Abstract
Endometrial carcinoma is the most common gynecological malignancy and the fifth most common malignancy in women. The worldwide incidence is 15.9 new cases per 100,000 women per year, and the incidence in Europe is 22.7 new cases. Minority of cases are diagnosed at an advanced stage of the disease. Cutaneous metastases are very rare with a prevalence of 0.8%. If cutaneous metastases are present, the prognosis is poor with an overall survival of up to 12 months. In this review, we presented clinical data on treatment of gynecological cancers with electrochemotherapy, with focus on treatment of cutaneous vulvar metastases from endometrial cancer. Further, we present our data on the case of a 64-year-old woman with recurrent endometrial adenocarcinoma with vulvar skin metastases. Treatment of endometrial carcinoma metastases is multimodal with surgery, chemotherapy, radiotherapy and hormone treatment. There is still no consensus about the specific treatment of cutaneous metastases from endometrial cancer, in particular in order to release symptoms. Electrochemotherapy may be a treatment option to reduce pain and bleeding and a safe option to treat multiple skin metastases.
Keywords: electrochemotherapy, endometrial cancer, cutaneous metastases, skin metastases, bleomycin
Introduction
Endometrial carcinoma is the most common gynecological malignancy and the fifth most common malignancy in women. The worldwide incidence is 15.9 new cases per 100,000 women per year, and the incidence in Europe is 22.7 new cases.1 The incidence in Slovenia in 2017 was 351 new cases (33.7 new cases per 100,000 women). Ninety-three percent of the affected women were over 50 years old. The majority of cases were diagnosed in a localized stage (69.5%), 19.9% in a regional stage and 7.7% with distant metastases.2 The most common sites where metastases are formed are vaginal cuff, pelvic and paraaortic lymph nodes, peritoneum, lungs, and liver. Cutaneous metastases are rare with a prevalence of 0.8%, often at the site of the primary incision (laparotomy or laparoscopy).3
Minimally invasive surgery has become increasingly prevalent in recent years and is considered a standard treatment for endometrial cancer.4 Studies comparing laparoscopy and laparotomy for surgical staging of endometrial cancer reported no difference in overall disease detection in advanced stages, equal or fewer intra- and postoperative complications with laparoscopic access and shorter hospital stays.5,6 As adjuvant therapy for advanced stage carcinomas radiotherapy is most commonly used.7 Chemotherapy is mainly used as primary treatment for unresectable advanced, metastatic or recurrent disease.8 Combination of paclitaxel and carboplatin-based agents is the first line chemotherapy. The purpose of chemotherapy is to prevent the occurrence of distant metastases and the purpose of radiation or multimodal adjuvant treatment combining radiotherapy and chemotherapy is to reduce the likelihood of local recurrence.8-10
Search Strategy
A systematic search of 3 bibliographic databases (PubMed, Web of Science, and ScienceDirect) was performed to obtain articles regarding clinical electrochemotherapy (ECT), using search terms “electrochemotherapy,” “vulvar cancer,” and “gynecologal cancer.” The search was performed in November 2020. Language restriction to English was applied. Some references cited in these articles were screened to identify additional potentially eligible studies. Unpublished studies, abstracts, posters, reviews, editorials, lectures, and commentaries were not included in this review.
Electrochemotherapy and Its Application in Gynecological Cancers
Electrochemotherapy (ECT) is a local ablative therapy that is used to treat various types of tumors such as melanoma, sarcoma, squamous cell carcinoma, basal cell carcinoma, skin metastases from breast cancer and others.11-15 In addition to superficial tumors, deep-seated tumors such as primary hepatocellular carcinoma and liver metastases from colorectal cancer are also treated effectively.16-18
Only a small number of papers describing the use of ECT for the treatment of gynecological cancers and mostly squamous cell vulvar carcinoma have been presented (Table 1). Safety and local efficacy after ECT with bleomycin in locoregional cutaneous recurrences of vulvar carcinomas previously treated with chemotherapy, radiotherapy and surgery or unsuitable for standard treatments have been observed. Objective response (complete response (CR) + partial response (PR)) in local tumor control was achieved in 60% of patients. After a mean follow-up period of 12 months, 6 out of 10 patients (60%) were still alive. Two patients with CR relapsed after 4 and 7 months, respectively, and were further treated with ECT.19 ECT was also used in 2 studies as a palliative therapy in older patients with locally recurrent vulvar cancer.20,21 Perrone et al first reported a study involving response of 8 patients with 62.5% CR, 12.5% PR and 12.5% SD and later a study involving 25 patients with 52% CR, 28% PR and 12% SD, without serious adverse events and with a high control rate of pain and other symptoms.20,21 A recent study of 9 patients included reported a similar objective response, 77.8% CR. A reduced tumor volume allows for more conservative surgery in 66.7% of patients who responded to ECT.22 With a median follow-up of 8 months (range 2-32 months), all patients were alive, without disease. Sersa et al reported a case of skin metastases from dedifferentiated papillary ovarian adenocarcinoma. The nodules were treated palliatively by ECT with cisplatin combined with irradiation. It was a short observation period of only 11 days, but the nodules did not grow, and a positive interaction was described.23
Table 1.
Summary of the Studies and Characteristics of the Patients Included in the Review.
| First author, year published (reference) | No. of patients included/analyzed | Diagnosis stage according to FIGO | Histology | Drug/route of administration | Response according to RECIST after 1 month | |
|---|---|---|---|---|---|---|
| CR (No. of patients/%) | PR (No. of patients/%) | |||||
| Pellegrino, 201619 | 10/10 | Stage IB 40% Stage II 40% Stage IIIA 10% Paget cancer 10% |
Recurrence of vulvar cancer | i.v. bleomycin | 2 (20%) | 4 (40%) |
| Perrone, 201320 | 9/8 | Stage IA 11% Stage IB 11% Stage II 33% Stage IIIA 33% Stage IIIB 11% |
Vulvar SCC | i.v. bleomycin | 5 (62.5%) | 1 (12.5%) |
| Perrone, 201521 | 25/25 | Stage IA 20% Stage IB 32% Stage IIA 32% Stage IIIA 8% Stage IIIB 4% Stage IV 4% |
Vulvar SCC | i.v. bleomycin | 13 (52%) | 7 (28%) |
| Perrone, 201822 | 9/9 | Stage I-II 100% | Vulvar SCC | i.v. bleomycin | 1 (11%) | 6 (67%) |
| Sersa, 199923 | 1/1 | / | Dedifferentiated papillary ovarian adenocarcinoma | i.t. cisplatin | / | / |
| Schiavi, 201724 | 1/1 | Stage II | Endometrial carcinoma | i.v. bleomycin | / | 1* |
Abbreviations: SCC, squamous cell carcinoma; i.v., intra venous; i.t., intra tumoral administration.
* Response 4 months post electrochemotherapy.
ECT for the Treatment of Treatment of Skin Metastases of Endometrial Carcinoma
To the best of our knowledge, only 1 case report has been published that describes the treatment of skin metastases of endometrial carcinoma with ECT.24 One of the reasons for lack of literature is also that less than 10% of endometrial carcinoma are diagnosed with distant metastases at an advanced stage and that vaginal skin metastases are extremely rare and occur mainly 2 to 3 years after the primary diagnosis.25 Moreover, in the literature also combination of ECT and brachytherapy (8 Gy) for recurrence inoperable endometrial cancer was mentioned.26 However, the ECT was performed intravaginal and not on skin metastases. Nevertheless, CR was observed after 2 months but tumor recurred 9 months later.
Schiavi et al presented the case of distant metastases from endometrial carcinoma treated with ECT and reported reduced pain and bleeding of nodules.24 The main symptoms of our patient after the second relapse were bleeding from vulvar nodules and pain, which was rated 7/10 on the visual analog scale (VAS). At this time, pain was controlled with an opioid analgetics administered through a spinal catheter. Four weeks after the ECT, the patient described mild pain, which was rated 2/10 on VAS, with the spinal catheter removed and pain controlled with only oral non-opioid analgesics. There was no more vaginal bleeding from metastases.
To facilitate the application of ECT also for the treatment of skin metastases of endometrial carcinoma we presented our experience in the following paragraphs.
A 64-year-old white female, gravid 3, para 3, was diagnosed with a moderately differentiated endometrioid adenocarcinoma of the endometrium. The diagnosis was confirmed with curettage due to postmenopausal bleeding. Her medical history included early childhood poliomyelitis and hypertension. Her family history was negative for malignant disease. A laparoscopic hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy was performed. Pathohistological analysis showed an endometrioid tumor with a diameter of 52 millimeters, which infiltrated more than half of the myometrium. There was a lymphatic invasion and 1 pelvic lymph node on the right side was metastatic. She was staged FIGO IIIC1 endometrial cancer with no residual disease. According to ESMO-ESGO-ESTRO consensus on endometrial cancer, she was classified as high-risk group to guide adjuvant therapy use. External beam radiation therapy in combination with chemotherapy was proposed.27
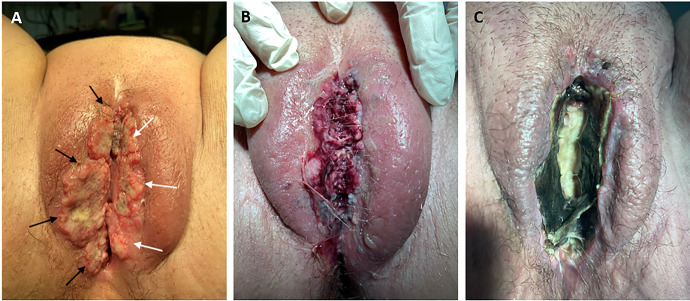
The patient continued treatment with adjuvant pelvic radiotherapy. Fractionation regimen of 50 Gray (Gy) in daily 2 Gy fraction over 5 weeks was used. Then 4 cycles of chemotherapy with carboplatin and paclitaxel were administered. Then, 11 months after treatment, abdominal magnetic resonance and positron emission tomography computed tomography showed a local recurrence at the proximal end of the vagina with infiltration of the distal urethra. An anterior exenteration with Bricker bladder formation and right hemivulvectomy was performed. Patohystological analysis showed a poorly differentiated endometrioid adenocarcinoma with tumor-free surgical margins and positivity of estrogen and progesterone receptors. Hormone treatment with megestrol acetate was initiated for 23 months when a second relapse of the disease was detected on the clitoris. A vulvectomy was performed and the tumor was found at the surgical margins. Radiotherapy of clitoral region (fractionation regimen of 45 Gy in daily 1.8 Gy fraction over 5 weeks) was carried out and megestrol acetate was replaced with an aromatase inhibitor. Fifty-two months after the initial diagnosis, 2 multicenter vulvar metastases of 100 and 70 millimeters in diameter, respectively, appeared (Figure 1A). The main symptom of the patient was pain in the area of the vulvar metastases. Palliative and symptomatic therapy with ECT was suggested at multidisciplinary team meeting. The patient signed informed consent and was treated in the frame of the clinical study (ISRCTN16726595) approved by the National Ethics Committee of the Republic of Slovenia (0120-692/2017/4).
Figure 1.
Photography of tumor nodules (100 (black arrows) and 70 millimeters in diameter (white arrows)) on vulva, before treatment (A), next day after the treatment, where edema of the soft vulvar tissue is seen (B) and 28 days after the treatment, showing ulcer with central necrosis (C).
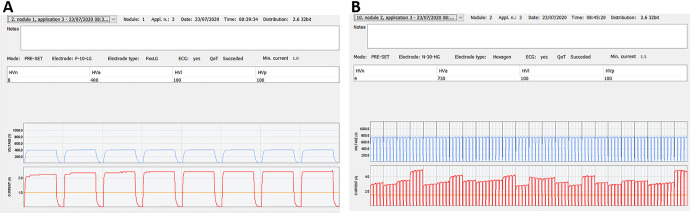
The patient was prepared under general anesthesia and her vital signs were monitored during the procedure. An intravenous bolus injection of 28,200 IU of bleomycin (15,000 IU/m2, Bleomycin medac, Medac, Hamburg, Germany) was administered to the patient 8 minutes before the delivery of electric pulses by Cliniporator® VITAE (IGEA SpA, Carpi, Italy). Two nodules were treated with ECT. First, the part of the bigger nodule, involving the inner part of vagina was treated with finger electrodes, consisting of 6 electrodes of 1 cm length in 2 rows (2 x 3 electrodes) with 4 mm distance between them. This part was treated with 7 electric application of 8 electric pulses of 100 µs duration, 400 V amplitude and frequency 5 kHz. The electrical current of the delivered pulses was in the range of 1.8 to 2.5 A (Figure 2A). Then, the rest of the bigger nodule involving the right labium with the largest diameter of 100 mm and the other nodule on the left labium with the largest diameter of 70 mm were treated with altogether 38 applications of electric pulses. The electrical currents of applied pulses were in the range between 3 to 10 A (Figure 2B). The pulses were delivered by 1 cm hexagonal electrodes in length with 7 electrodes arranged in hexagonal geometry.28 Ninety-six pulses, delivered in 24 trains of 4 pulses, of 100 µs duration, 720 V amplitude at a frequency of 5 kHz were delivered successively. The entire tumor including the safety margins was covered with the electric field. The treatment was performed according to Standard Operating Procedures for ECT.29
Figure 2.
Representative graph of voltages and currents of delivered electric pulses to part of the bigger nodule (A). Electrical currents were in a range of 1.8 to 2.5 A. representative graph of voltages and currents of delivered electric pulses to the other part of both nodules (B). Electrical currents were in a range of 3 to 10 A. The tumors were adequately covered with sufficient current for effective ECT, higher than 1.5 A.30
The patient had no residual pain, fever, or discomfort after the treatment. Edema of the soft vulvar tissue was seen next day after the treatment (Figure 1B). Patient was discharged from hospital first day after the procedure. After ECT hormone treatment with an aromatase inhibitor was continued. Four weeks after the treatment with ECT, the lesions had clinically disappeared and turned into a desiccated necrosis with a central ulcer (Figure 1B). Five months after treatment, the patient is still alive and describes the pain as 2/10 on the VAS scale. The pain is controlled with metamizole and non-opioid analgesics per os. There are no visible signs of disease progression in the vulvar region, the lesions have had a complete response. She has no problems with urination and defecation. She performs her daily hygiene independently and her quality of life has improved compared to before treatment with ECT.
Conclusions
Endometrial carcinoma is the most common gynecological malignancy, which is usually diagnosed at an early stage. Less than 10% of cases are diagnosed with distant metastases at an advanced stage and vaginal skin metastases are extremely rare.
In the case of recurrent disease surgery should only be considered if a complete gross resection can be achieved without macroscopic residual disease. In the case of vaginal recurrence, radiotherapy may be considered. The role of chemotherapy within radio-chemotherapy and its additional benefits are still unclear.27 Hormone therapy is an option in selected patients with recurrent endometrial carcinoma of the endometrioid histology. However, the response to hormone treatment is highly variable and depends very much on the histological type and on hormone receptor-positive tumors.31
The use of ECT in an advanced endometrial cancer with vulvar skin metastases showed that ECT can be proposed as a safe and effective locoregional symptomatic treatment. Our experience showed that ECT might be an alternative to conventional treatment options, especially if the patient is presented with multiple lesions. The main purpose of ECT is to reduce pain and bleeding and thus improve the quality of life. It has been proven that treatment with ECT improves the quality of life of patients and reduces symptoms such as bleeding and pain.
Abbreviations
- CR
complete response
- ECT
electrochemotherapy
- PR
partial response
- SD
stable disease.
Footnotes
Authors' Note: This work was performed at Department of Gynecological Oncology, Institute of Oncology Ljubljana. The patient signed informed consent and was treated in the frame of the clinical study (ISRCTN16726595) approved by the National Ethics Committee of the Republic of Slovenia (0120-692/2017/4).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Slovenian Research Agency (ARRS), grant No. P3-0003.
ORCID iD: Maša Bošnjak, PhD  https://orcid.org/0000-0001-7288-6582
https://orcid.org/0000-0001-7288-6582
Maja Čemažar, PhD  https://orcid.org/0000-0002-1418-1928
https://orcid.org/0000-0002-1418-1928
Gregor Serša, PhD  https://orcid.org/0000-0002-7641-5670
https://orcid.org/0000-0002-7641-5670
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Cancer in Slovenia 2017. Epidemiology and Cancer Registry, Slovenian Cancer Registry. Institute of Oncology Ljubljana; 2020. [Google Scholar]
- 3. Atallah D, el Kassis N, Lutfallah F. et al. Cutaneous metastasis in endometrial cancer: Once in a blue moon—case report. World J Surg Oncol. 2014;2:86. doi:10.1186/1477-7819-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falcone F, Balbi G, Di Martino L, Grauso F, Salzillo ME, Messalli EM. Surgical management of early endometrial cancer: an update and proposal of a therapeutic algorithm. Med Sci Monit. 2014;20:1298–1313. doi:10.12659/MSM.890478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker JL, Piedmonte MR, Spirtos NM. et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: gynecologic oncology group LAP2 study. J Clin Oncol. 2012;30(7):695–700. doi:10.1200/JCO.2011.38.8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zullo F, Falbo A, Palomba S. Safety of laparoscopy vs laparotomy in the surgical staging of endometrial cancer: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2012;207(2):94–100. doi:10.1016/j.ajog.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 7. Rovirosa A, Cortés KS, Ascaso C. et al. Are endometrial cancer radiotherapy results age related? Clin Transl Oncol. 2018;20(11):1416–1421. doi:10.1007/s12094-018-1872-x [DOI] [PubMed] [Google Scholar]
- 8. Neri M, Peiretti M, Melis GB. et al. Systemic therapy for the treatment of endometrial cancer. Expert Opin Pharmacother. 2019;20(16):2019–2032. doi:10.1080/14656566.2019.1654996 [DOI] [PubMed] [Google Scholar]
- 9. Bestvina CM, Fleming GF. Chemotherapy for endometrial cancer in adjuvant and advanced disease settings. Oncologist. 2016;21(10):1250–1259. doi:10.1634/theoncologist.2016-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooks RA, Fleming GF, Lastra RR. et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258–279. doi:10.3322/caac.21561 [DOI] [PubMed] [Google Scholar]
- 11. Groselj A, Bosnjak M, Strojan P, Krzan M, Cemazar M, Sersa G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: preliminary results. Head Neck. 2018;40(1):120–125. doi:10.1002/hed.24991 [DOI] [PubMed] [Google Scholar]
- 12. Rotunno R, Campana LG, Quaglino P. et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: analysis of 57 patients from the international network for sharing practices of electrochemotherapy registry. J Eur Acad Dermatology Venereol. 2018;32(7):1147–1154. doi:10.1111/jdv.14708 [DOI] [PubMed] [Google Scholar]
- 13. Clover AJP, Bertino G, Curatolo P. et al. Electrochemotherapy in the treatment of cutaneous malignancy; outcomes and subgroup analysis from the cumulative results from the pan-European InspECT database for 1478 lesions in 691 patients (2008-2018). Eur J Surg Oncol. 2019;138(2):30–40. doi:10.1016/j.ejca.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 14. Matthiessen LW, Johannesen HH, Hendel HW, Moss T, Kamby C, Gehl J. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51(6):713–721. doi:10.3109/0284186X.2012.685524 [DOI] [PubMed] [Google Scholar]
- 15. Campana LG, Edhemovic I, Soden D. et al. Electrochemotherapy—emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur J Surg Oncol. 2019;45(2):92–102. doi:10.1016/j.ejso.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 16. Edhemovic I, Brecelj E, Cemazar M. et al. Intraoperative electrochemotherapy of colorectal liver metastases: a prospective phase II study. Eur J Surg Ocol 2020;46(9):1628–1633. doi:10.1016/j.ejso.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 17. Djokic M, Cemazar M, Popovic P. et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol. 2018;44(5):651–657. doi:10.1016/j.ejso.2018.01.090 [DOI] [PubMed] [Google Scholar]
- 18. Djokic M, Dezman R, Cemazar M. et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: technological advancement. Radiol Oncol. 2020;54(3):347–352. doi:10.2478/raon-2020-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pellegrino A, Damiani GR, Mangioni C. et al. Outcomes of bleomycin-based electrochemotherapy in patients with repeated loco-regional recurrences of vulvar cancer. Acta Oncol. 2016;55(5):619–624. doi:10.3109/0284186X.2015.1117134 [DOI] [PubMed] [Google Scholar]
- 20. Perrone AM, Galuppi A, Cima S. et al. Electrochemotherapy can be used as palliative treatment in patients with repeated loco-regional recurrence of squamous vulvar cancer: a preliminary study. Gynecol Oncol. 2013;130(3):550–553. doi:10.1016/j.ygyno.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 21. Perrone AM, Cima S, Pozzati F. et al. Palliative electro-chemotherapy in elderly patients with vulvar cancer: a phase II trial. J Surg Oncol. 2015;112(5):529–532. doi:10.1002/jso.24036 [DOI] [PubMed] [Google Scholar]
- 22. Perrone AM, Galuppi A, Borghese G. et al. Electrochemotherapy pre-treatment in primary squamous vulvar cancer. Our preliminary experience. J Surg Oncol. 2018;117(8):1813–1817. doi:10.1002/jso.25072 [DOI] [PubMed] [Google Scholar]
- 23. Sersa G, Cemazar M, Rudolf Z, Fras A. Adenocarcinoma skin metastases treated by electrochemo therapy with cisplatin combined with radiation. Radiol Oncol. 1999;33(4):283–291. [Google Scholar]
- 24. Schiavi MC, Di Tucci C, Rotunno R. et al. Skin metastases from endometrial cancer treated with electrochemotherapy: case report and review of literature. Eur J Gynaecol Oncol. 2017;38(5):800–805. doi:10.12892/ejgo3823.2017 [Google Scholar]
- 25. Wong CY, Helm MA, Kalb RE, Helm TN, Zeitouni NC. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5(9):499–504. doi:10.4103/1947-2714.118918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skarlatos I, Kyrgias G, Mosa E. et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In Vivo. 2011;25(2):265–274. [PubMed] [Google Scholar]
- 27. Miklavčič D, Mali B, Kos B, Heller R, Serša G. Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13(1):29. doi:10.1186/1475-925X-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gehl J, Sersa G, Matthiessen LW. et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57(7):874–882. doi:10.1080/0284186X.2018.1454602 [DOI] [PubMed] [Google Scholar]
- 29. Colombo N, Creutzberg C, Amant F. et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi:10.1093/annonc/mdv484 [DOI] [PubMed] [Google Scholar]
- 30. Marty M, Sersa G, Garbay JR. et al. Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl. 2006;4(11):3–13. doi:10.1016/j.ejcsup.2006.08.002 [Google Scholar]
- 31. Yunokawa M, Yoshida H, Watanabe R. et al. Allred score is a promising predictor of prognosis and medroxyprogesterone acetate efficacy in patients with endometrial cancer. Cancer Chemother Pharmacol. 2017;80(1):127–134. doi:10.1007/s00280-017-3342-5 [DOI] [PubMed] [Google Scholar]