Abstract
Animal models are still indispensable for understanding the basic principles of glioma development and invasion. Preclinical approaches aim to analyze the treatment efficacy of new drugs before translation into clinical trials is possible. Various animal disease models are available, but not every approach is useful for addressing specific questions. In recent years, it has become increasingly evident that the tumor microenvironment plays a key role in the nature of glioma. In addition to providing an overview, this review evaluates available rodent models in terms of usability for research on the glioma microenvironment.
Keywords: tumor microenvironment, glioma, rodent model, brain tumor, synthetic biology, preclinical oncology
Introduction
Especially for high-grade gliomas, survival prognosis is still limited. These tumors show a pronounced tendency for recurrence, even after aggressive therapies, which is caused by glioma heterogeneity and the presence of glioma stem cells, which might support the resilience to cell death and the evasion of the host's immune response (Prager et al., 2020). Moreover, they show a strong invasive growth, which often makes radical surgical resections impossible and, therefore, affects the overall survival time (Brown et al., 2016). The interaction of the tumor microenvironment with the so-called “niches” that comprise all components of a tumor, except for the tumor cells themselves, also appears to play a crucial role in the limited therapeutic efficacy (Plaks et al., 2015). A variety of in vitro and in vivo pre-clinical models are available for the investigation of the pathophysiology of gliomas, and they provide important tools for the development of new therapies and treatment strategies. Gliomas occupy a special position among tumors due to their enormous heterogeneity, their special location within the blood–brain barrier, and their unique immunosuppressive tumor microenvironment. The latter is formed by a variety of different actors such as microglia, astrocytes, cytokines, extracellular vesicles, tumor-associated macrophages, and tumor-infiltrating dendritic cells, but also by non-neoplastic cells, such as astrocytes, and endothelial cells. This large variation renders it difficult to find the ideal glioma model for studying the tumor microenvironment, since each model affects it differentially. In vitro models, for example, can be useful for studying the intrinsic glioma properties in relation to cell biology and genetics. However, they reflect the complex interactions between the host and glioma to a limited extent, even though three-dimensional in vitro models already provide a more precise picture of the interactions between glioma cells and the tumor microenvironment.
In vivo models are currently superior and more suitable for replicating the complex interactions between gliomas and the tumor microenvironment. Investigating these interactions of gliomas with the tumor microenvironment in an organism that mimics human patients’ conditions as closely as possible is an important prerequisite for rodent glioma models. For instance, potential therapies and their outcomes can already be investigated in an organism, and thus, clinical studies may be carried out as efficiently and safely as possible. In recent years, many studies have shown the importance of the interaction of the tumor microenvironment with tumor cells in, for example, tumor progression, invasion, and angiogenesis. Against this background, the present review briefly discusses the advantages and disadvantages of in vivo models regarding the investigation of glioma, with a special focus on the tumor microenvironment.
Rodent Models
Xenograft and Syngeneic Models
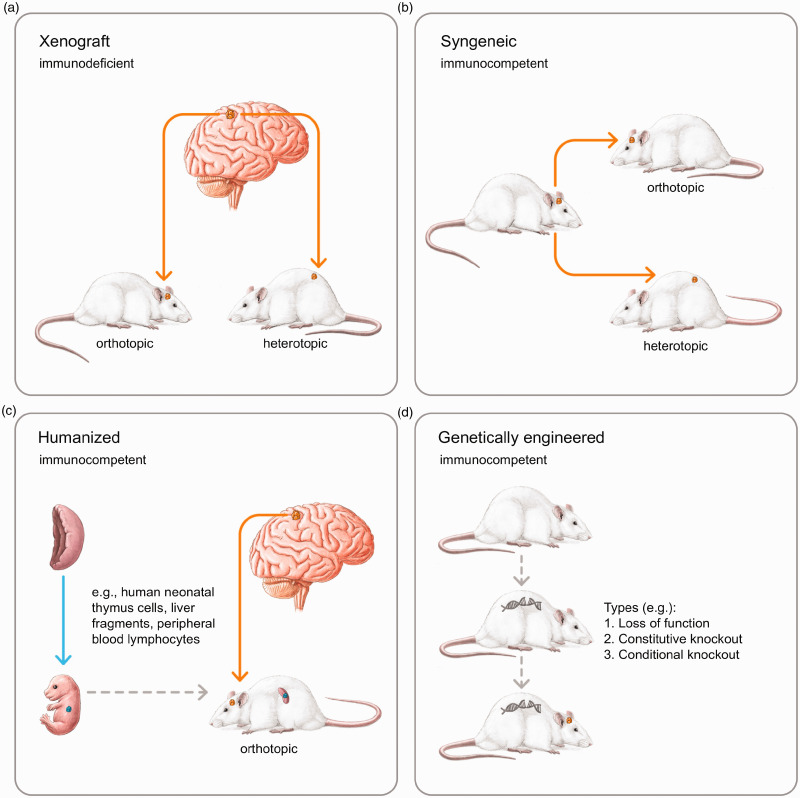
Most preclinical glioma research is carried out in rodent glioma models. Xenograft and syngeneic models are probably the most used and, therefore, the most cited ones. In xenograft models, patient-derived cells or tissues (patient-derived xenograft (PDX)) are implanted into an immunocompromised or humanized animal model (Figure 1A). In syngeneic models, tumor cells are immunologically compatible with an immunocompetent host (inbred mouse strain), and are thus called allograft models (Figure 1B). The advantage of xenograft models is that human glioma cells can be examined together with their surroundings at a defined point in time. The human glioma cells are transplanted under the skin (heterotopic) or into the brain (orthotopic) of an immunodeficient rodent. The disadvantages of this rodent glioma model are the surgical procedure required for tumor implantation and the cells are implanted in an immunosuppressed host. This immunosuppressed situation is a major limitation of using xenograft models for studying glioma microenvironment interactions, since immune cells play a decisive role in this phenomenon.
Figure 1.
In xenograft models, glioma cells of human origin are transplanted subcutaneously (heterotopic) or into the brain of an immunodeficient rodent (orthotopic) at a particular point in time. The tumor microenvironment is immunosuppressed and graft versus host reactions may occur (A). In syngeneic glioma models, the graft and host are syngeneic (heterotopic and orthotopic) and no graft versus host reactions occur due to an immunocompetent tumor microenvironment. This immunological advantage might be a disadvantage for translational research, because no human glioma cells are transplanted. In orthotopic models, glioma cells are examined in their natural TME, while in heterotopic models, the tumor microenvironment is unspecific, but implantation is much easier (B). In humanized glioma models, human immune cells and/or tissues are transplanted into rodents, thereby “humanizing” the murine immune system. If glioma cells and immune cells are derived from the same donor, graft versus host reactions are unlikely in an immunocompetent tumor microenvironment with a human character (C). In the genetic models, the rodents form the glioma endogenously due to genetic techniques. A surgery is not necessary, the immune properties of the tumor microenvironment remain unaffected, and graft versus host reactions do not occur (D). The following applies to all representations: Arrows that show a time sequence are dashed. If it is the same individual, the animals look in the same direction. If there are two different individuals, the animals look in opposite directions. Different individuals within an image have different tail positions.
The choice of the PDX model is decisive with regard to further influences on the translational evaluability. Most rodent PDX glioma models are carried out in mice that are immunosuppressed. Frequently used models are, for example, non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice, NOD/SCID gamma mice, and nude mice. In addition to the immunocompromised conditions, the respective models have further disadvantages that must be weighed for each specific model, and not only in relation to the investigation of the glioma microenvironment. In NOD/SCID mice, the SCID mutation (severe combined immunodeficiency) is combined with the NOD type (non-obese diabetic). SCID mutation homozygous animals do not form any T- or B-cells (with preserved myelopoiesis), and myeloid cells and natural killer (NK) cells functions can also be restricted In NOD mice, diabetes is caused by autoimmune reactions that cause the destruction of insulin-producing beta cells (Aldrich et al., 2020). Furthermore, polymorphisms of the Idd3 locus exist, that lead to increased IL-2 (IL = interleukin) and IL-21 levels, which can influence the function of dendritic cells, B-cells, T-cells, and NK-cells (Wendt et al., 2007), and a mutation of the CTLA-4 gene (cytotoxic T-lymphocyte-associated Protein 4) results in an attenuated T-cell immune response (Ueda et al., 2003). This immunocompromised situation is intensified in NOD/SCID gamma mice, which have no mature T-cells, NK-cells, or B-cells and show aberrated cytokine signaling pathways with accompanying deficits in innate immunity and a missing complementary hemolytic system. Additional immune cells are affected, as dendritic cells are reduced and macrophages are inactive (Shultz et al., 1995, 2005). Nude mice, on the other hand, are thymus aplastic (caused by a mutation of the FOXN1 gene [FOXN1 gene encodes the forkhead box protein N1]), and no CD4 + T helper cells (cluster of differentiation 4 - glycoprotein) are formed; as a result, no antibodies or cytotoxic T-cells are produced (Pantelouris, 1968). Obviously, these aspects crucially affect the interactions between the host and glioma with special regard to the immune microenvironment.
The technique of the transplantation itself can also have a decisive influence on glioma morphology and glioma behavior. Due to prolonged cultivation times, the morphological properties of glioma cells can be lost. It has been described, for example, that EGFR (epidermal growth factor receptor) and MGMT (O (6) -Methylguanine-DNA methyltransferase) can be altered (Carlson et al., 2011). These disadvantages can be countered by implanting tumor cells from patients directly into mice via PDX models. Serial implantation in xenograft orthotopic models could prevent alterations of molecular properties – the histopathological characteristics corresponded to those of primary gliomas (Pandita et al., 2004; Giannini et al., 2005; Carlson et al., 2009). However, orthotopic PDX models do not guarantee a clinical phenotype. For example, U251-cell line xenografts show very similar characteristic properties — including invasive growths, such as human glioblastomas, U87-cell line xenografts, on the other hand, do not show this typical growth behavior (Lenting et al., 2017). PDX models can be implemented in different ways: either through direct transfer from humans into the model or where the glioma cells are first cultivated before implantation. These different approaches may have a direct influence on the glioma properties of the model, since long-term cultivation can cause this drift of culture, which means that the genome structure and transcriptomics can be altered and the glioma cells no longer have the properties of the parental tumor (Simeonova and Huillard, 2014). Serum-free spheroid cultures are possibly a more suitable approach to cultivate human tumor cells under fixed growth factor conditions. The advantage is that the maternal tumor cell characteristics are preserved much more precisely (Seidel et al., 2015). There is evidence that glioblastoma stem cells have a hierarchical organization of stem cells (Prager et al., 2020). Besides, glioblastomas can have phenotypically different stem cells. Glioma neural stem cell lines from different gliomas have different gene expression signatures of the neuronal progenitors subtypes (Pollard et al., 2009). Preserving these properties in cell culture is necessary if molecular targets are to be examined or genetic screening is carried out. Even if low-passage patient-derived tumor cells and serum-free cultivations offer an alternative to represent maternal tumor properties better, maternal glioma cells are subject to constant changes in their properties, triggered by interaction with the different tumor microenvironment areas. Thus, more prolonged cultivation will not permanently reflect the maternal tumor changes (Sandén et al., 2015). However, for all conventional PDX models, an immunodeficient system is required, with the resulting limitations for investigations of the tumor microenvironment’s immunological patterns. Moreover, local inflammations caused by tumor cell implantation may change the immune composition of the tumor microenvironment, as in xenograft models, and the implantation of a cell mass does not correspond to a natural carcinogenic process. These problems can be countered if the tumor cells are not implanted orthotopically, but still grow in the brain. A xenograft model was recently described in which human glioblastoma cells were transplanted into the uterus and then integrated into the brains of mouse embryos (Hoffmann et al., 2020). The developed glioblastomas showed typical properties, such as infiltration and angiogenesis, and formed a complex tumor microenvironment that was like that of the donor tumor. One of the interesting things about this model is that transplanted glioma cells reached the central nervous system even if they were transplanted outside the blood–brain barrier (possibly associated with stem cells), and the thusly induced glioblastomas grew in an immunocompetent environment. However, the limitation for a translational consideration may also be the strength of this methodological approach: Glioblastomas are tumors of older people, and thus show an adult immune system and adult neuronal differentiation. Microglia play a central role in the development of the central nervous system and exist in all brain regions, but the distribution is dissimilar – even in rodents, with a greater presence in gray matter than in white matter – suggesting that microglial distribution is associated with the microenvironment (Lawson et al., 1990). It is known that in the perinatal phase, the brain is especially sensitive to immune-associated damage (Christensen et al., 2014); can modulate these inflammatory processes and can even act neuroprotectively in the early development phase. The microglia properties already differ between neonatal and juvenile rats, for example, with regard to the expression of genes involved in the cell cycle and migration (Parakalan et al., 2012). These complex aspects can influence the translational interpretation of the embryonic model. However, as long as a host individual has functioning components of the immune system, graft-versus-host reactions can still occur, which, in turn, can influence the immune situation or status of the tumor microenvironment. A translation to clinical questions is restricted, as human glioma cells are embedded in a rodent tumor microenvironment. Syngeneic models, on the other hand, do not have this disadvantage, given that tumor cells from rodents can be transplanted into genetically identical animals with the same immune patterns. Thus, rodent glioma models have already been used for many studies, such as vaccine, chemotherapy, immunobiological, and genetic studies (Wei et al., 2015; Buqué and Galluzzi, 2018). Even though syngeneic models might be the most appropriate for studying the glioma microenvironment, they have only limited benefits for translational studies. It applies to both the xenograft and syngeneic glioma models that the orthotopic approach is superior to the heterotopic approach for investigations of the tumor microenvironment, as it better depicts the specific immune situation of the central nervous system.
Humanized Tumor Mouse Models
To bridge these differences, there are several approaches, which comprise so-called humanized tumor mouse models (Figure 1C). The employed mouse strains are immunodeficient and lack NK-cells, macrophages, and T- and B-cells. Human hematopoietic stem cells are transplanted into mice, which subsequently develop a human immune system (Morton et al., 2016), whereby human glioma cells can be investigated in an immunocompetent tumor microenvironment. In these models, not only human tissue is engrafted, but solid and hematologic tumors are as well. Additionally, these models are a useful tool for studying graft-versus-host reactions, infectious diseases, and immunity (Walsh et al., 2017), as well as immunotherapeutic drug development in order to reduce failures in clinical settings (De La Rochere et al., 2018). The disadvantage of humanized tumor mouse models is, however, that immunocompetent and tumor cells are derived from different patients, which may trigger immune responses that, in turn, affect the immune patterns of the tumor microenvironment. To overcome this problem, immunocompetent and tumor cells can be grafted from the same patient (Morton et al., 2016). For example, a PDX model, in which the mouse genetic and immunohistological properties are identical to those of a specific patient. However, this is very complex and could be difficult to implement in the clinical process. However, humanized models can be very promising for the ability to better describe patient-specific tumor characteristics. Combining the properties of the tumor microenvironment with those of specific patient glioma cells could avoid immunological interactions of glioma cells and the tumor microenvironment (Walsh et al., 2017).
Genetically Engineered Models
In another approach, gliomas are initiated or modeled through genetic manipulation (genetically engineered models; Figure 1D) in the sense of loss of function (deletion) or gain of function (transgenic) (Fomchenko and Holland, 2006; Stylli et al., 2015). Genetically engineered models offer the possibility of enabling genetic factors in the development of brain tumors, particularly through oncogene and tumor suppressor analyses (Noorani, 2019). Other models are not able to precisely carry out genetic analyses with respect to the molecular basis of tumorigenesis. One problem of genetically engineered models is that developmental abnormalities that can result in (embryonic) lethality arise, even before tumor growth occurs (Janbazian et al., 2014), especially with gain-of-function approaches (Huszthy et al., 2012; Stylli et al., 2015). However, time- and tissue-specific control of gene expressions or the deletion of tumor suppressor genes can represent a strategy for counteracting lethality and tumor growth instability (Stylli et al., 2015). Based on these techniques, a large number of genetically engineered models were created with various patterns in histology, genotype penetrance, and expression behavior (Llaguno and Parada, 2016), each with a specific translational value. The greatest advantage of genetically engineered models for glioma tumor microenvironment research is likely that the blood–brain barrier is intact, and there are no changes in the immune milieu. In addition, invasive methods for tumor cell implantation can be dispensed. Longitudinal studies are difficult to carry out, as glioma growth does not start at a specific point in time. In order to monitor tumor growth, further techniques are necessary, such as magnetic resonance tomography or genetically engineered mouse models with bioluminescent or fluorescent reporter genes (Uhrbom et al., 2004; Yao et al., 2016). Xenograft and syngeneic models are, therefore, currently superior for this study design, since the tumor is implanted at a defined time point and, depending on this, selective data can be collected. The engineering of human cells to develop new cancer therapies is promising and will probably play an increasingly important role in tumor research in the coming years. Currently, T-cell engineering provides success with tumor-targeting receptors – so-called chimeric antigen receptor (CAR) T-cells – and hematologic cancer therapies have been successfully implemented (Dolberg et al., 2018; Caliendo et al., 2019). For rodent glioma models, this approach is extremely promising when combined with humanized models. Thus, the effects of artificially produced immune cells in a human immune environment could already be investigated in animal models. Going a step further, artificial immune systems and their influence on glioma microenvironment behavior could already be examined in rodent models before a clinical study is carried out, thereby increasing patient safety. There are already interesting approaches to engineering tumor cells to reduce the influence of the immunosuppressive tumor microenvironment. For instance, targeting tumor cells with the tumor necrosis factor (TNF)-α or CD40 ligand genes could provide a more anticancerogenic view of tumor microenvironment antitumor immune responses (Daneshmandi and Shahrokhi, 2019). A comparison of the advantages and disadvantages of the individual models is shown in Table 1. However, the translational use of results in genetically engineered models is limited by the fact that differences in genetics, histo-anatomical structures, and physiology between humans and rodents cannot be bridged (Bian et al., 2018).
Table 1.
Comparison of the Advantages and Disadvantages of the Individual Models.
| Model | Xenograft | Syngeneic | Humanized | Genetically engineered |
|---|---|---|---|---|
| Operation | Yes | Yes | Yes | No |
| Human cells | Yes | No | Yes | No |
| Longitudinal study | Yes | Yes | Yes | No |
| Immunosuppression | Yes | No | No | No |
| Graft vs. host reactions | Yes | No | No | No |
| Tumor microenvironment (human/mice) | Rodent | Rodent | Human (possible residual parts of the rodent) | Rodent |
| Suitable study design |
•Longitudinal studies •Molecular studies(serum-free condition) •Potential for personalized anticancer treatment •Clinical relevance •Biomarker studies •Stem cell studies (serum-free condition) |
•Longitudinal studies •Immune studies |
•Patient-oriented investigations |
•Molecular investigations •Studies that need an intact tumor microenvironment •Tumorigenesis studies |
| Limitations |
•Restricted translation to humans •Immune studies (immunodeficient rodents) •Tumorigenesis studies |
•Restricted translation to humans •Non-human glioblastoma •Tumorigenesis studies |
•Short time windows when examinations are to be carried out for specific patients •Tumorigenesis studies |
•Translation to human biology restricted •Different molecular structure from human glioblastoma •Limited number of mutations •Longitudinal studies and (immune) therapeutic studies |
| References | (Lenting et al., 2017) | (Huszthy et al., 2012) | (Morton et al., 2016) | (Noorani, 2019) |
The main general advantages and disadvantages of the orthotopic and xenograft approaches are additionally listed.
Discussion
A major limitation is that the rodent glioma models themselves affect the tumor microenvironment. The implantation of tumor cells into the brain can cause local inflammatory processes, which, in turn, may disrupt the natural composition of the tumor microenvironment. In particular, heterotopic models, such as subcutaneous implantation of glioma cells, are less suitable here because the special central neurological immune system does not exist, which means that conclusions about interactions between the microenvironment and glioma cells are only possible to a limited extent. The actors of the immune system can also be altered if the graft and host are genetically different or even if the glioma cells are donated from a different species. Orthotopic xenograft models are of value to investigate the heterogeneity of gliomas of specific patient, and syngeneic models have their strength in analyzing the glioma microenvironment in longitudinal studies where an immunocompetent host is needed. Humanized models can be a bridge to combine the advantages of both models, but they are very complex to incorporate into clinical routine, in particular to carry out timely investigations for individual patients. For cross-sectional studies, genetically engineered models may be superior, since the blood–brain barrier remains intact during tumor growth until a specific time point at which the experiment is carried out.
Representation of Tumor Heterogeneity
The genetic heterogeneity that gliomas show – even within a tumor – is difficult to depict in models, especially with respect to interactions with the glioma microenvironment. PDX models, in which cells from fresh human tumor samples are transferred directly into mice, represent a step forward toward analyzing the intrinsic glioma patterns, and the gene expression patterns can be maintained by serial passages from mouse to mouse (Xu et al., 2019). To date, PDX models are most likely to depict the morphological, histologic, and genetic characteristics of human gliomas (Patrizii et al., 2018). Conventional PDX models require immunodeficient mouse strains (Jung et al., 2018), which means that the tumor microenvironment and interactions with intrinsic glioma-cell properties cannot be entirely mapped. Moreover, the transplantation of human cancer cells into rodents also affects the tumor microenvironment, and the tumor microenvironment can, in turn, induce phenotypic and genotypic changes in the transplanted tumor cells (Varna et al., 2014). It has to be kept in mind that genetic heterogeneities are also temporally and spatially different within individual tumors (Tammela and Sage, 2020); thus, the glioma cells in a model may not necessarily represent the entire molecular landscape of a patient's glioma.
The Niches in the Microenvironment
Glioblastomas are characterized by their rapid, invasive growth; the tumor microenvironment, with its special immune situation, plays an important role here. The latter can be divided into so-called niches. These microanatomical structures within the microenvironment are crucial for the metabolic transformation of glioma cells and in stem cell differentiation (Hambardzumyan and Bergers, 2015). Derived on these microstructures', three niches can, in principle, be distinguished from one another: the invasive, hypoxic, and perivascular ones (Hambardzumyan and Bergers, 2015). In all niches, the presence of glioma stem cells is crucial for invasiveness, glioma stability, and the development of resistance to therapies (Lathia et al., 2015). Scherer, a German neuropathologist, already characterized the invasiveness of gliomas on the basis of different morphological growth patterns in relation to the meninges, white matter tract, and blood vessels (Scherer, 1938). Above all, the perivascular accumulation of glioma cells along the vascular structures outside the Virchow–Robin spaces (the fluid-filled spaces surrounding blood vessels, which have immunological functions and are important for blood-derived messengers) has been described, which is, in principal, coherent with the invasive/perivascular niches (Civita et al., 2020). The importance of perivascular growth is particularly clear when studies that show that >85% of glioma cells grow along vessels are considered (Cuddapah et al., 2014). The basis of the glioma cell spread within the niches seems to be supported by chemokines, such as SDf1-alpha (stromal cell-derived factor 1), which, in turn, is associated with vascular growth factors, such as VEGF (vascular endothelial growth factor) (Zagzag et al., 2008). The tumor microenvironment is, therefore, crucial in many ways for understanding the mechanisms involved in glioma spread and resistance development. Finding the appropriate animal model for investigating all of the manifold aspects of the niches – for example, regarding immunological, vascular, and molecular patterns – is a particular challenge. Syngeneic models seem to have advantages here, as they can best reflect the important immunological properties of the tumor microenvironment, especially in the case of orthotopic models. This model's limitation might be that the blood-brain barrier is interrupted more intensely by the implantation of tumor cells than by the tumor itself, which changes the peritumoral immune situation more intensely. The investigation of the perivascular niche in terms of the spread of glioma cells along the Scherer structures also seems to be possible in xenograft models. Cell-line-based orthotopic implantation of glioma stem cells into immunodeficient mice showed that the gliomas revealed the molecular biological characteristics of the donor gliomas and the tumors formed secondary structures, as described by Scherer in the sense of perivascular, perineuronal, and meningeal accumulation (Larsson et al., 2018). Humanized PDX models seem to have an advantage here, as the representation of Scherer structures/glioma niches could be examined in a (human) immunocompetent environment. Genetically engineered models also seem to be able to trace the growth of tumor cells along the Scherer structures, since morphological characteristics, such as palisade formations, also develop (Hara et al., 2019). The observation that genetically engineered models mimic glioma-specific growth properties along the Scherer spaces was also made by Jun et al. (2018). In a PDGFRα-driven model (platelet-derived growth factor receptor), tumor cells showed characteristics that corresponded to high-grade gliomas, even with pseudo-palisading necrosis and the pronounced invasive growth along existing brain structures with the formation of secondary structures in the sense of Scherer's structures. However, human genome structures are not reflected by this model, and tumor initiation cannot be controlled. The advantage, in turn, is that genetic conclusions can be drawn about the behavior of the glioma (stem) cells in Scherer’s structures.
Stem Cells
During glioma growth, a mesenchymal phenotype usually develops due to an epithelial–mesenchymal transition, with stem-cell-like properties of differentiated glioma cells, subsequent formation of resistance to genotoxic drugs, and increased invasiveness. This transition seems to be dependent on an hypoxic microenvironment and growth factors (Iwadate, 2016). The stem-cell-like properties associated with mesenchymal transition are particularly interesting, since tumors release exosomes (small membrane vesicles) and circulating tumor cells into the periphery. Exosomes carry DNA (exoDNA) that reflects the entire genome and the mutation status of the parental tumors (Thakur et al., 2014), and participate in many procancerogenic processes as they can initiate or suppress signaling pathways in other tumor cells (Tao and Guo, 2020). Circulating tumor cells show an increased mesenchymal transcription profile in both patients and PDX models (Behnan et al., 2019). Cancer stem cells from invasive mesenchymal glioma cell types grow significantly better in PDX models than cancer stem cells from the less aggressive proneural glioma cell type (da Hora et al., 2019) in the sense of a selective growth towards gliomas with a mesenchymal character. As cancer stem cells occur in all niches of the tumor microenvironment, with a momentous role in the invasiveness of glioblastomas (Schiffer et al., 2014), the selection of donor glioblastoma cells can have a decisive influence on growth and interactions with the tumor microenvironment.
Methodological Aspects
The respective technologies not only have possible effects on the intrinsic properties, such as the molecular profile of the glioma cells (for example, for culture drifting during prolonged culturing times, as mentioned above), but also enable a more selective choice of which glioma cells are to be transplanted. Tanaka et al. established a glioma stem cell culture that was dependent on the EGFR status and was cultivated at different times during EGFR-targeted therapies and recurrences. They were able to show that glioma stem cells from tumors of a second recurrence (after administration of the irreversible EGFR tyrosine kinase inhibitor, dacomitinib) presented tumors without an EGFR amplification or EGFR overexpression in the orthotopic PDX model (SCID mice). In addition, PDX gliomas that were developed from stem cells that were removed from patients before treatment with dacomitinib showed a higher proliferation, while the expression of the mesenchymal markers CD44 and YKL-40 (also known as chitinase-3-like protein 1) was increased in post-PDX gliomas. Their findings agreed with those obtained from the corresponding clinical samples.
The advantages and drawbacks of the transplantation of human glioma cells into PDX models were mentioned above. However, the PDX glioma properties can be influenced not only by the decision on the method with which cells are transplanted, but also by the selection of the cells themselves. Stem cells can influence the function of T-cells and tumor-associated microglia, as well as macrophages (Zhou et al., 2015), and they cause PD1-mediated (programmed cell death protein 1) decline of cytotoxic T-cells. In addition, cytokines, such as TGF-ß (tansforming growth factor beta) and IL-10, are expressed, which increases the immunosuppressive situation (Lathia et al., 2015), and glioma stem cells show certain patterns of transcription factors, such as STAT3 (signal transducers and activators of transcription) and FOXM1 (forkhead box protein M1) (Lathia et al., 2015). STAT3, for example, is involved in many prooncogenic signaling pathways, is activated in immune cells of the tumor microenvironment, and suppresses their anticancerogenic immune competence (Yu et al., 2007). It can, therefore, be assumed that the increased presence of glioma stem cells triggers an immunosuppressive tumor microenvironment. The choice of cells that are transplanted in xenograft models can, therefore, presumably have an influence on the properties of the glioma microenvironment.
Physiological Differences Between Humans and Rodents
A common problem of all rodent models is that investigations are carried out in an organism that has completely different physiological parameters (Davies and Morris, 1993) and metabolic rates (Terpstra, 2001) from those of humans. It needs to be considered that in every rodent model, the host metabolism is higher and is completely different from that in humans; naturally, the tumor sizes also strongly differ. The lifespan of animals is also significantly shorter than that of humans, which can influence, for example, the presence of growth factors (Azzu and Valencak, 2017). In contrast to tumors that grow slowly over a long period of time, high-grade gliomas grow rapidly, which means that they may be better represented in rodent glioma models in terms of the growth properties and the associated immune changes in the tumor microenvironment. For glioma stem cells, it has been shown that they are less glycolytic and have less lactate, but simultaneously increased ATP levels. These aspects, which initially appear to be of secondary importance, become relevant for the interpretation of study results if the glioma stem cells concomitantly have a higher mitochondrial reserve capacity and, thus, have different oxidative metabolic properties (Vlashi et al., 2011; Strickland and Stoll, 2017). The glioma metabolism also depends on the interaction between the glioma genotype and the microenvironment. For example, growth factor signaling, such as for IDH (isocitrate dehydrogenase), is altered, which, in turn, can control metabolic traits (Bi et al., 2020). IDH wild-type glioma cells grow better than IDH mutant cells (Zeng et al., 2020). IDH wild-type glioma cells produce NAPD + α-KG (nicotinamide adenine dinucleotide + alpha ketoglutarate) and NADPH (nicotinamide adenine dinucleotide phosphate) from isocitrate. IDH mutant cells instead utilize α-KG and NADPH and produce 2-hydroxyglutarat (D-2-HG), an oncometabolite (Lenting et al., 2017). The metabolic rate of immune functions is significant when examining the tumor microenvironment, especially concerning immunotherapy studies. However, T-cells have an increased turnover in activity, and can thus be impaired in their function by a reduced supply of nutrients – as in the hypoxic niche, which is nutritionally undersupplied – whereby the tumor microenvironment represents a metabolic barrier for (effector) T-cells (Lim et al., 2020). Thus, the size of a tumor with pronounced necrotic and undersupplied areas has an influence on the tumor microenvironment and, consequently, on anti-tumor immunity (Lim et al., 2020). Last but not least, a change in metabolism can also lead to an overproduction of lactic acid and can influence important glioma properties, such as invasion, resistance to therapy, or immunosuppression (Choi et al., 2014). The problem of the metabolic differences between humans and rodents affects all xenograft models, included humanized ones, and cannot be neglected, as the different conversions influence the immunological properties of the tumor microenvironment.
Conclusion
To date, there is still no ideal rodent glioma model for study glioma microenvironment interactions; depending on the rationale of the study and the available resources, a decision on which model to use must be made. New combinations of synthetic cells and humanized tumor models currently appear to be promising approaches for investigating the mechanisms and interactions between gliomas and their tumor microenvironment.
Summary
We discuss the benefits and drawbacks of using in vivo models to study gliomas, with a particular focus on the tumor microenvironment. Novel combinations of synthetic cells with humanized tumor models are currently promising approaches.
Supplemental Material
Supplemental material, sj-pdf-1-asn-10.1177_17590914211005074 for Rodent Models to Analyze the Glioma Microenvironment by Susann Hetze, Ulrich Sure, Manfred Schedlowski, Martin Hadamitzky and Lennart Barthel in ASN Neuro
Acknowledgment
We would like to thank Nadja Baltensweiler Illustration & Grafik, Ebikon (Lucerne), Switzerland for creating the graphic.
Footnotes
Author Contributions: SH wrote, reviewed, edited and conceptualized the manuscript and carried out the funding acquisition and administration. MH, US, and MS carried out critical reviews and commentary. LB wrote, reviewed, edited and conceptualized the manuscript and illustration and carried out the funding acquisition and administration.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This work was supported by the Internal Research Funding (IFORES) of the University Hospital Essen (D/107-41030 to SH) and the University Medicine Essen Clinician Scientist Academy and Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) (D/107-21930 to LB). This study was also supported by the DFG, SFB1280 (Sonderforschungsbereich; TP A18, project number 316803389, to MH and MS). The funding sources had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
ORCID iD: Lennart Barthel https://orcid.org/0000-0002-0503-8628
References
- Aldrich V. R., Hernandez-Rovira B. B., Chandwani A., Abdulreda M. H. (2020). NOD mice—Good model for T1D but not without limitations. Cell Transplantation, 29, 096368972093912–096368972093910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliendo F., Dukhinova M., Siciliano V. (2019). Engineered cell-based therapeutics: Synthetic biology meets immunology. Frontiers in Bioengineering and Biotechnology, 7, 43. https://www.frontiersin.org/article/10.3389/fbioe.2019.00043/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzu V., Valencak T. G. (2017). Energy metabolism and ageing in the mouse: A mini-review. Gerontology, 63(4), 327–336. https://www.karger.com/Article/FullText/454924 [DOI] [PubMed] [Google Scholar]
- Behnan J., Finocchiaro G., Hanna G. (2019). The landscape of the mesenchymal signature in brain tumours. Brain: A Journal of Neurology, 142(4), 847–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J., Chowdhry S., Wu S., Zhang W., Masui K., Mischel P. S. (2020). Altered cellular metabolism in gliomas—An emerging landscape of actionable co-dependency targets. Nature Reviews. Cancer, 20(1), 57–70. 10.1038/s41568-019-0226-5 [DOI] [PubMed] [Google Scholar]
- Bian S., Repic M., Guo Z., Kavirayani A., Burkard T., Bagley J. A., Krauditsch C., Knoblich J. A. (2018). Genetically engineered cerebral organoids model brain tumor formation. Nature Methods, 15(8), 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. J., Brennan M. C., Li M., Church E. W., Brandmeir N. J., Rakszawski K. L., Patel A. S., Rizk E. B., Suki D., Sawaya R., Glantz M. (2016). Association of the extent of resection with survival in glioblastoma a systematic review and meta-analysis. JAMA Oncology, 2(11), 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buqué A., Galluzzi L. (2018). Modeling tumor immunology and immunotherapy in mice. Trends in Cancer, 4(9), 599–601. https://pubmed.ncbi.nlm.nih.gov/30149876/ [DOI] [PubMed] [Google Scholar]
- Carlson B. L., Grogan P. T., Mladek A. C., Schroeder M. A., Kitange G. J., Decker P. A., Giannini C., Wu W., Ballman K. A., James C. D., Sarkaria J. N. (2009). Radiosensitizing effects of temozolomide observed in vivo only in a subset of O6-methylguanine-DNA methyltransferase methylated glioblastoma multiforme xenografts. International Journal of Radiation Oncology*Biology*Physics, 75(1), 212–219. https://pubmed.ncbi.nlm.nih.gov/19695438/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B. L., Pokorny J. L., Schroeder M. A., Sarkaria J. N. (2011). Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Current Protocols in Pharmacology, 52(1), 14–16. [DOI] [PMC free article] [PubMed]
- Choi S. Y. C., Lin D., Gout P. W., Collins C. C., Xu Y., Wang Y. (2014). Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Advanced Drug Delivery Reviews, 79–80, 222–237. https://pubmed.ncbi.nlm.nih.gov/25305336/ [DOI] [PubMed] [Google Scholar]
- Christensen L. B., Woods T. A., Carmody A. B., Caughey B., Peterson K. E. (2014). Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. Journal of Neuroinflammation, 11(1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civita P., Valerio O., Naccarato A. G., Gumbleton M., Pilkington G. J. (2020). Satellitosis, a crosstalk between neurons, vascular structures and neoplastic cells in brain tumours; early manifestation of invasive behaviour. Cancers, 12(12), 3720–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah V. A., Robel S., Watkins S., Sontheimer H. (2014). A neurocentric perspective on glioma invasion. Nature Reviews Neuroscience, 15(7), 455–465. 10.1038/nrn3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Hora C. C., Schweiger M. W., Wurdinger T., Tannous B. A. (2019). Patient-derived glioma models: From patients to dish to animals. Cells, 8(10), 1177. www.cbtrus.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmandi S., Shahrokhi S. (2019). Engineering tumor cells with tumor necrosis factor α (TNF-α) or CD40 ligand (CD40L) genes induce anti-tumor immune responses. International Journal of Peptide Research and Therapeutics, 25(2), 427–436. [Google Scholar]
- Davies B., Morris T. (1993). Physiological parameters in laboratory animals and humans. Pharmaceutical Research, 10(7), 1093–1095. https://link.springer.com/article/10.1023/A:1018943613122 [DOI] [PubMed] [Google Scholar]
- De La Rochere P., Guil-Luna S., Decaudin D., Azar G., Sidhu S. S., Piaggio E. (2018). Humanized mice for the study of immuno-oncology. Trends in Immunology, 39(9), 748–763. 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Dolberg T. B., Donahue P. S., Leonard J. N. (2018). Synthetic biology: Reframing cell therapy for cancer. Nature Chemical Biology, 14(3), 204–205. 10.1038/nchembio.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomchenko E. I., Holland E. C. (2006). Mouse models of brain tumors and their applications in preclinical trials. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 12(18), 5288–5297. https://pubmed.ncbi.nlm.nih.gov/17000661/ [DOI] [PubMed] [Google Scholar]
- Giannini C., Sarkaria J. N., Saito A., Uhm J. H., Galanis E., Carlson B. L., Schroeder M. A., James C. D. (2005). Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-Oncology, 7(2), 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D., Bergers G. (2015). Glioblastoma: Defining tumor niches. TRENDS in Cancer, 1(4), 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Kanayama T., Noguchi K., Niwa A., Miyai M., Kawaguchi M., Ishida K., Hatano Y., Niwa M., Tomita H. (2019). Treatment strategies based on histological targets against invasive and resistant glioblastoma. Journal of Oncology, 2019, 2964783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N., Ferná Ndez V., Cruz P. R., Rancati S., Pelizzoli R., De Pietri Tonelli D. (2020). A xenotransplant model of human brain tumors in wild-type mice. Iscience, 23(1), 100813. 10.1016/j.isci [DOI] [PMC free article] [PubMed]
- Huszthy P. C., Daphu I., Niclou S. P., Stieber D., Nigro J. M., Sakariassen P. O., Miletic H., Thorsen F., Bjerkvig R. (2012). In vivo models of primary brain tumors: Pitfalls and perspectives. Neuro-Oncology, 14(8), 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwadate Y. (2016). Epithelial-mesenchymal transition in glioblastoma progression. Oncology Letters, 11(3), 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbazian L., Karamchandani J., Das S. (2014). Mouse models of glioblastoma: Lessons learned and questions to be answered. Journal of Neuro-Oncology, 118(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Jun H. J., Appleman V. A., Wu H.-J., Rose C. M., Pineda J. J., Yeo A. T., Delcuze B., Lee C., Gyuris A., Zhu H., Woolfenden S., Bronisz A., Nakano I., Chiocca E. A., Bronson R. T., Ligon K. L., Sarkaria J. N., Gygi S. P., Michor F., Mitchison T. J., Charest A. (2018). A PDGFRα-driven mouse model of glioblastoma reveals a stathmin1-mediated mechanism of sensitivity to vinblastine. Nature Communications, 9(1), 1–13. 10.1038/s41467-018-05036-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Seol H. S., Chang S. (2018). The generation and application of patient-derived xenograft model for cancer research. Cancer Research and Treatment, 50(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima N., Kanemura Y. (2017). Mouse models of glioblastoma. In De Vleeschouwer S. (Ed.), Glioblastoma (pp. 131–139). Codon Publications. https://pubmed.ncbi.nlm.nih.gov/29251866/ [PubMed] [Google Scholar]
- Larsson S., Wenger A., Dósa S., Sabel M., Kling T., Carén H. (2018). Cell line-based xenograft mouse model of paediatric glioma stem cells mirrors the clinical course of the patient. Carcinogenesis, 39(10), 1304–1309. https://academic.oup.com/carcin/article/39/10/1304/5047402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia J. D., Mack S. C., Mulkearns-Hubert E. E., Valentim C. L. L., Rich J. N. (2015). Cancer stem cells in glioblastoma. Genes & Development, 29(12), 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson L. J., Perry V. H., Dri P., Gordon S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39(1), 151–170. [DOI] [PubMed] [Google Scholar]
- Lenting K., Verhaak R., ter Laan M., Wesseling P., Leenders W. (2017). Glioma: Experimental models and reality. Acta Neuropathologica, 133(2), 263–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A. R., Rathmell W. K., Rathmell J. C. (2020). The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaguno S. R. A., Parada L. F. (2016). Cell of origin of glioma: Biological and clinical implications. British Journal of Cancer, 115(12), 1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. J., Bird G., Refaeli Y., Jimeno A., Biology S. C. (2016). Humanized mouse xenograft models: Narrowing the tumor-microenvironment gap. CANCER Research, 76(21), 6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorani I. (2019). Genetically engineered mouse models of gliomas: Technological developments for translational discoveries. Cancers, 11(9), 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita A., Aldape K. D., Zadeh G., Guha A., James C. D. (2004). Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes, Chromosomes & Cancer, 39(1), 29–36. https://onlinelibrary.wiley.com/doi/full/10.1002/gcc.10300 [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M. (1968). Absence of thymus in a mouse mutant. Nature, 217(5126), 370–371. [DOI] [PubMed] [Google Scholar]
- Parakalan R., Jiang B., Nimmi B., Janani M., Jayapal M., Lu J., Tay S. S. W., Ling E. A., Dheen S. T. (2012). Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neuroscience, 13(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizii M. Bartucci M. Pine S. R. ?0026; Sabaawy He, (2018). Utility of glioblastoma patient-derived orthotopic xenografts in drug discovery and personalized therapy. Frontiers in Oncology, 8, 23. [DOI] [PMC free article] [PubMed]
- Plaks V., Kong N., Werb Z. (2015). The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell, 16(3), 225–238. 10.1016/j.stem.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. M., Yoshikawa K., Clarke I. D., Danovi D., Stricker S., Russell R., Bayani J., Head R., Lee M., Bernstein M., Squire J. A., Smith A., Dirks P. (2009). Resource glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell, 4(6), 568–580. 10.1016/j.stem.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Prager B. C., Bhargava S., Mahadev V., Hubert C. G., Rich J. N. (2020). Glioblastoma stem cells: Driving resilience through chaos. Trends in Cancer, 6(3), 223–235. 10.1016/j.trecan.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandén E., Eberstål S., Visse E., Siesjö P., Darabi A. (2015). A standardized and reproducible protocol for serum-free monolayer culturing of primary paediatric brain tumours to be utilized for therapeutic assays. Scientific Reports, 5(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer H. J. (1938). Structural development in gliomas. Am J Cancer, 34, 333–351. https://cancerres.aacrjournals.org/content/34/3/333 [Google Scholar]
- Schiffer D., Mellai M., Annovazzi L., Caldera V., Piazzi A., Denysenko T., Melcarne A. (2014). Stem cell niches in glioblastoma: A neuropathological view. BioMed Research International, 2014, 725921. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Seidel S., Garvalov B. K., Acker T. (2015). Chapter 19 isolation and culture of primary glioblastoma. Cells from Human Tumor Specimens, 1235, 263–275. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Lyons B. L., Burzenski L. M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S. D., King M., Mangada J., Greiner D. L., Handgretinger R. (2005). Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. Journal of Immunology (Baltimore, Md.: 1950), 174(10), 6477–6489. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Christianson S. W., Gott B., Schweitzer I. B., Tennent B., McKenna S., Mobraaten L., Rajan T. V., Greiner D. L. (1995). Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. Journal of Immunology (Baltimore, Md.: 1950), 154(1), 180–191. http://www.ncbi.nlm.nih.gov/pubmed/7995938 [PubMed] [Google Scholar]
- Simeonova I, Huillard E. In vivo models of brain tumors: roles of genetically engineered mouse models in understanding tumor biology and use in preclinical studies. Cell Mol Life Sci 2014 Oct; 71(20): 4007–26. doi: 10.1007/s00018-014-1675-3. Epub 2014 Jul 10. PMID: 25008045; PMCID: PMC4175043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland M., Stoll E. A. (2017). /pmc/articles/PMC5405080/?report=abstract. Metabolic Reprogramming in Glioma. Frontiers in Cell and Developmental Biology, 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylli S. S., Luwor R. B., Ware T. M. B., Tan F., Kaye A. H. (2015). Mouse models of glioma. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 22(4), 619–626. 10.1016/j.jocn.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Tammela T., Sage J. (2020). Investigating tumor heterogeneity in mouse models. Annual Review of Cancer Biology, 4(1), 99–119. 10.1146/annurev-cancerbio-030419-033413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Guo S-CG. (2020). Role of extracellular vesicles in tumour microenvironment. Cell Communication and Signaling, 18(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra A. H. M. (2001). Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: Role of metabolic rate. The Journal of Nutrition, 131(7), 2067–2068. https://academic.oup.com/jn/article/131/7/2067/4686907 [DOI] [PubMed] [Google Scholar]
- Thakur B. K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., Xiang J., Williams C., Rodriguez-Barrueco R., Silva J. M., Zhang W., Hearn S., Elemento O., Paknejad N., Manova-Todorova K., Welte K., . . . Lyden D. (2014). Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Research, 24(6), 766–769. www.cell-research.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Howson J. M. M., Esposito L., Heward J., Snook H., Chamberlain G., Rainbow D. B., Hunter K. M. D., Smith A. N., Di Genova G., Herr M. H., Dahlman I., Payne F., Smyth D., Lowe C., Twells R. C. J., Howlett S., Healy B., Nutland S., … Gough S. C. L. (2003). Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature, 423(6939), 506–511. [DOI] [PubMed] [Google Scholar]
- Uhrbom L., Nerio E., Holland E. C. (2004). Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nature Medicine, 10(11), 1257–1260. https://pubmed.ncbi.nlm.nih.gov/15502845/ [DOI] [PubMed] [Google Scholar]
- Varna M., Bertheau P., Legrès L. G. (2014). Tumor microenvironment in human tumor xenografted mouse models. Journal of Analytical Oncology, 3(3), 159–166. [Google Scholar]
- Vlashi E., Lagadec C., Vergnes L., Matsutani T., Masui K., Poulou M., Popescu R., Della Donna L., Evers P., Dekmezian C., Reue K., Christofk H., Mischel P. S., Pajonk F. (2011). Metabolic state of glioma stem cells and nontumorigenic cells. Proceedings of the National Academy of Sciences of the United States of America, 108(38), 16062–16067. /pmc/articles/PMC3179043/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N. C., Kenney L. L., Jangalwe S., Aryee K.-E., Greiner D. L., Brehm M. A., Shultz L. D. (2017). Humanized mouse models of clinical disease. Annual Review of Pathology, 12, 187–215. http://www.annualreviews.org/doi/10.1146/annurev-pathol-052016-100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W. Z., Jones R. F., Juhasz C., Gibson H., Veenstra J. (2015). Evolution of animal models in cancer vaccine development. Vaccine, 33(51), 7401–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt K., Wilk E., Buyny S., Schmidt R. E., Jacobs R. (2007). Interleukin-21 differentially affects human natural killer cell subsets. Immunology, 122(4), 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Li X., Liu P., Li M., Luo F. (2019). Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine (review). Oncology Letters, 17(1), 3–10. http://www.spandidos-publications.com/10.3892/ol.2018.9583/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N. W., Chang C., Lin H. T., Yen C. T., Chen J. Y. (2016). Functional assessment of glioma pathogenesis by in vivo multi-parametric magnetic resonance imaging and in vitro analyses. Scientific Reports, 6(1), 26050. www.nature.com/scientificreports [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kortylewski M., Pardoll D. (2007). Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nature Reviews. Immunology, 7(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Esencay M., Mendez O., Yee H., Smirnova I., Huang Y., Chiriboga L., Lukyanov E., Liu M., Newcomb E. W. (2008). Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1α/CXCR4 expression in glioblastomas: One plausible explanation of Scherer’s structures. The American Journal of Pathology, 173(2), 545–560. 10.2353/ajpath.2008.071197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Tang Z., Li Y., Yin G., Liu Z., Gao J., Chen Y., Chen F. (2020). Patient-derived xenografts of different grade gliomas retain the heterogeneous histological and genetic features of human gliomas. Cancer Cell International, 20, 1–12. https://cancerci.biomedcentral.com/articles/10.1186/s12935-019-1086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Ke S. Q., Huang Z., Flavahan W., Fang X., Paul J., Wu L., Sloan A. E., McLendon R. E., Li X., Rich J. N., Bao S. (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumor-associated macrophages and promotes malignant growth. Nature Cell Biology, 17(2), 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-asn-10.1177_17590914211005074 for Rodent Models to Analyze the Glioma Microenvironment by Susann Hetze, Ulrich Sure, Manfred Schedlowski, Martin Hadamitzky and Lennart Barthel in ASN Neuro