Abstract
The craniofacial region consists of several different tissue types. These tissues are quite commonly affected by traumatic/pathologic tissue loss which has so far been traditionally treated by grafting procedures. With the complications and drawbacks of grafting procedures, the emerging field of regenerative medicine has proved potential. Tissue engineering advancements and the application in the craniofacial region is quickly gaining momentum although most research is still at early in vitro/in vivo stages. We aim to provide an overview on where research stands now in tissue engineering of craniofacial tissue; namely bone, cartilage muscle, skin, periodontal ligament, and mucosa. Abstracts and full-text English articles discussing techniques used for tissue engineering/regeneration of these tissue types were summarized in this article. The future perspectives and how current technological advancements and different material applications are enhancing tissue engineering procedures are also highlighted. Clinically, patients with craniofacial defects need hybrid reconstruction techniques to overcome the complexity of these defects. Cost-effectiveness and cost-efficiency are also required in such defects. The results of the studies covered in this review confirm the potential of craniofacial tissue engineering strategies as an alternative to avoid the problems of currently employed techniques. Furthermore, 3D printing advances may allow for fabrication of patient-specific tissue engineered constructs which should improve post-operative esthetic results of reconstruction. There are on the other hand still many challenges that clearly require further research in order to catch up with engineering of other parts of the human body.
Keywords: Craniofacial, tissue engineering, craniofacial bone
The craniofacial region is a complex network of several tissue types, including bone, cartilage, muscle, salivary glands, nerve tissue, teeth and the surrounding periodontium, and skin/mucosa. The loss of craniofacial tissue may be due to congenital causes, such as clefting1 and craniofacial microsomia, or acquired conditions, such as facial trauma or tumor resection. The result of this loss is significant aesthetic, functional, and psychological affliction.2
Craniofacial reconstruction is challenging due to the complexity of the various structures involved. The various sources of infection in the region (oral/nasal infections) are also to be taken care of. Reconstruction of such defects has conventionally been approached using autologous, allogenic, or xenogeneic grafts to restore the missing tissue with the hope for long-term functional rehabilitation. Complications of this approach range from graft rejection, infection, increased morbidity, and prolonged hospital stays along with the economic burden of such complications.3–5 With continually evolving tissue-engineering and stem cell technologies, the application of regenerative strategies has gained momentum among many research groups worldwide. Regenerative medicine is a broad term encompassing all efforts to reach the ultimate goal of tissue replacement clinically. Tissue engineering is used to denote production of the target tissue using one of many approaches, which all follow similar principals. The most common pillars of the tissue engineering process are cells, scaffolds, growth factors, and gene modification to guide cellular differentiation and proliferation6 (Figure 1). The use of drug molecules for tissue engineering and regenerative procedures has also been reported to be a logical and successful regenerative mechanism.7 Cell transplantation is often used when the defect is challenging to repair using only the natural regenerative process. Cells are isolated from a donor biopsy and expanded in vitro before transplanting into the defect to effect repair and regeneration. Alternatively, expanded cells may be loaded into a bioactive scaffold. Following cellular adhesion and proliferation, the engineered tissue is then implanted into the defect. The scaffold gradually degrades to allow space for the regenerated tissue to fully integrate with the host to restore structure and function.8,9 Different scaffolding techniques are used for tissue engineering applications, including the use of decellularized extracellular matrix (ECM) and sheets of cells on ECM (Figure 2). An added advantage of the regenerative procedures is the ability of incorporation of antimicrobial materials to fight infection which may attack the implanted constructs from nasal or oral infections.10
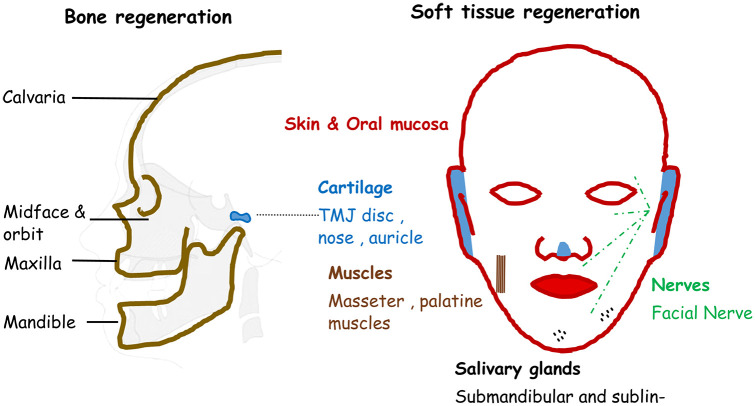
Figure 1.
Diagrammatic representation of different craniofacial tissues.
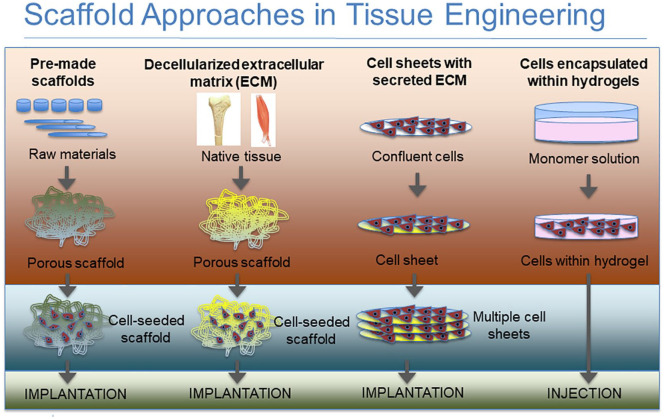
Figure 2.
Diagrammatic representation of the different scaffold approaches used in tissue engineering.
The specific application of such strategies in the craniofacial region is relatively recent compared to the limb region. Craniofacial reconstructive procedures have transitioned from grafting of tissue to 3D-printed implants to tissue engineering techniques, or a combination of these.11 This review aims to provide clinicians and researchers with a wide overview of what has been reported so far in the field of craniofacial tissue engineering. The latest evidence for each of the craniofacial tissue types will be presented with a representative image from discussed literature when possible (table 1). Tooth regeneration was left out of this review as it has been covered in depth in other previously published reviews.12,13
Table 1.
Summary of the tissue engineering approaches in the craniofacial region.
| Authors | Study type | Year | Regenerative construct | Study model | Reported results | References |
|---|---|---|---|---|---|---|
| Bone regeneration | ||||||
| Maliha et al. | In vivo | 2020 | Dipyridamole coated 3D printed B-tricalcium phosphate with varying pore dimensions (220, 330, and 500 µm) | Calvarial defects in rabbits | Large pore scaffolds with Dipyridamole coating showed most bone growth | 18 |
| Francis et al. | Clinical retrospective trial | 2012 | Endoscopic craniofacial reconstruction with injectable calcium phosphate cement | Secondary craniofacial reconstruction | The study group showed efficacious, cost-effective reconstruction | 31 |
| Kirschner et al. | In vivo | 2020 | Carbonated calcium phosphate cement in craniectomy defects | Frontal cranial defects in immature piglets | The study group with the CRS showed promising bone healing without growth hinderance when compared to the negative control | 32 |
| Mediero et al. | In vivo | 2016 | Collagen sponge with CAM/ticagrelor 1 µm/10 µm | Calvarial defect in mice | Ticagrelor and CAM both showed more bone formation three dimensionally as compared to negative control (scaffold with saline). Comparable to the amount of bone when BMP was used | 35 |
| In vivo | 2016 | 3D printed collagen coated hydroxyapatite—βtricalcium phosphate scaffolds with ticagrelor 1 mM or CAM 1 mM | Calvarial defect in mice | Both ticagrelor and CAM showed significantly more bone formation than scaffold alone and comparable amount to the BMP treated defect | ||
| Nokhbatolfoghahaei et al. | In vitro | 2020 | Gelatin/β-tricalcium phosphate scaffolds loaded with mesenchymal cells from the buccal fat pad and rotating-perfusion versus perfusion bioreactors | – | Rotating-perfusion bioreactor group showed higher RUNX2, OCN expressions and ALP and collagen one production increase when compared to the static and perfusion bioreactor | 36 |
| Lopez et al. | In vivo | 2019 | 3D-printed bioceramic scaffolds with 1000 μm of dipyridamole/10,000 μm of dipyridamole/0.2 mg/ml of rhBMP-2 | Alveolar clefts in white immature rabbits | Dipyridamole allowed bone healing comparable to the BMP group with the early suture closure seen with the latter. The formed bone in both groups were of mechanical properties comparable to that of the native bone | 37 |
| Wang et al. | In vivo | 2019 | Dipyridamole loaded 3D printed β-tricalcium phosphate scaffolds | Calvarial and alveolar defects in immature rabbits | The scaffolds showed significant bone formation in comparison to the gold standard bone graft | 38 |
| Zhao et al. | In vitro | 2009 | β-tricalcium phosphate mixed with fibrinogen and thrombin to make injectable scaffolds | – | Human mesenchymal stem cells showed cytoviability and cellular number increase in the scaffold. Increased β-TCP content enabled a higher elastic modulus of the final scaffold | 41 |
| Wang et al. | In vitro | 2016 | Injectable calcium phosphate cement scaffolds with different cell types hDPSCs, hiPSC-MSCs from bone marrow (BM-hiPSC-MSCs) and from foreskin (FS-hiPSC-MSCs) and hBMSCs | – | The scaffolds supported cell viability, osteogenic differentiation. All cell types showed expression of bone forming genes. FS-hiPSC-MSCs were reported to be relatively inferior to the rest of the cell types in osteogenesis | 42 |
| Hasani-Sadrabadi et al. | In vivo | 2020 | Injectable alginate-based hydrogel scaffold (AdhHG) with mesenchymal stem cells | Subcutaneous implantation in mice | The hydrogel was proven to be biocompatibility, biodegradable and osteoconductive | 43 |
| 2020 | Injectable Alginate-based hydrogel scaffold (AdhHG) with gingival mesenchymal stem cells | Rat peri-implantitis models | Complete bone regeneration was achieved around failing dental implants | |||
| Chen et al. | In vivo | 2019 | DBBM/collagen gel/DBBM + collagen gel | Rabbit calvarial model | Addition of DBBM significantly improved immature bone formation while the Gel group improved soft tissue healing. The combination treatment is the best way to manage multi-tissue regeneration | 47 |
| Salamanca et al. | In vivo | 2016 | Freeze-dried porcine collagen membrane with bovine xenograft | Lateral alveolar ridge defects in beagle dogs | The new collagen membrane improves osteoconduction and reduces alveolar height resorption rate | 48 |
| Salamanca et al. | In vitro | 2020 | Collagenated porcine graft compared to porcine graft, HA/β-tricalcium phosphate with MG-63 osteoblast-like cell line | – | CPG group showed greater cell proliferation and osteoblastic differentiation. Gene sequencing showed stable bone formation markers and reduction of resorption makers | 49 |
| In vivo | 2020 | Collagenated porcine graft compared to porcine graft, HA/β-tricalcium phosphate | Calvarial defects in adult male white rabbits | CPG group showed the highest new bone regeneration by osteoconduction | ||
| Cassetta et al. | Clinical trial | 2015 | Augmentation using 100% autologous bone, 100% porcine graft, 50:50 mixture of both | Sinus augmentation | Porcine bone alone and with autologous bone showed osteoconductivty and biocompatibility | 50 |
| Ning et al. | In vivo | 2019 | LAGG-PM composite hydrogels with rat adipose-derived stem cells (rADSCs) | MRONJ induced rat model | LAGG-PM composite hydrogels were found to promote mucosal recovery, bone tissue reconstruction, and osteoclastogenesis | 52 |
| Rodrigues-Lozano et al. | In vivo | 2020 | Bone marrow derived-MSCs cultured on β-Tri calcium phosphate | MRONJ induced mouse model (maxillary alveolar sockets) | No MRONJ-related bone exposure was detected in the study group versus 33% exposure in the control (β-TCP and saline) | 53 |
| Sallstrom et al. | In vitro | 2020 | Zwitterionic sulfobetaine hydrogel with direct culture of neuroblastoma cell line VS indirect culture | The material seemed to support cellular growth and proliferation and that was supported by the appearance of extended neurites on the hydrogel surface | 54 | |
| Diez-Escudero et al. | In vitro | 2020 | Porous polylactic acid scaffolds with Diamon/Gyroid/Schwarz internal configuration with pre-osteoblastic cell lines | No cytotoxicity was reported. The larger and multimodal porosity supported differentiation better | 55 | |
| Muscle regeneration | ||||||
| Manchineella et al. | In vitro | 2016 | Silk fibroin/melanin films and electrospun fiber sheets as scaffolds with C2C12 myoblast cell line | – | The scaffolds promoted the myoblast’s assembly and differentiation and proved thermal stability provided by melanin | 61 |
| Vandenburgh et al. | In vitro | 2008 | Primary mouse myoblasts on polydimethylsiloxane (PDMS) attached to flexible microposts of varying diameters (300–800 µm), 4–5 mm tall, and 4 mm apart | – | The miniature bioartificial muscles generated active forces upon electric stimulation | 62 |
| Abou Neel et al. | In vitro | 2005 | Phosphate-based glass fibers (PGF) with different iron oxide (Fe2O3) molarity | – | PGF with larger diameters and 3–5 mol% Fe2O3 are more durable scaffolds that should allow for better initial myoblast attachment than others with 1 or 2 mol% Fe2O3 | 63 |
| Farano et al. | In vitro | 2018 | Melt-quenched phosphate glasses were combined as powders with collagen fibers from bovine achilles tendon to make degradable scaffolds | Scaffold characterization | Characterization of the fabricated scaffolds showed interconnected porous structures and biodegradability. Bioactivity was proven by finding a Ca-P rich layer on all scaffolds’ surfaces—whish was comparable to that formed by HA in one sample | 64 |
| Guo et al. | In vitro | 2019 | Injectable electroactive degradable hydrogels (dextran-graft-tetraaniline and N-carboxyethyl chitosan) with C2C12 myoblasts and human umbilical vein endothelial cells | Biocompatibility was confirmed Myoblasts showed linear like release | 65 | |
| In vivo | 2019 | Injectable electroactive degradable hydrogels (dextran-graft-tetraaniline and N-carboxyethyl chitosan) with C2C12 myoblasts and human umbilical vein endothelial cells | 200 µL were injected subcutaneously in rat tibialis anterior defects | Due to it’s injectability, the hydrogel allows non-surgical implantation high myofiber density, more capillaries, and centronucleated myofibers in the defect were detected in all study groups with significantly higher numbers of centronucleated myofibers in the 3% AT scaffolds | ||
| Jung et al. | In vivo | 2017 | Pulp cells extracted from adult human premolars treated with 5-Aza | Gastrocnemius and masseter muscles of male mice | The epigenetic modification with 5-Aza stimulated muscle regeneration in vivo | 70 |
| Brady et al. | In vitro | 2008 | Human myogenic and non-myogenic muscle-derived cells (MDC) seeded in 3D collagen constructs | Non-myogenic cells can be used for 3D myogenic differentiation, force generation and matrix remodelling | 71 | |
| The mix of cell origins had a synergistic effect on peak force and MMP-2 mRNA expression | ||||||
| Shah et al. | In vitro | 2004 | Human masseter derived cells cultured on phosphate-based glass fibers of different orientations | 3D mesh arrangement of the glass fibers supported the best cell attachment and proliferation | 72 | |
| Increasing seeding density and adding ILGF-1 and Matrigel enhanced prototypic muscle fiber formation | ||||||
| Zhang et al. | In vivo | 2019 | Human amniotic mesenchymal cells with the DNA demethylating agent 5-azacytidine | Volumetric muscle loss in rat tibialis anterior muscle | The rat model showed improved local tissue repair and increased angiogenesis | 74 |
| Cartilage regeneration | ||||||
| Vinatier et al. | In vivo | 2009 | Autologous rabbit nasal chondrocytes (RNC) associated with an injectable self-setting cellulose-based hydrogel (Si-HPMC) | Rabbit articular cartilage defect | The defect treated with RNC showed formation of repair tissue organized similar to normal cartilage | 77 |
| The regenerated tissue was histologically hyaline-like cartilage | ||||||
| Ahtiainen et al. | In vivo | 2013 | Bi-layer polylactide (PLA) discs and autologous adipose stem cells (ASCs) with TGF-β1 for TMJ disc regeneration | Rabbit temporomandibular joints | ASC—PLA discs pre-treated with TGF-β1 improved condylar integrity | 78 |
| Histologically, no inflammation, infection or foreign body reactions were detected | ||||||
| Vapniarsky et al. | In vivo | 2018 | Scaffold-free tissue constructs from passaged costal chondrocytes | Intralaminar implantation in TMJ discs of minipigs | The tissue engineered construct group showed better healing of the defect than the empty control. Histologically the cartilaginous formation and collagen content change was noted, while the mechanical properties of the constructs were also acceptable. Necropsy revealed no signs of cell damage/inflammation/neoplastic changes | 79 |
| Cakmak et al. | In vivo | 2013 | Injectable tissue engineered cartilage within a fibrin glue with/without aprotinin, different concentrations of thrombin and fibrinogen. (chondrocytes harvested from auricle/costa/nasal septum) | Subcutaneous injection interocular and forehead of white rabbits | Inflammatory reactions, abscess formation, and foreign body reactions around the new cartilage tissue of tissue-engineered cartilage | 80 |
| The different groups (concentrations of constituents/cell sources) showed no statistically significant differences | ||||||
| Kim et al. | In vivo | 2019 | Human umbilical cord matrix-mesenchymal stem cells (hUCM-MSCs) for the treatment of TMJ-osteoarthritis in comparison to other MSCs origins | Intra-articular injection in rabbit models with induced TMJ osteoarthritis | Regenerative and anti-inflammatory capacity of the hUCM-MSCs was clear | 82 |
| hUCM-MScs anti-inflammatory effect was comparable to that of dexamethasone | ||||||
| Moreover, only hUCM-MSCs showed potential for chondrogenesis. | ||||||
| Cui et al. | In vivo | 2020 | Human dental pulp stem cells (DPSCs) were injected into the articular cavity to treat rat TMJ arthritis | Local injection in arthritic temporomandibular joints of female rats | Local injection of DPSCs in rats with arthritic joints of rats relieved hyperalgesia, synovial inflammation, reduced cartilage degradation, and enhanced bone regeneration | 83 |
| Ogasawara et al. | In vivo | 2020 | IV injection of conditioned media from human exfoliated deciduous teeth stem cells (SHED-CM) | Injection in induced osteoarthritic mouse model | Suppressed temporal muscle inflammation, and improved bone integrity and surface smoothness of the destroyed condylar cartilage | 84 |
| Zhang et al. | In vivo | 2019 | Mesenchymal stem cells’ exosomes injection | Intra-articular injection in 8-week old rats’ osteoarthritic TMJ | MSC exosomes promoted TMJ repair and regeneration in OA The cell-free ready-to-use exosome-based therapeutic potential for treating TMJ pain and degeneration is significant | 85 |
| Kuznetsov et al. | In vivo | 2019 | Undifferentiated bone marrow stromal cells (BMSCs) on fibrin microbeads (FMBs) | Subcutaneous injection in immunocompromised mice | Significant amounts of hyaline-like cartilage were reported when BMSCs were attached to hyaluronic acid coated FMBs | 86 |
| Chen et al. | In vivo | 2020 | 3D fabricated decellularized bone scaffolds with autologous adipose-derived chondrogenic and osteogenic cells. | Ramus-condyle defect models in minipigs | The fabricated RCUs maintained their structure and cartilage was regenerated over the underlying bone more than the bone only and acellular scaffold comparators | 87 |
| Park et al. | In vivo | 2017 | 3D-printed PolyCaproLactone implants | Septal grafting for nasal reshaping in white rabbits | The implants retained their location | 89 |
| Histologically, the implant retained its morphology with significant fibrovascular ingrowth and minimal inflammation | ||||||
| Reuther et al. | In vitro | 2014 | Human septal chondrocytes expanded and resuspended in alginate on transwell clear polyester membrane insert | The expanded constructs were histologically similar to those of the standard size | 90 | |
| Mendelson et al. | In vivo | 2014 | Alginate containing gelatin microspheres encapsulating cytokines on PLGA base (with r-TGFβ3 at different concentrations) | Rhinoplasty model in rats | Cartilage-like tissue formation was enhanced by increasing doses of TGFβ3 | 91 |
| This technique may be a successful alternative for augmentative and reconstructive rhinoplasty | ||||||
| Yi et al. | In vivo | 2019 | 3D model of customized nasal implant with injected hydrogel containing human adipose-derived stem cells | Subcutaneous implantation in mice | Maintenance of the exquisite shape and structure, and striking formation of the cartilaginous tissues for 12 weeks | 92 |
| Cao et al. | In vivo | 1996 | PGA-PLA scaffolds with chondrocytes isolated from bovine articular cartilage | Subcutaneous pockets on dorsa of athymic mice | Morphologic and histologic assessment showed the formation of new cartilage | 94 |
| The overall geometry resembled that of an infant auricle | ||||||
| Morrison et al. | In vivo | 2016 | Human auricular chondrocytes (hAuC) and human mesenchymal stem cells (hMSC) encapsulated into type I collagen hydrogels shaped like full scale-ear constructs | Subcutaneously implanted in mice dorsa | The construct showed cartilage microstructure | 95 |
| The human ear constructs maintained shape, projection, and flexibility | ||||||
| Kagimoto et al. | In vivo | 2016 | Xenotransplantation of progenitor cells to reconstruct ear cartilage. | Subcutaneous region of a craniofacial defect in a monkey | Elastic cartilage was regenerated | 96 |
| Mature elastic cartilage with newly formed perichondrium was successfully detected | ||||||
| Liao et al. | In vivo | 2015 | A chondrocyte membrane on an ear-shaped Ti model | Implanted in dorsal pockets of nude mice | Histologically the newly formed tissue was confirmed to be elastic cartilage | 97 |
| Matuska et al. | In vitro | 2018 | Effect of delipidation on decellularized porcine TMJ disc with seeded human MSCc | A combination of solvents and surfactant treatment no cytotoxicity or residual lipid content was noted | 98 | |
| Nerve regeneration | ||||||
| Binnetoglu et al. | In vivo | 2019 | Bacterial cellulose conduits for nerve regeneration with or without primary suturing | Main trunk of facial nerve in female rats | The number of myelinated fibres was significantly higher with the placement of bacterial cellulose conduits | 107 |
| Piao et al. | In vivo | 2020 | Collagen conduits with collagen-binding domain (CBD)-human basic fibroblast growth factor (bFGF) | Buccal branch of facial nerve injury model in white rabbits | CBD-bFGF enhanced functional facial nerve regeneration | 108 |
| Watanabe et al. | In vivo | 2017 | Silicone conduits with differentiated and undifferentiated Adipose derived stem cells (ADSCs) embedded in a collagen gel | Nerve defect in the buccal branch of the facial nerve of rats | Functional nerve regeneration was evident in all groups comparable to results of autologous nerve grafts | 110 |
| Sasaki et al. | In vivo | 2011 | Degradable PLGA tubes filled with dental pulp cells (DPCs) embedded in collagen gel | Nerve defects in the buccal branch of mandibular nerve of adult rats | The PLGA tubes resorbed in vivo Tuj-1 positive axons were noted 2 months after transplantation | 111 |
| Costa et al. | In vivo | 2013 | Bone marrow stem cells in Polyglycolic acid tube conduits with BMSCs/Schwann-like cells differentiated from BMSCs | Mandibular branch of facial nerve defects in rats | Facial nerve regeneration was improved by PGAt and the Schwann-like cells enhanced the regeneration potential | 112 |
| Xiao et al. | In vitro | 2017 | Dental pulp cell spheroids on matrigel in vitro | DPCs differentiated into neuronal lineage under neuronal inductive conditions | 113 | |
| They can stimulate neurogenesis in mouse hippocampal slices in vitro | ||||||
| Salivary gland regeneration | ||||||
| Joraku et al. | In vivo | 2005 | Primary human salivary gland cells grown expanded and seeded on Polyglycolic acid scaffolds | Subcutaneous implantation in mice | Histologically acinar gland-like structures were noted in the regenerated tissue | 121 |
| Expression of human salivary type of α-amylase mRNA was confirmed | ||||||
| Joraku et al. | In vitro | 2007 | Human salivary cells cultured, expanded and seeded on a 3D collagen-based gel scaffold | – | Functional, differentiated salivary units containing acini and ducts were reported | 122 |
| Nam et al. | In vivo | 2019 | Submandibular gland cell sheets (single vs multiple layers) | Direct placement into the wounded submandibular glands of mice | Single layer cells retained the cell-to-cell junctions. The double layer sheets formed glandular like structures in vitro. | 125 |
| Ogawa et al. | In vivo | 2013 | Bioengineered gland germ from cells from submandibular, sublingual and parotid glands of mice with PGA extension into the parotid duct | Implanted atop the masstere muscle after extraction of salivary glands in female mice | Salivary flow and content was comparable of that in normal mice | 124 |
| Nam et al. | In vivo | 2017 | Submandibular gland cells on Fibrin Hydrogels with L1 peptide conjugation | Submandibular gland wound models in mice | Organized salivary tissue was formed with good collagen organization was noted in the group with the FH scaffolds | 126 |
| Maruyama et al. | In vitro | 2015 | Combination of laminin and a feeder layer of human hair follicle derived mesenchymal stem cells (hHF-MSCs) | – | hHF-MSC conditioned medium improved cellular orientation and allowed acinar and ductal structure formation | 127 |
| Su et al. | In vivo | 2020 | Labial stem cells from human labial glands were extracted and expanded, the extract (LSCE) after centrifugation was used to regenerate irradiated salivary glands | Irradiated mice were injected with the LSCE through the tail vein | 50%–60% increase in salivary flow was noted in LSCE treated mice in comparison to the control group | 129 |
| Histologically a comparable number of acinar and neurovascular components was noted | ||||||
| Skin, mucosa, and periodontal regeneration | ||||||
| Gielkins et al. | In vivo | 2008 | Poly (DL-lactide-e-caprolactone) (PDLLCL) membrane versus collagen and expanded polytetrafluoroethylene (ePTFE) membranes in implant defects | Mandibular angle defects in male rats | PDLLCL membranes showed less bone formation than the collagen and ePTFE membranes | 132 |
| Duskova et al. | In vivo | 2006 | Resorbable collagen membranes (single-layer and double-layer); porcine collagen type I and III membrane versus atelocollagen membrane | Clinical alveolar defects with cancellous bone grafts | No statistically significant difference was found between the groups although the double membrane was more expensive | 134 |
| Cortellini et al. | Clinical in vivo | 2011 | Non-resorbable/bio-resorbable barrier membranes; enamel matrix derivative (EMD)/a combination of bio-resorbable membranes and a bovine xenograft of bovine origin/a combination of EMD and alloplastic biomaterials/a combination of bio-resorbable membranes and EMD versus extraction and restoration of hopeless teeth | Hopeless teeth with perio-endo lesions | 92% of the teeth treated with regeneration protocols lasted throughout the 5-year follow-up | 135 |
| Most of the regenerated teeth showed reduction in mobility | ||||||
| Liu et al. | In vitro | 2020 | Assessment of potential use of Human periodontal ligament stem cells (hPDLSCs) to differentiate into different cell lineage | – | hPDLSCs were able to differentiate into bone-, fiber- and cementum-forming cells, and so can be used for regeneration of periodontium—bone-PDL-cementum complex specifically | 136 |
| Guo et al. | In vivo | 2017 | Dental follicle cell (DFC) sheets and periodontal ligament cell (PDLC) sheets in periodontal defects | Healthy beagle dogs with simulated periodontal defects | Periodontal attachment was noted in both groups. Periodontal ligament–cementum complex structure and better alveolar bone height was only noted in the DFC sheet group | 137 |
| DFC sheets are more effective for periodontal regeneration | ||||||
| Xue et al. | Clinical trial | 2018 | Human acellular amniotic membrane (HAAM) with Vaseline gauze | Full-thickness defects in the lower third of the nose in humans | HAAM improved hemostasis and accelerated pain reduction. Lower infection rates and scar incidence were also noted | 140 |
| Chen et al. | Clinical trial | 2018 | Bioengineered dermal substitute (dermal regeneration template) | Human traumatic periocular tissue loss | Defects either healed completely (50%), one case showed significant improvement not requiring secondary reconstructive procedures, and one other case showed significant reduction in defect size | 142 |
| Rhee et al. | Clinical trial | 1998 | Acellular dermal matrix in comparison to split thickness skin grafting | Intraoral mucosal defects in humans | Graft take was successful in 90% of the cases | 143 |
| Seol et al. | In vivo | 2018 | BioMask—a customized bioengineered skin substitute which fits perfectly onto facial wounds | Face defects in mice | Skin regeneration was noted at the dermis and epidermis levels | 144 |
| According to patient’s CT; wound dressing material and cell-laden hydrogels are accurately printed in a layer-by-layer way | ||||||
| John et al. | In vitro | 2019 | De-epithelialization of human amniotic membrane as a cellular scaffold as a skin substitute | – | Trypsin and cell scraper provided best de-epithelialization results but showed tissue strain | 145 |
| Culturing of keratinocytes and fibroblasts on the membrane was successful and resulted in a mostly keratinized surface | ||||||
| Roh et al. | In vivo | 2017 | Mucosa and skin equivalents were produced from cultured fibroblasts and autologous fibrin and seeding keratinocytes | Full-thickness excisional wounds of rat skin | The cell sheets enhanced healing with earlier wound closure and less scarring | 147 |
| Lower TGF-β1, α-smooth muscle actin, and fibronectin mRNA expression was also noted | ||||||
| Suzuki et al. | In vitro | 2020 | Fish scale type I collagen scaffolds as oral mucosa equivalent | – | Histologically, a fully differentiated epithelial layer was noted indicating that the microstructured fish scale collagen scaffolds can be used to fabricate tissue-engineered oral mucosa equivalents for clinical use | 150 |
| Engineering of multiple tissues | ||||||
| Costa et al. | In vivo | 2014 | Biphasic scaffold with a bone compartment (coated with a calcium phosphate (CaP) layer) and a periodontal PCL compartment | Subcutaneous implantation dorsally in nude male rats | The CaP compartment showed significant ALP activity while the PCL compartment showed with the larger pores allowed better vascularization and periodontal attachment | 153 |
| Lee et al. | In vivo | 2014 | PCL-HA scaffolds with three phases (100 mm microchannels for cementum/dentin interface, 600 mm microchannels for PDL, and 300 mm microchannels for alveolar bone) with DPSCs, PDLSCs, and ABSCs | Subcutaneous pouches in immunodeficient mice | Properly oriented PDL-like collagen fibers, bone sialoprotein-positive bone-like tissue and putative cementum matrix/dentin tissues were found indicating success of the multiphasic scaffold | 154 |
Craniofacial bone regeneration
The current “gold standard” for craniofacial bone tissue replacement is the use of autologous bone grafts from the rib, cranium, or iliac crest. These procedures are associated with several complications, including bone resorption, infection, donor site complication, and can only be used in relatively small defects.14 Bone regeneration is a popular research topic and numerous reports have been published on novel approaches to promote the best outcomes.15–17 The investigation of such techniques in the craniofacial region has been reported clinically, but is still in its infancy due to the difficulty of developing a robust in vivo model of a craniofacial bone defect18 (Figure 3).
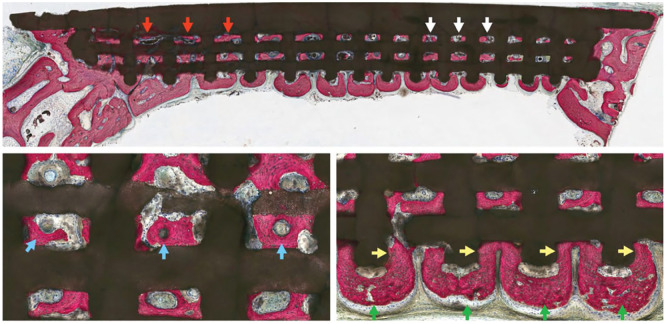
Figure 3.
Dipyridamole coated β-TCP scaffold assessment: quadrant scaffold demonstrates bone regeneration through scaffold porosity, at both larger (red arrows) and smaller (white arrows) pore dimensions. (Below, left) Highly cellular and vascularized bone formation is seen within scaffold interstices. Intramembranous-like healing is observed with regions of mature, lamellar-like bone formation (blue arrows). (Below, right) Bone formation is guided by highly osteoconductive scaffold dimensions as new bone formation is directed from scaffold pore-to-pore (green arrows) while interacting with scaffold struts (yellow arrows). Adapted from Maliha et al.18
The innate regenerative process of healthy bone has been reported as early as 1992.19 The role of the cellular constituents of the periosteum and their distinct functions at the time of bone injury dictate the regenerative process.20 The resident bone-forming stem cells of the periosteum differ according to the type of bone, such that the craniofacial periosteum triggers intramembranous bone formation while long bone periosteum promotes endochondral ossification.21 The employability of the endochondral route to craniofacial bone formation is also a logical approach according to the type of bone to regenerate.22 The proven regenerative power of the periosteum has led to its use in the management of maxillofacial defects of relatively small size.23–26 Bone engineering techniques may use a combination of cells (especially mesenchymal stem cells—MSCs), scaffolds and growth factors to manage larger defects. Although several sources of stem cells are known (embryonic; ESCs, Umbilical cord cells; UCSCs, Adult somatic; iPSCs and adult tissue cells) BM-MSCs and ADSCs remain the major sources of stem cells. MSCs are usually harvested from the bone marrow (BM-MSCs) or can be adipose-derived (ADSCs).27 Bioactive scaffolds provide the proliferating cells with a framework for adhesion, proliferation, and consolidation. 3D printing technologies may be used to produce these scaffolds from materials, such as natural and synthetic polymers. Other recent technologies for scaffold fabrication reported include gas foaming, cryogelation, material extrusion, photopolymerization, electrospinning each with a set of materials that work with it.28 The use of specific agents/materials/growth factors to enhance the bone-forming activity of differentiated osteoblasts is regularly reported in literature. These agents may enhance cellular recruitment and adhesion to the scaffold, promote proliferation then differentiation of specific cells important for bone regeneration and inhibit antagonistic activity (such as that of osteoclasts).15–18,29,30
Calcium phosphates are widely used for bone reconstruction.31,32 3D-printed β-tricalcium phosphate (β-TCP) scaffolds soaked in collagen and coated with dipyridamole have been investigated for bone regenerative purposes. Dipyridamole is a known osteogenic agent that increases osteoblastic differentiation and inhibits osteoclastic activity and inflammatory responses.33–35 Scaffolds of varying pore dimensions (220, 330, and 500 μm) impregnated with different concentrations of dipyridamole (100, 1000, and 10,000 μm) were placed within critical-sized calvarial defects in 5-week-old rabbits. Optimal bone growth and scaffold biodegradation were reported with larger pore sizes and the highest dipyridamole concentration. Histological and radiographical assessment showed vascularized woven and lamellar bone along with initial formation of vascular canals (denoting angiogenesis) within the scaffold lattice and patent calvarial sutures, an important requirement for calvarial reconstruction/regeneration.35 Other researchers have also reported success with 3D-printed β-TCP scaffolds coated with dipyridamole.36,37 Wang et al.38 compared autologous bone grafts with 3D-printed β-TCP dipyridamole-coated scaffolds in alveolar clefts and calvarial defects created in immature rabbits. At 24 weeks, the bioactive scaffolds showed better osteogenic generation than the autologous graft in both the alveolar and calvarial defect sites. In addition, the regenerated bone in both sites showed resemblance to the native bone in terms of organization of trabeculae and mechanical characteristics. The patency of the sutures was validated radiographically at 6 months.
A firm, solid cell-scaffold construct can prove difficult to handle and may not fit a defect easily. Injectable scaffolds have several advantages over stiffer scaffolds.39 The ability to inject a semi-solid or gel-like material into a defect and have it set in position in vivo supports a less invasive clinical approach.40 Injectable tricalcium phosphate scaffolds were tested in vitro within a fibrin gel and were proposed as an osteogenic property-enhancer.41 Injectable hydrogels containing calcium phosphate cement have proven to possess superior mechanical properties and allow better cellular adhesion in vitro.42 Research on methods to enhance the mechanical properties of the setting scaffolds is currently ongoing. Such bioactive hydrogels have been investigated for craniofacial bone regeneration applications.43 Injectable alginate hydrogels loaded with hydroxyapatite, bone morphogenic proteins, and gingival mesenchymal cells were used in peri-implantitis defects created in the maxilla of mice. Once injected, final setting of the alginate hydrogel was achieved by photopolymerization. In vivo assessment showed that more than 50% of the alginate had dissociated by 6 weeks. Clinical and micro-CT assessment revealed fewer inflammatory mediators, better bone recovery and implant survival in defects managed with the hydrogel compared to controls. The option to alter the degradation rate of the hydrogel by changing its molecular weight opens up the potential for regeneration of tissues other than bone.43 Hydroxyapatite (HA)—which is a naturally occurring calcium phosphate—has been commonly used to enhance osteoconductivty of regenerative constructs is a commonly followed strategy.44 HA and other biphasic calcium phosphates have been reported to be a successful addition to β-TCP in experimental maxillofacial models, clinical socket preservation, sinus lift procedures, and crestal height and width augmentation.45 The use of synthetic biomimetic calcium phosphate (SBCP) granules in rat calvarial defects showed bone regrowth that was comparable to that resulting from deproteinized bovine bone material (DBBM). SBCP led to faster bone regeneration. This was thought to be due to the microstructure and higher total porosity of SBCP. The SBCP showed superior results in terms of vertical bone growth which is of great clinical importance in alveolar augmentation.46
Collagen is an integral constituent of bone. Thus, many groups have utilized this natural biomaterial combined with xenogeneic bone material to mimic bone structure and facilitate regeneration. Collagen gels combined with DBBM were used to reconstruct critical-sized calvarial defects in adult rabbits. The presence of DBBM allowed maturation of the formed bone such that it resembled the composition and mechanical properties of native bone. The collagen gel supported better soft tissue healing (in the calvarial skin flap) when compared to the autologous bone grafting; which adds to the scaffold advantages regarding multi-tissue regeneration.47 Collagen combined with porcine bone particles was studied and reported to enhance bone regeneration and reduce bone loss in alveolar defects in beagle dogs.48 Moreover, the collagen-porcine bone particle scaffolds demonstrated greater bone regeneration of critical-sized calvarial defects in adult white rabbits as compared to β-TCP/hydroxyapatite scaffolds.49,50
In cases of bone osteonecrosis-specifically MRONJ (medication related osteonecrosis of the jaw) the use of stem cell therapies to reduce the effect of the drug on the bone, reducing inflammatory reactions and promoting healing. One study identified that MSCs in a mouse MRONJ model improved the healing capacity of the affected MSCs and so provided a better therapeutic benefit. The effect of MSCs in MRONJ treatment may be attributed to their capability of secreting immunomodulatory factors and being immunopriviliged.51 On the other hand, the local delivery of ASCs and BMP-2 in a hydrogel vehicle reinforced with hemicellulose polysaccharide fibers, showed better mucosal recovery, bony reconstruction, and formation of new osteoclasts in a rat model.52 A more recent report also confirmed the effectiveness of BM-MSCs cultured on β-TCP in improving mucosal coverage with no bone exposure and better bony healing in comparison to the negative control in rat models.53 This gives hope for future better management of MRONJ cases which are a clinical dilemma for craniofacial surgeons. The application of bone engineering technologies in the craniofacial region has had reasonable success. The multiple cell types, scaffolds and additions (e.g. growth factors) allow for testing of different combinations to promote bone deposition and maturation. The use of 3D printing technologies and computer-aided design of scaffolds with specific micro- and macro-structure of the scaffolds is now possible and has a promising future. A recent study reported acceptable primary results using a syringe-extruded hydrogel scaffold which was then cured to final setting. Although elastic modulus and tensile strengths of the final material still needs to be improved; the cellular viability and proliferation of the used neuronal cells in this study prove potential for further use.54 Moreover, the further utilization of additive manufacturing and computer-aided designing (CAD) technologies will allow the specific designing of internal micro and macro porosity which could be tweaked according to the necessary tissue types. This was tested on a PLA printed scaffold with different internal pore morphologies and the seeded pre-osteoblastic line showed promising proliferation and activity.55
Further research on the application of these technologies is expected soon. The possibilities of specific designing of the internal architecture of scaffolds to mimic the extracellular matrix of target defects in patients are encouraging. Combining these methods with more conventional grafting procedures or with vascularized grafts may also be a possibility in large defects to combine the advantages of each of the techniques. Clinically oriented research should soon be able to provide evidence on techniques suitable for the different types of craniofacial bony defects.
Craniofacial skeletal muscle regeneration
Muscles of the craniofacial region are of great importance for function and esthetic appearance.56,57 These muscles may be subject to trauma, cancer, generalized muscle disorders, surgical resections, or autoimmune diseases necessitating grafting/regenerative procedures. Orofacial congenital defects, such as cleft lip and/or palate, are associated with impaired muscle regeneration and fibrosis after surgery also requiring repair.58
For engineered muscle to be an acceptable option for muscle regeneration, the tissue has to have similar to native architecture and possess acceptable mechanical properties. The muscle healing process starts by activation of quiescent myogenic progenitor cells followed by proliferation and differentiation.59 To resemble the regenerative process on a larger scale, muscle progenitor cells can be extracted and expanded in vitro. The confluent cells may then be loaded onto a scaffold, which guides and supports early muscle formation and maturation.30 Growth factors may also be used to support the muscle regenerative process and include fibroblast growth factor (FGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and stromal cell–derived factor (SDF).60
The fabrication of a scaffold mimicking the ECM of the native muscle tissue is necessary to enable proper growth and angiogenic activity of the central core to provide nutrients to the newly formed muscle and the proliferating cells. There are several different biomaterials that have been investigated for muscle tissue engineering. Biodegradable polyester scaffolds used for muscle regeneration include natural compounds, such as α-hydroxy acids (polyglycolic acid, poly-L-lactic acid, and polycaprolactone) and silk-fibroin.61 Synthetic biomaterial scaffolds, such as polyurethanes, polypropylene, silicone,62 and phosphate-based glasses63,64 are alternative options.65 Injectable hydrogels have further allowed for simpler delivery to sites of defect.65
Different cell sources have also been investigated for muscle regenerative approaches including satellite cells, ADSCs, BM-MSCs, PVSCs, iPSCs, ESCs, and UC-MSCs.66 Umbilical cord mesenchymal stem cells (UC-MSC) are a promising source. These can be harvested from the umbilical cord without the need for a separate biopsy procedure from the child. The umbilical cord blood and tissue contain a heterogeneous mixture of stem and progenitor cells at different stages of differentiation.67,68 The use of UCMSCs in cleft lip and palate surgery together with anti-inflammatory and antifibrotic agents is a promising method.69
Pulp stem cells were also reported to have myogenic potential when pretreated with 5-Aza (5-aza-2’-deoxycytidine; a modified demethylation agent) in vitro. Treatment of the pulp cells with 5-Aza stimulated myotube formation, myogenic differentiation associated with desmin and myogenin expression, and a degree of scaffold contraction. The epigenetic modification of these cells (collected from premolars) stimulated craniofacial muscle regeneration in the masseter and gastrocnemius of adult mice in vivo.70
Human myogenic and non-myogenic craniofacial muscle-derived cells (MDC) extracted from biopsies and seeded onto 3D collagen constructs expressed myogenin, indicative of myogenic differentiation. Furthermore, there was a synergistic effect as the heterogeneous co-culture of myogenic and non-myogenic cells generated the highest peak force (muscle function) and expressed the most MMP-2 mRNA compared to isolated individual cell populations.71 This may guide further research on muscle regeneration by starting off with a mixed co-culture if that enhances the final engineered muscle activity. Human masseter muscle-derived cells were also used with phosphate-based glass fiber scaffolds in vitro. The scaffolds were fabricated with different internal configurations; bundle alignment, spread-out, and mesh-like arrangement. Microscopic imaging showed that the mesh arrangement led to optimal cell attachment and proliferation which may have been due to the macrotopography of the scaffolds which provided more delicate spaces allowing better cellular grouping and adhesion.72
Creation of a robust craniofacial muscle defect in animal models has proved challenging—for example, a soft palate surgical site was tested in adult rats, but proved too small and difficult to handle.58 Therefore, the most common region to test muscle engineering applications are the limb muscles. Although the structural similarities of craniofacial and limb muscle are undeniable, cellular constituents vary.
Clinical application of muscle regeneration spans a wide range of defects with volumetric muscle loss being the type of defect immensely requiring intervention whether as in grafting with the complications of that73 or regeneration.66,74 A recent study reported encouraging results in Volumetric Muscle Loss (VML) of rat tibialis anterior muscle using amniotic mesenchymal cells. Muscle specific markers (MyoD and desmin) were detected at the end of the study along with improved angiogenesis and local tissue repair74 (Figure 4).
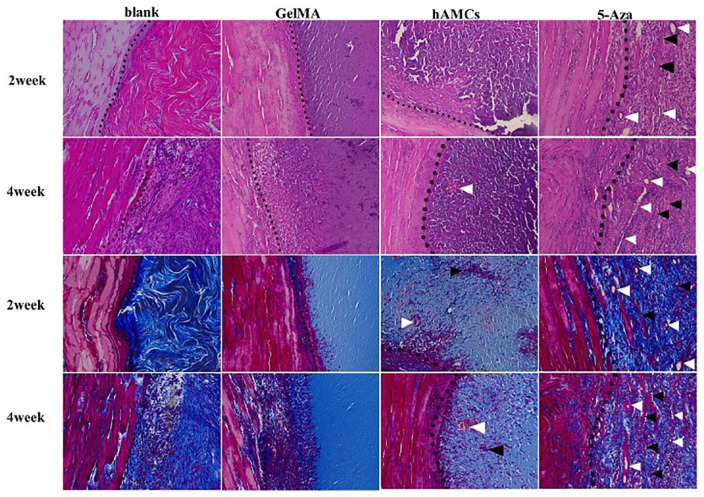
Figure 4.
hAMCs improved tissue repair on VML. H&E and Masson trichrome staining of 2 and 4 weeks after establishment of VML model (blank group), implantation of GelMA gel (GelMA group), GelMA + hAMCs (hAMCs group), and GelMA + 5-Aza-inducted hAMCs (5-Aza group). The dotted line is the boundary between normal muscle tissue and defect. White arrow shows the neovascularization, black arrow shows fused hAMCs and fiber-like tissue. 200×. Adapted from Zhang et al.74
Craniofacial muscle engineering efforts are starting to gain more attention with the important clinical solutions they offer. Challenges still exist regarding the conductivity of scaffolds, vascular ingrowth within the engineered constructs and transfer of the in vitro outcomes to the in vivo environment to further test the proposed methods.
Cartilage
The craniofacial region has several cartilaginous structures, such as the articular disc of the temporomandibular joint (TMJ), the nose and ears. The avascular nature of cartilage and its resulting poor regenerative capacity has made the reconstruction of cartilage defects/deformities an important area of research.
The TMJ disc is a cartilaginous structure, which is integral to the normal mandibular movements pertaining to function. Traditional approaches to manage common destructive conditions of the TMJ disc, include non-invasive and invasive joint procedures associated with high complication and failure rates including diminished mobility, prolonged pain episodes, scarring, numbness, and bleeding.75 Therefore, investigation into disc regeneration utilizing tissue engineering approaches has become essential. The direct injection of human mesenchymal cells into arthritic joints showed better articular cartilage repair and slowing down of the arthritic progression.76 However, there is still a need to produce cartilage discs in the presence of advanced destruction, and many approaches have also utilized scaffolds loaded with cells. Nasal chondrocytes extracted from nasal cartilage of adult female white rabbits were reported to successfully treat a knee articular disc defect in an adult rabbit model when used with an injectable hydrogel scaffold. Although this was tested in an osteochondral knee defect; the principle was reported to be applicable in other similar joints.77 Moreover, Chondrogenic differentiation of rabbit adipose stem cells seeded on PLA scaffolds enabled fabrication of articular discs.78
Scaffold-free tissue engineered implants were constructed from costal chondrocytes of minipig ribs. After ex vivo testing of the technique of the tissue-engineered construct implantation; the constructs were placed in a designed intralaminar defects of TMJ discs of minipigs. The efficacy of these constructs to repair thinning discs was assessed by gross inspection, histologic assessment, and osteoarthritis scoring. All these criteria showed better disc repair using the tissue engineered constructs as opposed to the empty controls.79
An injectable mixture of fibrin, thrombin, and differentiated chondrocytes was reported to enable fabrication of cartilage-like structures in adult rabbit heads. The origin of these chondrocytes was septal/auricular and the recipient sites were the forehead and interocular regions.80 The inflammatory events that occur within the temporomandibular joint are now proven to be a primary cause of the pain, functional limitation, and degenerative procedures that occur. The use of cell-laden biomaterials as vehicles for drug delivery intra-articularly has shown superior results to conventional injections due to the longevity of its action and the regenerative capacity it enforces81 (Figure 5). Moreover, hUC-MSCs injected into osteoarthritic rabbit joints, showed significant regenerative capacity and anti-inflammatory action comparable to that of the Dexamethasone injection control. Growth factors, ECM markers and anti-inflammatory cytokines exhibited upregulated expression while pro-inflammatory cytokines expression was reduced.82 This was also proven with Dental Pulp stem cells in arthritic rat joints83,84 human shed deciduous SCs in mice arthritic joints84 and human-derived ESCs in rats.85 Although clinical trials have not yet reported the use of these technologies to fabricate human cartilage clinically; reports of the differentiation and implantation of human bone marrow cells to engineer cartilage have emerged.86
Figure 5.
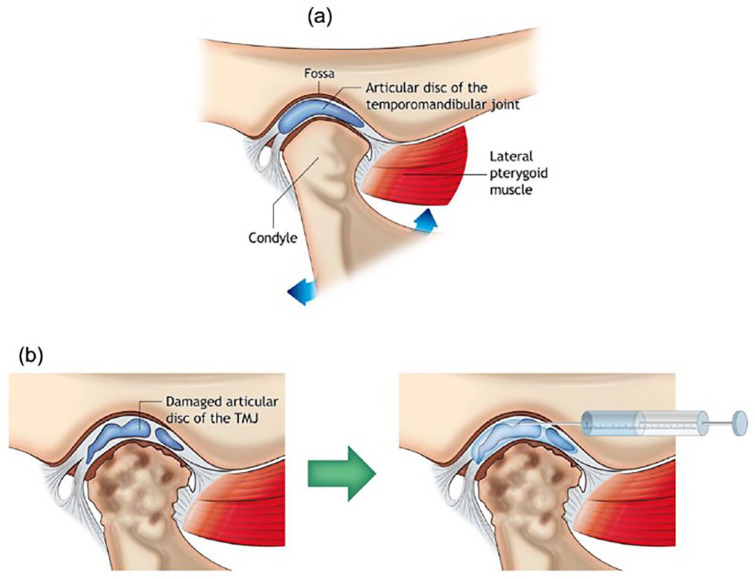
(a) Schematic image of the anatomical structure of temporomandibular joint (TMJ) and the most common target sites for treating temporomandibular disorder (TMD). The image shows components of normal joint anatomy, including the articular disk of TMJ, mandibular fossa, the head of the mandibular condyle, lateral pterygoid muscle, and TMJ capsule enclosing the disk. (b) TMD morphology; the head of the mandibular condyle and the articular disk lose their structures and functions. Intra-articular injection: injection with syringe and needle can deliver proper biomolecules into TMJ capsule for treating TMD as adapted from Dashnyam et al.81
The regeneration of ramus-condyle units (RCU) has been reported to be the solution in cases of TMJ degenerative disease. Scaffolds were milled into anatomically identical forms using decellularized bone matrix and impregnated with adipose-derived chondrogenic and osteogenic cells. The constructs were implanted in minipig’s jaws after culturing for 5 weeks. The constructs maintained their forms and showed better full-thickness regeneration and mechanically comparable cartilage formed over a bony stump than acellular scaffolds and bone-only engineered grafts. This provided an opportunity for multiple tissue regeneration. Inclusion of the adjacent tissues such as soft connective tissues and the TMJ disc could further extend the functional integration of engineered RCUs with the host.87
Nasal reconstruction historically involved autogenous grafting to attain an aesthetic alar/nostril configuration especially in cleft patients.88 This has evolved recently with the use of 3D printing technologies to improve post-surgical outcome.89 Septal chondrocytes harvested from healthy human candidates showed regenerative capacity and resulted in neocartilage constructs of significant volume in vitro.90 A cell-homing procedure was reported where nasal dorsum progenitor cells were recruited and chondrogenically differentiated onto a bi-layered alginate and PGLA scaffold leading to the formation of cartilage-like constructs 10 weeks later in rat models.91 A recent report on a 3D printed nasal cartilage augmentation technique has shown promising results when the printed scaffold impregnated with hASCs implanted subcutaneously in female athymic mouse backs. The use of such technology carries a promise for better postoperative esthetics and shorter healing periods which are crucial in esthetic surgery.92
With the improved esthetics and patient satisfaction, similar techniques were used for auricular cartilage reconstruction.93,94 Human auricular chondrocytes (hAuC),95 monkey-derived perichondrium progenitor cells,96 and porcine chondrocytes were all tested for their regenerative capacity.97 The cells in these studies were harvested, grown in vitro then implanted in vivo. The harvested tissue showed organized arrangement of cartilage mimicking auricular composition. Ex vivo testing of scaffolds versus scaffold-less injectable techniques showed primary results of the possibility of using such techniques for articular regeneration.98
Although research is targeted towards cartilage regeneration in several parts of the human body (in vitro and in vivo preclinical testing), the applications in the craniofacial region are in the early stages. Clinical reports on TMJ disc regeneration are yet to provide evidence of success. Improving mechanical properties of the engineered cartilage and further testing of its long-term survival and function is also yet to be improved.
Craniofacial nerve tissue engineering
Peripheral nerve injuries generally require a complicated grafting procedure to return to pre-injury sensation/function in sensory and motor neurons, respectively. The grafting procedures carry increased risk of infection, graft rejection, and donor site morbidity.99,100 Craniofacial nerves are of specific importance. They are either sensory, motor, or carry both types of fibers. These nerves play an integral part in the sensory detection and reaction to internal and external stimuli. They also have a key role in motor control of the masticatory muscles, extraocular muscles, and muscles of facial expression.101 Craniofacial nerve injury is not uncommon due to the prevalence of facial trauma/tumors affecting the related neural structures.102 The facial nerve, in particular, is responsible for facial expression—injury of the facial nerve causes facial asymmetry and esthetic deficits, which are managed by primary surgical nerve repair or nerve grafting.103,104 These techniques are quite complex and require expensive equipment and training to enable the surgeon to perform the delicate microsurgical anastomosis.105,106
An intact endoneurium is necessary to bridge gaps between the distal and proximal edges of a nerve injury. The use of conduits for neural repair is a common strategy where different materials and/or grafts are implanted at the injury site to guide the neuronal regeneration.107 Different additives have been tested on the fabricated scaffolds for neuronal regeneration, such as fibroblast growth factor,108 IL-6, neurotrophins, glial-derived neurotrophins, and persephin.109 Most of the reported studies have used pluripotent stem cells because they are easy to harvest and readily differentiate to nerve tissue. A few studies have reported the use of ADSCs, BMSCs, or dental pulp cells in facial nerve regeneration with varying success.110–112 Dental pulp stem cells have a common ectodermal origin making these cells a logical source for nerve tissue engineering. These cells were tested in vitro and showed encouraging results in terms of stimulating neurogenesis in adult mice ex vivo.113,114 Isolated muscle stem cells have also shown ability to regenerate nerve cells in murine xenotransplantation models.115,116 Moreover, recent murine studies have demonstrated that cells of the immune system, specifically regulatory T cells, play a critical role in nerve regeneration following acute chemical injury.115–118 The utilization of recent technologies has not been disregarded in neural regeneration attempts; recently a 3D engineered functional human peripheral nerve was tested on a novel nerve-on-a-chip platform with very encouraging results119 (Figure 6). With most of these trials, especially targeting craniofacial nerve engineering, being in vitro; translational and in vivo trials are much needed. Further assessment of the proposed methods, their longevity and long-term results and whether they may also aid in myogenic regeneration of target muscles is crucial. Moreover, clinical application remains largely unattained to with it being the most important target of all neural regeneration research.
Figure 6.
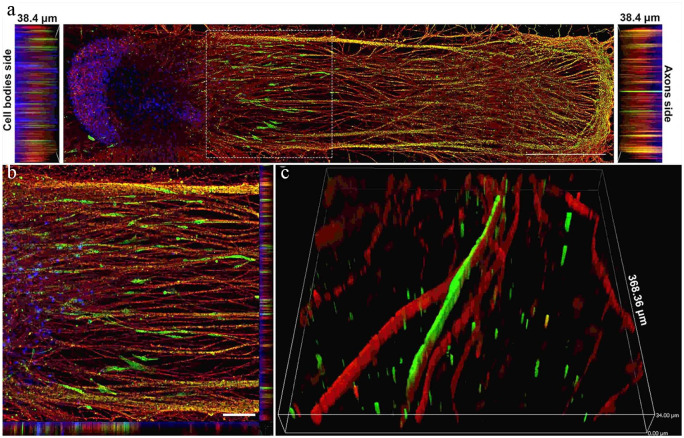
Schwann cells migrated out of the spheroid and elongated along the axons. (a) Image showing how human Schwann cells (hSCs) stained for the hSC marker S100 (green) migrated out of the spheroid along with growing axons stained for βIII-tubulin (red) over a period of 4 weeks. Nuclei were labeled with DAPI (blue). Scale bar: 1000 µm. (b) High-magnification image of inset from image A. Scale bar: 25 µm. (c) 3D image showing close-up of the relationship between hSCs (green) and myelinated axons (red). Slice size was 368.36 × 368.36 × 34.00 µm. Adapted from Sharma et al.119
Engineering the salivary glands
The craniofacial region has multiple major and minor salivary glands, which may be affected by conditions, such as auto-immune disorders (Sjogren’s disorder) and tumors. The result of these conditions is salivary gland dysfunction leading to hyposalivation or xerostomia. Patients have a poorer quality of life, because of poor masticatory and taste ability, and the increased risk of fungal infections and dental caries due to loss of the protective and lubricant quality of saliva. Conventional management is with sialogogues and salivary substitutes, which require strict patient compliance. Sialogogues are associated with complications, such as muscle aches and pains.120
Attempts to regenerate the damaged salivary parenchyma started by isolation of primary human-salivary cells; the culture of isolated cells was reported as early as 2007.121,122 Parotid specimens of healthy consenting adults were collected and the epithelial cells were isolated and cultured on collagen scaffolds. A rise in amylase production was noted in the cultured salivary epithelial cells indicating the organization and activity of acinar cells. These were compared with human bladder cells as a control group over a 6-day culture period. Ogawa et al. (2015) isolated germ cells from the submandibular, parotid, and sublingual glands of mice and demonstrated epithelial bud formation after 2 days in organ culture.123 When transplanted back into salivary gland defects in mice, better salivary flow was noted when compared to the no-intervention control.123 A mixture of epithelial and mesenchymal cells extracted from submandibular, parotid, and sublingual salivary glands of adult mice were used to regenerate an organ germ which was then implanted onto a masseter defect in female mice after extraction of their submandibular, sublingual, and parotid glands. The implanted gland had an extending Polyglycolic acid (PGA) guide which was inserted into the host parotid duct. Saliva was collected from the oral cavity with and without stimulation proving the functional capacity of the bioengineered salivary glands. No significant difference in salivary flow and content between the bioengineered glands and those in natural mice was noted (Figure 7).124 Correct acinar/glandular cellular orientation is crucial to provide proper salivary function. Researchers have been able to achieve this through the layering of cells. When implanted into a mouse model, a double layer of cultured submandibular gland cells showed superior regenerative properties with organization of the cells into acini-like structures compared to a single layer of cells.125
Figure 7.
(a) Schematic representation of the transplantation procedure using the interepithelial tissue-connecting plastic method with the bioengineered salivary gland germ. (b) Phase-contrast images of the bioengineered salivary gland germ containing a PGA monofilament guide. Scale bar, 200 μm. (c) Photographs of bioengineered salivary gland germ transplantations in salivary gland defect mice. The three major salivary glands were extracted and a bioengineered salivary gland germ was transplanted. Scale bar, 1 mm. (d) Photographs of the natural submandibular gland (left) and the bioengineered salivary gland at days 0 and 30 after transplantation (second and third figure from the left). FITC-gelatine conjugate was injected into the bioengineered submandibular gland from the host parotid duct (right). Scale bar, 1 mm. (e) Histological images of the duct connection between the host duct and epithelial duct of the GFP-labelled bioengineered salivary gland (left). Higher magnification images in the box area are shown (right). Bioengineered salivary glands developed in vivo with the correct connection to the recipient parotid gland duct (arrowhead). Scale bar, 150 μm. (f) Photographs of the bioengineered salivary gland, which was reconstituted from GFP-transgenic mice-derived epithelial cells and normal mice-derived mesenchymal cells (left: merged with the stereomicroscope image and the GFP image, second figure from the left: GFP image). Scale bar, 1 mm. The section images of hematoxylin and eosin (HE) staining (third figure from the left) and GFP fluorescence (right) are shown. Scale bar, 200 μm. (g) Histological analysis of the submandibular gland (upper columns) and the sublingual gland (lower columns), including the natural (upper) and bioengineered (lower) salivary glands. Images of HE staining (left three) and periodic acid and Schiff (PAS) staining (right two) are shown. Higher magnification images in each box area are shown (second and third panels from the left, right figure). Scale bar, 100 μm in the left column and 25 μm in the second and subsequent columns. (h) Wet weights of natural and bioengineered salivary glands. The data are presented as the median ± max, min; n = 6 for the natural parotid, submandibular and sublingual glands, n = 20 for the bioengineered submandibular glands and n = 9 for the bioengineered sublingual glands. PG: parotid gland; SLG: sublingual gland; SMG: submandibular gland. Adapted from Ogawa et al.124
Hydrogel scaffolds were used in mouse submandibular gland injury models to prove their efficiency in promoting regeneration of salivary gland injury. Laminin-fibrin hydrogel scaffolds were used in a wound-healing model of mouse submandibular glands.126 Laminin is a protein of the extracellular matrix that showed capacity to improve growth organization and differentiation of salivary cells in vitro.127 Histologic assessment showed that the laminin-fibrin hydrogels guided organized salivary regeneration as opposed to the disorganized collagen formation in the untreated model. The defect treated with fibrin hydrogel alone showed some salivary organization, but was said to be worse than that seen with the laminin-fibrin group.126 Any scaffolds to be used for salivary regeneration must allow for proper structural integrity for the heterogenic population the naturally occurring salivary glands. This was reported to be achieved by nature-inspired catechol-conjugated hyaluronic acid environment (NiCHE) formation. This was said to mimic the hyaluronic acid rich mesenchymal environment in embryonic submandibular glands. When tested on previously discussed scaffolds (PCL, hydrogels, polycarbonate membrane) led to better cellular adhesion, proliferation, angiogenesis, and structural branching in vitro.128 Surgical access to cells of the minor salivary glands of the lips and cheeks is easier and safer than those of the parotid, submandibular, and sublingual salivary glands. Recently, cultured stem cells extracted from human labial tissue discarded during dental surgery were injected into the blood stream of irradiated mice. Better salivary flow was noted in the salivary glands of the injected subjects at 9 weeks than those of the sham control group.129
The use of different stem cell reservoirs to enable salivary differentiation and the combinations with different scaffold materials allows for a great deal of flexibility in research design. With the promising results seen in large animal studies, the transition into clinical trials is in the near-future and may finally provide a satisfactory solution for patients with salivary hypofunction.
Mucosa, periodontal, and skin tissue engineering
The periodontal interface consists of bone, dentin, cementum, and the periodontal ligament. Periodontitis is a chronic disorder with progressive inflammation and damage of the tooth-supporting structures, eventually leading to tooth loss.130 Treatment of the resulting defects must include regeneration of the alveolar bone and adjacent periodontal ligament (PDL). One of the earliest forms of craniofacial tissue engineering was the guided regeneration achieved by using membranes (such as GorTEX) in periodontal defects whereby unwanted cells were excluded from the regenerative field and the healing process was “guided” as needed. Several reports claim it a possible method of PDL regeneration.131–134 A 5-year clinical trial reported the successful restoration of periodontal defects with the use of GorTEX membranes, bovine xenograft, enamel derivative, or a combination.135 Regeneration of periodontal defects is a distinct process due to the complexity of the structures of the periodontal interface. Young native PDL stem cells (PDLSCs) derived during tooth extraction were investigated for their ability to regenerate the PDL structures. These cultured cells showed the ability to differentiate into osteogenic, cementogenic, and fibrogenic lineages enabling periodontal regeneration.136 PDLSCs were also compared with dental follicle cells (DFCs) in their ability to restore periodontal defects in adult dogs. Although periodontal reattachment was seen in both groups, it was reported that the DFC group showed more organized periodontal regeneration.137
Regeneration of facial skin has a great impact on the patient’s quality of life and psychological health. Skin engineering was one of the earliest tissues investigated. As a result, skin tissue grafts are commercially available reducing the need for autologous grafts from other sites and the associated morbidity.138,139 However, limitations will always exist necessitating continual improvement. Acellular membranes, such as human acellular amniotic membranes separated from consenting mother placentas, have been used in clinical trials. When compared to Vaseline® gauze treatment, the human acellular membranes showed better hemostasis and pain scores.140 Unfortunately, due to their acellular nature, these grafts show high resorption and infection rates.141 Dermal substitutes, such as Integra®, have been used successfully in cases of traumatic defects of large areas of facial skin. Defect size shrinkage of up to 40% have been reported, but graft take, infection, time to treatment, and cost are some of the disadvantages.142 Allograft dermal tissue is another method for mucosal defect coverage in various locations, such as the tongue, vestibule, floor of the mouth, palate, and lips. Although 90% of the patient population showed complete graft take and epithelialization, contracture, scarring, pain, and infection were also reported.143 To further improve facial aesthetics and patient satisfaction, CAD/CAM applications and recent 3D bioprinting were used to produce patient-specific facial skin based on a CT images. Researchers have bioprinted “facial masks” composed of a tri-layer of polyurethane (PU), a keratinocyte-laden hydrogel, and a fibroblast-laden hydrogel and tested it within facial defects in adult mice with promising results (Figure 8).144 Recently, culturing of keratinocytes and fibroblasts was reported on de-epithelialized human amniotic membranes with favorable results. A largely keratinized layer was achieved on the epidermal surface of the membrane with a structured fibrous surface on the other side.145
Figure 8.
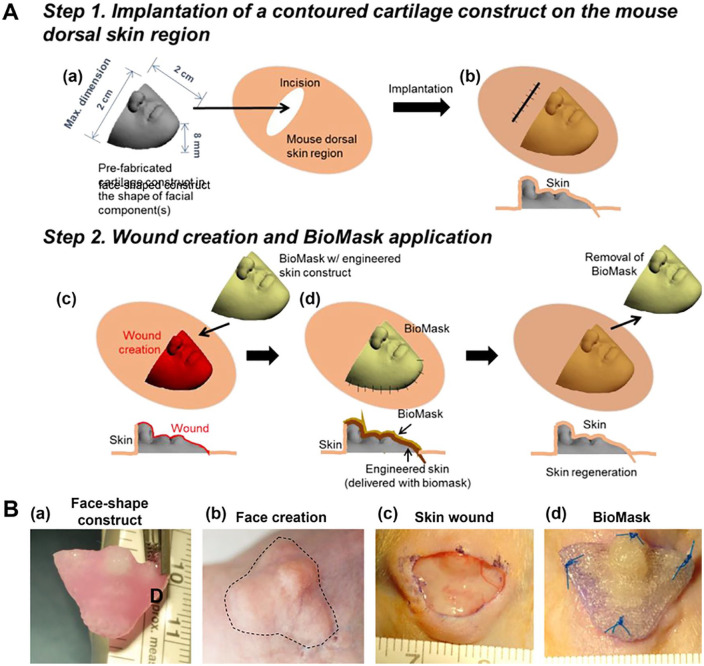
(A) Schematic illustration of facial skin wound animal model creation and implantation: (a) fabrication and (b) implantation of pre-fabricated face-shaped construct, (c) wound creation on the face-shaped construct after 4-week implantation, and (d) BioMask application. (B) Surgical procedure of BioMask application: (a) face-shape construct, (b) face creation after 4-week implantation, (c) 70% skin wound on the face-shaped construct, and (d) BioMask application. Adapted from Seol et al.144
Wound healing of the oral mucosa is generally faster and associated with less scarring than in the skin. This may be attributed to the simple epithelial differentiation and lower inflammatory cascades that occur.146 Culture of mucosal cells (whether in vitro or in vivo) are therefore a logical goal. Oral mucosa cell sheets were grown from human and rat donor keratinocytes and fibroblasts. These were isolated from oral mucosal, pharyngeal, esophageal, and neck skin biopsies. Implantation in a skin model wound of adult rats demonstrated enhanced primary wound healing with less scar tissue formation in comparison to the no intervention control group.147 Cell feeder systems were also proposed to fabricate mucosal tissue148 with long-term cryogenic storage (up to 204 days) of fabricated oral mucosa epithelial cell sheets opening up more possibilities in clinical application.149
Recently, a biomimetic engineered human mucosal equivalent was produced from fish collagen. Histologic assessment of the produced construct showed a fully differentiated and stratified epithelial layer and a dermal-epidermal junction similar to that of human oral mucosal tissue.150 The enhancement of the physical properties of this construct remains an issue that must be solved before clinical use.
Although there has been expansive research in skin/mucosal tissue engineering; the integration of these techniques clinically will only be acceptable when issues of infection, scarring, and cost are completely resolved. On the other hand, periodontal tissue regeneration is becoming more and more predictable in preventing tooth loss secondary to periodontal disease.
Engineering of multiple tissues
The craniofacial region as discussed earlier consists of several different tissue types. Regeneration procedures in many of the clinical scenarios requires regeneration of more than a single tissue type. The most common explain for this is the engineering of the periodontium. In this case a bone, periodontal ligament, dentin, and cementum should be regenerated. Research has proven that is possible especially with the advances in scaffold fabrication. Multilayering of scaffolds to fit the different necessities for each tissue type has proven to be of acceptable results. Different materials and fabrication technologies have been used to fabricate multilayered scaffolds and all showed primary promising results in vitro.151,152 An earlier attempt reported the use of a biphasic scaffold to engineer the bony and periodontal components of a periodontal defect. The scaffold consisted of an FDM fabricated part coated with Calcium phosphate, seeded with osteoblasts and cultured for 6 weeks. The resulting construct was then augmented with PDL cell sheets onto the electrospun scaffold surface and implanted into rats for a total of 8 weeks. The results showed good regeneration in the bony and periodontal compartments and high vascularization.153 Fabricating scaffolds such that each part fits the target tissue needed. This was tested by fabricating PCL-HA scaffolds in three different phases each with internal porosities differing according to the tissue type and DPSCs, PDL stem cells, ABSCs were cultured. The results of this study also supported the evidence suggesting that multi-layered/configured scaffolds can indeed be used to produce constructs of different tissue types.154
The concept of simultaneous multi-tissue engineering is of great clinical importance. Most craniofacial defect caused by tumor ablation or trauma consist of several tissue types requiring regeneration whether bone, PDL, and mucosa or muscle and skin for example. Providing clinicians with well tested choices to regenerate these tissues rather than resort to much dreaded grafting procedures is target to lots of ongoing research.
Conclusions and future perspectives
The clinical condition of patients with craniofacial defects necessitates reconstruction utilizing the most cost-efficient and cost-effective approaches. Complexity of the reconstruction is paramount due to the different tissue types usually involved in such defects. The necessity for simple effective regenerative procedures is paramount and the results discussed in this review confirm the potential of craniofacial tissue engineering strategies as an alternative to avoid the problems of currently employed reconstructive/grafting techniques (Table 1). With the variety of scaffold materials, cell origins, growth factors, drug molecules, and gene modification possibilities; a wide range of application is feasible. Research targeting specific tissue type or a multi-tissue engineering are to be proven in vivo. Furthermore, recent advances in 3D printing and scanning technologies open the opportunity for fabrication of patient-specific tissue engineered constructs. This should allow launching of in vivo and clinical trials assessing what has already been proven in vitro. The translation of the research has shown that some of the successful in vitro approaches do not really work in vivo due to the complexity of natural cellular and extracellular microenvironments. The importance of vascular ingrowth (angiogenesis) in vivo is another area that needs more work. It remains one of the main reasons scaffold-cell construct implantations fail due to the necrosis of the inner portions of the constructs not receiving sufficient blood supply from the surrounding host tissue. There are still many challenges ahead and it is clear that further research is essential in order to catch up with engineering of other parts of the human body.
Acknowledgments
This review was completed during a Fulbright Visiting Scholar fellowship sponsored by the Fulbright Commission in Egypt.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Part of Dr Emara’s Fulbright Visiting Scholar Fellowship
ORCID iD: Aala’a Emara  https://orcid.org/0000-0001-8104-1282
https://orcid.org/0000-0001-8104-1282
References
- 1. Sousa AD, Devare S, Ghanshani J. Psychological issues in cleft lip and cleft palate. J Indian Assoc Pediatr Surg 2009; 14: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrovic V, Zivkovic P, Petrovic D, et al. Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: e1–e9. [DOI] [PubMed] [Google Scholar]
- 3. Chen B, Gao Q, Song H, et al. Retrospective study of experience of craniofacial reconstruction. Int Wound J 2017; 14: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sawin PDTV, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg 1998; 88: 255–265. [DOI] [PubMed] [Google Scholar]
- 5. Warren SM, Fong KD, Chen CM, et al. Tools and techniques for craniofacial tissue engineering. Tissue Eng 2004; 9: 187– 200 [DOI] [PubMed] [Google Scholar]
- 6. Lanza R. Regenerative medicine the last 10 years. Regen Med 2016; 8: 745–746. [DOI] [PubMed] [Google Scholar]
- 7. Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release 2014; 189: 108–122. [DOI] [PubMed] [Google Scholar]
- 8. Robert Langer JV. Tissue engineering. Science 1993; 260: 920–926. [DOI] [PubMed] [Google Scholar]
- 9. Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med 1996; 2: 824–826. [DOI] [PubMed] [Google Scholar]
- 10. Mountziaris PM, Shah SR, Lam J, et al. A rapid, flexible method for incorporating controlled antibiotic release into porous polymethylmethacrylate space maintainers for craniofacial reconstruction. Biomater Sci 2016; 4: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarsitano A, Del Corso G, Ciocca L, et al. Mandibular reconstructions using computer-aided design/computer-aided manufacturing: a systematic review of a defect-based reconstructive algorithm. J Craniomaxillofac Surg 2015; 43: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 12. Morsczeck C, Reichert TE. Dental stem cells in tooth regeneration and repair in the future. Expert Opin Biol Ther 2018; 18: 187–196. [DOI] [PubMed] [Google Scholar]
- 13. Hu L, Liu Y, Wang S. Stem cell-based tooth and periodontal regeneration. Oral Dis 2018; 24: 696–705. [DOI] [PubMed] [Google Scholar]
- 14. Broyles JM, Abt NB, Shridharani SM, et al. The fusion of craniofacial reconstruction and microsurgery: a functional and aesthetic approach. Plast Reconstr Surg 2014; 134: 760–769. [DOI] [PubMed] [Google Scholar]
- 15. McGovern JA, Griffin M, Hutmacher DW. Animal models for bone tissue engineering and modelling disease. Dis Model Mech 2018; 11(4): dmm033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vieira S, Vial S, Reis RL, et al. Nanoparticles for bone tissue engineering. Biotechnol Prog 2017; 33: 590–611. [DOI] [PubMed] [Google Scholar]
- 17. Roseti L, Parisi V, Petretta M, et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl 2017; 78: 1246–1262. [DOI] [PubMed] [Google Scholar]
- 18. Maliha SG, Lopez CD, Coelho PG, et al. Bone tissue engineering in the growing calvaria using dipyridamole-coated, three-dimensionally–printed bioceramic scaffolds. Plast Reconstr Surg 2020; 145: 337e–347e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollinger J, Chaudhari A. Bone regeneration materials for the mandibular and craniofacial complex. Cells Mater 1992; 2: 143–151. [Google Scholar]
- 20. Lin Z, Fateh A, Salem DM, et al. Periosteum: biology and applications in craniofacial bone regeneration. J Dent Res 2014; 93: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leucht P, Kim JB, Amasha R, et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 2008; 135: 2845–2854. [DOI] [PubMed] [Google Scholar]
- 22. Kruijt Spanjer EC, Bittermann GKP, van Hooijdonk IEM, et al. Taking the endochondral route to craniomaxillofacial bone regeneration: a logical approach? J Craniomaxillofac Surg 2017; 45: 1099-1106. [DOI] [PubMed] [Google Scholar]
- 23. Debelmas A, Picard A, Kadlub N, et al. Contribution of the periosteum to mandibular distraction. PLoS One 2018; 13: e0199116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glowacki J, Shusterman EM, Troulis M, et al. Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone. Plast Reconstr Surg 2004; 113: 566–573. [DOI] [PubMed] [Google Scholar]
- 25. Kadlub N, Debelmas A, Dallard J, et al. Modeling of the human mandibular periosteum material properties and comparison with the calvarial periosteum. Biomech Model Mechanobiol 2019; 19(2): 461–470. [DOI] [PubMed] [Google Scholar]
- 26. Nakahara K, Haga-Tsujimura M, Iizuka T, et al. Periosteum-induced bone formation by distraction osteogenesis: histologic and microcomputed tomography analysis. Int J Oral Maxillofac Implants 2016; 31: 785–792. [DOI] [PubMed] [Google Scholar]
- 27. Paduano F, Marrelli M, Amantea M, et al. Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: a topical review on the most promising craniomaxillofacial applications. Int J Mol Sci 2017; 18: 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wubneh A, Tsekoura EK, Ayranci C, et al. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater 2018; 80: 1–30. [DOI] [PubMed] [Google Scholar]
- 29. Vacanti CA, Bonassar LJ. Tissue engineering the first decade and beyond. J Cell Biochem 30: 297–303. [DOI] [PubMed] [Google Scholar]
- 30. Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med 2001; 12: 64–75. [DOI] [PubMed] [Google Scholar]
- 31. Francis CS, Wong RK, Cohen SR. Endoscopic delivery of calcium phosphate cement for secondary craniofacial reconstruction. J Craniofac Surg 2012; 23: 2057–2060. [DOI] [PubMed] [Google Scholar]
- 32. Kirschner RE, Karmacharya J, Ong G, et al. Repair of the immature craniofacial skeleton with a calcium phosphate cement: quantitative assessment of craniofacial growth. Ann Plast Surg 2020; 49: 33–38. [DOI] [PubMed] [Google Scholar]
- 33. Mediero A, Kara FM, Wilder T, et al. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol 2012; 180: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Costa MA, Barbosa A, Neto E, et al. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J Cell Physiol 2011; 226: 1353–1366. [DOI] [PubMed] [Google Scholar]
- 35. Mediero A, Wilder T, Reddy VS, et al. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J 2016; 30: 3887–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nokhbatolfoghahaei HBM, Paknejad Z, Rad MR, et al. Bioreactor cultivation condition for engineered bone tissue: effect of various bioreactor designs on extra cellular matrix synthesis. J Biomed Mater Res A 2020; 108(8): 1662–1672. [DOI] [PubMed] [Google Scholar]
- 37. Lopez CD, Coelho PG, Witek L, et al. Regeneration of a pediatric alveolar cleft model using three-dimensionally printed bioceramic scaffolds and osteogenic agents: comparison of dipyridamole and rhBMP-2. Plast Reconstr Surg 2019; 144: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang MM, Flores RL, Witek L, et al. Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity. Sci Rep 2019; 9: 18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thrivikraman G, Athirasala A, Twohig C, et al. Biomaterials for craniofacial bone regeneration. Dent Clin North Am 2017; 61: 835–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang Y, Zhao Y, Li Y, et al. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing nanoparticles for drug delivery. Polym Bull 2009; 64: 791–804. [Google Scholar]
- 41. Zhao H, Ma L, Gao C, et al. Fabrication and properties of injectable β-tricalcium phosphate particles/fibrin gel composite scaffolds for bone tissue engineering. Mater Sci Eng C 2009; 29: 836–842. [Google Scholar]
- 42. Wang L, Zhang C, Li C, et al. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater Sci Eng C Mater Biol Appl 2016; 69: 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med 2020; 12(534): eaay6853. [DOI] [PubMed] [Google Scholar]
- 44. Govindaraj S, Costantino PD, Friedman CD. Current use of bone substitutes in maxillofacial surgery. Facial Plast Surg 1999; 15: 73–81. [DOI] [PubMed] [Google Scholar]
- 45. Sanz M, Vignoletti F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent Mater 2015; 31: 640–647. [DOI] [PubMed] [Google Scholar]
- 46. Hoornaert A, Maazouz Y, Pastorino D, et al. Vertical bone regeneration with synthetic biomimetic calcium phosphate onto the calvaria of rats. Tissue Eng Part C Methods 2019; 25: 1–11. [DOI] [PubMed] [Google Scholar]
- 47. Chen C-L, Tien H-W, Chuang C-H, et al. A comparison of the bone regeneration and soft-tissue-formation capabilities of various injectable-grafting materials in a rabbit calvarial defect model. J Biomed Mater Res B Appl Biomater 2019; 107: 529–544. [DOI] [PubMed] [Google Scholar]
- 48. Salamanca E, Tsai CY, Pan YH, et al. In vitro and in vivo study of a novel porcine collagen membrane for guided bone regeneration. Materials (Basel) 2016; 9: 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salamanca E, Hsu CC, Yao WL, et al. Porcine collagen-bone composite induced osteoblast differentiation and bone regeneration in vitro and in vivo. Polymers (Basel) 2020; 12: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cassetta M, Perrotti V, Calasso S, et al. Bone formation in sinus augmentation procedures using autologous bone, porcine bone, and a 50: 50 mixture: a human clinical and histological evaluation at 2 months. Clin Oral Implants Res 2015; 26: 1180–1184. [DOI] [PubMed] [Google Scholar]
- 51. Matsuura Y, Atsuta I, Ayukawa Y, et al. Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw. Stem Cell Res Ther 2016; 7: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ning H, Wu X, Wu Q, et al. Microfiber-reinforced composite hydrogels loaded with rat adipose-derived stem cells and BMP-2 for the treatment of medication-related osteonecrosis of the jaw in a rat model. ACS Biomater Sci Eng 2019; 5: 2430–2443. [DOI] [PubMed] [Google Scholar]
- 53. Rodriguez-Lozano FJ, Onate-Sanchez R, Gonzalvez-Garcia M, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in tooth extractions sites ameliorates the incidence of osteonecrotic jaw-like lesions in zoledronic acid-treated rats. J Clin Med 2020; 9: 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sallstrom N, Capel A, Lewis MP, et al. 3D-printable zwitterionic nano-composite hydrogel system for biomedical applications. J Tissue Eng 2020; 11: 2041731420967294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Diez-Escudero A, Harlin H, Isaksson P, et al. Porous polylactic acid scaffolds for bone regeneration A study of additively manufactured triply periodic minimal surfaces and their osteogenic potential. J Tissue Eng 2020; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hopper RA, Tse R, Smartt J, et al. Cleft palate repair and velopharyngeal dysfunction. Plast Reconstr Surg 2014; 133: 852e–864e. [DOI] [PubMed] [Google Scholar]
- 57. Arunachalam D, Pendem S, Ravi P, et al. Abnormalities of the muscles of the soft palate and their impact on auditory function in patients operated on for cleft palate: a case-control study. Br J Oral Maxillofac Surg 2019; 57: 566–571. [DOI] [PubMed] [Google Scholar]
- 58. Carvajal Monroy PL, Grefte S, Kuijpers-Jagtman AM, et al. A rat model for muscle regeneration in the soft palate. PLoS One 2013; 8: e59193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robinson DCL, Dilworth FJ. Epigenetic regulation of adult myogenesis. Curr Top Dev Biol 2018; 126: 235–284. [DOI] [PubMed] [Google Scholar]
- 60. Rosero Salazar DH, Wagener F, Von den Hoff JW. Orofacial congenital defects such as cleft lip and/or palate are associated with impaired muscle regeneration and fibrosis after surgery. Also, other orofacial reconstructions or trauma may end up in defective muscle regeneration and fibrosis. 2020; 2: 125–135. [Google Scholar]
- 61. Manchineella S, Thrivikraman G, Khanum KK, et al. Pigmented silk nanofibrous composite for skeletal muscle tissue engineering. Adv Healthc Mater 2016; 5: 1222–1232. [DOI] [PubMed] [Google Scholar]
- 62. Vandenburgh H, Shansky J, Benesch-Lee F, et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 2008; 37: 438–447. [DOI] [PubMed] [Google Scholar]
- 63. Abou Neel EA, Ahmed I, Blaker JJ, et al. Effect of iron on the surface, degradation and ion release properties of phosphate-based glass fibres. Acta Biomater 2005; 1: 553–563. [DOI] [PubMed] [Google Scholar]
- 64. Farano V, Cresswell M, Gritsch K, et al. Bioactivity evaluation of collagen-based scaffolds containing a series of Sr-doped melt-quench derived phosphate-based glasses. J Mater Sci Mater Med 2018; 29: 101. [DOI] [PubMed] [Google Scholar]
- 65. Guo B, Qu J, Zhao X, et al. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater 2019; 84: 180–193. [DOI] [PubMed] [Google Scholar]
- 66. Pantelic MN, Larkin LM. Stem cells for skeletal muscle tissue engineering. Tissue Eng Part B Rev 2018; 24: 373–391. [DOI] [PubMed] [Google Scholar]
- 67. Harris DT. Stem cell banking for regenerative and personalized medicine. Biomedicines 2014; 2: 50–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Araujo AB, Salton GD, Furlan JM, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy 2017; 19: 577–585. [DOI] [PubMed] [Google Scholar]
- 69. Schreurs M, Suttorp CM, Mutsaers HAM, et al. Tissue engineering strategies combining molecular targets against inflammation and fibrosis, and umbilical cord blood stem cells to improve hampered muscle and skin regeneration following cleft repair. Med Res Rev 2020; 40: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jung JE, Song MJ, Shin S, et al. Local myogenic pulp-derived cell injection enhances craniofacial muscle regeneration in vivo. Orthod Craniofac Res 2017; 20: 35–43. [DOI] [PubMed] [Google Scholar]
- 71. Brady MA, Lewis MP, Mudera V. Synergy between myogenic and non-myogenic cells in a 3D tissue-engineered craniofacial skeletal muscle construct. J Tissue Eng Regen Med 2008; 2: 408–417. [DOI] [PubMed] [Google Scholar]
- 72. Shah R, Sinanan AC, Knowles JC, et al. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials 2005; 26: 1497–1505. [DOI] [PubMed] [Google Scholar]
- 73. Brian BF, Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg 2011; 19: s35–s37. [DOI] [PubMed] [Google Scholar]
- 74. Zhang D, Yan K, Zhou J, et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J Tissue Eng 2019; 10: 2041731419887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lowe J, Almarza AJ. A review of in-vitro fibrocartilage tissue engineered therapies with a focus on the temporomandibular joint. Arch Oral Biol 2017; 83: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. N. Serakinci GS. Modeling mesenchymal stem cells in TMJ rheumatoid arthritis and osteoarthritis therapy. Crit Rev Eukaryot Gene Expr 2017; 27: 205–210. [DOI] [PubMed] [Google Scholar]
- 77. Vinatier C, Gauthier O, Fatimi A, et al. An injectable cellulose-based hydrogel for the transfer of autologous nasal chondrocytes in articular cartilage defects. Biotechnol Bioeng 2009; 102: 1259–1267. [DOI] [PubMed] [Google Scholar]
- 78. Ahtiainen K, Mauno J, Ella V, et al. Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. J R Soc Interface 2013; 10: 20130287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vapniarsky N, Huwe LW, Arzi B, et al. Tissue engineering toward temporomandibular joint disc regeneration. Sci Transl Med 2018; 10: eaaq1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cakmak O, Babakurban ST, Akkuzu HG, et al. Injectable tissue-engineered cartilage using commercially available fibrin glue. Laryngoscope 2013; 123: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 81. Dashnyam LJ, Mandakhbayar N, Jin GZ, et al. Intra-articular biomaterials-assisted delivery to treat temporomandibular joint disorders. J Tissue Eng 2018; 13: 2041731418776514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim H, Yang G, Park J, et al. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci Rep 2019; 9: 13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cui SJ, Zhang T, Fu Y, et al. DPSCs attenuate experimental progressive TMJ arthritis by inhibiting the STAT1 pathway. J Dent Res 2020; 99: 446–455. [DOI] [PubMed] [Google Scholar]
- 84. Ogasawara N, Kano F, Hashimoto N, et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthritis Cartilage 2020; 28: 831–841. [DOI] [PubMed] [Google Scholar]
- 85. Zhang S, Teo KYW, Chuah SJ, et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019; 200: 35–47. [DOI] [PubMed] [Google Scholar]
- 86. Kuznetsov SA, Hailu-Lazmi A, Cherman N, et al. In vivo formation of stable hyaline cartilage by naive human bone marrow stromal cells with modified fibrin microbeads. Stem Cells Transl Med 2019; 8: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. David Chen JYW, Kennedy KM, Yeager K, et al. Tissue engineered autologous cartilage-bone grafts for temporomandibular joint regeneration. Sci Transl Med 2020; 12: eabb6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu CS-W, Hsiao Y-C, Huang J-J, et al. Secondary unilateral cleft rhinoplasty using natural curvature of rib cartilage as alar rim graft. Plast Reconstr Surg 2020; 145: 775–779. [DOI] [PubMed] [Google Scholar]
- 89. Park SH, Yun BG, Won JY, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope 2017; 127: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 90. Reuther MS, Briggs KK, Neuman MK, et al. Volume expansion of tissue engineered human nasal septal cartilage. J Otol Rhinol 2014; 3: 1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mendelson A, Ahn JM, Paluch K, et al. Engineered nasal cartilage by cell homing: a model for augmentative and reconstructive rhinoplasty. Plast Reconstr Surg 2014; 133: 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yi HG, Choi YJ, Jung JW, et al. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J Tissue Eng 2019; 10: 2041731418824797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Berens AM, Newman S, Bhrany AD, et al. Computer-aided design and 3d printing to produce a costal cartilage model for simulation of auricular reconstruction. Otolaryngol Head Neck Surg 2016; 155: 356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cao JPV, Paige KT, Upton J, et al. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg 1997; 100: 297–304. [DOI] [PubMed] [Google Scholar]
- 95. Morrison KAC, Benjamin P, Dong X, et al. Utilizing a novel cell sourcing strategy to fabricate the first full-scale tissue engineered human ear scaffold. Plast Reconstr Surg 2016; 4: 1051. [Google Scholar]
- 96. Kagimoto S, Takebe T, Kobayashi S, et al. Autotrans-plantation of monkey ear perichondrium-derived progenitor cells for cartilage reconstruction. Cell Transplant 2016; 25: 951–962. [DOI] [PubMed] [Google Scholar]
- 97. Liao HT, Zheng R, Liu W, et al. Prefabricated, ear-shaped cartilage tissue engineering by scaffold-free porcine chondrocyte membrane. Plast Reconstr Surg 2015; 135: 313e–321e. [DOI] [PubMed] [Google Scholar]
- 98. Matuska AM, Dolwick MF, McFetridge PS. Approaches to improve integration and regeneration of an ex vivo derived temporomandibular joint disc scaffold with variable matrix composition. J Mater Sci Mater Med 2018; 29: 152. [DOI] [PubMed] [Google Scholar]
- 99. Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol 2008; 119: 1951–1965. [DOI] [PubMed] [Google Scholar]
- 100. Sullivan R, Dailey T, Duncan K, et al. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci 2016; 17: 2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moskow J, Ferrigno B, Mistry N, et al. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact Mater 2019; 4: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang H, Liu H, Zhang S, et al. Traumatic fractures resulting from collisions in children and adolescents: A retrospective observational study. Medicine (Baltimore) 2018; 97: e10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ali YH, Al Sheikh AE. Nonmicrosurgical grafting for facial nerve branches with permanent sensational functional outcome. Plast Reconstr Surg Glob Open 2019; 7: e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sanchez-Ocando M, Gavilan J, Penarrocha J, et al. Facial nerve repair: the impact of technical variations on the final outcome. Eur Arch Otorhinolaryngol 2019; 276: 3301–3308. [DOI] [PubMed] [Google Scholar]
- 105. Johnson EO, Zoubos AB, Soucacos PN. Regeneration and repair of peripheral nerves. Injury 2005; 36(Suppl 4): S24–S29. [DOI] [PubMed] [Google Scholar]
- 106. Dellon AL. Wound healing in nerve. Clin Plast Surg 1990; 17: 545–570. [PubMed] [Google Scholar]
- 107. Binnetoglu A, Demir B, Akakin D, et al. Bacterial cellulose tubes as a nerve conduit for repairing complete facial nerve transection in a rat model. Eur Arch Otorhinolaryngol 2020; 277: 277–283. [DOI] [PubMed] [Google Scholar]
- 108. Piao Wang HZ, Yao Y, Lu C, et al. Repair of facial nerve crush injury in rabbits using collagen plus basic fibroblast growth factor. J Biomed Mater Res A 2020; 108: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 109. Onger ME, Delibas B, Turkmen AP, et al. The role of growth factors in nerve regeneration. Drug Discov Ther 2017; 10: 285–291. [DOI] [PubMed] [Google Scholar]
- 110. Watanabe Y, Sasaki R, Matsumine H, et al. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med 2017; 11: 362–374. [DOI] [PubMed] [Google Scholar]
- 111. Sasaki R, Aoki S, Yamato M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 2011; 5: 823–830. [DOI] [PubMed] [Google Scholar]
- 112. Costa HJ, Bento RF, Salomone R, et al. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res 2013; 1510: 10–21. [DOI] [PubMed] [Google Scholar]
- 113. Xiao L, Ide R, Saiki C, et al. Human dental pulp cells differentiate toward neuronal cells and promote neuroregeneration in adult organotypic hippocampal slices in vitro. Int J Mol Sci 2017; 18: 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Marinkovic M, Dybdal-Hargreaves NF, Block TJ, et al. Oral and craniofacial stem cells: an untapped source for neural tissue regeneration. Tissue Eng Part A 2020; 26: 935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Castiglioni A, Corna G, Rigamonti E, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One 2015; 10: e0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013; 155: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Krieger JR, Tellier LE, Ollukaren MT, et al. Quantitative analysis of immune cell subset infiltration of supraspinatus muscle after severe rotator cuff injury. Regen Eng Transl Med 2017; 3: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wong A, Pomerantz JH. The role of muscle stem cells in regeneration and recovery after denervation: a review. Plast Reconstr Surg 2019; 143: 779–788. [DOI] [PubMed] [Google Scholar]
- 119. Sharma AD, McCoy L, Jacobs E, et al. Engineering a 3D functional human peripheral nerve in vitro using the Nerve-on-a-Chip platform. Sci Rep 2019; 9: 8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract 2010; 64: 404–407. [DOI] [PubMed] [Google Scholar]
- 121. Joraku A, Sullivan CA, Yoo JJ, et al. Tissue engineering of functional salivary gland tissue. Laryngoscope 2005; 115: 244–248. [DOI] [PubMed] [Google Scholar]
- 122. Joraku A, Sullivan CA, Yoo J, et al. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation 2007; 75: 318–324. [DOI] [PubMed] [Google Scholar]
- 123. Ogawa M, Tsuji T. Functional salivary gland regeneration as the next generation of organ replacement regenerative therapy. Odontology 2015; 103: 248–257. [DOI] [PubMed] [Google Scholar]
- 124. Ogawa M, Oshima M, Imamura A, et al. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 2013; 4: 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nam K, Kim K, Dean SM, et al. Using cell sheets to regenerate mouse submandibular glands. NPJ Regen Med 2019; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nam K, Wang CS, Maruyama CLM, et al. L1 peptide-conjugated fibrin hydrogels promote salivary gland regeneration. J Dent Res 2017; 96: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Maruyama CL, Leigh NJ, Nelson JW, et al. Stem cell-soluble signals enhance multilumen formation in SMG cell clusters. J Dent Res 2015; 94: 1610–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lee SW, Ryu JH, Do MJ, et al. NiCHE platform: nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl Mater Interfaces 2020; 12: 4285–4294. [DOI] [PubMed] [Google Scholar]
- 129. Su X, Liu Y, Bakkar M, et al. Labial stem cell extract mitigates injury to irradiated salivary glands. J Dent Res 2020; 99(3): 293–301. [DOI] [PubMed] [Google Scholar]
- 130. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. The Lancet 2005; 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 131. Menicanin D, Hynes K, Han J, et al. Cementum and periodontal ligament regeneration. In: Bertassoni LE, Coelho PG. (eds.) Engineering mineralized and load bearing tissues. Cham, Switzerland: Springer International Publishing, 2015, pp.207–236. [Google Scholar]
- 132. Gielkens PF, Schortinghuis J, de Jong JR, et al. Vivosorb, Bio-Gide, and Gore-Tex as barrier membranes in rat mandibular defects: an evaluation by microradiography and micro-CT. Clin Oral Implants Res 2008; 19: 516–521. [DOI] [PubMed] [Google Scholar]
- 133. Bunyaratavej P, Wang HL. Collagen membranes a review. J Periodontol 2001; 72: 215–229. [DOI] [PubMed] [Google Scholar]
- 134. Duskova M, Leamerov E, Sosna B, et al. Guided tissue regeneration, barrier membranes and reconstruction of the cleft maxillary alveolus. J Craniofac Surg 2006; 17: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 135. Cortellini P, Stalpers G, Mollo A, et al. Periodontal regeneration versus extraction and prosthetic replacement of teeth severely compromised by attachment loss to the apex: 5-year results of an ongoing randomized clinical trial. J Clin Periodontol 2011; 38: 915–924. [DOI] [PubMed] [Google Scholar]
- 136. Liu J, Zhao Z, Ruan J, et al. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J Dent 2020; 92: 103259. [DOI] [PubMed] [Google Scholar]
- 137. Guo S, Kang J, Ji B, et al. Periodontal-derived mesenchymal cell sheets promote periodontal regeneration in inflammatory microenvironment. Tissue Eng Part A 2017; 23: 585–596. [DOI] [PubMed] [Google Scholar]
- 138. Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci 2016; 17: 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Suhail S, Sardashti N, Jaiswal D, et al. Engineered skin tissue equivalents for product evaluation and therapeutic applications. Biotechnol J 2019; 14: e1900022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xue SL, Liu K, Parolini O, et al. Human acellular amniotic membrane implantation for lower third nasal reconstruction: a promising therapy to promote wound healing. Burns Trauma 2018; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bassuner JK, Rice DC, Antonoff MB, et al. Polytetrafluoroethylene or acellular dermal matrix for diaphragmatic reconstruction? Ann Thorac Surg 2017; 103: 1710–1714. [DOI] [PubMed] [Google Scholar]
- 142. Chen TA, Ayala-Haedo JA, Blessing NW, et al. Bioengineered dermal substitutes for the management of traumatic periocular tissue loss. Orbit 2018; 37: 115–120. [DOI] [PubMed] [Google Scholar]
- 143. Rhee CDF, Ridge JA, Kusiak J. The use of processed allograft dermal matrix for intraoral resurfacing an alternative to split-thickness skin grafts. Arch Otolaryngol Head Neck Surg 1998; 124: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 144. Seol YJ, Lee H, Copus JS, et al. 3D bioprinted biomask for facial skin reconstruction. Bioprinting 2018; 10: e00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. John S, Kesting MR, Paulitschke P, et al. Development of a tissue-engineered skin substitute on a base of human amniotic membrane. J Tissue Eng 2019; 10: 2041731418825378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Iglesias-Bartolome R, Uchiyama A, Molinolo AA, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 2018; 10: 8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Roh JL, Lee J, Kim EH, et al. Plasticity of oral mucosal cell sheets for accelerated and scarless skin wound healing. Oral Oncol 2017; 75: 81–88. [DOI] [PubMed] [Google Scholar]
- 148. Oliva J, Ochiai K, Florentino A, et al. Feeder cells free rabbit oral mucosa epithelial cell sheet engineering. Tissue Eng Regen Med 2018; 15: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Oliva J, Florentino A, Bardag-Gorce F, et al. Vitrification and storage of oral mucosa epithelial cell sheets. J Tissue Eng Regen Med 2019; 13: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Suzuki A, Kato H, Kawakami T, et al. Development of microstructured fish scale collagen scaffolds to manufacture a tissue-engineered oral mucosa equivalent. J Biomater Sci Polym Ed 2020; 31: 578–600. [DOI] [PubMed] [Google Scholar]
- 151. Bittner SM, Guo JL, Melchiorri A, et al. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater Today (Kidlington) 2018; 21: 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ivanovski S, Vaquette C, Gronthos S, et al. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 2014; 93: 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Costa PF, Vaquette C, Zhang Q, et al. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 2014; 41: 283–294. [DOI] [PubMed] [Google Scholar]
- 154. Lee CH, Hajibandeh J, Suzuki T, et al. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A 2014; 20: 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]