Abstract
Eltrombopag is an orally administered, non-peptide, thrombopoietin receptor agonist which initiates thrombopoietin signaling and stimulates the production of normally functioning platelet. We aimed to do a systematic review and meta-analysis of currently available published data to verify whether eltrombopag treatment in patients with chronic immune-mediated thrombocytopenia can prolong survival. We searched for published, randomized, controlled trials in PubMed, Cochrane and Scopus databases using the following search strategy (“Eltrombopag” OR “Benzoates” OR “Hydrazines”) AND (“Idiopathic Thrombocytopenic Purpura” OR “immune thrombocytopenia” OR “Idiopathic Thrombocytopenic Purpuras” OR “Immune Thrombocytopenia” OR “Autoimmune Thrombocytopenia” OR “Werlhof”). The pooled relative risk (RR) showed that eltrombopag group has significantly higher overall platelet response than placebo group (MD = 3.42, 95% CI [2.51, 4.65], P > .0001); pooled results were homogenous (P = .27, I2 = 22%). The pooled relative risk showed that eltrombopag group has lower incidence of any bleeding than placebo group (MD = 0.65, 95% CI [0.48, 0.87], P = .003); pooled results were heterogenous (P = .001, I2 = 75%) and the detected heterogeneity was best resolved after excluding Bussel et al (P = .10). Homogeneous results were still favored eltrombopag group (MD = 0.75, 95% CI [0.60, 0.93], P = .008).
Keywords: chronic immune thrombocytopenia, eltrombopag, thrombocytopenia, immune thrombocytopenic purpura, thrombopoietin
Introduction
Chronic Immune thrombocytopenia (ITP), is an immune-mediated disease of adults and children it is characterized by thrombocytopenia (platelet count less than 100,000/lL), and increased risk of bleeding.1 In chronic Immune thrombocytopenia antibody- and/or T cell-mediated immune responses participate in the development of thrombocytopenia which leads to platelet destruction and abnormal platelet production and increases the risk of severe bleeding such as intracranial hemorrhage and may lead to death. The diagnosis of ITP is based on the exclusion of any other possible causes of secondary thrombocytopenia.2 According to new guidelines, newly diagnosed ITP patients with a platelet count of less than 30 × 109/L is indicated for the treatment.3
There are various treatment modalities that have been involved in the management for ITP including corticosteroids and intravenous immunoglobulins which destroy the antibody-coated platelets and augment platelet production. The primary goal of treatment of this disease is to prevent the bleeding.4 However, approximately 30% of patients with chronic ITP patients remain refractory to these therapies, including splenectomy.5 Recent studies suggest that Thrombopoietin receptor (TPO-R) agonists which activate the receptor for thrombopoietin a platelet growth factor, that regulates platelet production are an effective treatment for patients with refractory chronic ITP.6
Eltrombopag is an orally administered, non-peptide, thrombopoietin receptor agonist which initiates thrombopoietin signaling and stimulates the production of normally functioning platelet and it has shown efficacy in the treatment of chronic immune thrombocytopenia patients.4 Some studies suggest that eltrombopag increased the production of platelets by interacting with the transmembrane domain of the TPO-R inducing the process of megakaryopoiesis and helps in reduce bleeding events.7–9
Because of the importance of eltrombopag therapy as an efficacious treatment for ITP, we aimed to do a systematic review and meta-analysis of currently available published data to verify whether eltrombopag treatment in patients with chronic immune-mediated thrombocytopenia can prolong survival.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines during the preparation of this systematic review and meta-analysis. Moreover, all steps of this study are conducted according to the Cochrane Handbook for Systematic Reviews of Interventions.10,11
Literature Search
We searched for published, randomized, controlled trials in PubMed, Cochrane and Scopus databases using the following search strategy (“Eltrombopag” OR “Benzoates” OR “Hydrazines” OR “Pyrazoles” OR “Revolade” OR “SB-497” OR “Promacta”) AND (“Idiopathic Thrombocytopenic Purpura” OR “immune thrombocytopenia” OR “Idiopathic Thrombocytopenic Purpuras” OR “Immune Thrombocytopenia” OR “Autoimmune Thrombocytopenia” OR “Werlhof”) from November 2019 to May 2020. We also checked the clinical trial registry (Clinicaltrials.gov) for any ongoing and unpublished studies.
Eligibility Criteria
We include the studies that met the following criteria:
Study design: clinical trials comparing Eltrombopag with placebo and observational studies.
-
Intervention:
Drug: Eltrombopag.
Dose: 30, 50 and 70 mg. Other doses were not adequately reported in the included studies.
Route of administration: oral route.
Comparator: any control group if present.
Population: patients with immune thrombocytopenia.
-
Outcomes:
The primary outcome: efficacy endpoints including Overall platelet response, Incidence of significant bleeding, Incidence of any bleeding, and Number of cases needed rescue treatment. In addition to safety endpoints including the incidence of any adverse events and the incidence of serious adverse events.
The secondary outcomes: detailed reported adverse events.
We excluded animal studies, secondary works, and conference abstracts.
Data Extraction
Two independent authors extracted the data using a formatted data extraction sheet. A consensus between the authors was obtained and any conflicts were resolved upon the opinion of a third reviewer. We extracted 3 categories of data: 1) Baseline and demographic data about the included participants including male ratio, age, Weight, Prior Therapy, Splenectomy, and baseline Platelet Count, 2) Criteria of study design, and data required for risk of bias assessment to ensure high-quality appraisal of studies, and 3) Data concerned with safety including the reported adverse events such as Headache, Aspartate aminotransferase elevation, Constipation, Fatigue, Rash, Diarrhea, Peripheral edema, Taste disturbance, Abdominal distention, Arthralgia, Epistaxis, Hemorrhoids, Pain, Nausea, Nasopharyngitis, Upper respiratory tract infection, Vomiting, Urinary tract infection, Myalgia, Pharyngitis, Influenza, Cough, Dizziness, Cataract, Anxiety, Increased ALT concentration, Patients with any adverse event, Serious adverse events, Transient ischemic attack, Pyrexia. Other data about the efficacy including Overall platelet response, Incidence of significant bleeding, and Incidence of any bleeding.
Quality Assessment
We performed this meta-analysis according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines. Two independent authors assessed the risk of bias in included studies, in accordance with the Cochrane handbook of systematic reviews of interventions.12 The tool depends on the following domains for assessment of the risk of bias: 1) proper randomization of patients, 2) the blinding of allocation of patients into the intended treatment arms (Allocation concealment), 3) Blinding of patients only (termed single blinding), or blinding of both personnel and participants (double-blinding), 4) Attrition bias, 5) whether the outcomes mentioned in the protocol are all reported or not (Selection bias), 6) blinding of outcome assessors to prevent over- and/or under-estimation of outcome values, and 7) other bias. We assessed the quality of the included single arm and observational studies using the quality assessment tools of the National Heart, Lung, and Blood Institute (NHLBI).1 We used a tool for observational cohort and cross-sectional studies and another tool for single-arm studies. Each tool composed of some questions to assess the possible risks of bias and confounders. Each question was answered by “yes,” “no,” “not applicable,” “cannot determine,” or “not reported” then each study was given a score to guide the overall rating of the quality as “good,” “fair,” or “poor” quality.
Data Synthesis
All outcomes were dichotomous and they were expressed as event and total. Efficacy endpoints were pooled as a relative risk using Review Manager Software (version 5.3) for windows under the Mantel-Hanszel method. For safety outcomes analysis, we used OpenMeta [Analyst] software for windows to pool the data as a single-arm analysis in order to increase the power and the number of included studies in each outcome. The data were pooled under the binary random-effects method.
The heterogeneity was assessed by the Chi-square test and its extent was determined by I-square. In Chi-square test, P < .1 or I2 > 50% were significant indicators of heterogeneity. Whenever the heterogeneity was detected, we used the random-effects model and performed a sensitivity and subgroup analysis to solve it.
Results
Literature Search
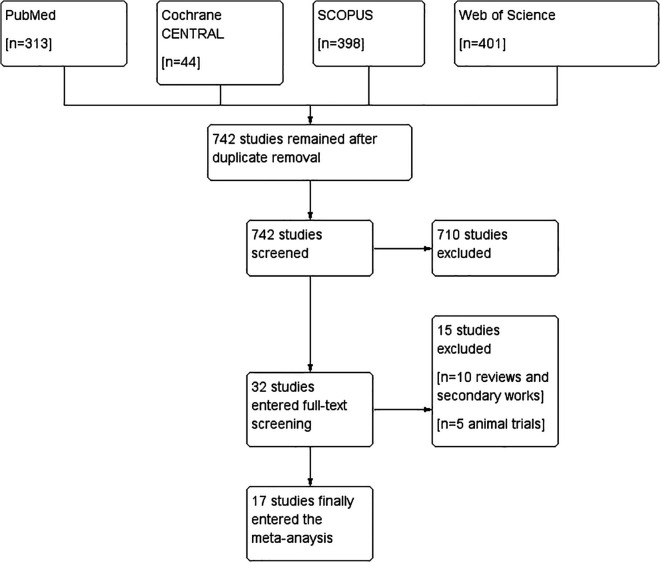
After searching PubMed, Web of Science, Scopus, and Cochrane Central Register of Controlled Trials (CENTRAL), we identified 1156 records. We removed 414 duplicates and the remaining 742 studies were screened for eligibility. We excluded 710 studies and only 32 studies were further included for full-text screening. We didn’t find any missing paper after the screening of the references of the included studies. We finally included 17 studies,2,4,6,13-26 all of them were included in the meta-analysis. The literature search process is described in a PRISMA flow diagram in (Figure 1).
Figure 1.
PRISMA.
Characteristics of the Included Studies
We included 8 RCTs17,18-22 and 3 single-arm trials,16,24,25 and 6 observational studies.17-22 Summary of the included studies and their study design and results in addition to baseline characteristics of their patients are shown in Supplementary Sheet 1.
Results of Risk of Bias Assessment
According to the Cochrane tool for assessment of the risk of bias, the quality of the included RCTs was ranged from moderate to high quality. A summary of the risk of bias domains is shown in (Figure 2). All single-arm trials were fair in quality according to the NIH quality assessment tool for before-after (pre-post) studies with no control group.
Figure 2.
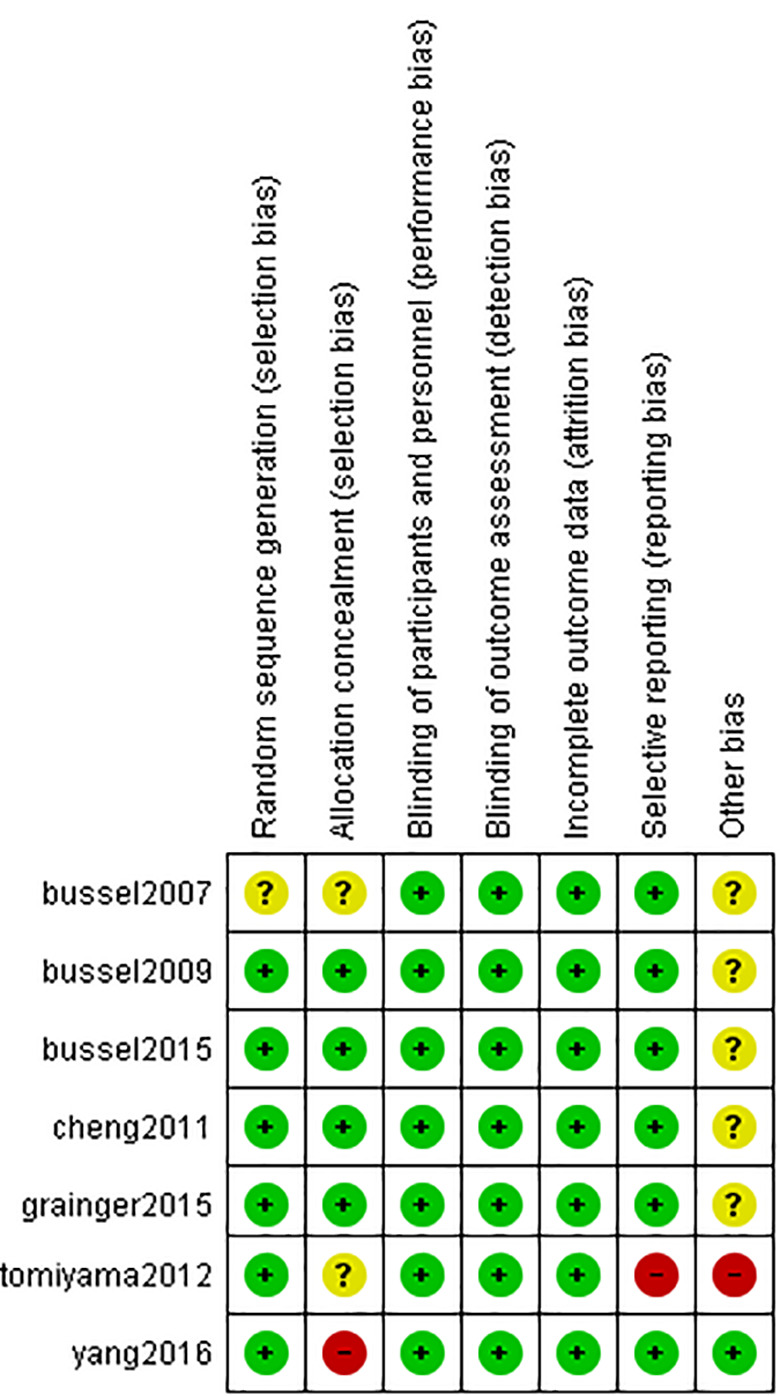
ROB. Risk of bias domain.
According to the NIH quality assessment tool for Observational Cohort and Cross-Sectional Studies, the rest 6 observational studies were poor in quality. A summary of the risk of bias assessment domains and authors’ judgments with justifications are shown in Supplementary Tables 1, 2, 3, respectively.
Analysis of Efficacy Outcomes
1. Overall platelet response
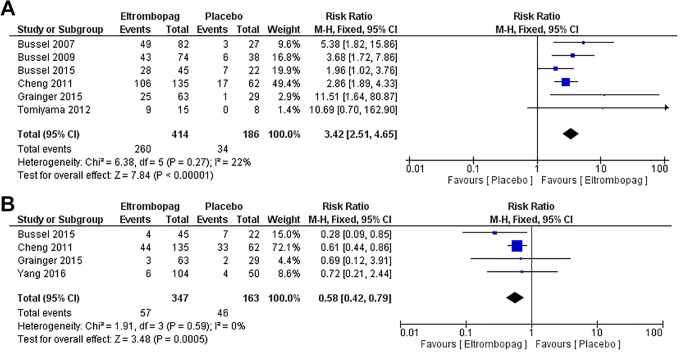
Six studies2,4,6,14,15,23 with 600 patients reported the overall platelet response. The pooled relative risk (RR) showed that eltrombopag group has significantly higher overall platelet response than placebo group (MD = 3.42, 95% CI [2.51, 4.65], P > .0001); (Figure 3A). Pooled results were homogenous (P = .27, I2 = 22%).
Figure 3.
Platelet response and significant bleeding. (A) Overall platelet response and (B) incidence of significant bleeding.
2. Incidence of significant bleeding
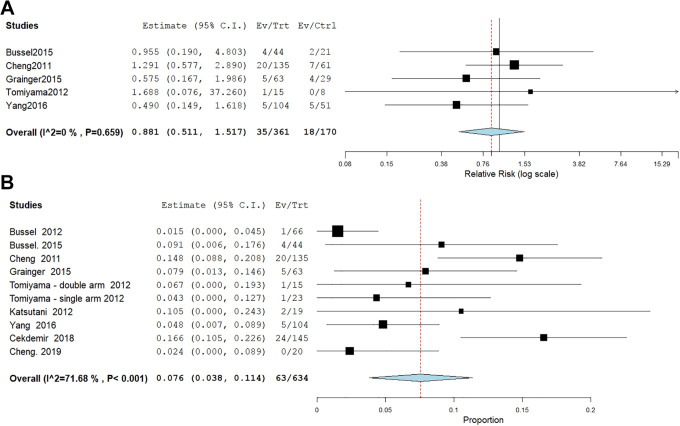
Four studies4,14,23,26 with 510 patients reported the incidence of significant bleeding. The pooled effect estimate revealed that eltrombopag group has lower incidence of significant bleeding than placebo group (MD = 0.58, 95% CI [0.42, 0.79], P = .0005); (Figure 3B). Pooled results were homogenous (P = .59, I2 = 0%).
3. Incidence of any bleeding
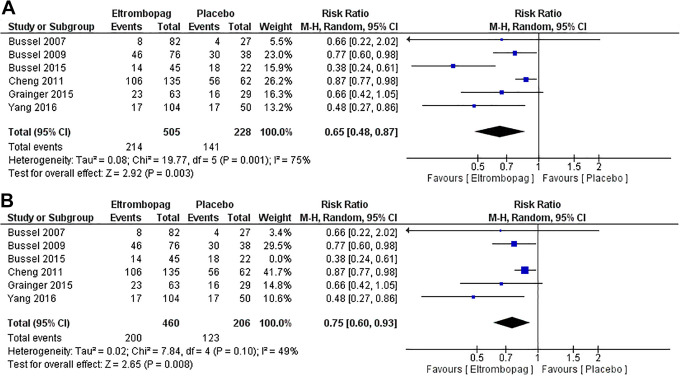
Five studies2,4,14,15,23,26 with 666 patients reported the incidence of any bleeding. The pooled relative risk showed that eltrombopag group has lower incidence of any bleeding than placebo group (MD = 0.65, 95% CI [0.48, 0.87], P = .003); (Figure 4A). Pooled results were heterogenous (P = 0.001, I2 = 75%) and the detected heterogeneity was best resolved after excluding Bussel et al 2015 (P = .10). Homogeneous results were still favored eltrombopag group (MD = 0.75, 95% CI [0.60, 0.93], P = .008) (Figure 4B).
Figure 4.
A and B, Incidence of any bleeding.
4. Number of cases needed rescue treatment
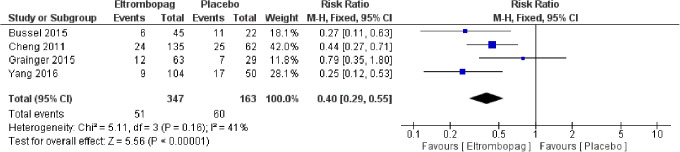
Four studies4,14,23,26 with 510 patients reported the number of cases needed rescue treatment. The pooled relative risk (RR) showed that eltrombopag was associated with less cases needed rescue treatment than placebo (MD = 0.40, 95% CI [0.29, 0.55], P < .0001); (Figure 5). Pooled results were homogenous (P = .16, I2 = 41%).
Figure 5.
Rescue treatment. Number of cases needed rescue treatment.
Analysis of Safety Outcomes
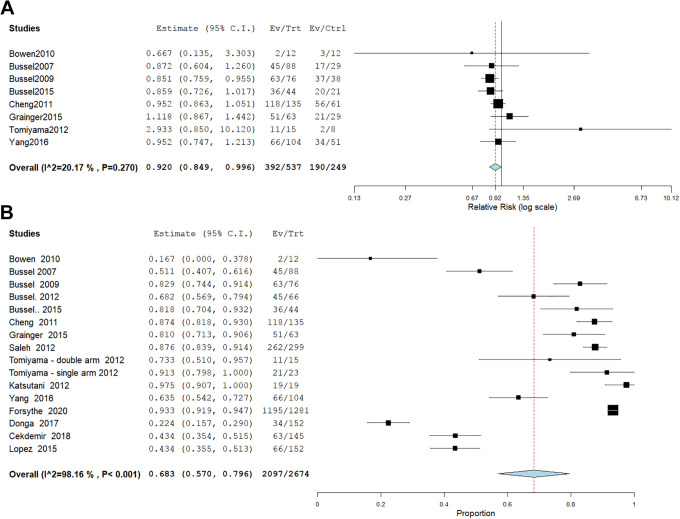
1. Any adverse event
The overall risk ratio of any adverse side effect revealed a significant difference between Eltrombopag and placebo (RR = 0.9, 95% CI [0.849, 0.996], P = .04). Pooled results were homogeneous (I2 = 20%, P = .2). (Figure 6A). Fifteen studies2,4,6,13-17,19,20,22-26 with 2674 patients receiving eltrombopag were eligible for single arm analysis. The mean incidence of any adverse event was 68.3%, 95% CI [57.0%, 79.6%], P < .001 with significant heterogeneity (P < .001, I2 = 98.16%,) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure 6B).
Figure 6.
A and B, Any adverse event.
2. Serious adverse events
The combined effect estimate of severe adverse events revealed no significant difference between Eltrombopag and placebo (RR = 0.9, 95% CI [0.511, 1.517], P = .6). Pooled results were homogeneous (I2 = 0%, P = .7). (Figure 7A). Nine studies4,6,14,16-18,23,24,26 with 634 patients receiving eltrombopag were eligible for single arm analysis. The mean incidence of serious adverse events was 7.6%, 95% CI [3.8%, 11.4%], P < .001 with significant heterogeneity (P < .001, I2 = 71.68%,) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure 7B).
Figure 7.
A and B, Serious adverse events.
Incidence of Each Side Effect
1. Headache
Seventeen studies2,4,6,13-26 with 2765 patients received eltrombopag reported headache incidence which was 11.7%, 95% CI [8.0%, 15.5%], P < .001 with significant heterogeneity (P < .001, I2 = 89.12%,) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S1).
2. Aspartate aminotransferase elevation
Nine studies2,4,6,15,18,23-26 with 842 patients received eltrombopag reported aspartate aminotransferase elevation incidence which was 3.6%, 95% CI [1.9%, 5.2%], P < .001. Pooled results were homogenous (P = .17, I2 = 29.96%) (Figure S2).
3. Fatigue
Ten studies2,4,6,13-16,20,24,25 with 2058 patients received eltrombopag reported fatigue incidence which was 8.4, 95% CI [5.6%, 11.2%], P < .001 with significant heterogeneity (P = .001, I2 = 65.18%). which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S3).
4. Anemia
Four studies2,15,24,25 with 482 patients received eltrombopag reported anemia incidence which was 2.2%, 95% CI [0.3%, 4.1%], P = .024. Pooled results were homogenous (P = .244, I2 = 28.01%) (Figure S4).
5. Diarrhea
Twelve studies2,4,6,14-17,19,20,22,24,25 with 2495 patients received eltrombopag reported diarrhea incidence which was 9.2%, 95% CI [4.3%, 14.1%], P < .001 with significant heterogeneity (P < .001, I2 = 96.15%) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S5).
6. Arthralgia
Nine studies2,6,15-17,19,20,22,25 with 2297 patients received eltrombopag reported arthralgia incidence which was 5.6, 95% CI [2.5%, 8.8%], P < .001 with significant heterogeneity (P < .001, I2 = 88.48%) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S6).
7. Epistaxis
Four studies4,14,20,23 with 1523 patients received eltrombopag reported epistaxis incidence which was 5.8%, 95% CI [1.9%, 9.7%], P = .003 with significant heterogeneity (P < .001, I2 = 81.96%) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S1).
8. Abdominal pain
Nine studies2,4,13-15,17,23-25 with 881 patients received eltrombopag reported abdominal pain incidence which was 2.0%, 95% CI [0.7%, 3.2%], P = .002. Pooled results were homogenous (P = .145, I2 = 34.09%) (Figure S8).
9. Nausea
Seven studies4,6,13-16,25 with 670 patients received eltrombopag reported nausea incidence which was 9.6%, 95% CI [7.4%, 11.8%], P < .001. Pooled results were homogenous (P = .655, I2 = 0%) (Figure S9).
10. Nasopharyngitis
Eight studies4,6,13,15,16,23-25 with 708 patients received eltrombopag reported nasopharyngitis incidence which was 19.3%, 95% CI [11.7%, 26.8%], P < .001 with significant heterogeneity (P < .001, I2 = 85.01%,) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S10).
11. Upper respiratory tract infection
Nine studies4,14-16,20,22-25 with 2135 patients received eltrombopag reported upper respiratory tract infection incidence which was 11.0%, 95% CI [5.8%, 16.2%], P < .001 with significant heterogeneity (P < .001, I2 = 92.31%,) which couldn’t be solved by leave-one-out or subgroup analysis according to study design, population, dose, or follow-up duration (Figure S11).
12. Vomiting
Five studies4,14-16,23 with 384 patients received eltrombopag reported vomiting incidence which was 5.7%, 95% CI [3.4, 8.0%], P < .001. Pooled results were homogenous (P = .638, I2 = 0%) (Figure S12).
13. Myalgia
Five studies4,6,15,22,24 with 420 patients received eltrombopag reported nausea incidence which was 4.4%, 95% CI [2.4%, 6.3%], P < .001. Pooled results were homogenous (P = .598, I2 = 0%) (Figure S13).
14. Cough
Four studies4,14,23,24 with 261 patients received eltrombopag reported nausea incidence which was 6.0%, 95% CI [3.1%, 8.8%], P < .001. Pooled results were homogenous (P = .408, I2 = 0%) (Figure S14).
15. Increased alanine aminotransferase (ALT) concentration
Three studies6,15,26 with 218 patients received eltrombopag reported increased ALT concentration incidence which was 6.9%, 95% CI [1.3%, 12.5%], P = .015 with significant heterogeneity (P = .082, I2 = 55.33%,); Figure S15, which could be resolved after excluding Bussel et al 2009 (P = .22) and the incidence was still significant (10.9%, 95% CI [2.5%, 19.3%], P = .011) (Figure S16).
Discussion
Although this meta-analysis shows that Eltrombopag has a great efficacy as reported by the higher overall platelet response, lower incidence of significant bleeding, lower incidence of any bleeding, and decreased number of patients needed rescue treatment compared to placebo. The study raises suspicions about the long-term incidence of adverse events of the drug. The analysis revealed a significant difference regarding the incidence of any adverse events between Eltrombopag and placebo. We also reported a detailed analysis of side effects from the most prevalent to the least; the drug is associated with a 68.3% risk of incidence of any adverse event; 7.6% for serious adverse events; 19.3% for nasopharyngitis; 11.7% for headache; 11.0% for upper respiratory tract infection; 9.6% for nausea; 9.2% for diarrhea; 8.4% for fatigue; 6.9% for increased ALT concentration; 6% for cough; 5.8% for epistaxis; 5.7% for vomiting; 5.6 for arthralgia; 4.4% for myalgia; 3.6% for aspartate aminotransferase elevation; 2.0% for abdominal pain; and 2.2% for anemia.
ITP is associated with antiplatelet antibodies that increase the rate of destruction of platelets which increase the incidence of bleeding.27 Eltrombopag initiates signaling of thrombopoietin and accelerates the proliferation and differentiation of cells in megakaryocytic lineage, which leads to increased production of platelets.28 The target of the treatment of ITP is to provide enough platelet levels to avoid serious bleeding and to diminish treatment-related toxicity. Patients with platelet counts of ≥30,000/µL are expected to have suitable hemostasis and generally do not require treatment in the absence of a history of bleeding.29 ITP patients who have platelet counts above or below the normal range may have a risk of thrombotic or thromboembolic complications.30 Our meta-analysis exhibits that Eltrombopag has a significant effect on the treatment of ITP patients as a result of decreasing the risk of significant bleeding or any bleeding.
Our results are consistent with other studies in the literature. Elgebaly et al9 conducted a meta-analysis that showed Eltrombopag has a significant result in terms of the overall platelet response, incidence of significant bleeding, number of cases needed to rescue treatment, and incidence of any bleeding, and found no significant difference in total adverse events compared with placebo. A recent meta-analysis31 of 2 trials using Eltrombopag reported that Eltrombopag significantly reduces the incidence of bleeding and decreases the rescue medications. However, results revealed no significant favoring between the 2 arms regarding platelet response and the incidence of any side effects. Yasuyuki Arai et al32 conducted a network meta-analysis and demonstrated that both TPO-RAs can be the first selection for treating patients with persistent ITP, rather than RTX, as substitutes to splenectomy. Furthermore, no significant side effects were noticed among the 2 groups. In another network meta-analysis, Ran Yang et al33 reported that romiplostim looks to be the best selection for cases who fail to respond to first-line ITP medication or relapse subsequently, avatrombopag and Eltrombopag are reasonable alternatives, while RTX monotherapy is not recommended because it yields the lowest OR and ER rates. Our results agreed with the previous studies which were conducted in ITP patients.
Our work has some strengths, all steps were performed in strict accordance with the Cochrane Handbook of Systematic Reviews of Interventions. Another point is the large included sample size from 17 unique studies. This strength was most evident in our results where our analysis of a larger sample size has revealed a significant incidence of any adverse events compared with placebo. The main limitation of our study is the severe inconsistency found in the data. We tried to solve this heterogeneity and although managed to solve some outcomes, other outcomes could not be solved by either subgroup analysis or leave-one-out meta-analysis. Additionally, 6 studies of included papers were observational and 3 were single-arm, which are not of the highest evidence according to GRADE.
Conclusion
Eltrombopag is an effective drug for the management of patients with immune thrombocytopenia and is associated with some side effects as nasopharyngitis, headache, upper respiratory tract infection, nausea, and diarrhea. The drug is associated with an overall significant incidence of any adverse events compared with placebo. Larger trials with larger sample sizes are required to provide a clearer safety profile for the drug.
Supplemental Material
Supplemental Material, sj-pdf-1-cat-10.1177_10760296211005555 for Eltrombopag Effectiveness and Tolerability in Chronic Immune Thrombocytopenia: A Meta-Analysis by Hafiz Abdul Waqas Ahmed, Ahmed Taher Masoud, Jia Han, Ahmed Adel Sofy, Ahmed Saeed Ahmed, Ahmed Taha Abdesattart, Emmanuel Kwateng Drokow and Kai Sun in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, sj-xlsx-1-cat-10.1177_10760296211005555 for Eltrombopag Effectiveness and Tolerability in Chronic Immune Thrombocytopenia: A Meta-Analysis by Hafiz Abdul Waqas Ahmed, Ahmed Taher Masoud, Jia Han, Ahmed Adel Sofy, Ahmed Saeed Ahmed, Ahmed Taha Abdesattart, Emmanuel Kwateng Drokow and Kai Sun in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was partially supported by the National Natural Science Foundation of China (No. 81971502, No. 81471589 and No. 81273259).
ORCID iD: Hafiz Abdul Waqas Ahmed  https://orcid.org/0000-0002-5071-8251
https://orcid.org/0000-0002-5071-8251
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an International Working Group. Blood. 2009;113(11):2386–2393. [DOI] [PubMed] [Google Scholar]
- 2. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New Engl J Med. 2007;357(22):2237–2247. [DOI] [PubMed] [Google Scholar]
- 3. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. [DOI] [PubMed] [Google Scholar]
- 5. McMillan R, Durette C. Long-term outcomes in adults with chronic ITP after splenectomy failure. Blood. 2004;104(4):956–960. [DOI] [PubMed] [Google Scholar]
- 6. Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799–806. [DOI] [PubMed] [Google Scholar]
- 7. Shi M, Xu F, Yang X, et al. The synergistic antileukemic effects of eltrombopag and decitabine in myeloid leukemia cells. Cancer Manag Res. 2019;11:8229–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. New Engl J Med. 2012;367(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elgebaly AS, Ashal GE, Elfil M, Menshawy A. Tolerability and efficacy of eltrombopag in chronic immune thrombocytopenia: meta-analysis of randomized controlled trials. Clin Appl Thromb Hemost. 2017;23(8):928–937. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thomas J, Chandler J, et al. , editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 12. The Cochrane Collaboration RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. Published October 2020. Accessed June 15, 2020. bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 13. Bowen CJ, Lobb KM, Park JW, Sanderson B, Ferguson J. Eltrombopag (75 mg) does not induce photosensitivity: results of a clinical pharmacology trial. Photodermatol Photoimmunol Photomed. 2010;26(5):243–249. [DOI] [PubMed] [Google Scholar]
- 14. Bussel JB, De Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2(8):e315–325. [DOI] [PubMed] [Google Scholar]
- 15. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. The Lancet. 2009;373(9664):641–648. [DOI] [PubMed] [Google Scholar]
- 16. Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Brit J Haematol. 2013;160(4):538–546. [DOI] [PubMed] [Google Scholar]
- 17. Çekdemir D, Güvenç S, Özdemirkıran F, et al. A multi-center study on the efficacy of eltrombopag in management of refractory chronic immune thrombocytopenia: a real-life experience. Turk J Hematol. 2019;36(4):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng X, Yan K, Ma J, et al. Efficacy and safety of eltrombopag in the treatment of severe chronic immune thrombocytopenia in children of China: a single-center observational study. Int J Immunopathol Pharmacol. 2019;33:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donga PZ, Bilir SP, Little G, Babinchak T, Munakata J. Comparative treatment-related adverse event cost burden in immune thrombocytopenic purpura. J Med Econ. 2017;20(11):1200–1206. [DOI] [PubMed] [Google Scholar]
- 20. Forsythe A, Schneider J, Pham T, et al. Real-world evidence on clinical outcomes in immune thrombocytopenia treated with thrombopoietin receptor agonists. J Comp Eff Res. 2020;9(7):447–457. [DOI] [PubMed] [Google Scholar]
- 21. Giordano P, Lassandro G, Barone A, et al. Use of eltrombopag in children with chronic immune thrombocytopenia (ITP): a real life retrospective multicenter experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Front Med. 2020;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. González-López TJ, Alvarez-Román MT, Pascual C, et al. Eltrombopag safety and efficacy for primary chronic immune thrombocytopenia in clinical practice. Eur J Haematol. 2016;97(3):297–302. [DOI] [PubMed] [Google Scholar]
- 23. Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. The Lancet. 2015;386(10004):1649–1658. [DOI] [PubMed] [Google Scholar]
- 24. Katsutani S, Tomiyama Y, Kimura A, et al. Oral eltrombopag for up to three years is safe and well-tolerated in Japanese patients with previously treated chronic immune thrombocytopenia: an open-label, extension study. Int J Hematol. 2013;98(3):323–330. [DOI] [PubMed] [Google Scholar]
- 25. Saleh MN, Bussel JB, Cheng G. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Transfuze a Hematologie Dnes. 2013;19(2):191. [DOI] [PubMed] [Google Scholar]
- 26. Yang R, Li J, Jin J, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Brit J Haematol. 2017;176(1):101–110. [DOI] [PubMed] [Google Scholar]
- 27. Nakhoul IN, Kozuch P, Varma M. Management of adult idiopathic thrombocytopenic purpura. Clin Adv Hematol Oncol. 2006;4(2):136–144. [PubMed] [Google Scholar]
- 28. Erickson-Miller CL, Delorme E, Tian S-S, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cell. 2009;27(2):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chouhan JD, Herrington JD. Treatment options for chronic refractory idiopathic thrombocytopenic purpura in adults: focus on romiplostim and eltrombopag. Pharmacotherapy. 2010;30(7):666–683. [DOI] [PubMed] [Google Scholar]
- 30. Garnock-Jones KP. Eltrombopag: a review of its use in treatment-refractory chronic primary immune thrombocytopenia. Drugs. 2011;71(10):1333–1353. [DOI] [PubMed] [Google Scholar]
- 31. Tumaini Massaro J, Chen Y, Ke Z. Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenic purpura: meta-analysis. Platelets. 2019;30(7):828–835. [DOI] [PubMed] [Google Scholar]
- 32. Arai Y, Matsui H, Jo T, Kondo T, Takaori-Kondo A. Comparison of treatments for persistent/chronic immune thrombocytopenia: a systematic review and network meta-analysis. Platelets. 2019;30(8):946–956. [DOI] [PubMed] [Google Scholar]
- 33. Yang R, Lin L, Yao H, Ji O, Shen Q. Therapeutic options for adult patients with previously treated immune thrombocytopenia—a systematic review and network meta-analysis. Hematology (United Kingdom). 2019;24(1):290–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cat-10.1177_10760296211005555 for Eltrombopag Effectiveness and Tolerability in Chronic Immune Thrombocytopenia: A Meta-Analysis by Hafiz Abdul Waqas Ahmed, Ahmed Taher Masoud, Jia Han, Ahmed Adel Sofy, Ahmed Saeed Ahmed, Ahmed Taha Abdesattart, Emmanuel Kwateng Drokow and Kai Sun in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, sj-xlsx-1-cat-10.1177_10760296211005555 for Eltrombopag Effectiveness and Tolerability in Chronic Immune Thrombocytopenia: A Meta-Analysis by Hafiz Abdul Waqas Ahmed, Ahmed Taher Masoud, Jia Han, Ahmed Adel Sofy, Ahmed Saeed Ahmed, Ahmed Taha Abdesattart, Emmanuel Kwateng Drokow and Kai Sun in Clinical and Applied Thrombosis/Hemostasis