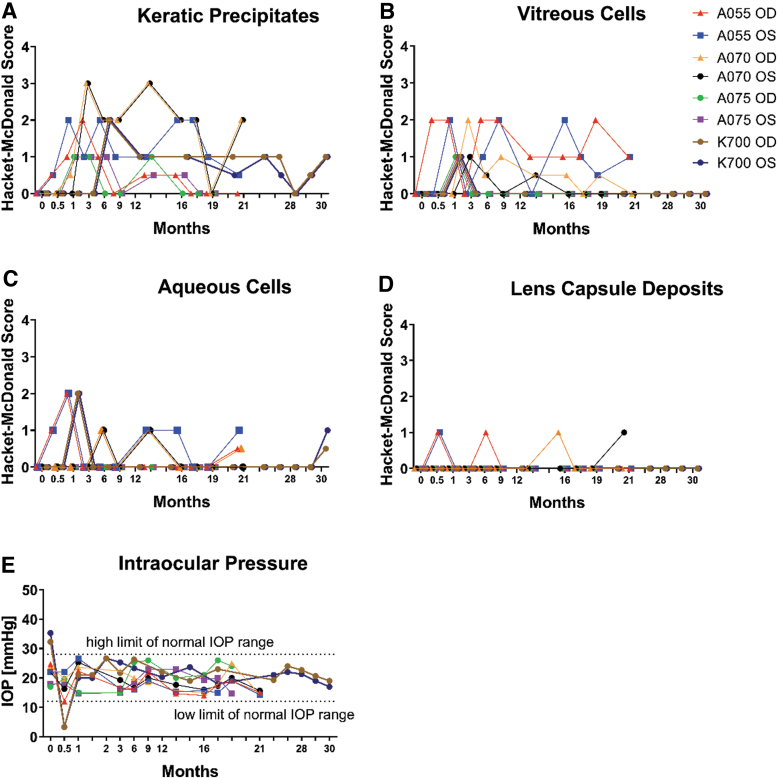
As shown in Fig. 7, signs of inflammation were limited to mild aqueous cell infiltrates, mild vitreous cell infiltrates, mild to moderate keratic precipitates, and incidental mild lens capsule deposits. There was no aqueous flare observed throughout the study in any of the eyes. A single case of mild vitreous haze was detected in one eye (A055 OD) at 12.5 months and resolved without anti-inflammatory treatment by 17.5 months as assessed by slit lamp examination and color fundus imaging. Additional parameters of inflammation and safety were evaluated and no obvious anterior or posterior inflammation was observed in these studies. Intraocular pressure (IOP) remained within normal limits in all eyes, except OD and OS of K700, which experienced a transient IOP decrease 14 days post-treatment (Fig. 2E), which was completely resolved by the next time point (1 month postdose). After that time point, the IOP remained normal for all the eyes for the duration of the study. These long-term safety assessments demonstrate that ADVM-022 was well tolerated throughout the study periods. In addition, no clinical sign indicative of ADVM-022-related systemic effects was observed.13 However promising, caution should be taken in extrapolating these data into human clinical trials.
FIG. 7.
(A–D) Ocular clinical pathology and (E) IOP in the individual eyes treated with ADVM-022 were assessed throughout the course of 2 studies at the indicated time points and out to 30 months after intravitreal injection. Examinations revealed mild-to-moderate inflammation with resolving trend throughout the time course without anti-inflammatory intervention. The parameters scored by the Hackett-McDonald irritation and inflammation scoring system are shown: (A) KPs, (B) VCs, (C) ACs, (D) LCDs, and (E) IOP. No VH has been detected, except in one eye (A055, OD) at only one time point (12.5 months) at grade 1+. (E) IOP was within normal range, except for both eyes in animal K700 on D14 (when it dropped to 3.3 mmHg). The IOP in both eyes of K700 returned to normal at the next time point assessed (D28). Normal lower and upper IOP limits were determined based on the IOP measurements conducted on 148 eyes at baseline. Lower and upper IOP limits are designated as 2 standard deviations of the mean value (μ ± 2σ). Scores are represented for each eye in the study for every time point assessed and each symbol represents one individual eye.13 Printed with permission from Kiss et al.13 IOP, intraocular pressure.
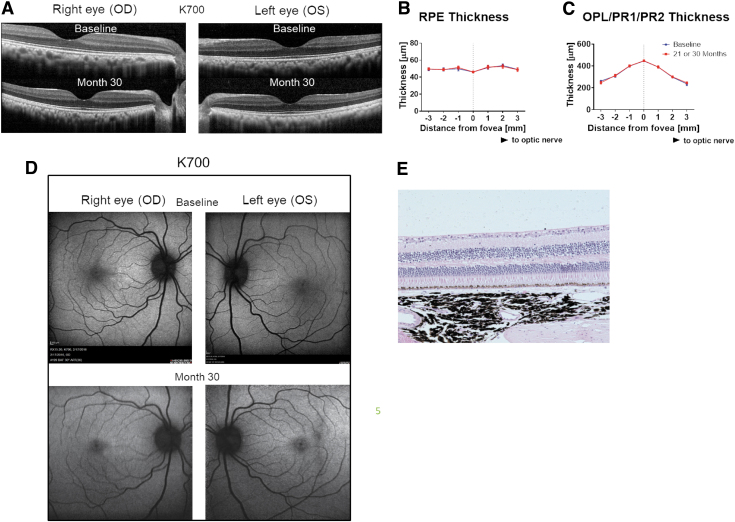
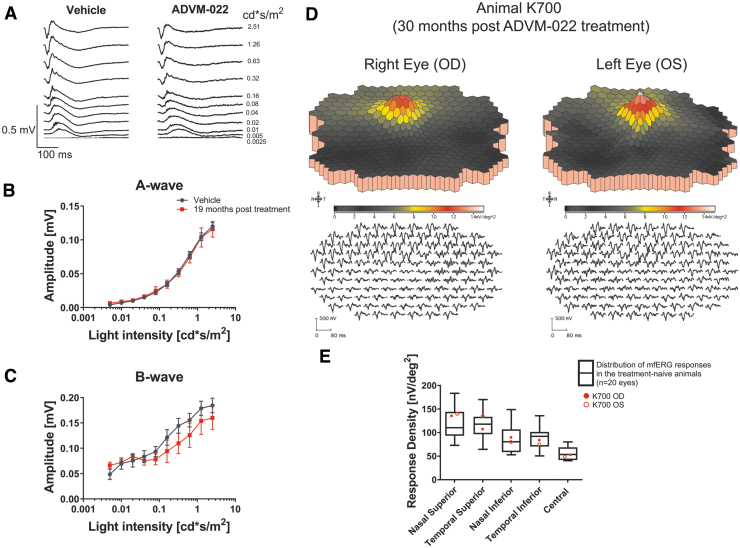
Retinal morphology was also assessed in these same 4 animals at 21 (n = 3) and 30 months (n = 1).13 As shown in Fig. 8, long-term expression of aflibercept in the retina from ADVM-022-treated animals did not result in retinal toxicity, as evidenced by normal retinal morphology and histology features evaluated by OCT and hematoxylin and eosin (H&E) staining, respectively (Fig. 8A, E), assessment of retinal pigmented epithelium (RPE) thickness (Fig. 8B), or assessment of outer nuclear-photoreceptor 1-photoreceptor 2 (ONL-PR1-PR2) thickness (Fig. 8C). Representative fundus autofluorescence micrographs of animal K700 at baseline and 30 months post-ADVM-022 treatment are shown in Fig. 8D.13
FIG. 8.
Long-term expression of aflibercept in the retina from ADVM-022-treated animals does not affect retinal morphology features as evaluated by the OCT or H&E staining. (A) OCT images of both eyes from a representative animal (K700) treated with ADVM-022 were analyzed at baseline and at 30 months post-treatment. (B) RPE and (C) ONL/PR1/PR2 layer thickness measurement revealed no difference in any of the segments analyzed. Analysis is performed at baseline and at respective terminal time points (21 or 30 months postdosing) (student t test, P = 0.99 for RPE analysis and P = 0.79 for ONL/PR1/PR2 analysis). Please note that some of the error bars are smaller than the symbol size. (D) Fundus autofluorescence micrographs of animal K700 at baseline and 30 months post-ADVM-022 treatment. (E) H&E staining of animal K700 OS retinal tissue 30 months post-treatment. Note the integrity of all retinal layers and absence of any morphological abnormality.13 Printed with permission from Kiss et al.13 H&E, hematoxylin and eosin; RPE, retinal pigmented epithelium.
ERG responses assessed at 19 months postinjection in 2 treated animals (A055 and A075, n = 4 eyes) were normal and showed no difference compared with 6 vehicle-treated eyes (Fig. 9A–C). First-order three-dimensional multifocal ERG topography density at 30 months post-treatment (K700) also revealed normal recordings (Fig. 9D), and ERG response density at different retinal regions from both eyes of animal K700 30 months postdose was not significantly different than those observed in treatment-naive animals (Fig. 9E).13
FIG. 9.
ERG analysis of the ADVM-022-treated animals at the late time points reveals normal ERG responses. (A) Scotopic ERG recordings were performed on 2 animals (A055 and A075, n = 4 eyes) treated with ADVM-022 19 months postinjection and compared to vehicle-treated animals (n = 6 eyes). (B) Analysis of scotopic A-wave and (C) B-wave revealed no statistically significant difference between ADVM-022 and vehicle-treated animals (RM-ANOVA A-wave P = 0.95 and B-wave P = 0.48). (D) First-order three-dimensional multifocal ERG topography density at 30 months post-treatment (K700) revealed normal recordings. The stimulus configuration is an array of 103 hexagons scaled with retinal eccentricity. A local response was derived for each of the 103 stimulus elements. First-order ERG recording traces are shown on the lower panels. The mfERG traces are shown equally spaced for clarity. (E) ERG response density at different regions of retina from both eyes for the animal treated with ADVM-022 (K700) at 30 months postdose was plotted against distribution of the ERG responses in treatment-naive, healthy animals (white boxes, n = 20 eyes), and were within limits of responses recorded in the treatment-naive animals.13 Printed with permission from Kiss et al.13 ERG, electroretinography; RM-ANOVA, repeated measures-analysis of variance.
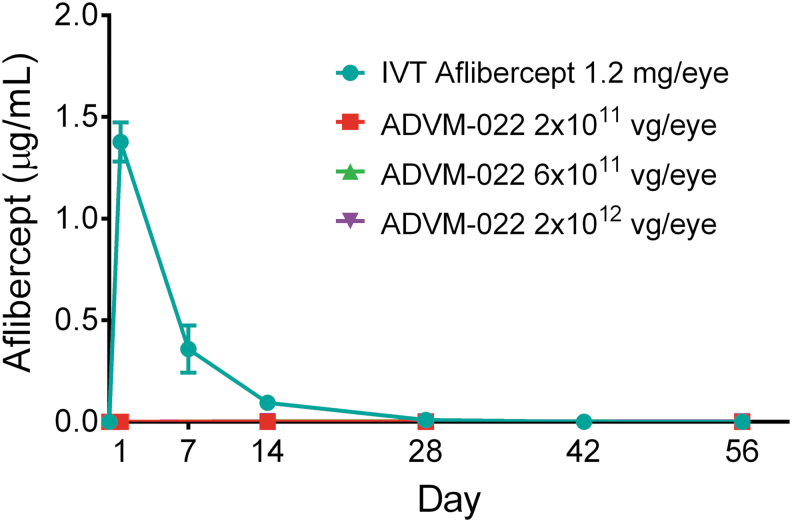
The potential for systemic exposure to aflibercept is a possible safety concern, given the persistent expression of the protein following IVT injection of ADVM-022. The presence of aflibercept protein in serum was assessed out to 56 days post-IVT injection of ADVM-022 (2 × 1011, 6 × 1011, or 2 × 1012 vg/eye) or aflibercept (1.2 mg/eye). As shown in Fig. 10, aflibercept expression in the eye following IVT injection of ADVM-022 did not result in quantifiable levels of aflibercept in the systemic circulation at any point in the study at all 3 doses evaluated. Conversely, IVT injection of aflibercept recombinant protein resulted in significant levels of the protein observed immediately postinjection, which remained detectable out to 28 days postdosing.14
FIG. 10.
Serum levels of aflibercept following IVT delivery of either aflibercept recombinant protein or increasing doses of ADVM-022. Mean ± SEM for treatment groups is shown. Note that the data points for each ADVM-022 dose are superimposed on the x axis. For ADVM-022-dosed animals, n = 2 animals per dose at each time point. Due to the serum and other tissue collection schedule, the number of serum specimens from animals dosed with aflibercept was different at each of the time points [n = 12 at baseline (all aflibercept-treated subgroups); n = 2 on day 1 (subgroup 1a); n = 2 on day 7 (subgroup 1b); n = 8 on day 14 (subgroups 1c, d, e, and f); n = 6 on day 28 (subgroups 1d, e, and f); n = 4 on day 42 (subgroups 1e and f); and n = 2 on day 56 (subgroup 1f)]. Printed with permission from Kiss et al.14
Discussion
The comprehensive preclinical expression, ocular safety, and efficacy data generated to date for ADVM-022 in nonhuman primates13–15 reviewed in this study demonstrate the potential of a single administration of ADVM-022 as an alternative to frequent bolus injections of VEGF inhibitor proteins for the treatment of nAMD and DME. Indeed, these data show that a single IVT injection of ADVM-022 results in sustained aflibercept expression out to 30 months postdose at levels that are consistent with therapeutic levels achieved following a bolus administration of aflibercept recombinant protein. In addition, the levels of aflibercept achieved following a single IVT injection of ADVM-022 (2 × 1012 vg/eye) have demonstrated efficacy in the laser-induced CNV model more than a year (13 months) postdosing.
These promising expression and efficacy results were achieved with a favorable safety profile, and without the need for intervention of an anti-inflammatory agent. Long-term (21 and 30 months) safety assessments demonstrated that inflammatory responses were generally mild to moderate and self-resolving, and that the long-term continuous expression of ADVM-022-derived aflibercept did not impact the retina structure or its function.13 The lack of systemic aflibercept exposure following IVT injection of ADVM-022 may also provide safety benefits compared with bolus IVT injection of anti-VEGF protein therapies. Injections of aflibercept or bevacizumab recombinant proteins can be associated with a reduction in systemic VEGFA levels.24–27 While the clinical implications of this suppression are not fully understood, some data suggest that certain patient subpopulations may exhibit an increased risk for systemic serious adverse events, including those with diabetes, recent myocardial infarction, or cerebrovascular accidents.24–27 Given the reduced compliance with the recommended dosing schedules of approved anti-VEGF protein therapies that are associated with frequent IVT injections9 and the sub-optimal outcomes associated with this lack of compliance,6–8 single-dose ADVM-022 has the potential to improve clinical outcomes in patients with nAMD and DME.
As anti-VEGF therapeutics have been used to treat ocular neovascular diseases for over 2 decades, it was necessary to conduct a comprehensive preclinical program to allow for early scrutiny and a thorough analysis of this clearly disruptive technology, which has the potential to be transformative for nAMD and DME patients and caregivers alike. The rigorous approach described in this study was undertaken to establish the efficacy in a relevant disease model, and long-term safety and pharmacokinetic analysis in a relevant animal species, the nonhuman primate. The robust data set obtained from these studies supported the evaluation of ADVM-022 in human studies, that is, the OPTIC Phase 1 for nAMD and INFINITY Phase 2 trials in DME, which are currently ongoing, and in addition allowed ADVM-022 to be granted Fast Track Designation from the FDA in recognition of the need for new nAMD therapies. As ADVM-022 and other gene therapy-based approaches gain traction in the clinic, the preclinical path for each should be shared with the medical and scientific community to increase the chances of success in identifying innovative treatments for patients with neovascular retinal disease.
Acknowledgments
Authors would like to thank Aaron Osborne, MD, for critical discussions and article review, and Stephanie Seiler, PhD, for editorial support.
Author Disclosure Statement
C.M.G., R.G., K.O.B., A.N., J.G., P.S., and J.N. are employees of Adverum Biotechnologies and hold stock grants; S.K. and M.G. are paid consultants of Adverum Biotechnologies and hold grants of Adverum Biotechnologies stock.
Funding Information
This study was fully funded by Adverum Biotechnologies.
References
- 1. Wang, W., and Lo, A.C.Y.. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 19:1816, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenfeld, P.J., Rich, R.M., and Lalwani, G.A.. Ranibizumab: phase III clinical trial results. Ophthalmol. Clin. North Am. 19:361–372, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Lynch, S.S., and Cheng, C.M.. Bevacizumab for neovascular ocular diseases. Ann. Pharmacother. 41:614–625, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Heier, J.S., Brown, D.M., Chong, V., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 119:2537–2548, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Holekamp, N.M., Liu, Y., Yeh, W.S., et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am. J. Ophthalmol. 157:825–833.e1, 2014 [DOI] [PubMed] [Google Scholar]
- 6. Boulanger-Scemama, E., Querques, G., About, F., et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J. Fr. Ophtalmol. 38:620–627, 2015 [DOI] [PubMed] [Google Scholar]
- 7. Droege, K.M., Muether, P.S., Hermann, M.M., et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch. Clin. Exp. Ophthalmol. 251:1281–1284, 2013 [DOI] [PubMed] [Google Scholar]
- 8. Hussain, R.M., Hariprasad, S.M., and Ciulla, T.A.. Treatment burden in neovascular AMD: visual acuity outcomes are associated with anti-VEGF injection frequency. Ophthalmic Surg. Lasers Imaging Retina. 48:780–784, 2017 [DOI] [PubMed] [Google Scholar]
- 9. Boyle, J., Vukicevic, M., Koklanis, K., Itsiopoulos, C., and Rees, G.. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol. Health Med. 23:127–140, 2018 [DOI] [PubMed] [Google Scholar]
- 10. Avery, R.L., Castellarin, A.A., Steinle, N.C., et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98:1636–1641, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Do, D.V., Rhoades, W., and Nguyen, Q.D.. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina. 40:643–647, 2020 [DOI] [PubMed] [Google Scholar]
- 12. Picaud, S., Dalkara, D., Marazova, K., Goureau, O., Roska, B., and Sahel, J-A.. The primate model for understanding and restoring vision. Proc. Natl. Acad. Sci. U. S. A. 116:26280–26287, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiss, S., Bender, K.O., Grishanin, R., et al. Long-term safety evaluation of continuous intraocular delivery of aflibercept in the intravitreal gene therapy candidate ADVM-022 in nonhuman primates. Trans. Vis. Sci. Technol. 10:34, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiss, S., Grishanin, R., Nguyen, A., et al. Analysis of aflibercept expression in NHPs following intravitreal administration of ADVM-022, a potential gene therapy for nAMD. Mol. Ther. Methods Clin. Dev. 18:345–353, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grishanin, R., Vuillemenot, B., Sharma, P., et al. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol. Ther. 27:118–129, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalkara, D., Byrne, L.C., Klimczak, R.R., et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5:189ra76, 2013 [DOI] [PubMed] [Google Scholar]
- 17. Goody, R.J., Hu, W., Shafiee, A., et al. Optimization of laser-induced choroidal neovascularization in African green monkeys. Exp. Eye Res. 92:464–472, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Goody, R.J., Banerjee, A., and Lawrence, M.S.. Validating a scoring methodology for drug-induced anterior segment inflammation in nonhuman primate eyes. Society of Toxicology Annual Meeting. Poster P708, 2018 [Google Scholar]
- 19. Nork, T.M., Dubielzig, R.R., Christian, B.J., et al. Prevention of experimental choroidal neovascularization and resolution of active lesions by VEGF trap in nonhuman primates. Arch. Ophthalmol. 129:1042–1052, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Giani, A., Luiselli, C., Esmaili, D.D., et al. Spectral-domain optical coherence tomography as an indicator of fluorescein angiography leakage from choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 52:5579–5586, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Giani, A., Thanos, A., Roh, M.I., et al. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52:3880–3887, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Cloutier, F., Lawrence, M., Goody, R., et al. Antiangiogenic activity of aganirsen in nonhuman primate and rodent models of retinal neovascular disease after topical administration. Invest. Ophthalmol. Vis. Sci. 53:1195–1203, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Sidman, R.L., Li, J., Lawrence, M., et al. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 7:309ra165, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avery, R.L., and Gordon, G.M.. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol.134:21–29, 2016 [DOI] [PubMed] [Google Scholar]
- 25. Wang, X., Sawada, T., Sawada, O., Saishin, Y., Liu, P., and Ohji, M.. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am. J. Ophthalmol. 158:738–744.e1, 2014 [DOI] [PubMed] [Google Scholar]
- 26. Zehetner, C., Kirchmair, R., Huber, S., Kralinger, M.T., and Kieselbach, G.F.. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br. J. Ophthalmol. 97:454–459, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Avery, R.L., Castellarin, A.A., Steinle, N.C., Dhoot, D.S., Pieramici, D.J., See, R., Couvillion, S., Nasir, M.A., Rabena, M.D., Le, K., Maia, M., and Visich, J.E.. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 98:1636–1641, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]