Abstract
CRISPR-derived biotechnologies have revolutionized the genetic engineering field and have been widely applied in basic plant research and crop improvement. Commonly used Agrobacterium- or particle bombardment-mediated transformation approaches for the delivery of plasmid-encoded CRISPR reagents can result in the integration of exogenous recombinant DNA and potential off-target mutagenesis. Editing efficiency is also highly dependent on the design of the expression cassette and its genomic insertion site. Genetic engineering using CRISPR ribonucleoproteins (RNPs) has become an attractive approach with many advantages: DNA/transgene-free editing, minimal off-target effects, and reduced toxicity due to the rapid degradation of RNPs and the ability to titrate their dosage while maintaining high editing efficiency. Although RNP-mediated genetic engineering has been demonstrated in many plant species, its editing efficiency remains modest, and its application in many species is limited by difficulties in plant regeneration and selection. In this review, we summarize current developments and challenges in RNP-mediated genetic engineering of plants and provide future research directions to broaden the use of this technology.
Keywords: CRISPR, RNP, genetic engineering, genome editing, transgene free
CRISPR-derived genome engineering technologies have been widely applied in plant science. Delivery of CRISPR reagents as RNPs is a promising approach for the generation of transgene-free genome-edited crops. This review summarizes recent progress, discusses present challenges, and presents some future perspectives on this topic.
Introduction
Plant genetic engineering has been revolutionized by the rapid development of CRISPR-derived biotechnologies because of their simple, inexpensive, and efficient application in many plant species (Li et al., 2013; Nekrasov et al., 2013; Shan et al., 2013; Zhang et al., 2019). CRISPR relies on the nuclease activity of CRISPR-associated proteins (Cas) and their specific binding to the genome directed by guide RNAs (gRNAs). CRISPR-Cas generates DNA double-strand breaks and triggers endogenous repair pathways. Insertions and deletions (Indels) can be introduced through the nonhomologous end joining (NHEJ) repair pathway, the predominant repair pathway in plant somatic tissues, resulting in random mutagenesis at the target site (Puchta, 2005; Schmidt et al., 2019). Precise genome editing can be achieved through the homology-directed repair (HDR) pathway by introducing repair templates. Cas proteins can be further engineered to recruit effector protein domains in order to achieve base editing (Kim, 2018), prime editing (Li et al., 2020b; Butt et al., 2020; Lin et al., 2020; Lu et al., 2020; Tang et al., 2020; Xu et al., 2020), epigenome editing (Gallego-Bartolomé et al., 2018; Papikian et al., 2019), and transcriptional regulation (Lowder et al., 2015, 2018; Piatek et al., 2015; Li et al., 2017; Tang et al., 2017). To date, three major CRISPR systems for plant genome editing have been successfully demonstrated: Cas9, Cas12a, and Cas12b (Zhang et al., 2019; Ming et al., 2020). Diverse Cas orthologs and variants are available with different protospacer adjacent motif (PAM) requirements, gRNA scaffolds, temperature tolerances, and editing efficiencies. All these tools enable the broad application of CRISPR technology to plant breeding and fundamental research (Chen et al., 2019; Pramanik et al., 2020; Veillet et al., 2020).
Agrobacterium- or particle bombardment-mediated transformation with DNA harboring CRISPR expression cassettes is the most common approach for the delivery of CRISPR reagents, including Cas proteins and gRNAs, into plant cells and tissues. The random integration of CRISPR cassettes into genomes raises concerns and regulatory burdens. Although these transgene elements can be segregated from the editing events of interest through breeding, this process is usually time- and labor-consuming, and it is not a viable option for species and varieties that have a lengthy juvenile growth period (e.g., trees) or are typically vegetatively propagated. To avoid the integration of foreign DNA, the transient expression of transgenes without selection has been used to generate transgene-free genome-edited plants (Iaffaldano et al., 2016; Zhang et al., 2016). Although particle bombardment can be used to deliver various types of cargo, it can cause random DNA integrations into the plant genome as well as genome damage (Banakar et al., 2019; Liu et al., 2019), making it difficult to monitor and eliminate transgenes. Another concern is that the genomic DNA will be continuously exposed to CRISPR reagents if the integrated transgene cassettes are continuously expressed, increasing the possibility of off-target mutagenesis and chimeric mutants (Fu et al., 2013; Pattanayak et al., 2013; Hashimoto et al., 2016). Plasmid-based CRISPR genome editing also requires efficient species-specific expression systems, including optimal promoters and terminators, as well as codon optimization. An efficient multiplexing system is also critical when editing multiple targets and is especially important for polyploid plants in which more than one gRNA may be needed to knock out a single gene. The expression of transgenes can also be affected by their integration position (Matzke and Matzke, 1998). In addition, DNA-based Cas protein expression is toxic in some mammalian cell types and organisms (Jiang et al., 2014; Morgens et al., 2017; Foster et al., 2018).
To mitigate the potential problems associated with DNA delivery, mRNA encoding Cas proteins can be co-delivered with the gRNA(s) into plants by particle bombardment (Zhang et al., 2016). Alternatively, the Cas protein and gRNA(s), two components of the CRISPR system, can be preassembled to form ribonucleoproteins (RNPs) and introduced into plants. Because no recombinant DNA is involved in this process if the gRNAs are chemically synthesized, plants edited by RNPs can be considered transgene free. Moreover, RNPs are only transiently present in plant cells and are degraded by endogenous proteases and nucleases (Kim et al., 2014), thus minimizing off-target effects and mosaicism caused by prolonged exposure of genomic DNA to CRISPR reagents (Kim et al., 2017; Liang et al., 2017). RNPs can be used for all organisms that do not have delivery barriers without considering promoter compatibility or multiplexing strategies. Multiple gRNAs can be used simultaneously to target multiple genomic regions. Different types of CRISPR-Cas systems and orthogonal Cas proteins can also be used simultaneously if there is no crosstalk between them, allowing multiple editing outcomes to be achieved (Najm et al., 2018). Furthermore, RNP-mediated genome editing has been demonstrated in cells and organisms that cannot be edited with CRISPR delivered by plasmids because of toxicity (Jiang et al., 2014; Shin et al., 2016; Foster et al., 2018).
RNP-mediated genome editing has been demonstrated in many organisms, including Caenorhabditis elegans (Cho et al., 2013), human cells (Kim et al., 2014), mice (Zuris et al., 2015), fungi (Foster et al., 2018), and plants (Woo et al., 2015; Li et al., 2020a). RNPs have been widely used for gRNA screening in vitro and in vivo. RNP-mediated genome editing can be achieved shortly after cell transfection because transcription or translation is not required (Kim et al., 2014). In plants, RNP-mediated genetic engineering has been demonstrated in protoplasts, and RNP-edited plants have been generated. Although RNP-based CRISPR technology holds promise for generating transgene-free and improved germplasm that can be more readily commercialized (Metje-Sprink et al., 2019), it still presents many technological limitations and challenges. Here, we review current advances in RNP-mediated transgene-free genome editing in plants. We also highlight challenges and future research directions in this field.
RNP production
CRISPR-endonuclease production
Multiple Cas protein versions, including Cas9, HiFi Cas9, Cas9 nickase, catalytically dead Cas9 (dCas9), Cas9 fusion proteins, and Cas12a, are currently commercially available, providing a highly accessible avenue for conducting RNP-mediated genetic engineering. However, many CRISPR applications cannot be performed using commercially available Cas proteins. For example, many unique Cas variants harboring mutations that yield altered PAM requirements and increase fidelity or activity are not currently available. Cas fusion proteins that can achieve base editing, prime editing, and epigenome editing in plants have not been developed for RNP delivery. To broaden the application of RNP-mediated genetic engineering and potentially reduce cost, CRISPR endonucleases can also be produced using E. coli expression systems. One commonly used approach is to transform a plasmid encoding a Cas gene driven by the T7 promoter into E. coli. The Cas gene sequence is usually flanked by nuclear localization signals and tagged with the hexahistidine-maltose binding protein (6-His-MBP) for downstream purification (Kim et al., 2014). Such plasmids for Cas9 expression and purification are available from Addgene and include pMJ915 (plasmid no. 69090) (Lin et al., 2014), pET-28b-Cas9-His (plasmid no. 47327) (Gagnon et al., 2014), and pET28a-Cas9-His (plasmid no. 98158) (Liang et al., 2017). In addition, Cas12a can be produced using the plasmid 6-His-MBP-TEV-FnCpf1 (plasmid no. 90094) (Zetsche et al., 2015). Different Cas proteins, protein variants, and fusion proteins can be customized based on existing plasmids and produced using a similar procedure. To date, Cas protein production and purification are still a significant challenge for many labs, limiting the diversity of CRISPR applications that involve Cas fusion proteins and unique Cas variants.
Guide RNA production
Similarly, gRNA can be produced by in vitro transcription (IVT) or synthesized commercially. The transcription template is first prepared with a T7 promoter in front of the gRNA sequence that includes the target-specific protospacer sequence and the gRNA scaffold. The gRNA is then transcribed by T7 RNA polymerase in vitro, a step that can be performed using commercial RNA synthesis kits. The gRNA is then purified and ready for RNP assembly. Although gRNA can be produced inexpensively using IVT, studies have shown that DNA contamination from gRNA produced by IVT can integrate into the genome at the CRISPR cutting site, resulting in undesired DNA insertions (Kim et al., 2017; Andersson et al., 2018). This problem cannot be fully resolved by DNase treatment. Commercially synthesized gRNA exhibits high purity and eliminates this DNA contamination problem (Kim et al., 2017). For the CRISPR-Cas9 system, a gRNA can be synthesized as a single guide RNA (sgRNA) or as a CRISPR RNA (crRNA):trans-activating CRISPR RNA (tracrRNA) duplex; the latter is more economically viable. Only crRNA is required for the Cas12a system, lowering the cost of gRNA synthesis compared with the Cas9 and Cas12b systems. When gRNAs are made by direct synthesis, chemical modifications can also be added to enhance their stability in cells (Hendel et al., 2015).
RNP assembly
The RNP complex can be assembled by simply mixing the Cas and the gRNA with the reaction buffer, followed by incubation at room temperature for 10 min (Liang et al., 2017; Liu et al., 2020). If a crRNA:tracrRNA duplex is used instead of a single gRNA, an annealing step is required before RNP assembly. Theoretically, a Cas:gRNA ratio of 1:1 can be used for RNP assembly (Kim et al., 2014), and Cas:gRNA ratios of 3:1, 1:1, 1:2, 1:3, and 1:6 have been used in plants (Malnoy et al., 2016; Subburaj et al., 2016; Svitashev et al., 2016; Kim et al., 2017). Providing more Cas or gRNA does not consistently improve editing efficiency (Malnoy et al., 2016). At this point, the assembled RNPs are ready for plant transformation.
RNP delivery into plants
PEG-mediated protoplast or zygote transfection
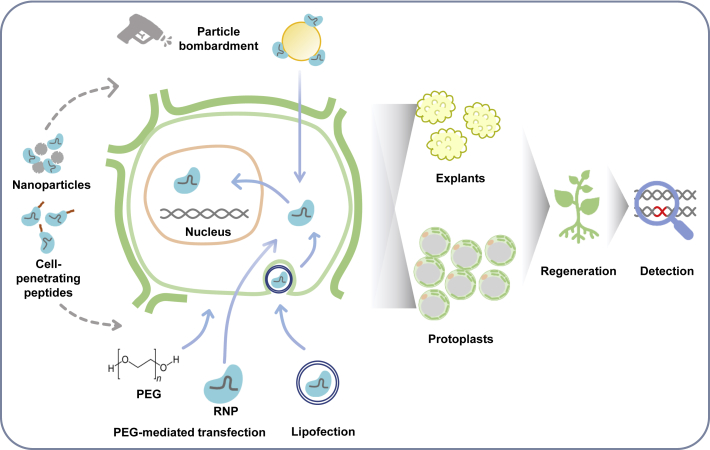
Four methods have been used previously to deliver RNPs into plants: polyethylene glycol (PEG)-mediated cell transfection, particle bombardment, electroporation, and lipofection (Figure 1). PEG-mediated cell transfection requires the pre-removal of the cell wall using cellulase and pectinase. Editing efficiencies can be assessed 1–3 days after transfection. Efficiencies of different Cas systems and gRNAs can be quickly evaluated using this method. PEG-mediated protoplast assays have been developed for many plant species, including Arabidopsis thaliana, rice (Oryza sativa), lettuce (Lactuca sativa) (Woo et al., 2015), tobacco (Nicotiana tabacum and N. attenuata) (Woo et al., 2015; Kim et al., 2017), petunia (Petunia × hybrida) (Subburaj et al., 2016; Yu et al., 2020), maize (Zea mays) (Sant’Ana et al., 2020), grapevine (Vitis vinifera), apple (Malus × domestica) (Malnoy et al., 2016), wheat (Triticum aestivum) (Liang et al., 2017), soybean (Glycine max) (Kim et al., 2017; Kim and Choi, 2020), potato (Solanum tuberosum) (Andersson et al., 2018; González et al., 2020), cabbage (Brassica oleracea), Chinese cabbage (Brassica rapa) (Murovec et al., 2018), and banana (Musa spp.) (Wu et al., 2020). Up to 71% editing efficiency was achieved in Arabidopsis (Woo et al., 2015). Because plant protoplasts can be totipotent, it is possible to regenerate edited plants from transfected protoplasts. In lettuce, 46% editing efficiency was observed in microcalli regenerated from protoplasts treated with CRISPR-Cas9 RNPs (Woo et al., 2015). In potato, 1%–25% editing efficiency was obtained in regenerated shoots (Andersson et al., 2018). Recently, up to 68% editing efficiency was obtained in potato plants regenerated from protoplasts, 24% of which were edited in all four alleles (González et al., 2020). In petunia, a mutation frequency of 11.9% was observed in T0 plants regenerated from RNP transfected protoplasts (Yu et al., 2020). In cabbage, edits were detected in 8 out of 46 protoplast-regenerated plants (Park et al., 2019). To date, methods to regenerate whole plants from protoplasts have been developed in many species, including lettuce (Woo et al., 2015), potato (Andersson et al., 2018; González et al., 2020), tobacco (Lin et al., 2018), carrot (Daucus carota) (Grzebelus et al., 2012), petunia (Yu et al., 2020), cabbage (Park et al., 2019), maize (Rhodes et al., 1988; He et al., 2006), and rice (Li and Murai, 1990). However, the regeneration procedure for many of these species is challenging, and the efficiency remains low (Eeckhaut et al., 2013; Altpeter et al., 2016). Moreover, plants regenerated from protoplasts contain somaclonal variations and may have unstable genomes (Kawata and Oono, 1998; Tang et al., 2018; Fossi et al., 2019), raising concerns about undesired mutations and phenotypic changes. To overcome difficulties in protoplast regeneration, Toda et al. (2019) used PEG to introduce CRISPR-Cas9 RNPs into rice zygotes. Editing efficiencies of 14%–64% were observed in mature plants derived from the transformed zygotes, providing a new avenue for RNP delivery.
Figure 1.
CRISPR RNP-mediated genetic engineering in plants.
CRISPR reagents can be delivered into plant cells as ribonucleoproteins (RNPs). Particle bombardment can be used to deliver RNPs into explants, and polyethylene glycol (PEG)-mediated transfection and lipofection can be used to deliver RNPs into protoplasts. Nanoparticles and cell-penetrating peptides are emerging methods for RNP delivery into plants. Transformed cells and tissues are used for plant regeneration and edit detection.
Particle bombardment
Particle bombardment is another commonly used approach to deliver RNPs into plant tissues and cells. Explants that are typically used for plant regeneration, including embryos and leaf discs, can be used as targets of RNP-coated particles. The explants can then be used for plant regeneration, with or without selection. RNP-mediated genome editing using particle bombardment has been successfully demonstrated in rice (Banakar et al., 2020), maize (Svitashev et al., 2016), and wheat (Liang et al., 2017). Without any selection, the editing efficiency is usually less than 10%. To enhance editing efficiency, plasmids encoding a selective marker gene can be co-transformed with RNPs into plants (Svitashev et al., 2016; Banakar et al., 2020). Although the editing efficiency can be improved significantly, high-frequency random DNA insertions have also been detected (Banakar et al., 2019), defeating the main purpose of RNP delivery. Notably, particle bombardment can result in a small or large degree of genomic damage, including chromosome truncations, DNA fragment deletions, and genomic rearrangement, potentially altering phenotypes and complicating commercialization (Liu et al., 2019).
Emerging transformation methods
Recently, RNPs have been delivered into tobacco (N. tabacum) BY2 protoplasts by lipofection, and ~6% editing efficiency has been achieved (Liu et al., 2020). Plant cell walls must be degraded to enable lipofection. Preassembled RNPs can be mixed with cationic lipids, which are positively charged and therefore attracted to the negatively charged surfaces of plant protoplasts. RNPs and lipids can form a liposome structure that can easily merge with cell membranes, resulting in RNP transfection. Lipofection is considered an easy and inexpensive method for cell transfection that can potentially be applied to other plant species (Yu et al., 2016; Liu et al., 2020). In addition, electroporation was used for Chlamydomonas reinhardtii transformation, and up to 1.1% editing efficiency was achieved (Baek et al., 2016). Recently, electro-transfection has also been demonstrated in cabbage, and editing efficiency as high as 3.4% was obtained (Lee et al., 2020). This method may improve the regeneration ability of protoplasts and increase editing efficiency compared with the PEG method. It is also potentially applicable to other plant species.
RNP applications in plant genome editing
RNP-mediated genome editing has been demonstrated in many plant species, targeting genes of agricultural importance, including those involved in grain yield (Liang et al., 2017; Toda et al., 2019), disease resistance (Malnoy et al., 2016), nutritional composition (Kim et al., 2017; Andersson et al., 2018), herbicide resistance, male fertility (Svitashev et al., 2016), and other traits (Table 1). These studies provide a foundation for crop improvement using RNP-mediated CRISPR technologies. To date, most studies have used Cas9 for NHEJ-mediated genome editing, and editing efficiencies have varied depending on species, transformation method, transformation efficiency, detection method, RNP purity and amount, gRNA efficiency, and other factors. In addition, Cas12a has also been used in several species. LbCas12a produced less than 1% editing efficiency in wild tobacco (N. attenuata) protoplasts (Kim et al., 2017). In soybean, up to 17.5% editing efficiency was observed in protoplasts using LbCas12a, and the highest editing efficiency obtained using AsCas12a was only 1.6% (Kim et al., 2017; Kim and Choi, 2020). Up to 19.3% editing efficiency was observed in pepper (Capsicum annuum) using LbCas12a (Kim et al., 2020). These low editing efficiencies could potentially be explained by the temperature sensitivity of Cas12a (Malzahn et al., 2019), the formation of undesirable crRNA secondary structures (Lee et al., 2019), and the low activity of Cas12a in certain species. When Cas12a and Cas9 were compared in parallel using overlapping gRNAs in rice, higher editing efficiency was obtained with LbCas12a (32.3%) than with wild-type Cas9 (3.6%) and HiFi Cas9 (8.8%) (Banakar et al., 2020). However, no edited plants were recovered using AsCas12a (Banakar et al., 2020). We summarize studies that have used RNP-delivered CRISPR reagents for genome engineering in Table 1. It is notable that the editing efficiencies reported in these studies vary greatly among different plant species, RNP delivery methods, and sample types (e.g., protoplasts versus regenerated calli or plants). There is room for further improvement to achieve much higher editing efficiencies, making RNP-based CRISPR genome editing more practical for a variety of plant species.
Table 1.
Summary of CRISPR RNP-mediated genetic engineering in plants.
| Species | Target genes | CRISPR system | Plant material | Transformation method | Detection method | Editing efficiency | References |
|---|---|---|---|---|---|---|---|
| Protoplast transformationa | |||||||
| Arabidopsis thaliana (Columbia-0) | Allene oxide cyclase, AOC | Cas9 | protoplast | PEG | targeted deep sequencing | 16% | Woo et al. (2015) |
| BRASSINOSTEROID INSENSITIVE 1 (BRI1) | Cas9 with two gRNAs simultaneously | T7E1b | 54%–71% (A 223 bp deletion is observed) | ||||
| Oilseed rape (Brassica napus cv. Topaz) | Phytoene desaturase (PDS) | Cas9 | protoplast | PEG | targeted deep sequencing | 0 | Murovec et al. (2018) |
| FRIGIDA (FRI) | 0 | ||||||
| Cabbage (Brassica oleracea var. capitata f. alba) | Phytoene desaturase (PDS) | Cas9 | protoplast | PEG | targeted deep sequencing | 0.14%–1.33% | Murovec et al. (2018) |
| FRIGIDA (FRI) | 0.09%–2.25% | ||||||
| Cabbage (Brassica oleracea var. capitata f. alba) | GIGANTEA (GI) | Cas9 | protoplast | PEG | targeted deep sequencing | 2% | Park et al. (2019) |
| Cabbage (Brassica oleracea var. capitata cv. Dongbok) | Phytoene desaturase 1 (PDS1) | Cas9 | protoplast | PEG | targeted deep sequencing | 1.8% | Lee et al. (2020) |
| electro-transfection | 0%–3.4% | ||||||
| Chinese cabbage (Brassica rapa subsp. pekinensis) | Phytoene desaturase (PDS) | Cas9 | protoplast | PEG | targeted deep sequencing | 3.78%–24.51% | Murovec et al. (2018) |
| FRIGIDA (FRI) | 1.15%–12.58% | ||||||
| Hot pepper (Capsicum annuum cv. CM334) | Mildew locus O 2 (MLO2) | Cas9 | callus protoplast | PEG | targeted deep sequencing | 0.2%–17.6% | Kim et al. (2020) |
| Sweet pepper (Capsicum annuum cv. Dempsey) | leaf protoplast | 0.5%–11.3% | |||||
| Apple (Malus domestica cv. Golden Delicious) | DspA/E-interacting proteins of Malus × domestica 1, 2, 4 (DIPM1, 2, 4) | Cas9 | protoplast | PEG | targeted deep sequencing | 0.5%–6.9% | Malnoy et al. (2016) |
| Cavendish banana (Musa spp. Cavendish; AAA Group cv. Baxi) | Phytoene desaturase (PDS) | Cas9 | protoplast | PEG | targeted deep sequencing | 0.19%–0.92% | Wu et al. (2020) |
| Wild tobacco (Nicotiana attenuata) | Phytochrome B, PHYB | Cas9 | protoplast | PEG | targeted deep sequencing | 44% | Woo et al. (2015) |
| Tobacco (Nicotiana tabacum cv. Bright Yellow-2) | ppor-RFP reporter | Cas9 | BY2 protoplast | lipofectamine 3000 | Sanger sequencing | 6% | Liu et al. (2020) |
| Rice (Oryza sativa cv. Dongjin) | P450 | Cas9 | protoplast | PEG | targeted deep sequencing | 19% | Woo et al. (2015) |
| DWD1 | 8.4% | ||||||
| Garden petunia (Petunia × hybrida) | Nitrate reductase (NR) | Cas9 Four gRNAs |
protoplast | PEG | targeted deep sequencing | 5.30%–17.83% (average 11.5 ± 2%) | Subburaj et al. (2016) |
| Garden petunia (Petunia × hybrida cv. Madness Midnight) | Flavanone 3′-hydroxylase (F3HA and F3HB) | Cas9 | protoplast | PEG | targeted deep sequencing | 9.99%–26.27% | Yu et al. (2020) |
| Bread wheat (Triticum aestivum cv. Kenong 199) | Grain width and weight 2 (GW2) | Cas9 | protoplast | PEG | PCR-RE | 33.4% (TaGW2-B1) 21.8% (TaGW2-D1) | Liang et al. (2017) |
| GASR7 | 45.3% | ||||||
| Grapevine (Vitis vinifera cv. Chardonnay) | Mildew locus O 7 (MLO-7) | Cas9 | protoplast | PEG | targeted deep sequencing | 0.1% | Malnoy et al. (2016) |
| Maize (Zea mays) | Inositol phosphate kinase (IPK) | Cas9 | protoplast | PEG | Sanger sequencing | 0.85%–5.85% | Sant’Ana et al. (2020) |
| Hot pepper (Capsicum annuum cv. CM334) | Mildew locus O 2 (MLO2) | LbCas12a | callus protoplast | PEG | targeted deep sequencing | 9.9%–19.3% | Kim et al. (2020) |
| Soybean (Glycine max cv. Williams 82) | Fatty acid desaturase 2-1A (FAD2-1A) | LbCas12a | protoplast | PEG | targeted deep sequencing | 0%–11.7% | Kim et al. (2017) |
| Fatty acid desaturase 2-1B (FAD2-1B) | 0%–9.1% | ||||||
| Fatty acid desaturase 2-1A (FAD2-1A) | AsCas12a | 0%–1.6% | |||||
| Fatty acid desaturase 2-1B (FAD2-1B) | 0%–0.6% | ||||||
| Fatty acid desaturase 2-1A (FAD2-1A) | LbCas12a | cotyledon protoplast | PEG | targeted deep sequencing | 10.5%–11.7% | Kim and Choi (2020) | |
| Fatty acid desaturase 2-1B (FAD2-1B) | 6.7%–9.1% | ||||||
| Fatty acid desaturase 2-1A (FAD2-1A) | callus protoplast | 7.4% | |||||
| Fatty acid desaturase 2-1B (FAD2-1B) | 6.9% | ||||||
| Soybean (Glycine max cv. Kwangan) | Fatty acid desaturase 2-1A (FAD2-1A) | 2.1%–11.8% | |||||
| Fatty acid desaturase 2-1B (FAD2-1B) | 1.9%–9.6% | ||||||
| Soybean (Glycine max cv. Daewon) | Fatty acid desaturase 2-1A (FAD2-1A) | 4.2%–17.5% | |||||
| Fatty acid desaturase 2-1B (FAD2-1B) | 2.0%–10.3% | ||||||
| Wild tobacco (Nicotiana attenuata) | Allene oxide cyclase (AOC) | LbCas12a | protoplast | PEG | targeted deep sequencing | ~0.08%–0.8% | Kim et al. (2017) |
| AsCas12a | ~0.01%–0.9% | ||||||
| Protoplast transformation and plant regenerationc | |||||||
| Cabbage (Brassica oleracea var. capitata f. alba) | GIGANTEA (GI) | Cas9 | protoplast | PEG | Sanger sequencing of plants regenerated from protoplasts | 17.4% (8/46) | Park et al. (2019) |
| Lettuce (Lactuca sativa cv. Cheongchima) | Homolog of A. thaliana BRASSINOSTEROID INSENSITIVE 2 (BIN2) | Cas9 | protoplast | PEG | PCR-REd and targeted deep sequencing using microcalli regenerated from protoplasts | 46% (5.7% monoallelic mutations; 40% biallelic mutations) | Woo et al. (2015) |
| Garden petunia (Petunia × hybrida cv. Madness Midnight) | Flavanone 3′-hydroxylase (F3HA and F3HB) | Cas9 | protoplast | PEG | targeted deep sequencing using regenerated plants | 11.9% | Yu et al. (2020) |
| Potato (Solanum tuberosum cv. Desiree) | Polyphenol oxidase 2 (PPO2) | Cas9 | protoplast | PEG | high-resolution fragment analysis (HRFA) and Sanger sequencing of plants regenerated from protoplasts | 68% (40% PEG) 27% (25% PEG) |
González et al. (2020) |
| Potato (Solanum tuberosum cv. Kuras) | Granule bound starch synthase (GBSS) | Cas9 (synthetically produced gRNA) | protoplast | PEG | HRFA and Sanger sequencing of shoots regenerated from protoplasts | 9% (40% PEG, 30 min) 1% (25% PEG, 3 min) |
Andersson et al. (2018) |
| Cas9 (in vitro transcribed gRNA) | 22% (40% PEG, 30 min) 25% (25% PEG, 3 min) |
||||||
| Particle bombardment of embryoe | |||||||
| Rice (Oryza sativa cv. Nipponbare) | Phytoene desaturase (PDS) | Cas9 with the plasmid encoding hygromycin phosphotransferase (hpt) | Scutellum-derived embryos | particle bombardment | targeted deep sequencing of proliferating hygromycin-resistant callus | 3.6% | Banakar et al. (2020) |
| HiFi Cas9 with the plasmid encoding hpt | 8.8% | ||||||
| Cas9 D10A with two gRNAs and the plasmid encoding hpt | 0 | ||||||
| Cas9 with two gRNAs and the plasmid encoding hygromycin phosphotransferase (hpt) | scutellum-derived embryos | particle bombardment | Sanger sequencing of regenerated lines | 62.9% | Banakar et al. (2019) | ||
| Bread wheat (Triticum aestivum cv. Kenong 199) | Grain width and weight 2 (GW2) | Cas9 | immature embryo | particle bombardment | PCR-RE and Sanger sequencing | 2.2% (TaGW2-B1) 4.4% (TaGW2-D1) | Liang et al. (2017) |
| Bread wheat (Triticum aestivum cv. YZ814) | Grain width and weight 2 (GW2) | Cas9 | immature embryo | particle bombardment | PCR-RE | 1.3% | Liang et al. (2017) |
| GASR7 | 1.8% | ||||||
| Maize (Zea mays) | Male fertility gene MS45 | Cas9 RNP with DNA vectors encoding “helper genes”-cell division-promoting transcription factors (maize ovule developmental protein 2 [ODP2] and maize Wuschel [WUS]) and selectable and visible marker genes (MOPAT-DSRED fusion) | immature embryo | particle bombardment | targeted deep sequencing | 47% (28% monoallelic mutations; 19% biallelic mutations) | Svitashev et al. (2016) |
| Acetolactate synthase (ALS2) | Cas9 RNP with DNA vectors encoding helper genes; 127 nt single-stranded DNA donor |
~2%–2.5% (all monoallelic mutations) | |||||
| Male fertility gene MS45 | Cas9 RNP only | 4.0% (3.1% biallelic mutations) | |||||
| Male fertility gene MS26 | Cas9 RNP only | 2.4% (0.3% biallelic mutations) | |||||
| Liguleless1 (LIG) | Cas9 RNP only | 9.7% (0.9% biallelic mutations) | |||||
| Rice (Oryza sativa cv. Nipponbare) | Phytoene desaturase (PDS) | AsCas12a with the plasmid encoding hpt | scutellum-derived embryos | particle bombardment | targeted deep sequencing of proliferating hygromycin-resistant callus | 0 | Banakar et al. (2020) |
| LbCas12a with the plasmid encoding hpt | 32.3% | ||||||
| Other transformation methods | |||||||
| Chlamydomonas reinhardtii | CpFTSY | Cas9 | Cell | electroporation | targeted deep sequencing | 0.56%–1.1% | Baek et al. (2016) |
| Zeaxanthin epoxidase (ZEP) | 0.46% | ||||||
| Beta subunit of tryptophan synthase (MAA7) | eight targeted mutations obtained | Shin et al. (2016) | |||||
| Chloroplast SRP43 (CpSRP43) | Cas9 RNP with the linearized plasmid encoding hygromycin resistance gene | knockin mutations obtained | |||||
| Mg-protoporphyrin IX S-adenosyl methionine O-methyl transferase (ChlM) | |||||||
| Tobacco (Nicotiana tabacum cv. Bright Yellow-2) | ppor-RFP reporter | Cas9 | BY2 cells | particle bombardment | Sanger sequencing | 3% | Liu et al. (2020) |
| Rice (Oryza sativa cv. Nipponbare) | DsRed2 | Cas9 | Zygotes produced by gamete fusion | PEG | Sanger sequencing using leaves regenerated from transfected zygotes | 25% | Toda et al. (2019) |
|
DROOPING LEAF (DL) |
13.6%–14.3% | ||||||
| GRAIN WIDTH 7 (GW7) | 21.4% | ||||||
|
GENERATIVE CELL SPECIFIC-1 (GCS1) |
64.3% | ||||||
Editing efficiencies were measured using protoplast DNA.
T7 endonuclease 1 assay.
Editing efficiencies were measured using DNA from protoplast-regenerated plants, shoots, or calli.
PCR-restriction enzyme assay.
Editing efficiencies were measured using DNA from embryo-regenerated plants or calli.
Challenges and future perspectives
RNP-mediated CRISPR genome engineering is a promising technique for quickly screening CRISPR components and CRISPR systems, as well as an effective platform for generating transgene-free genetically engineered plants. CRISPR RNPs have also been shown to be a sensitive and easy method for genotyping targeted mutagenesis in plants (Liang et al., 2018). However, RNP-delivered CRISPR has been limited to inducing targeted mutagenesis through the NHEJ repair pathway, leading to imprecise Indels and gene knockouts. Only one maize study used RNPs to achieve precise genome editing through the HDR pathway by introducing a single-stranded DNA template (Svitashev et al., 2016). Although the targeted edits were actively selected during plant regeneration, the editing efficiency was low, and no biallelic mutants were recovered (Svitashev et al., 2016). With the rapid development of technologies to enhance HDR-derived genome editing, higher editing efficiencies may be achieved using RNP delivery. In plants, more drastic changes may be needed for certain kinds of trait improvement, such as metabolic engineering, that involve targeted large DNA fragment deletion and insertion. A 223-bp deletion was achieved in Arabidopsis protoplasts using RNPs (Woo et al., 2015), opening new avenues for RNP-mediated genome engineering. In principle, multiplexed editing can be readily achieved with RNPs. Due to a lack of relevant studies, the number of target sites that can be efficiently edited through RNP-based multiplexed editing in plants remains unclear. Moreover, precise genome editing can also be achieved using base editors and prime editors, but these approaches have not been demonstrated in plants using RNP delivery. Although RNP-mediated gene regulation through the recruitment of regulatory effectors is probably not plausible because of its transient nature, RNPs can be used to edit regulatory elements or epigenetic marks to alter gene expression.
RNP editing efficiency depends largely on delivery efficiency (Figure 1). PEG can efficiently transfect RNPs into plant protoplasts, but this method is only suitable for plant species in which protoplast isolation and regeneration protocols are available. Without the ability to regenerate whole plants from protoplasts, transient experiments to evaluate the efficacy of CRISPR systems can still be conducted. Particle bombardment requires specific equipment and must be optimized for each species. An ideal delivery method would be able to carry large protein complexes such as RNPs and to penetrate plant cell walls and membranes. Nanotechnology provides a potential new method for cargo delivery into plants. Nanomaterials (size <100 nm) have a high surface area to volume ratio and unique physicochemical properties, potentially enabling the efficient delivery of cargos independent of plant species and tissue. In addition, nanomaterials can offer cargo protection and spatiotemporally controlled cargo release (Cunningham et al., 2018; Sanzari et al., 2019). Although the large size, high negative charge, and instability of CRISPR RNP may pose difficulties for nanoparticle delivery, genome editing using nanoparticle-delivered RNP has been achieved in mammalian cells (Wang et al., 2016; Lee et al., 2017; Mout et al., 2017). Nanoparticles have been used to successfully deliver genetic materials and proteins into plants (Cunningham et al., 2018; Sanzari et al., 2019). Nanoparticles were also successfully used to deliver DNA into the pollen of cotton (Gossypium hirsutum), pepper (C. annuum), pumpkin (Cucurbita moschata), ‘Cocozelle’ squash (Cucurbita pepo), and lily (Lilium brownii) by magnetofection, and a tissue culture-free transformation method was established (Zhao et al., 2017). However, this method has not been successfully demonstrated in the monocot species maize (Z. mays) and sorghum (Sorghum bicolor), and it could not be replicated in lily, raising concerns about its utility and efficiency in dicot species (Vejlupkova et al., 2020). In the future, nanoparticles may become new tools for the delivery of RNPs into plants for genetic engineering regardless of plant species. Other delivery methods demonstrated in plants include the modification of proteins with cell-penetrating materials, such as cell-penetrating peptides (Numata et al., 2016; Guo et al., 2019; Midorikawa et al., 2019). These could potentially be used for RNP delivery into plant cells, as has been demonstrated previously for human cells (Ramakrishna et al., 2014).
The application of RNP-mediated genome editing to a wide variety of plant species is limited largely by the absence of robust methods for the recovery of edited plants (Figure 1). Protoplast regeneration remains a challenge in many plant species, especially in monocot crops. Regeneration efficiency relies on numerous factors, including explant type, culture conditions (oxygen level and light), media composition (phytohormones and other growth factors, signaling molecules), and oxidative stress (Eeckhaut et al., 2013). In addition to optimizing regeneration systems for each species, the manipulation of cell differentiation and growth stimulating genes may offer another approach for boosting protoplast regeneration (Lowe et al., 2016; Mookkan et al., 2017). This approach may also enhance the regeneration ability of recalcitrant plants from other explants. For instance, two cell division-promoting transcription factors were co-introduced into maize immature embryos with RNPs to improve regeneration efficiency (Svitashev et al., 2016). Another challenge is the lack of ability to select edited plants, thus increasing the effort required to culture and screen the regenerated plants. Edited plants can only be selected if the targeted mutagenesis leads to phenotypic changes such as herbicide resistance or pigment content. In Phaeodactylum tricornutum, simultaneous targeting of the gene of interest and a gene that results in a phenotypic change when perturbed allows edited plants to be selected visually, enriching for plants with edited genes of interest (Serif et al., 2018). In the future, improved regeneration systems for RNP delivery can be developed without compromising the transgene-free nature of the delivery method. Other transformation methods can also be used to deliver RNPs while bypassing the regeneration steps, including pollen transfection (Zhao et al., 2017) and de novo meristem induction (Maher et al., 2020).
RNP-mediated genetic engineering technology provides a promising platform for crop improvement with minimal regulatory concerns. With the rapid development of CRISPR and other biotechnologies, the applications of RNP-mediated genetic engineering in plants will be broadened and improved, facilitating fundamental research, agricultural development, and food production.
Funding
This work was supported by the National Science Foundation Plant Genome Research Program (award nos. IOS-1758745 and IOS-2029889), the U.S. Department of Agriculture Biotechnology Risk Assessment Grant Program (award nos. 2018-33522-28789 and 2020-33522-32274), the Emergency Citrus Disease Research and Extension Program (award no. 2020-70029-33161), a Foundation for Food and Agriculture Research grant (award no. 593603) and Syngenta. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of these funding agencies.
Author contributions
Y.Z. and Y.Q. outlined the review. Y.Z. and B.I. wrote the original manuscript draft. Y.Z. prepared the figure and table. Y.Q. revised the manuscript. All authors reviewed and edited the manuscript.
Acknowledgments
We apologize to our colleagues whose work was not cited in this review due to limited space. No conflict of interest is declared.
Published: February 10, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
References
- Altpeter F., Springer N.M., Bartley L.E., Blechl A.E., Brutnell T.P., Citovsky V., Conrad L.J., Gelvin S.B., Jackson D.P., Kausch A.P. Advancing crop transformation in the era of genome editing. Plant Cell. 2016;28:1510–1520. doi: 10.1105/tpc.16.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Turesson H., Olsson N., Fält A.-S., Ohlsson P., Gonzalez M.N., Samuelsson M., Hofvander P. Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol. Plant. 2018;164:378–384. doi: 10.1111/ppl.12731. [DOI] [PubMed] [Google Scholar]
- Baek K., Kim D.H., Jeong J., Sim S.J., Melis A., Kim J.-S., Jin E., Bae S. DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 2016;6:30620. doi: 10.1038/srep30620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banakar R., Eggenberger A.L., Lee K., Wright D.A., Murugan K., Zarecor S., Lawrence-Dill C.J., Sashital D.G., Wang K. High-frequency random DNA insertions upon co-delivery of CRISPR-Cas9 ribonucleoprotein and selectable marker plasmid in rice. Sci. Rep. 2019;9:19902. doi: 10.1038/s41598-019-55681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banakar R., Schubert M., Collingwood M., Vakulskas C., Eggenberger A.L., Wang K. Comparison of CRISPR-Cas9/Cas12a ribonucleoprotein complexes for genome editing efficiency in the rice phytoene desaturase (OsPDS) gene. Rice. 2020;13:4. doi: 10.1186/s12284-019-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H., Rao G.S., Sedeek K., Aman R., Kamel R., Mahfouz M. Engineering herbicide resistance via prime editing in rice. Plant Biotechnol. J. 2020;18:2370–2372. doi: 10.1111/pbi.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Wang Y., Zhang R., Zhang H., Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019;70:28.1–28.31. doi: 10.1146/annurev-arplant-050718-100049. [DOI] [PubMed] [Google Scholar]
- Cho S.W., Lee J., Carroll D., Kim J.-S., Lee J. Heritable gene knockout in Caenorhabditis elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics. 2013;195:1177–1180. doi: 10.1534/genetics.113.155853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F.J., Goh N.S., Demirer G.S., Matos J.L., Landry M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36:882–897. doi: 10.1016/j.tibtech.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut T., Lakshmanan P.S., Deryckere D., Van Bockstaele E., Van Huylenbroeck J. Progress in plant protoplast research. Planta. 2013;238:991–1003. doi: 10.1007/s00425-013-1936-7. [DOI] [PubMed] [Google Scholar]
- Fossi M., Amundson K., Kuppu S., Britt A., Comai L. Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol. 2019;180:78–86. doi: 10.1104/pp.18.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A.J., Martin-Urdiroz M., Yan X., Wright H.S., Soanes D.M., Talbot N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018;8:14355. doi: 10.1038/s41598-018-32702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J.A., Valen E., Thyme S.B., Huang P., Akhmetova L., Ahkmetova L., Pauli A., Montague T.G., Zimmerman S., Richter C. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One. 2014;9:e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Gardiner J., Liu W., Papikian A., Ghoshal B., Kuo H.Y., Zhao J.M.-C., Segal D.J., Jacobsen S.E. Targeted DNA demethylation of the Arabidopsis genome using the human TET1 catalytic domain. Proc. Natl. Acad. Sci. U S A. 2018;115:E2125–E2134. doi: 10.1073/pnas.1716945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M.N., Massa G.A., Andersson M., Turesson H., Olsson N., Fält A.-S., Storani L., Décima Oneto C.A., Hofvander P., Feingold S.E. Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front. Plant Sci. 2020;10:1649. doi: 10.3389/fpls.2019.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzebelus E., Szklarczyk M., Baranski R. An improved protocol for plant regeneration from leaf- and hypocotyl-derived protoplasts of carrot. Plant Cell Tissue Organ Cult. 2012;109:101–109. [Google Scholar]
- Guo B., Itami J., Oikawa K., Motoda Y., Kigawa T., Numata K. Native protein delivery into rice callus using ionic complexes of protein and cell-penetrating peptides. PLoS One. 2019;14:e0214033. doi: 10.1371/journal.pone.0214033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Yamashita Y., Takemoto T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 2016;418:1–9. doi: 10.1016/j.ydbio.2016.07.017. [DOI] [PubMed] [Google Scholar]
- He G., Zhang J., Li K., Xiong Z., Chen M., Chang J., Wang Y., Yang G., Barnabás B. An improved system to establish highly embryogenic haploid cell and protoplast cultures from pollen calluses of maize (Zea mays L.) Plant Cell Tissue Organ Cult. 2006;86:15–25. [Google Scholar]
- Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaffaldano B., Zhang Y., Cornish K. CRISPR/Cas9 genome editing of rubber producing dandelion Taraxacum kok-saghyz using Agrobacterium rhizogenes without selection. Ind. Crops Prod. 2016;89:356–362. [Google Scholar]
- Jiang W., Brueggeman A.J., Horken K.M., Plucinak T.M., Weeks D.P. Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot. Cell. 2014;13:1465–1469. doi: 10.1128/EC.00213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Oono K. Protoclonal variation in crop improvement. In: Jain S.M., Brar D.S., Ahloowalia B.S., editors. Somaclonal Variation and Induced Mutations in Crop Improvement. Springer Netherlands; Dordrecht: 1998. pp. 135–148. [Google Scholar]
- Kim J.-S. Precision genome engineering through adenine and cytosine base editing. Nat. Plants. 2018;4:148–151. doi: 10.1038/s41477-018-0115-z. [DOI] [PubMed] [Google Scholar]
- Kim H., Choi J. A robust and practical CRISPR/crRNA screening system for soybean cultivar editing using LbCpf1 ribonucleoproteins. Plant Cell Rep. 2020 doi: 10.1007/s00299-020-02597-x. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim D., Cho S.W., Kim J., Kim J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim S.-T., Ryu J., Kang B.-C., Kim J.-S., Kim S.-G. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 2017;8:14406. doi: 10.1038/ncomms14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Choi J., Won K.-H. A stable DNA-free screening system for CRISPR/RNPs-mediated gene editing in hot and sweet cultivars of Capsicum annuum. BMC Plant Biol. 2020;20:449. doi: 10.1186/s12870-020-02665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Conboy M., Park H.M., Jiang F., Kim H.J., Dewitt M.A., Mackley V.A., Chang K., Rao A., Skinner C. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat. Biomed. Eng. 2017;1:889–901. doi: 10.1038/s41551-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Zhang Y., Kleinstiver B.P., Guo J.A., Aryee M.J., Miller J., Malzahn A., Zarecor S., Lawrence-Dill C.J., Joung J.K. Activities and specificities of CRISPR-Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol. J. 2019;17:362–372. doi: 10.1111/pbi.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Lee J., Choi S.A., Kim Y.-S., Koo O., Choi S.H., Ahn W.S., Jie E.Y., Kim S.W. Efficient genome editing using CRISPR-Cas9 RNP delivery into cabbage protoplasts via electro-transfection. Plant Biotechnol. Rep. 2020;14:695–702. [Google Scholar]
- Li Z., Murai N. Efficient plant regeneration from rice protoplasts in general medium. Plant Cell Rep. 1990;9:216–220. doi: 10.1007/BF00232183. [DOI] [PubMed] [Google Scholar]
- Li J.-F., Norville J.E., Aach J., McCormack M., Zhang D., Bush J., Church G.M., Sheen J. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013;31:688–691. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang D., Xiong X., Yan B., Xie W., Sheen J., Li J.-F. A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants. 2017;3:930–936. doi: 10.1038/s41477-017-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Shi W., Geng L., Xu J. Genome editing in plants directed by CRISPR/Cas ribonucleoprotein complexes. Hered. Beijing. 2020;42:556–564. doi: 10.16288/j.yczz.20-017. [DOI] [PubMed] [Google Scholar]
- Li H., Li J., Chen J., Yan L., Xia L. Precise modifications of both exogenous and endogenous genes in rice by prime editing. Mol. Plant. 2020;13:671–674. doi: 10.1016/j.molp.2020.03.011. [DOI] [PubMed] [Google Scholar]
- Liang Z., Chen K., Li T., Zhang Y., Wang Y., Zhao Q., Liu J., Zhang H., Liu C., Ran Y. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017;8:14261. doi: 10.1038/ncomms14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Chen K., Yan Y., Zhang Y., Gao C. Genotyping genome-edited mutations in plants using CRISPR ribonucleoprotein complexes. Plant Biotechnol. J. 2018;16:2053–2062. doi: 10.1111/pbi.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Staahl B.T., Alla R.K., Doudna J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife. 2014;3:e04766. doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-S., Hsu C.-T., Yang L.-H., Lee L.-Y., Fu J.-Y., Cheng Q.-W., Wu F.-H., Hsiao H.C.-W., Zhang Y., Zhang R. Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018;16:1295–1310. doi: 10.1111/pbi.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Zong Y., Xue C., Wang S., Jin S., Zhu Z., Wang Y., Anzalone A.V., Raguram A., Doman J.L. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020;38:582–585. doi: 10.1038/s41587-020-0455-x. [DOI] [PubMed] [Google Scholar]
- Liu J., Nannas N.J., Fu F., Shi J., Aspinwall B., Parrott W.A., Dawe R.K. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell. 2019;31:368–383. doi: 10.1105/tpc.18.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Rudis M.R., Cheplick M.H., Millwood R.J., Yang J.-P., Ondzighi-Assoume C.A., Montgomery G.A., Burris K.P., Mazarei M., Chesnut J.D. Lipofection-mediated genome editing using DNA-free delivery of the Cas9/gRNA ribonucleoprotein into plant cells. Plant Cell Rep. 2020;39:245–257. doi: 10.1007/s00299-019-02488-w. [DOI] [PubMed] [Google Scholar]
- Lowder L.G., Paul J.W., Baltes N.J., Voytas D.F., Zhang Y., Zhang D., Tang X., Zheng X., Hsieh T.-F., Qi Y. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 2015;169:971–985. doi: 10.1104/pp.15.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowder L.G., Zhou J., Zhang Y., Malzahn A., Zhong Z., Hsieh T.-F., Voytas D.F., Zhang Y., Qi Y. Robust transcriptional activation in plants using multiplexed CRISPR-Act2.0 and mTALE-Act systems. Mol. Plant. 2018;11:245–256. doi: 10.1016/j.molp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Lowe K., Wu E., Wang N., Hoerster G., Hastings C., Cho M.-J., Scelonge C., Lenderts B., Chamberlin M., Cushatt J. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Tian Y., Shen R., Yao Q., Zhong D., Zhang X., Zhu J.-K. Precise genome modification in tomato using an improved prime editing system. Plant Biotechnol. J. 2020 doi: 10.1111/pbi.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher M.F., Nasti R.A., Vollbrecht M., Starker C.G., Clark M.D., Voytas D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020;38:84–89. doi: 10.1038/s41587-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoy M., Viola R., Jung M.-H., Koo O.-J., Kim S., Kim J.-S., Velasco R., Nagamangala Kanchiswamy C. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016;7:1904. doi: 10.3389/fpls.2016.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malzahn A.A., Tang X., Lee K., Ren Q., Sretenovic S., Zhang Y., Chen H., Kang M., Bao Y., Zheng X. Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 2019;17:9. doi: 10.1186/s12915-019-0629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A.J.M., Matzke M.A. Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1998;1:142–148. doi: 10.1016/s1369-5266(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Metje-Sprink J., Menz J., Modrzejewski D., Sprink T. DNA-free genome editing: past, present and future. Front. Plant Sci. 2019;9:1957. doi: 10.3389/fpls.2018.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa K., Kodama Y., Numata K. Vacuum/compression infiltration-mediated permeation pathway of a peptide-pDNA complex as a non-viral carrier for gene delivery in planta. Sci. Rep. 2019;9:271. doi: 10.1038/s41598-018-36466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming M., Ren Q., Pan C., He Y., Zhang Y., Liu S., Zhong Z., Wang J., Malzahn A.A., Wu J. CRISPR-Cas12b enables efficient plant genome engineering. Nat. Plants. 2020;6:202–208. doi: 10.1038/s41477-020-0614-6. [DOI] [PubMed] [Google Scholar]
- Mookkan M., Nelson-Vasilchik K., Hague J., Zhang Z.J., Kausch A.P. Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Rep. 2017;36:1477–1491. doi: 10.1007/s00299-017-2169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgens D.W., Wainberg M., Boyle E.A., Ursu O., Araya C.L., Tsui C.K., Haney M.S., Hess G.T., Han K., Jeng E.E. Genome-scale measurement of off-target activity using Cas9 toxicity in high-throughput screens. Nat. Commun. 2017;8:15178. doi: 10.1038/ncomms15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mout R., Ray M., Yesilbag Tonga G., Lee Y.-W., Tay T., Sasaki K., Rotello V.M. Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano. 2017;11:2452–2458. doi: 10.1021/acsnano.6b07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murovec J., Guček K., Bohanec B., Avbelj M., Jerala R. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front. Plant Sci. 2018;9:1594. doi: 10.3389/fpls.2018.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F.J., Strand C., Donovan K.F., Hegde M., Sanson K.R., Vaimberg E.W., Sullender M.E., Hartenian E., Kalani Z., Fusi N. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat. Biotechnol. 2018;36:179–189. doi: 10.1038/nbt.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov V., Staskawicz B., Weigel D., Jones J.D.G., Kamoun S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013;31:691–693. doi: 10.1038/nbt.2655. [DOI] [PubMed] [Google Scholar]
- Numata K., Horii Y., Motoda Y., Hirai N., Nishitani C., Watanabe S., Kigawa T., Kodama Y. Direct introduction of neomycin phosphotransferase II protein into apple leaves to confer kanamycin resistance. Plant Biotechnol. 2016;33:403–407. doi: 10.5511/plantbiotechnology.16.0929a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papikian A., Liu W., Gallego-Bartolomé J., Jacobsen S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019;10:729. doi: 10.1038/s41467-019-08736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-C., Park S., Jeong Y.J., Lee S.B., Pyun J.W., Kim S., Kim T.H., Kim S.W., Jeong J.C., Kim C.Y. DNA-free mutagenesis of GIGANTEA in Brassica oleracea var. capitata using CRISPR/Cas9 ribonucleoprotein complexes. Plant Biotechnol. Rep. 2019;13:483–489. [Google Scholar]
- Pattanayak V., Lin S., Guilinger J.P., Ma E., Doudna J.A., Liu D.R. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatek A., Ali Z., Baazim H., Li L., Abulfaraj A., Al-Shareef S., Aouida M., Mahfouz M.M. RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant Biotechnol. J. 2015;13:578–589. doi: 10.1111/pbi.12284. [DOI] [PubMed] [Google Scholar]
- Pramanik D., Shelake R.M., Kim M.J., Kim J.-Y. CRISPR-mediated engineering across the central dogma in plant biology for basic research and crop improvement. Mol. Plant. 2020 doi: 10.1016/j.molp.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Puchta H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J. Exp. Bot. 2005;56:1–14. doi: 10.1093/jxb/eri025. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Dad A.-B.K., Beloor J., Gopalappa R., Lee S.-K., Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014;24:1020–1027. doi: 10.1101/gr.171264.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C.A., Lowe K.S., Ruby K.L. Plant regeneration from protoplasts isolated from embryogenic maize cell cultures. Nat. Biotechnol. 1988;6:56–60. [Google Scholar]
- Sant’Ana R.R.A., Caprestano C.A., Nodari R.O., Agapito-Tenfen S.Z. PEG-delivered CRISPR-Cas9 ribonucleoproteins system for gene-editing screening of maize protoplasts. Genes. 2020;11:1029. doi: 10.3390/genes11091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzari I., Leone A., Ambrosone A. Nanotechnology in plant science: to make a long story short. Front. Bioeng. Biotechnol. 2019;7:120. doi: 10.3389/fbioe.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Pacher M., Puchta H. DNA break repair in plants and its application for genome engineering. In: Kumar S., Barone P., Smith M., editors. Transgenic Plants: Methods and Protocols. Springer; New York, NY: 2019. pp. 237–266. [DOI] [PubMed] [Google Scholar]
- Serif M., Dubois G., Finoux A.-L., Teste M.-A., Jallet D., Daboussi F. One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing. Nat. Commun. 2018;9:3924. doi: 10.1038/s41467-018-06378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q., Wang Y., Li J., Zhang Y., Chen K., Liang Z., Zhang K., Liu J., Xi J.J., Qiu J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013;31:686–688. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- Shin S.-E., Lim J.-M., Koh H.G., Kim E.K., Kang N.K., Jeon S., Kwon S., Shin W.-S., Lee B., Hwangbo K. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016;6:27810. doi: 10.1038/srep27810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaj S., Chung S.J., Lee C., Ryu S.-M., Kim D.H., Kim J.-S., Bae S., Lee G.-J. Site-directed mutagenesis in Petunia × hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 2016;35:1535–1544. doi: 10.1007/s00299-016-1937-7. [DOI] [PubMed] [Google Scholar]
- Svitashev S., Schwartz C., Lenderts B., Young J.K., Cigan A.M. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 2016;7:13274. doi: 10.1038/ncomms13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Lowder L.G., Zhang T., Malzahn A.A., Zheng X., Voytas D.F., Zhong Z., Chen Y., Ren Q., Li Q. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants. 2017;3:17103. doi: 10.1038/nplants.2017.103. [DOI] [PubMed] [Google Scholar]
- Tang X., Liu G., Zhou J., Ren Q., You Q., Tian L., Xin X., Zhong Z., Liu B., Zheng X. A large-scale whole-genome sequencing analysis reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in rice. Genome Biol. 2018;19:84. doi: 10.1186/s13059-018-1458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Sretenovic S., Ren Q., Jia X., Li M., Fan T., Yin D., Xiang S., Guo Y., Liu L. Plant prime editors enable precise gene editing in rice cells. Mol. Plant. 2020;13:667–670. doi: 10.1016/j.molp.2020.03.010. [DOI] [PubMed] [Google Scholar]
- Toda E., Koiso N., Takebayashi A., Ichikawa M., Kiba T., Osakabe K., Osakabe Y., Sakakibara H., Kato N., Okamoto T. An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat. Plants. 2019;5:363–368. doi: 10.1038/s41477-019-0386-z. [DOI] [PubMed] [Google Scholar]
- Veillet F., Durand M., Kroj T., Cesari S., Gallois J.-L. Precision breeding made real with CRISPR: illustration through genetic resistance to pathogens. Plant Commun. 2020;1:100102. doi: 10.1016/j.xplc.2020.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejlupkova Z., Warman C., Sharma R., Scheller H.V., Mortimer J.C., Fowler J.E. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat. Plants. 2020;6:1323–1324. doi: 10.1038/s41477-020-00798-6. [DOI] [PubMed] [Google Scholar]
- Wang M., Zuris J.A., Meng F., Rees H., Sun S., Deng P., Han Y., Gao X., Pouli D., Wu Q. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl. Acad. Sci. U S A. 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.W., Kim J., Kwon S.I., Corvalán C., Cho S.W., Kim H., Kim S.-G., Kim S.-T., Choe S., Kim J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015;33:1162–1164. doi: 10.1038/nbt.3389. [DOI] [PubMed] [Google Scholar]
- Wu S., Zhu H., Liu J., Yang Q., Shao X., Bi F., Hu C., Huo H., Chen K., Yi G. Establishment of a PEG-mediated protoplast transformation system based on DNA and CRISPR/Cas9 ribonucleoprotein complexes for banana. BMC Plant Biol. 2020;20:425. doi: 10.1186/s12870-020-02609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Li J., Liu X., Shan T., Qin R., Wei P. Development of plant prime-editing systems for precise genome editing. Plant Commun. 2020;1:100043. doi: 10.1016/j.xplc.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Liang X., Xie H., Kumar S., Ravinder N., Potter J., de Mollerat du Jeu X., Chesnut J.D. Improved delivery of Cas9 protein/gRNA complexes using lipofectamine CRISPRMAX. Biotechnol. Lett. 2016;38:919–929. doi: 10.1007/s10529-016-2064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tu L., Subburaj S., Bae S., Lee G.-J. Simultaneous targeting of duplicated genes in Petunia protoplasts for flower color modification via CRISPR-Cas9 ribonucleoproteins. Plant Cell Rep. 2020 doi: 10.1007/s00299-020-02593-1. [DOI] [PubMed] [Google Scholar]
- Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liang Z., Zong Y., Wang Y., Liu J., Chen K., Qiu J.-L., Gao C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016;7:12617. doi: 10.1038/ncomms12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Malzahn A.A., Sretenovic S., Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants. 2019;5:778–794. doi: 10.1038/s41477-019-0461-5. [DOI] [PubMed] [Google Scholar]
- Zhao X., Meng Z., Wang Y., Chen W., Sun C., Cui B., Cui J., Yu M., Zeng Z., Guo S. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants. 2017;3:956. doi: 10.1038/s41477-017-0063-z. [DOI] [PubMed] [Google Scholar]
- Zuris J.A., Thompson D.B., Shu Y., Guilinger J.P., Bessen J.L., Hu J.H., Maeder M.L., Joung J.K., Chen Z.-Y., Liu D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]